Numerical Modeling of Combustion and Detonation in Aqueous Foams
Abstract
:1. Introduction
2. Mathematical Model and Problem Setup
2.1. Basic Two-Phase Model
2.2. Dynamic Processes
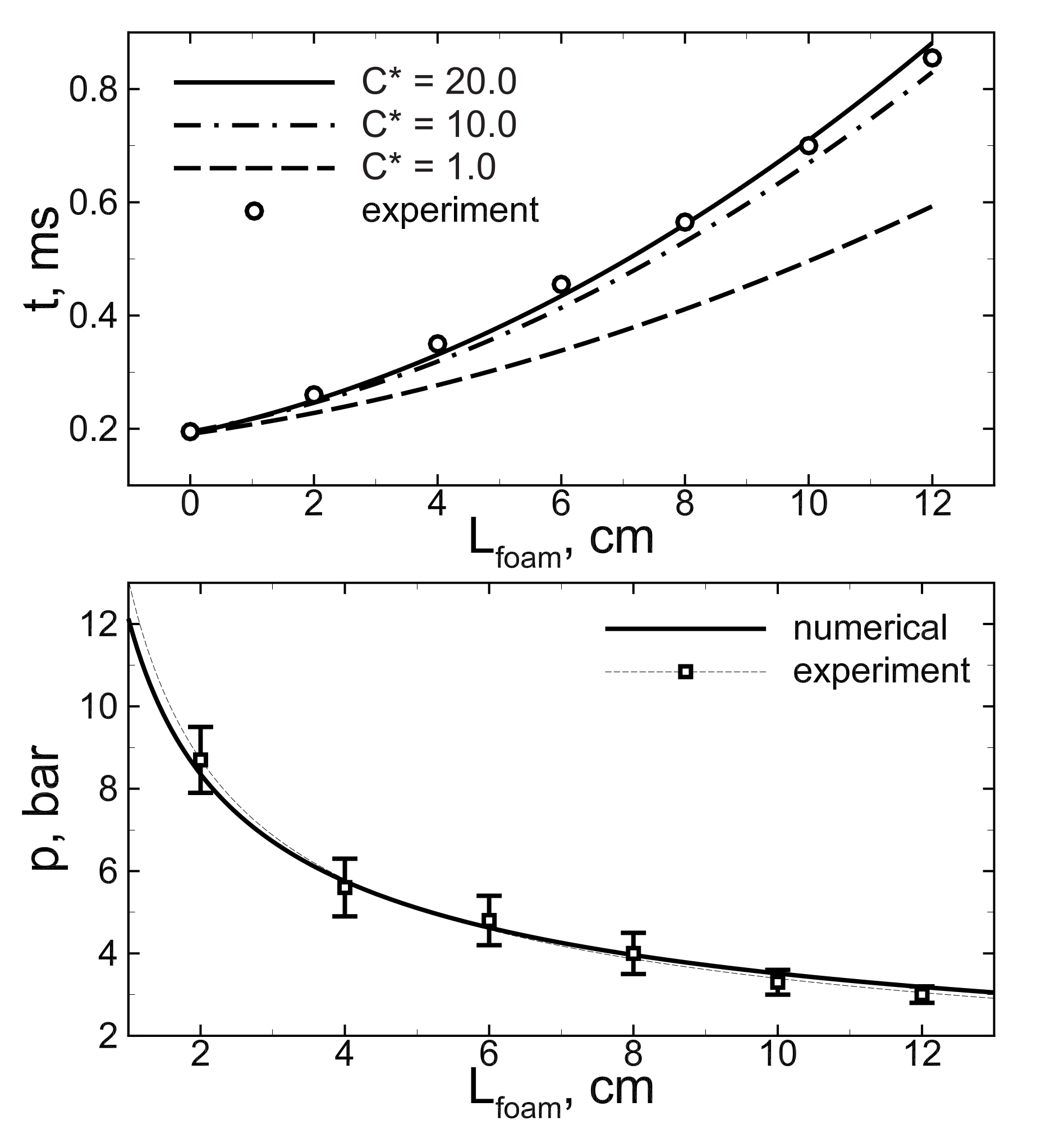
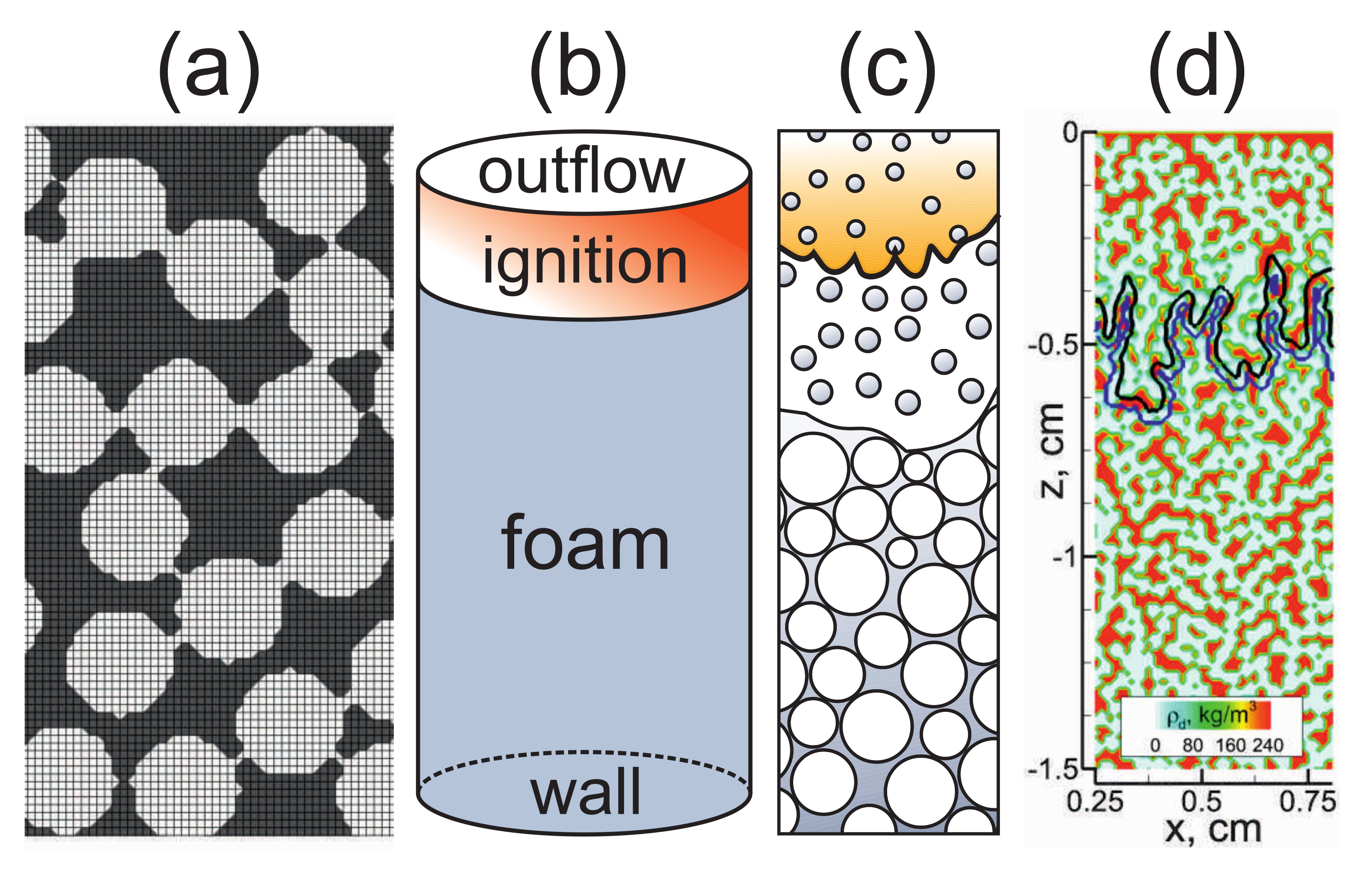
2.3. Peculiarities of the Problem Setup Related to the Foam Structure
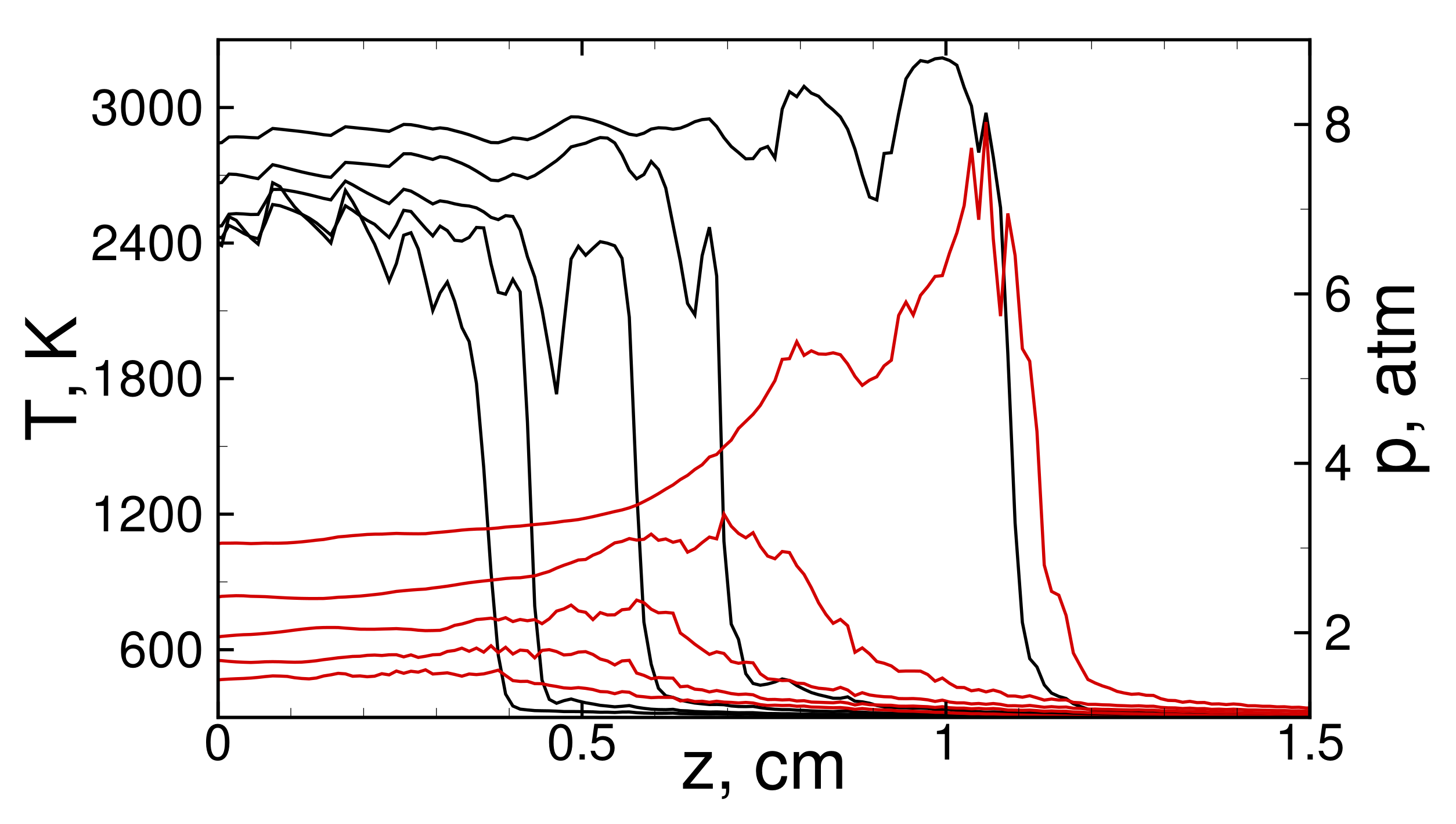
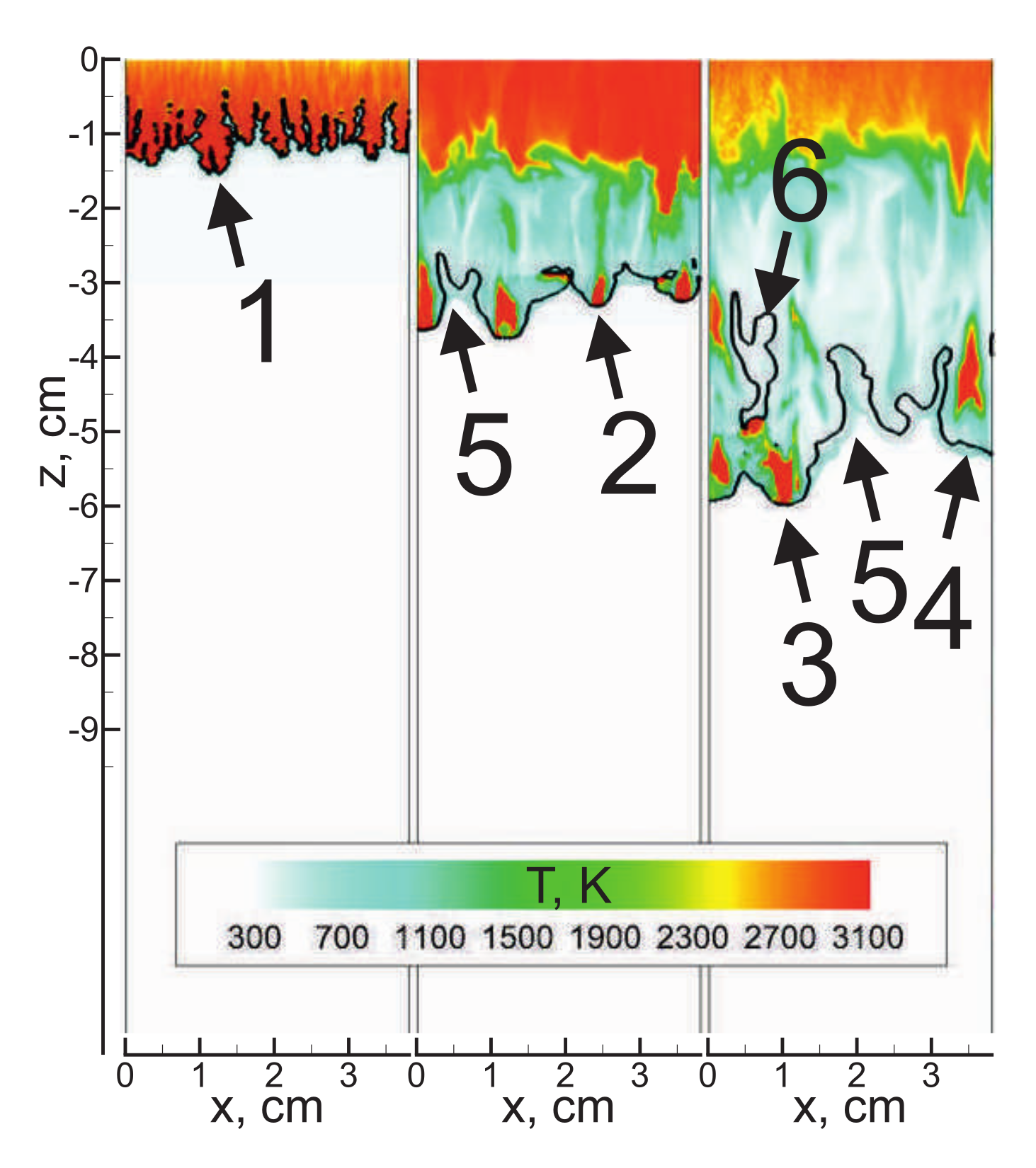
3. Analysis of Flame Dynamics
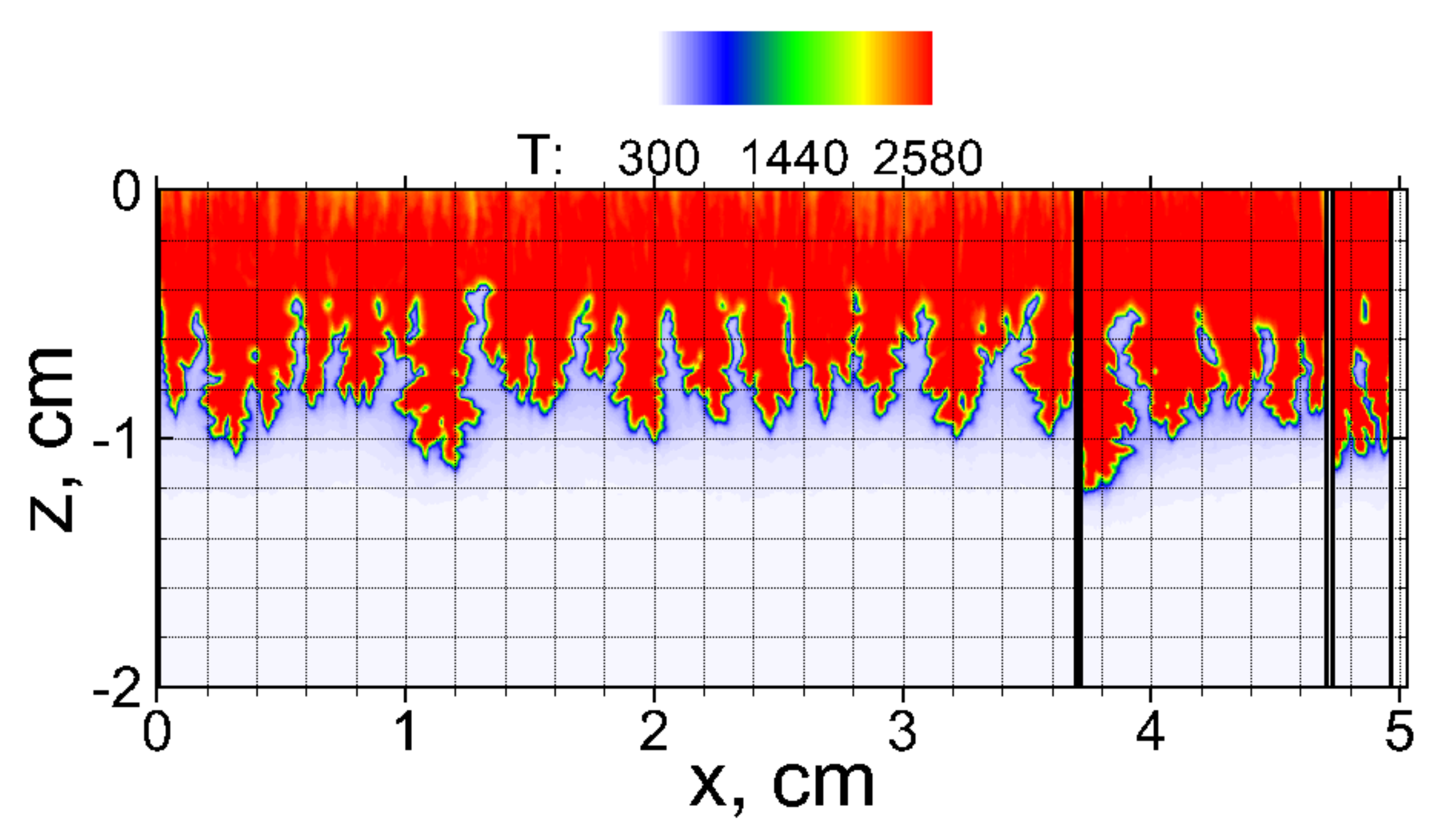
3.1. Flame Acceleration
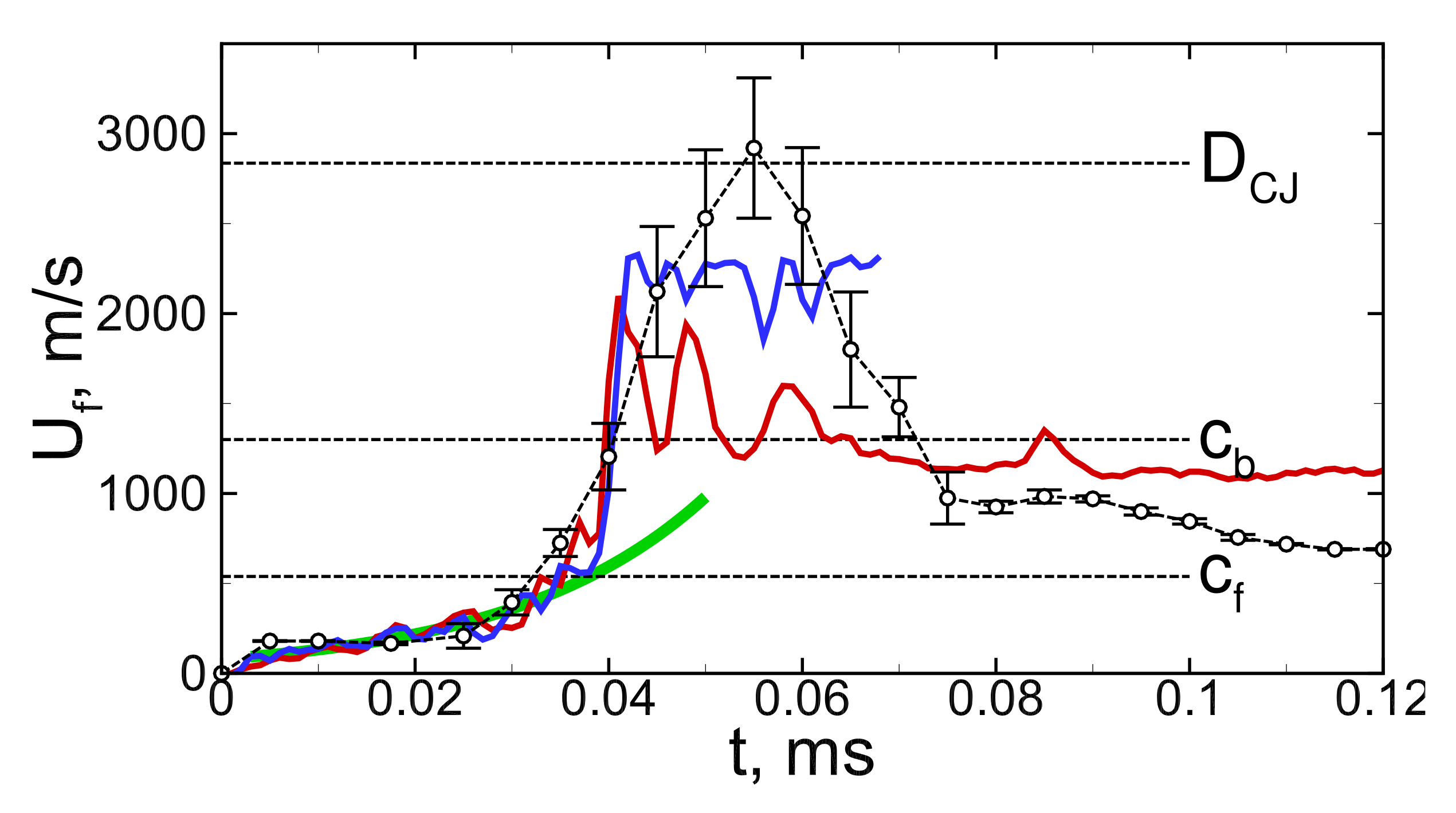
3.2. Detonation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abe, J.; Popoola, A.; Ajenifuja, E.; Popoola, O. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Verhelst, S. Recent progress in the use of hydrogen as a fuel for internal combustion engines. Int. J. Hydrog. Energy 2014, 39, 1071–1085. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Bi, M.; Li, B.; Zhou, Y.; Gao, W. Effects of hydrogen and initial pressure on flame characteristics and explosion pressure of methane/hydrogen fuels. Fuel 2018, 233, 269–282. [Google Scholar] [CrossRef]
