Exergetic Performance of a PEM Fuel Cell with Laser-Induced Graphene as the Microporous Layer
Abstract
:1. Introduction
- -
- The exergy analysis is employed to gain insight on the thermodynamical factors that drive the notable contrast in performance between the two models. Exergy analysis has been introduced as a remedy to limitations inherent in the classical energy efficiency analysis, which does not identify the generating factors of thermodynamical losses within systems nor quantify deviations from ideality. Within the analytical framework of exergy, thermodynamical imperfections are quantified in the concept of exergy destructions, representing losses in energy quality or usability. Shifting the focal point from the 1st to the 2nd Law of Thermodynamics, exergetic analysis monitors the thermodynamic irreversibility in FC energy conversion processes (i.e., from chemical to electrical) and tracks all associated losses, which are proportional to the production of enthalpy.
- -
- The exergetic analytical tools are reinforced by voltage efficiency monitoring as a direct metric of FC efficiency, which registers the correspondence between measured voltage and theoretical OCV and provides a strong indicator of the degree of reversibility in FC energy conversion processes by comparing the actual work against the maximum work potential [40,41,42,43,44].
- -
- The flow rate of water used to humidify the inlet gas streams was considered here for the general exergy balance of the fuel cell system. The effect of relative humidity on the standard chemical exergy values of the gas mixture species during an exergy study of a PEMFC was reported here for the first time.
2. Materials and Methods
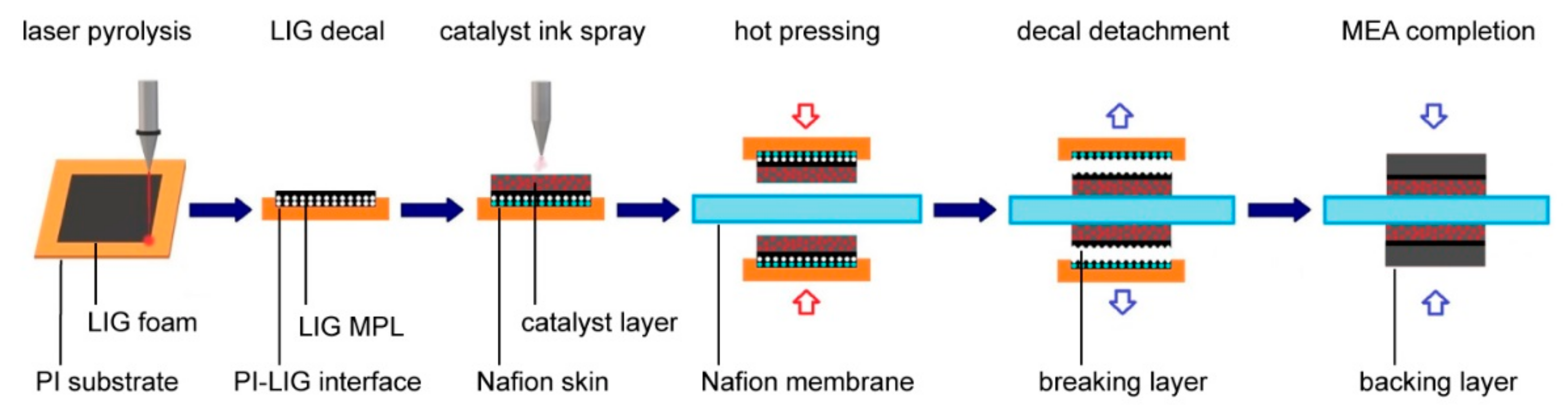
2.1. Overview of Laser Pyrolysis and Fuel Cell Assembly Procedures
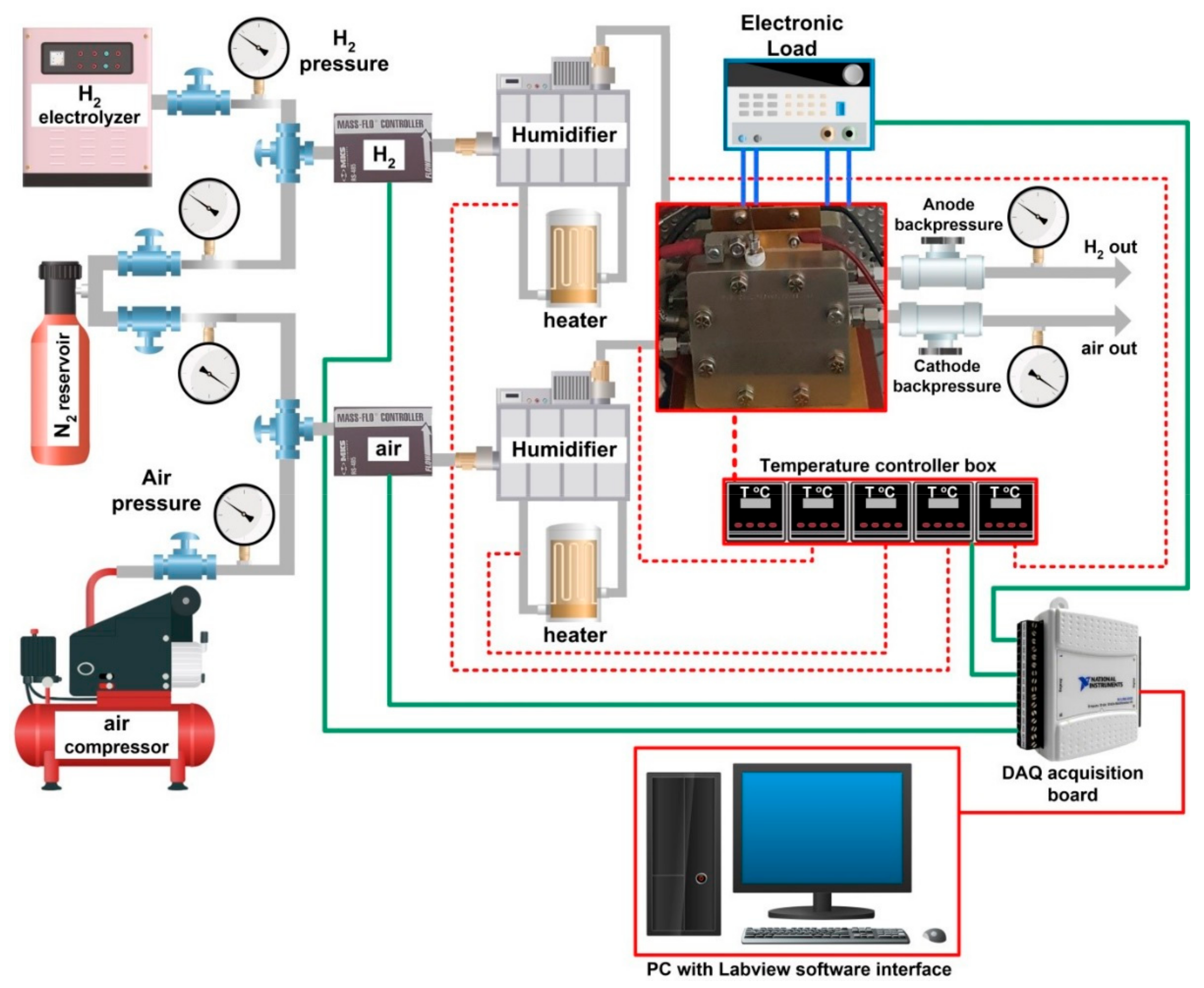
2.2. Instrumentation and Experimental Measurement Protocols
2.3. Physicomathematical Model for Fuel Cell Preformance Analysis
2.3.1. Voltage Efficiency Analysis
2.3.2. Exergy Analysis
- All reactant gases behave as ideal gases;
- Steady, incompressible, laminar flow for all reactant gases;
- Potential and kinetic exergies can be neglected;
- Dead state temperature and pressure are 298.15 K and 1 atm, respectively.
