Raw Biogas Desulphurization Using the Adsorption-Absorption Technique for a Pilot Production of Agricultural Biogas from Pig Slurry in Poland
Abstract
1. Introduction
- -
- biogas treatment consisting of two desulphurizers, each with a volume of 50 L, along with equipment for the regeneration of the bed,
- -
- loose bed, which consists of the modification of bog iron and activated carbon.
- -
- biogas composition;
- -
- amount of H2S in biogas produced from polydisperse substrate;
- -
- course of changes in the average daily temperature of the fermentation process;
- -
- biogas permeability characteristics (raw, desulphurized, treated) resulting from the pressure that forces this flow.
2. Aspects of Biogas Desulphurization in the Technical, Technological and Socio-Economic Context
3. Materials and Methods
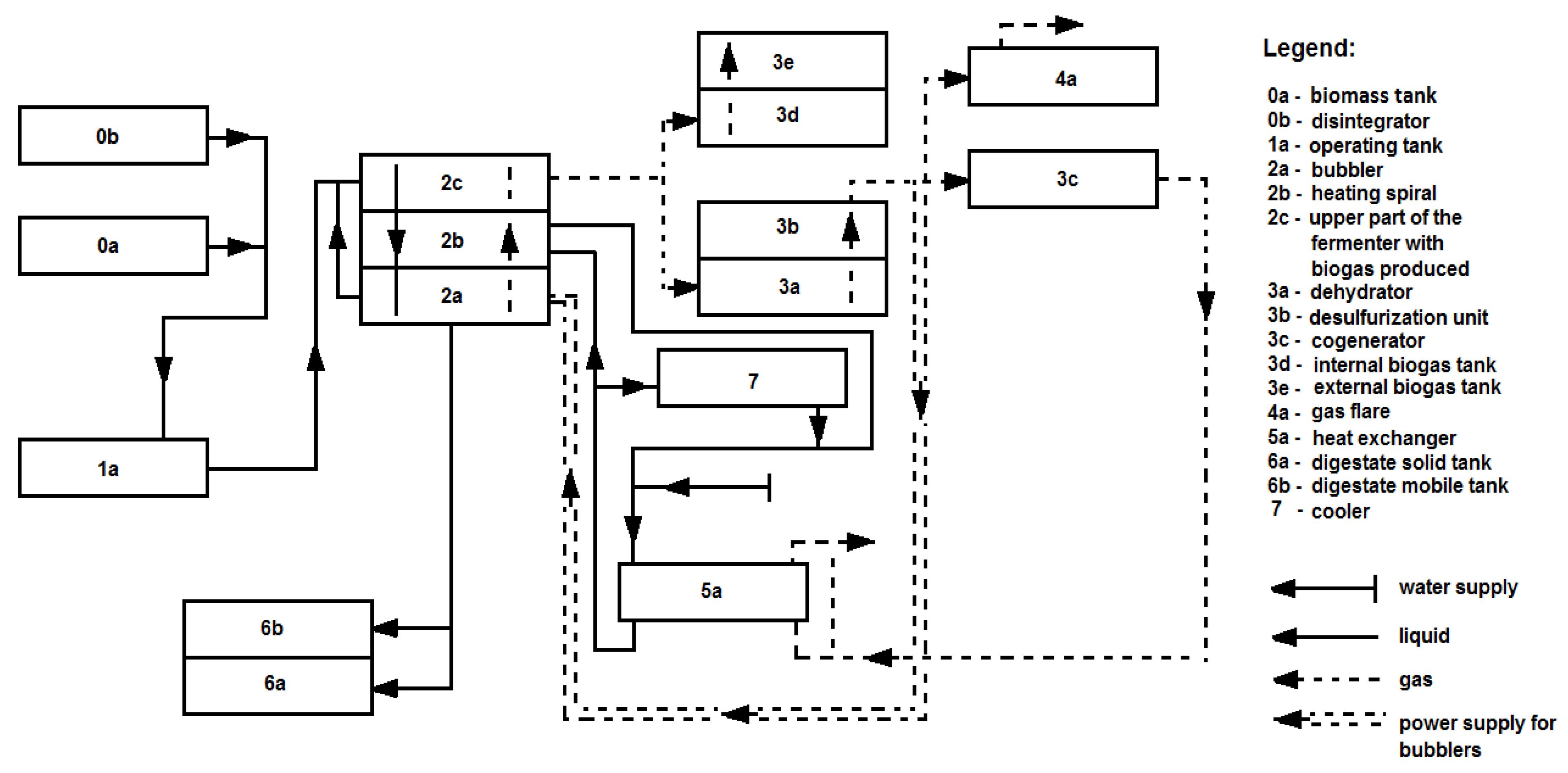
3.1. Agricultural Biogas Production Installation Using a Polydisperse Substrate (Pig Manure)-Research Position
3.2. Scope and Research Methodology
- -
- desulphurization of raw agricultural biogas using the granular bed adsorption-absorption technique.