- Taghavifar, H.; Anvari, S.; Parvishi, A. Benchmarking of water injection in a hydrogen-fueled diesel engine to reduce emissions. Int. J. Hydrog. Energy 2017, 42, 11962–11975. [Google Scholar] [CrossRef]
- Anufriev, I.S.; Kopyev, E.P. Diesel fuel combustion by spraying in a superheated steam jet. Fuel Process. Technol. 2019, 192, 154–169. [Google Scholar] [CrossRef]
- Huo, M.; Lin, S.; Liu, H.; Lee, C.f.F. Study on the spray and combustion characteristics of water–emulsified diesel. Fuel 2014, 123, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Kichatov, B.; Korshunov, A.; Kiverin, A.; Son, E. Foamed emulsion—Fuel on the base of water-saturated oils. Fuel 2017, 203, 261–268. [Google Scholar] [CrossRef]
- Kichatov, B.; Korshunov, A.; Kiverin, A.; Yakovenko, I.; Gubernov, V.; Khomik, S.V.; Medvedev, S.P. Detonation in the hydrogen-oxygen microfoam on the aqueous base. Int. J. Hydrog. Energy 2019, 44, 31567–31578. [Google Scholar] [CrossRef]
- Kichatov, B.; Korshunov, A.; Gubernov, V.; Kiverin, A.; Yakovenko, I. Combustion of heptane-in-water emulsion foamed with hydrogen-oxygen mixture. Fuel Process. Technol. 2020, 198, 106230. [Google Scholar] [CrossRef]