3. Results and Discussion
Polarization Profiles and Overall Power Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Experiment | |||
|---|---|---|---|
| Test 1 | 2.26 | 0.246 | 0.058 |
| Test 2 | 2.13 | 0.219 | 0.187 |
| Test 3 | 1.94 | 0.179 | 0.374 |
| Experiment | |||||
|---|---|---|---|---|---|
| Test 1 | 2.948·10−8 | 2.215·10−7 | 0.98922 | 0.77394 | 0.20573 |
| Test 2 | 1.458·10−8 | 1.096·10−7 | 0.99461 | 0.78197 | 0.20787 |
| Test 3 | 2.948·10−8 | 2.215·10−7 | 0.98922 | 0.77394 | 0.20573 |
Appendix B
References
- Kocha, S.S. Polymer Electrolyte Membrane (PEM) Fuel Cells: Automotive Applications. In Fuel Cells and Hydrogen Production; Lipman, T., Weber, A., Eds.; Springer: New York, NY, USA, 2019; pp. 135–171. [Google Scholar] [CrossRef]
- Pollet, B.G.; Kocha, S.S.; Staffell, I. Current status of automotive fuel cells for sustainable transport. Curr. Opin. Electrochem. 2019, 16, 90–95. [Google Scholar] [CrossRef]
- Gómez, J.C.; Serra, M.; Husar, A. Controller design for polymer electrolyte membrane fuel cell systems for automotive applications. Int. J. Hydrog. Energy. 2021, 46, 23263–23278. [Google Scholar] [CrossRef]
- Fathabadi, H. Novel fuel cell/battery/supercapacitor hybrid power source for fuel cell hybrid electric vehicles. Energy 2018, 143, 467–477. [Google Scholar] [CrossRef]
- Das, H.S.; Tan, C.W.; Yatim, A.H.M. Fuel cell hybrid electric vehicles: A review on power conditioning units and topologies. Renew. Sust. Energ. Rev. 2017, 76, 268–291. [Google Scholar] [CrossRef]
- Migliardini, F.; Veneri, O.; Corbo, P. Hydrogen and proton exchange membrane fuel cells for clean road transportation. J. Ind. Eng. Chem. 2011, 17, 633–641. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef] [Green Version]
- Li, X.J.; Allen, J.D.; Stager, J.A.; Ku, A.Y. Paths to low-cost hydrogen energy at a scale for transportation applications in the USA and China via liquid-hydrogen distribution networks. Clean Energy 2020, 4, 26–47. [Google Scholar] [CrossRef] [Green Version]
- Ahluwalia, R.K.; Wang, X.; Peng, J.; Konduru, V.; Arisetty, S.; Ramaswamy, N.; Kumaraguru, S. Achieving 5000-h and 8000-h low-PGM electrode durability on automotive drive cycles. J. Electrochem. Soc. 2021, 168, 044518. [Google Scholar] [CrossRef]
- Kitahara, T.; Nakajima, H.; Inamoto, M.; Shinto, K. Triple microporous layer coated gas diffusion layer for performance enhancement of polymer electrolyte fuel cells under both low and high humidity conditions. J. Power Sources 2014, 248, 1256–1263. [Google Scholar] [CrossRef]