- -
- Raw biogas was produced using an adhesive bed under mesophilic process conditions.
- -
- The criteria for the evaluation of biogas production were developed depending on:
- -
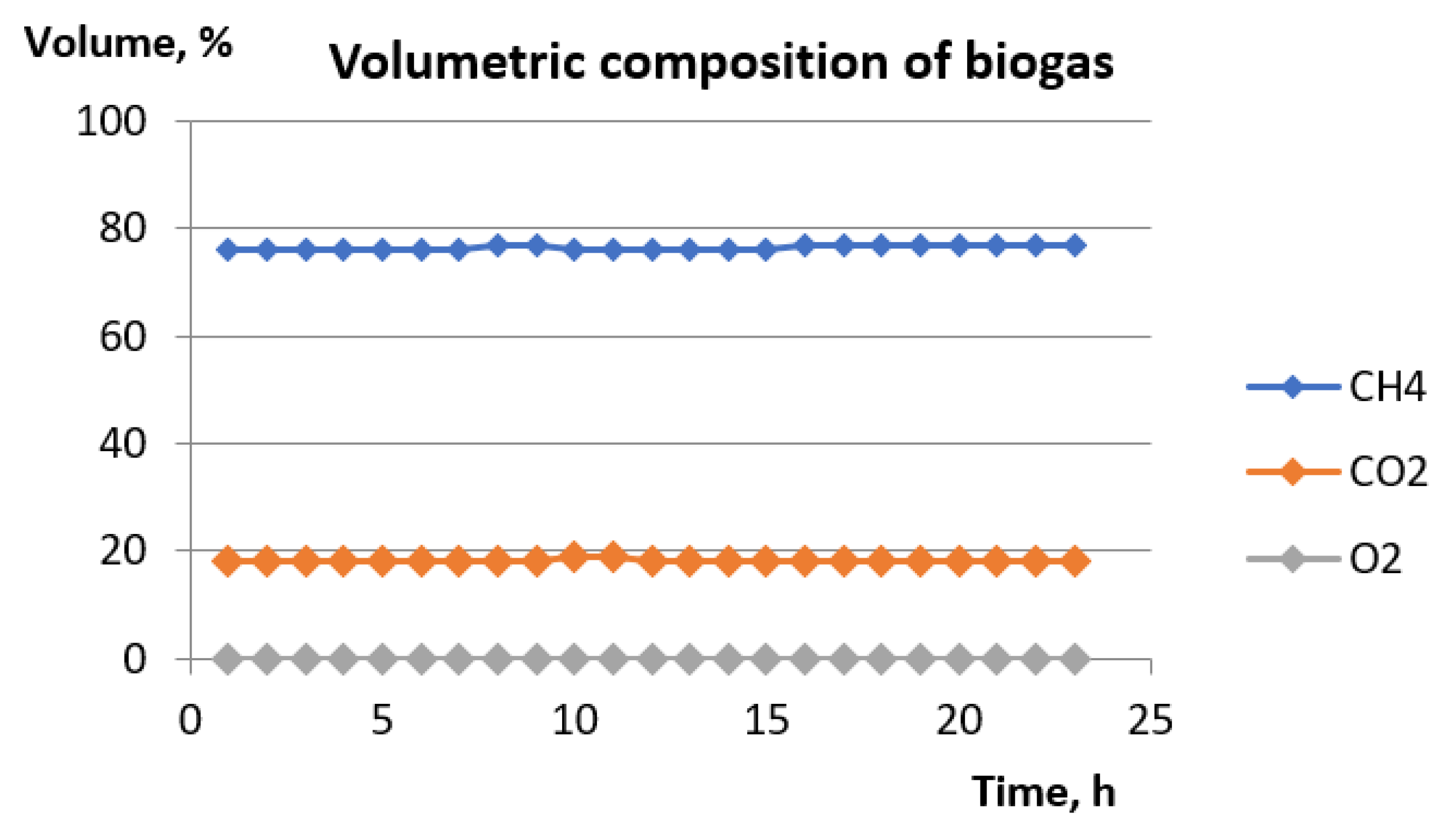
- the composition of crude and desulphurized gas in the flow in time;
- -
- gas temperature over time in the fermentor, before the desulfurizer, after the desulfurizer;
- -
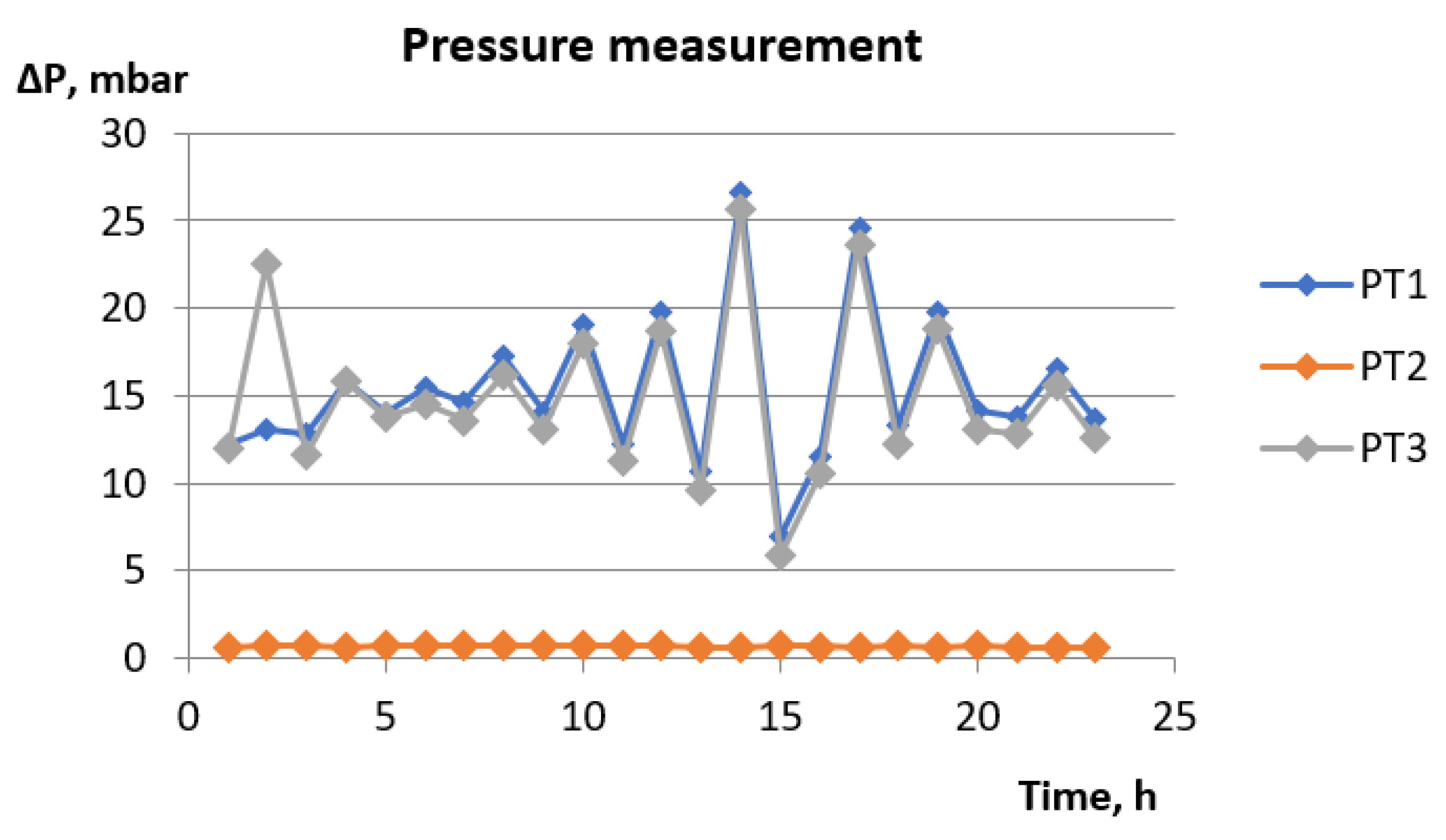
- gas permeability in time in the fermenter (adhesive bed), before the desulphurizer, after the desulphurizer (loose bed).
4. Results and Discussion
5. Conclusions
- (1)
- The applied technique of desulphurization of raw agricultural biogas significantly contributes to the treatment of purified biogas;
- (2)
- The method of using a granular bed in desulphurizers significantly improves the process of raw biogas desulphurization, for high-calorific biogas 100% desulphurized and in cogeneration into electricity and heat;
- (3)
- Raw biogas can be cleaned easily and reliably and produced close to the livestock building.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smolinski, A.; Howaniec, N.; Gasior, R.; Polański, J.; Magdziarczyk, M. Thermal conversion of low rank coal, flotation concentrate and refuse derived fuel in the process of steam co-gasification to hydrogen-rich gas. Energy 2021, 235, 121348. [Google Scholar] [CrossRef]