- Gostintsev, Y.A.; Istratov, A.G.; Shulenin, Y.V. Self-similar propagation of a free turbulent flame in mixed gas mixtures. Combust. Explos. Shock Waves 1988, 24, 563–569. [Google Scholar] [CrossRef]
- Yakovenko, I.; Kiverin, A. Intensification mechanisms of the lean hydrogen-air combustion via addition of suspended micro-droplets of water. Int. J. Hydrog. Energy 2021, 46, 1259–1272. [Google Scholar] [CrossRef]
- Sychev, A.; Pinaev, A. Self-sustaining detonation in liquids with bubbles of explosive gas. J. Appl. Mech. Tech. Phys. 1986, 27, 119–123. [Google Scholar] [CrossRef]
- Saint-Cloud, J.; Guerraud, C.; Moreau, M.; Manson, M. Experiences sur la propagation des detonations dans un milieu biphasique. Acta Astronaut. 1976, 3, 781–794. [Google Scholar] [CrossRef]
- Segev, G.; Hasson, A.; Siman, M.; Burcat, A. Detonation waves through foam. In Proceedings of the 22nd Symposium (International) on Combustion, Seattle, WA, USA, 14–19 August 1989; pp. 1751–1756. [Google Scholar]
- Subbotin, V.; Usol’tsev, S. Study of the mechanism of the transfer of gaseous detonation through films of liquid. Combust. Explos. Shock Waves 1984, 20, 224–230. [Google Scholar] [CrossRef]