- Hou, S.; Su, H.; Zou, H.; Dang, D.; Song, H.; Li, X.; Liao, S. Enhanced low-humidity performance in a proton exchange membrane fuel cell by the insertion of microcrystalline cellulose between the gas diffusion layer and the anode catalyst layer. Int. J. Hydrog. Energy 2015, 40, 15613–15621. [Google Scholar] [CrossRef]
- Blanco, M.; Wilkinson, D.P.; Wang, H. Application of water barrier layers in a proton exchange membrane fuel cell for improved water management at low humidity conditions. Int. J. Hydrog. Energy 2011, 36, 3635–3648. [Google Scholar] [CrossRef]
- Neyerlin, K.C.; Gasteiger, H.A.; Mittelsteadt, C.K.; Jorne, J.; Gu, W. Effect of relative humidity on oxygen reduction kinetics in a PEMFC. J. Electrochem. Soc. 2005, 152, A1073. [Google Scholar] [CrossRef]
- Zhang, J.; Song, C.; Zhang, J.; Baker, R.; Zhang, L. Understanding the effects of backpressure on PEM fuel cell reactions and performance. J. Electroanal. Chem. 2013, 688, 130–136. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, X.; Chang, H.; Chan, S.H.; Tu, Z. Effects of operating temperature on the carbon corrosion in a Proton Exchange Membrane Fuel Cell under high current density. Energy Convers. Manag. X 2021, 10, 100087. [Google Scholar] [CrossRef]
- Dai, W.; Wang, H.; Yuan, X.Z.; Martin, J.J.; Yang, D.; Qiao, J.; Ma, J. A review on water balance in the membrane electrode assembly of proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2009, 34, 9461–9478. [Google Scholar] [CrossRef]
- Yousfi-Steiner, N.; Moçotéguy, P.; Candusso, D.; Hissel, D.; Hernandez, A.; Aslanides, A. A review on PEM voltage degradation associated with water management: Impacts, influent factors and characterization. J. Power Sources 2008, 183, 260–274. [Google Scholar] [CrossRef]
- Park, J.; Oh, H.; Ha, T.; Lee, Y.I.; Min, K. A review of the gas diffusion layer in proton exchange membrane fuel cells: Durability and degradation. Appl. Energy 2015, 155, 866–880. [Google Scholar] [CrossRef]
- Cho, J.; Oh, H.; Park, J.; Min, K.; Lee, E.; Jyoung, J.Y. Effect of the microporous layer design on the dynamic performance of a proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2014, 39, 459–468. [Google Scholar] [CrossRef]
- Smirnova, A.; Dong, X.; Hara, H.; Vasiliev, A.; Sammes, N. Novel carbon aerogel-supported catalysts for PEM fuel cell application. Int. J. Hydrog. Energy 2015, 30, 149–158. [Google Scholar] [CrossRef]
- Trefilov, A.M.I.; Tiliakos, A.; Serban, E.C.; Ceaus, C.; Iordache, S.M.; Voinea, S.; Balan, A. Carbon xerogel as gas diffusion layer in PEM fuel cells. Int. J. Hydrog. Energy 2017, 42, 10448–10454. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, G.; Yu, X.; Hou, M.; Shao, Z.; Hong, S.; Mu, C. Carbon nanotubes grown in situ on carbon paper as a microporous layer for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2015, 40, 8958–8965. [Google Scholar] [CrossRef]