- Wałowski, G.; Borek, K.; Romaniuk, W.; Wardal, W.J.; Borusiewicz, A. Modern systems of obtaining energy-biogas. In Monograph; Wacław, R., Ed.; Publishing House of the University of Agribusiness in Łomża: Warsaw, Poland, 2019; ISBN 978-83-947669-9-3. [Google Scholar]
- Aleksandrow, S.; Michalak, D. Analysis of the potential of the Łódź region in terms of building a biogas plant. Acta Innov. 2013, 7, 28–44. [Google Scholar]
- Kowalski, Ł.; Smerkowska, B. A Polish case study for biogas to biomethane upgrading. Combustion. Engines 2012, 1, 15–24. [Google Scholar]
- Piskowska-Wasiak, J. Treatment of biogas to the parameters of high-methane gas. Nafta-Gaz 2014, 70, 94–105. [Google Scholar]
- Kociołek-Balawejder, E.; Wilk, Ł. Review of methods for removing hydrogen sulphide from biogas. Przemysł Chem. 2011, 90, 389–397. [Google Scholar]
- Zawadzka, A.; Imbierowicz, M. Biogas, p. 17–31 and Technologies and devices for biogas plants, p. 153–168. In Collective Work Investing in Renewable Energy. Ecological, Technological, Financial and Benchmarking Aspects; Polish Academy of Sciences, Łódź Branch, Environmental Protection Commission, Pro-Akademia Association of Economic Advisors: Łódź, Poland, 2010; ISBN 978-83-86492-59-6. [Google Scholar]
- Wilk, J. Use of sewage sludge for biogas production. Aura 2011, 5, 18–20. [Google Scholar]