- Faure, S.; Ghidaglia, J.M. Violent flows in aqueous foams I: Physical and numerical models. Eur. J. Mech. B/Fluids 2011, 30, 341–359. [Google Scholar] [CrossRef]
- Kuo, K.K.; Acharya, R. Fundamentals of Turbulent and Multiphase Combustion, 1st ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Shinjo, J.; Xia, J.; Ganippa, L.C.; Megaritis, A. Physics of puffing and microexplosion of emulsion fuel droplets. Phys. Fluids 2014, 26. [Google Scholar] [CrossRef]
- Tonini, S.; Gavaises, M.; Theodorakakos, A. The role of droplet fragmentation in high-pressure evaporating diesel sprays. Int. J. Therm. Sci. 2009, 48, 554–572. [Google Scholar] [CrossRef]
- Boiko, V.M.; Poplavski, S.V. Experimental study of two types of stripping breakup of the drop in the flow behind the shock wave. Combust. Explos. Shock Waves 2012, 48, 440–445. [Google Scholar] [CrossRef]
- Spalding, D.B. Combustion and Mass Transfer, 1st ed.; Pergamon Press Inc.: Elmsford, NY, USA, 1979. [Google Scholar]
- Kéromnès, A.; Metcalfe, W.K.; Heufer, K.A.; Donohoe, N.; Das, A.K.; Sung, C.J.; Herzler, J.; Naumann, C.; Griebel, P.; Mathieu, O.; et al. An experimental and detailed chemical kinetic modeling study of hydrogen and syngas mixture oxidation at elevated pressures. Combust. Flame 2013, 160, 995–1011. [Google Scholar] [CrossRef] [Green Version]
- Olm, C.; Zsély, I.G.; Pálvölgyi, R.; Varga, T.; Nagy, T.; Curran, H.J.; Turányi, T. Comparison of the performance of several recent hydrogen combustion mechanisms. Combust. Flame 2014, 161, 2219–2234. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.F.; Santner, J.; Dryer, F.L.; Padak, B.; Farouk, T.I. Computational Study of NOx Formation at Conditions Relevant to Gas Turbine Operation, Part 2: NOx in High Hydrogen Content Fuel Combustion at Elevated Pressure. Energy Fuels 2016, 30, 7691–7703. [Google Scholar] [CrossRef]
- Burke, M.P.; Chaos, M.; Ju, Y.; Dryer, F.L.; Klippenstein, S.J. Comprehensive H2/O2 kinetic model for high-pressure combustion. Int. J. Chem. Kinet. 2012, 44, 444–474. [Google Scholar] [CrossRef]