- Liu, J.; Takeshi, D.; Sasaki, K.; Lyth, S.M. Defective graphene foam: A platinum catalyst support for PEMFCs. J. Electrochem. Soc. 2014, 161, 838–844. [Google Scholar] [CrossRef]
- Liu, J.; Takeshi, D.; Sasaki, K.; Lyth, S.M. Platinum-decorated nitrogen-doped graphene foam electrocatalysts. Fuel Cells 2014, 14, 728–734. [Google Scholar] [CrossRef]
- Marinoiu, A.; Teodorescu, C.; Carcadea, E.; Raceanu, M.; Varlam, M.; Cobzaru, C.; Stefanescu, I. Graphene-based materials used as the catalyst support for PEMFC applications. Mater. Today Proc. 2015, 2, 3797–37805. [Google Scholar] [CrossRef]
- Marinoiu, A.; Andrulevicius, M.; Tamuleviciene, A.; Tamulevicius, T.; Raceanu, M.; Varlam, M. Synthesis of well dispersed gold nanoparticles on reduced graphene oxide and application in PEM fuel cells. Appl. Surf. Sci. 2020, 504, 144511. [Google Scholar] [CrossRef]
- Marinoiu, A.; Carcadea, E.; Sacca, A.; Carbone, A.; Sisu, C.; Dogaru, A.; Raceanu, M.; Varlam, M. One-step synthesis of graphene supported platinum nanoparticles as electrocatalyst for PEM fuel cells. Int. J. Hydrog. Energy 2021, 46, 12242–12253. [Google Scholar] [CrossRef]
- Najafabadi, A.T.; Leeuwner, M.J.; Wilkinson, D.P.; Gyenge, E.L. Electrochemically produced graphene for microporous layers in fuel cells. ChemSusChem 2016, 9, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Leeuwner, M.J.; Wilkinson, D.P.; Gyenge, E.L. Novel graphene foam microporous layers for PEM fuel cells: Interfacial characteristics and comparative performance. Fuel Cells 2015, 15, 790–801. [Google Scholar] [CrossRef]
- Leeuwner, M.J.; Patra, A.; Wilkinson, D.P.; Gyenge, E.L. Graphene and reduced graphene oxide based microporous layers for high performance proton-exchange membrane fuel cells under varied humidity operation. J. Power Sources 2019, 423, 192–202. [Google Scholar] [CrossRef]
- Latorrata, S.; Mariani, M.; Stampino, P.G.; Cristiani, C.; Dotelli, G. Graphene-based microporous layers for enhanced performance in PEM fuel cells. Mater. Today Proc. 2020, 31, 426–432. [Google Scholar] [CrossRef]
- Joo, Y.; Kim, M.; Kanimozhi, C.; Huang, P.; Wong, B.M.; Roy, S.S.; Arnold, M.S.; Gopalan, P. Effect of Dipolar Molecule Structure on the Mechanism of Graphene- Enhanced Raman Scattering. J. Phys. Chem. C 2016, 120, 13815–13824. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef]
- Tiliakos, A.; Trefilov, A.M.I.; Tanasă, E.; Balan, A.; Stamatin, I. Laser-induced graphene as the microporous layer in proton exchange membrane fuel cells. Appl. Surf. Sci. 2019, 504, 144096. [Google Scholar] [CrossRef]