- Cebula, J. Selected Methods of Agricultural and Landfill Biogas Treatment; Publishing House of the Silesian University of Technology: Gliwice, Poland, 2012; ISBN 978-83-7335-983-3. [Google Scholar]
- Aleksandrow, S.; Staniszewska, M. The importance of renewable energy sources in the global economy and their impact on the labor market. Acta Innov. 2013, 6, 41–45. [Google Scholar]
- Wawer, P. 1.6 MW biogas plant. Ekologia Tech. 2013, 21, 204–209. [Google Scholar]
- Pomykała, R.; Łyko, P. Biogas from waste as biofuel for transport—Barriers and prospects. Chemik 2013, 5, 454–457. [Google Scholar]
- Zagdański, D. Implementation and operation of an agricultural biogas plant. An example of a selected object. Aura 2014, 6, 16–18. [Google Scholar]
- Gaj, K.; Cybulska-Szulc, H. Time changeability model of the bog ore sorption ability. Ecol. Chem. Eng. 2014, 21, 113–123. [Google Scholar] [CrossRef]
- Żarczyński, A.; Rosiak, K.; Anielak, P.; Wolf, W. Practical methods of purifying biogas from hydrogen sulphide. Part. 1. Application of solid sorbents. Acta Innov. 2014, 12, 24–35. Available online: http://www.proakademia.eu/gfx/baza_wiedzy/255/praktyczne_metody_oczyszczania_biogazu_z_siarkowodoru.pdf (accessed on 3 December 2015).
- Fischer, E.; Gattermann, H.; Grope, J.; Scholwin, F.; Weidele, T.; Weithäuser, M. Gasaufbereitung und Verwertungsmöglichkeiten. In Leitfaden Biogas von der Gewinnung zur Nutzung; Fachagentur Nachwachsende Rohstoffe e. V. (FNR): Gülzow, Germany, 2013; pp. 106–127. Available online: http://mediathek.fnr.de/media/downloadable/files/samples/l/e/leitfadenbiogas2013_web_komp.pdf (accessed on 3 December 2015).