- Ivanov, M.F.; Kiverin, A.D.; Liberman, M.A. Ignition of deflagration and detonation ahead of the flame due to radiative preheating of suspended micro particles. Combust. Flame 2015, 162, 3612–3621. [Google Scholar] [CrossRef] [Green Version]
- Baer, M.R. A numerical study of shock wave reflections on low density foam. Shock Waves 1992, 2, 121–124. [Google Scholar] [CrossRef]
- Baer, M.R.; Nunziato, J.W. A two-phase mixture theory for the deflagration-to-detonation transition (ddt) in reactive granular materials. Int. J. Multiph. Flow 1986, 12, 861–889. [Google Scholar] [CrossRef]
- Sembian, S.; Liverts, M.; Apazidis, N. Attenuation of strong external blast by foam barriers. Phys. Fluids 2016, 28. [Google Scholar] [CrossRef]
- Kiverin, A.D.; Yakovenko, I.S.; Kichatov, B.V.; Korshunov, A.M. Cumulative effect in foams and mechanism of detonation development. J. Phys. Conf. Ser. 2020, 1686, 012079. [Google Scholar] [CrossRef]
- Roache, P.J. Perspective: A Method for Uniform Reporting of Grid Refinement Studies. J. Fluids Eng. 1994, 116, 405. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M. Fluid Mechanics, 2nd ed.; Butterworth-Heinemann: Burlington, MA, USA, 1987; Volume 6. [Google Scholar]