- Morjan, I.; Voicu, I.; Dumitrache, F.; Sandu, I.; Soare, I.; Alexandrescu, R.; Vasile, E.; Pasuk, I.; Brydson, R.M.; Daniels, H. Carbon nanopowders from the continuous-wave CO2 laser-induced pyrolysis of ethylene. Carbon 2003, 41, 2913–2921. [Google Scholar] [CrossRef]
- Gao, W.; Singh, N.; Song, L.; Liu, Z.; Reddy, A.L.M.; Ci, L.; Vajtai, R.; Zhang, Q.; Wei, B.; Ajayan, P.M. Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nat. Nanotechnol. 2011, 6, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Tiliakos, A.; Ceaus, C.; Iordache, S.M.; Vasile, E.; Stamatin, I. Morphic transitions of nanocarbons via laser pyrolysis of polyimide films. J. Anal. Appl. Pyrolysis 2016, 121, 275–286. [Google Scholar] [CrossRef]
- Tiliakos, A.; Trefilov, A.M.I.; Tanasă, E.; Balan, A.; Stamatin, I. Space-filling supercapacitor carpets: Highly scalable fractal architecture for energy storage. J. Power Sources 2018, 384, 145–155. [Google Scholar] [CrossRef]
- Hoogers, G. Fuel Cell Technology Handbook, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar] [CrossRef]
- Miansari, M.; Sedighi, K.; Amidpour, M.; Alizadeh, E.; Miansari, M.O. Experimental and thermodynamic approach on proton exchange membrane fuel cell performance. J. Power Sources 2009, 190, 356–361. [Google Scholar] [CrossRef]
- Ionescu, V.; Buzbuchi, N.; Andronie, A.; Stamatin, I.; Gîrleanu, V. Exergy analysis of a PEM fuel cell system with different bipolar plate flow fields. In Proceedings of the 11th International Symposium on Advanced Topics in Electrical Engineering (ATEE), Bucharest, Romania, 28–30 March 2019; IEEE: New York, NY, USA, 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Arshad, A.; Ali, H.M.; Habib, A.; Bashir, M.A.; Jabbal, M.; Yan, Y. Energy and exergy analysis of fuel cells: A review. Therm. Sci. Eng. Prog. 2019, 9, 308–321. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M.A. Chapter 3. Chemical Exergy. In Exergy: Energy, Environment and Sustainable Development, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 2021; p. 44. ISBN 9780128243725. [Google Scholar] [CrossRef]
- Wu, Q.X.; Zhao, T.S.; Chen, R.; Yang, W.W. Effects of anode microporous layers made of carbon powder and nanotubes on water transport in direct methanol fuel cells. J. Power Sources 2009, 191, 304–311. [Google Scholar] [CrossRef]
- Tsotridis, G.; Pilenga, A.; de Marco, G.; Malkow, T. EU Harmonised Test protocols for PEMFC MEA testing in single cell configuration for automotive applications. JRC Sci. Policy Rep. 2015, EUR 27632 EN. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC99115 (accessed on 25 September 2021). [CrossRef]