- Rejman-Burzyńska, A.; Jędrysik, E.; Gądek, M. Concept of the plant for upgrading biogas to biomethane. Przemysł Chem. 2013, 92, 68–72. [Google Scholar]
- Michalska, K.; Kacprzak, A. Swedish model of development of innovative biogas technologies based on waste management. Acta Innov. 2012, 3, 39–70. [Google Scholar]
- Biernat, K.; Gis, W.; Grzelak, P.; Żółtowski, A. Possibilities of using biomethane as fuel for city buses on the example of Poland and Sweden. Przemysł Chem. 2013, 92, 1280–1284. [Google Scholar]
- Electrigaz Technologies Inc. Feasibility Study—Biogas Upgrading and Grid Injection in the Fraser Halley. British Columbia. Final Report. Available online: http://www.lifesciencesbc.ca/files/PDF/feasibility_study_biogas.pdf (accessed on 3 December 2015).
- Kogut, P.; Piekarski, J.; Dąbrowski, T.; Kaczmarek, F. Biogas production plants as a method of utilisation of sewage sludge in relation to the Polish legislation. Annu. Set Environ. Prot. 2012, 14, 299–313. [Google Scholar]
- Klemba, K. Biogas plant as a potential source of odor emissions and preventive measures. Eliksir 2015, 2, 22–27. Available online: http://chemia.p.lodz.pl/Eliksir/Eliksir_nr2.pdf (accessed on 3 December 2015).