- Ogawa, T.; Gamezo, V.N.; Oran, E.S. Flame acceleration and transition to detonation in an array of square obstacles. J. Loss Prev. Process Ind. 2013, 26, 355–362. [Google Scholar] [CrossRef]
- Babkin, V.S. Filtrational combustion of gases. Present state of affairs and prospects. Pure Appl. Chem. 1993, 65, 335–344. [Google Scholar] [CrossRef]
- Kiverin, A.; Yakovenko, I.; Ivanov, M. On the structure and stability of supersonic hydrogen flames in channels. Int. J. Hydrog. Energy 2016, 41, 22465–22478. [Google Scholar] [CrossRef]
- Ivanov, M.F.; Kiverin, A.D.; Liberman, M.A.; Fortov, V.E. The flame-acceleration mechanism and transition to detonation of a hydrogen-oxygen mixture in a channel. Dokl. Phys. 2010, 55, 480–484. [Google Scholar] [CrossRef]
- Ivanov, M.; Kiverin, A.; Liberman, M.A. Flame acceleration and DDT of hydrogen–oxygen gaseous mixtures in channels with no-slip walls. Int. J. Hydrog. Energy 2011, 36, 7714–7727. [Google Scholar] [CrossRef]
- Saif, M.; Wang, W.; Pekalski, A.; Levin, M.; Radulescu, M.I. Chapman–Jouguet deflagrations and their transition to detonation. Proc. Combust. Inst. 2016, S1540748916303807. [Google Scholar] [CrossRef] [Green Version]