- Harrison, K.W.; Remick, R.; Hoskin, A.; Martin, G.D. Hydrogen Production: Fundamentals and Case Study Summaries; (No. NREL/CP-550-47302); National Renewable Energy Lab. (NREL): Golden, CO, USA, 2010. [Google Scholar]
- Ay, M.; Midilli, A.; Dincer, I. Exergetic performance analysis of a PEM fuel cell. Int. J. Energy Res. 2006, 30, 307–321. [Google Scholar] [CrossRef]
- Kazim, A. Exergy analysis of a PEM fuel cell at variable operating conditions. Energy Convers. Manag. 2004, 45, 1949–1961. [Google Scholar] [CrossRef]
- Özgür, T.; Yakaryilmaz, A.C. Thermodynamic analysis of a proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2018, 43, 18007–18013. [Google Scholar] [CrossRef]
- Taner, T. Energy and exergy analyze of PEM fuel cell: A case study of modeling and simulations. Energy 2018, 143, 284–294. [Google Scholar] [CrossRef]
- Ozden, A.; Shahgaldi, S.; Zhao, J.; Li, X.; Hamdullahpur, F. Assessment of graphene as an alternative microporous layer material for proton exchange membrane fuel cells. Fuel 2018, 215, 726–734. [Google Scholar] [CrossRef]
- Vilekar, S.A.; Datta, R. The effect of hydrogen crossover on open-circuit voltage in polymer electrolyte membrane fuel cells. J. Power Sources 2010, 195, 2241–2247. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, L.; Song, Z.; Li, J.; Ouyang, M. A semiempirical dynamic model of reversible open circuit voltage drop in a PEM fuel cell. Int. J. Energy Res. 2019, 43, 2550–2561. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Song, C.; Zhang, J.; Wang, H. PEM fuel cell open circuit voltage (OCV) in the temperature range of 23 °C to 120 °C. J. Power Sources 2006, 163, 532–537. [Google Scholar] [CrossRef]
- Huang, J. A Simple Accurate Formula for Calculating Saturation Vapor Pressure of Water and Ice. J. Appl. Meteorol. Climatol. 2018, 57, 1265–1272. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Wu, J.; Zhang, J. Chapter 4—The Effects of Temperature on PEM Fuel Cell Kinetics and Performance. In PEM Fuel Cell Testing and Diagnosis; Zhang, J., Zhang, H., Wu, J., Zhang, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 121–141. ISBN 9780444536884. [Google Scholar] [CrossRef]
- Pukrushpan, J.T.; Peng, H.; Stefanopoulou, A.G. Control-Oriented Modeling and Analysis for Automotive Fuel Cell Systems. J. Dyn. Sys., Meas. Control. 2004, 126, 14–25. [Google Scholar] [CrossRef]
- Mench, M.M. Chapter 3—Thermodynamics of Fuel Cell Systems. In Fuel Cell Engines; John Wiley & Sons: New Jersey, NJ, USA, 2008; pp. 62–120. [Google Scholar] [CrossRef]
- Larminie, J.; Dicks, A. Appendix 2—Useful Fuel Cell Equations. In Fuel Cell Systems Explained, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2003; pp. 395–399. [Google Scholar] [CrossRef]