- Żarczyński, A.; Rosiak, K.; Anielak, P.; Ziemiński, K.; Wolf, W. Practical methods of hydrogen sulphide removal from biogas. II. Application of sorption solutions and biological methods. Acta Innov. 2015, 15, 57–71. [Google Scholar]
- Vakili, M.; Gholami, Z.; Gholami, F. Removal of hydrogen sulfide from gaseous streams by a chemical method using ferric sulfate solution. World Appl. Sci. J. 2012, 19, 241–245. [Google Scholar]
- Kuo-Ling, H.; Wei-Chih, L.; Ying-Chien, C.; Yu-Pei, C.; Ching-Ping, T. Elimination of high concentration hydrogen sulfide and biogas purification by chemical–biological process. Chemosphere 2013, 92, 1396–1401. [Google Scholar]
- Zając, G.; Szyszlak-Bargłowicz, J.; Słowik, T. Production and use of biogas in the “Hajdów” sewage treatment plant. Gaz Woda Tech. Sanit. 2013, 2, 93–95. [Google Scholar]
- Zdeb, M. An Efficiency of H2S Removal from Biogas via Physicochemical and Biological Methods—A Case Study. Annu. Set Environ. Prot. 2013, 15, 551–563. [Google Scholar]
- Frare, L.M.; Bortoleto, R.M.; Mufalo, A.N., Jr.; Pereira, N.C.; Gimenes, M.L. Optimum liquid/gas ratio determination for removing H2S from biogas using Fe-EDTA solution. In Proceedings of the 4th Mercosur Congres on Process Systems Engineering: 2nd Mercosur Congres on Chemical Engineering, Costa Verde, Brasil; Available online: http://www.enpromer2005.eq.ufrj.br/nukleo/pdfs/0968_trabalho_968_revisado_final.pdf (accessed on 12 December 2015).
- Ermich, S.; Pruszyńska, E. Comprehensive biogas treatment—Biosulfex device: Operational results. Gaz, Woda Tech. Sanit. 2008, 4, 6–9. [Google Scholar]
- Paprota, E. Treatment of biogas as a way of its wider use. Autobusy. Tech. Eksploat. Syst. Transp. 2011, 10, 329–333. [Google Scholar]
- Steppa, M. Agricultural biogas plants. In Doc. dr inż. Wacław Romaniuk. Supplement and Update: Doc. dr inż.; Institute for Building Mechanization and Electrification of Agriculture, Department of Mechanization of Animal Breeding—Head of the Department, Wacław Romaniuk: Warsaw, Poland, 1992. [Google Scholar]
- Romaniuk, W.; Biskupska, K. An Agricultural Biogas Plant Step by Step; Hortpress: Warsaw, Poland, 2014; pp. 3–32. [Google Scholar]
- Aleszczyk, Ł. Biogas Desulphurization and Dewatering. Tank. Patent 230468, 25 June 2018. [Google Scholar]
- Agreement. Implementation Agreement No. AT-23/2019, ITP Falenty. 2019. Available online: https://res.mdpi.com/d_attachment/energies/energies-14-03538/article_deploy/energies-14-03538-v2.pdf (accessed on 1 April 2019).
- Pawłowska, M.; Zdeb, M. Comparison of the effectiveness of microbiological biogas desulphurization in bioscrubbers and biofilters with an irrigated layer. In Proceedings of the 3rd Congress of Environmental Engineering, Lublin, Poland, 13–17 September 2019; Volume 1, pp. 191–198. Available online: http://wis.pol.lublin.pl/kongres3/tom1/21.pdf (accessed on 3 December 2015).
- Biernat, K.; Samson-Bręk, I. Review of biogas purification technology to natural gas quality. Chemik 2011, 65, 435–444. [Google Scholar]
- Gaj, K.; Cybulska-Szulc, H.; Knop, F.; Steininger, M. Examination of biogas hydrogen sulphide sorption on a layer of activated bog ore. Environ. Prot. Eng. 2008, 4, 33–41. [Google Scholar]
- Cosoli, P.; Ferrone, M.; Pricl, S.; Fermeglia, M. Hydrogen sulphide removal from biogas by zeolite adsorption. Part I. GCMC molecular simulations. Chem. Eng. J. 2008, 145, 86–92. [Google Scholar] [CrossRef]
- Barbusiński, K. Calcium and magnesium peroxides—Application for commercial purposes and in environmental protection. Chemik 2006, 9, 433–438. [Google Scholar]
- Cebula, J.; Sołtys, J. Removal of Volatile Sulfur Compounds from Biogas Produced in an Agricultural Micro Biogas Plant with the Use of Halloysite Sorbent. Baltic Biogas Forum, Paper, September 2012. Available online: http://www.imp.gda.pl/BF2012/prezentacje/p254.pdf (accessed on 3 December 2015).