- Britan, A.; Shapiro, H.; Liverts, M.; Ben-Dor, G. Macro-mechanical modeling of blast-wave mitigation in foams. Part III: Verification of the models. Shock Waves 2014, 24, 241–256. [Google Scholar] [CrossRef]
| , m | , cm | Error, % |
|---|---|---|
| 100 | 1.13 | 24 |
| 50 | 1.36 | 8 |
| 25 | 1.45 | 2 |
| Exact value estimate: cm | ||
| Convergence rate: 1.87 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiverin, A.; Yakovenko, I. Numerical Modeling of Combustion and Detonation in Aqueous Foams. Energies 2021, 14, 6233. https://doi.org/10.3390/en14196233
Kiverin A, Yakovenko I. Numerical Modeling of Combustion and Detonation in Aqueous Foams. Energies. 2021; 14(19):6233. https://doi.org/10.3390/en14196233
Chicago/Turabian StyleKiverin, Alexey, and Ivan Yakovenko. 2021. "Numerical Modeling of Combustion and Detonation in Aqueous Foams" Energies 14, no. 19: 6233. https://doi.org/10.3390/en14196233
APA StyleKiverin, A., & Yakovenko, I. (2021). Numerical Modeling of Combustion and Detonation in Aqueous Foams. Energies, 14(19), 6233. https://doi.org/10.3390/en14196233