- Khazaee, I.; Ghazikhani, M.; Mohammadiun, M. Experimental and thermodynamic investigation of a triangular channel geometry PEM fuel cell at different operating conditions. Sci. Iran. 2012, 19, 585–593. [Google Scholar] [CrossRef] [Green Version]
- Yilanci, A.; Dincer, I.; Ozturk, H.K. Performance analysis of a PEM fuel cell unit in a solar–hydrogen system. Int. J. Hydrogen. Energ. 2008, 33, 7538–7552. [Google Scholar] [CrossRef]
- Valero, A. Exergy accounting: Capabilities and drawbacks. Energy 2006, 31, 164–180. [Google Scholar] [CrossRef]
- Amidpour, J.; Manesh, M.H.K. Chapter 5—Exergy And Thermoeconomic Evaluation of Cogeneration and Polygeneration Systems in Cogeneration and Polygeneration Systems; Academic Press: Cambridge, MA, USA, 2021; p. 62. [Google Scholar] [CrossRef]
- Lemmon, E.W.; McLinden, M.O.; Friend, D.G. Thermophysical Properties of Fluid Systems in NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2021; pp. 263–267. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Gallorini, F.; Ottaviano, A. An energetic–exergetic analysis of a residential CHP system based on PEM fuel cell. Appl. Energy 2011, 88, 4334–4342. [Google Scholar] [CrossRef]
- Ertesvag, I.S. Sensitivity of chemical exergy for atmospheric gases and gaseous fuels to variations in ambient conditions. Energy Convers. Manag. 2007, 48, 1983–1995. [Google Scholar] [CrossRef]

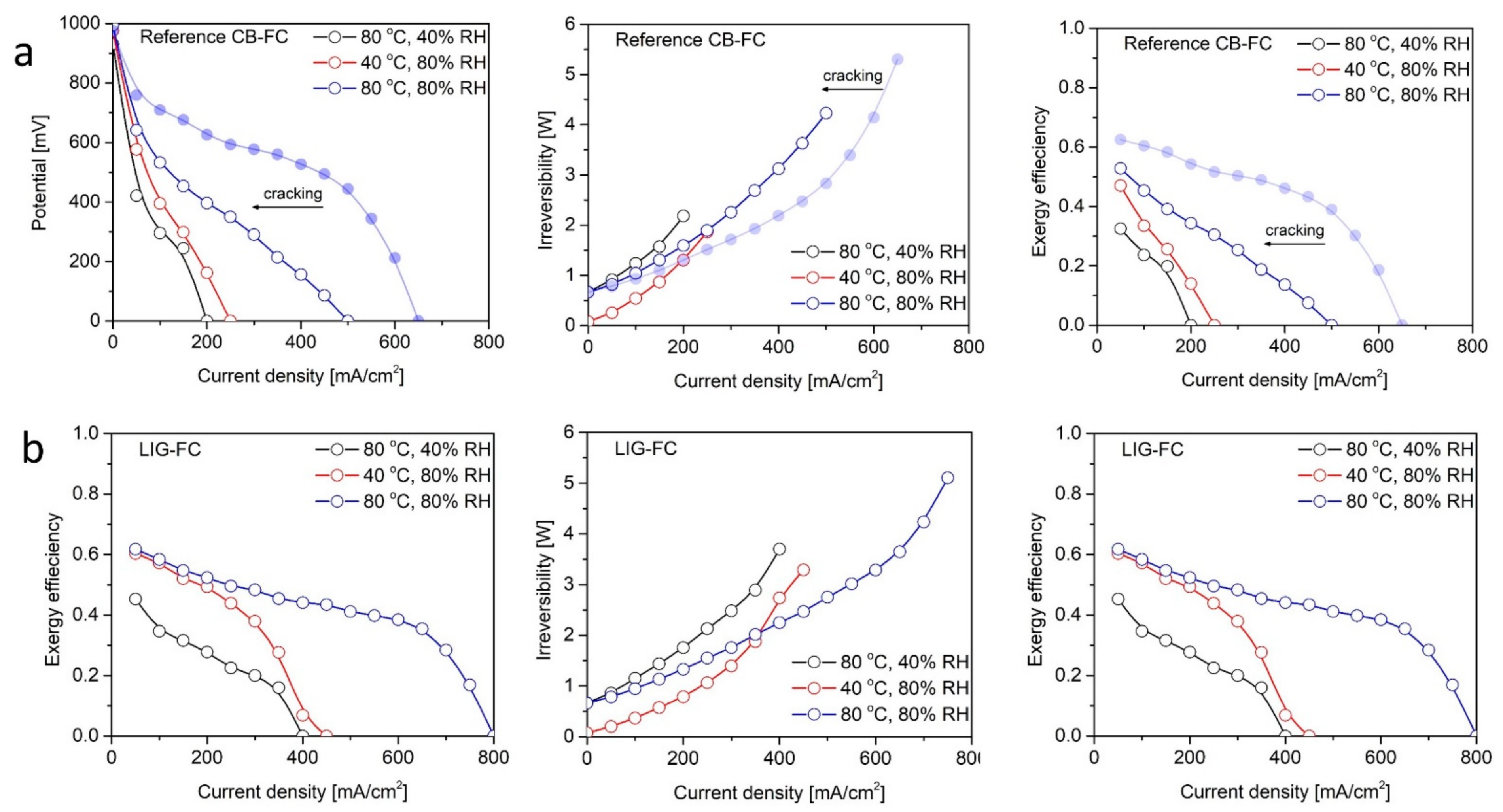

| MEA Samples | Potentials (mV) | Test 1 40 °C—80%RH | Test 2 80 °C—40%RH | Test 3 80 °C—80%RH |
|---|---|---|---|---|
| Reference CB-FC | 975 | 932 | 984 | |
| 287 | 296 | 230 | ||
| LIG-FC | 989 | 978 | 993 | |
| 272 | 249 | 221 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, V.; Balan, A.E.; Trefilov, A.M.I.; Stamatin, I. Exergetic Performance of a PEM Fuel Cell with Laser-Induced Graphene as the Microporous Layer. Energies 2021, 14, 6232. https://doi.org/10.3390/en14196232
Ionescu V, Balan AE, Trefilov AMI, Stamatin I. Exergetic Performance of a PEM Fuel Cell with Laser-Induced Graphene as the Microporous Layer. Energies. 2021; 14(19):6232. https://doi.org/10.3390/en14196232
Chicago/Turabian StyleIonescu, Viorel, Adriana Elena Balan, Alexandra Maria Isabel Trefilov, and Ioan Stamatin. 2021. "Exergetic Performance of a PEM Fuel Cell with Laser-Induced Graphene as the Microporous Layer" Energies 14, no. 19: 6232. https://doi.org/10.3390/en14196232
APA StyleIonescu, V., Balan, A. E., Trefilov, A. M. I., & Stamatin, I. (2021). Exergetic Performance of a PEM Fuel Cell with Laser-Induced Graphene as the Microporous Layer. Energies, 14(19), 6232. https://doi.org/10.3390/en14196232