- Mroczkowski, P.; Seiffert, M. Purification and Injection of Biogas on the Example of Germany. Possibilities of Implementing Technology in Poland. The Material Was Created during the Scholarship of the Federal Environment Foundation (DBU) and the Foundation of Prof. Nowicki at the German Biomass Center (DBFZ). Available online: www.cire.pl (accessed on 3 December 2011).
- Kwaśny, J.; Balcerzak, W.; Rezka, P. Biogas and characteristics of selected methods of its desulphurization. J. Civil Eng. Environ. Archit. 2016, 63, 129–141. [Google Scholar]
- De Arespacochaga, N.; Valderrama, C.; Mesa, C.; Bouchy, L.; Cortina, J.L. Biogas deep clean-up based on adsorption technologies for Solid Oxide Fuel Cell applications. Chem. Eng. J. 2014, 255, 593–603. [Google Scholar] [CrossRef]
- Petersson, A. Biogas Cleaning in the Biogas Hand Book; Wellinger, A., Murphy, J.P., Baxter, D., Eds.; Woodhead Publishing Limited: Sawston, UK, 2013; ISBN 978-0-85709-498-8. [Google Scholar] [CrossRef]
- Hernández, S.P.; Scarpa, F.; Fino, D.; Conti, R. Biogas purification for MCFC application. Int. J. Hydrog. Energy 2011, 36, 8112–8118. [Google Scholar] [CrossRef]
- Micoli, L.; Bagnasco, G.; Turco, M. H2S removal from biogas for fuelling MCFCs: New adsorbing materials. Int. J. Hydrog. Energy 2014, 39, 1783–1787. [Google Scholar] [CrossRef]
- Sisani, E.; Cinti, G.; Discepoli, G.; Penchini, D.; Desideri, U.; Marmottini, F. Adsorptive removal of H2S in biogas conditions for high temperature fuel cell systems. Int. J. Hydrog. Energy 2014, 39, 21753–21766. [Google Scholar] [CrossRef]
- Herr, M.; Lermen, A.; Rostek, S. Biogaspartner—A joint initiative. Dtsch. Energ. Agent. GmbH HP Druck Berlin 2010, 5, 1–72. [Google Scholar]
- Cybulska, H.; Gaj, K.; Knop, F.; Steininger, M. Research on the Sorption of Hydrogen Sulphide Contained in Biogas on Activated Turf Ore; Institute of Environmental Protection Engineering, Wrocław University of Technology: Wrocław, Poland, 2021. [Google Scholar]
- Jędrczak, A. Biological Waste Treatment; PWN: Warsaw, Poland, 2007; pp. 186–190. ISBN 978-83-01-15166-9. [Google Scholar]
- Więckol-Ryk, A.; Krzemień, A.; Smoliński, A.; Lasheras, F.S. Influence of wet flue gas desulfurization on amine based absorption plant for CO2 removal. Sustainability 2018, 10, 923. [Google Scholar] [CrossRef]
- Krzemień, A.; Więckol-Ryk, A.; Smoliński, A.; Koteras, A.; Więcław-Solny, L. Assessing the risk of corrosion in amine-based CO2 Capture Process. J. Loss Prev. Process Ind. 2016, 43, 189–197. [Google Scholar] [CrossRef]
- Ramos, I.; Fdz-Polanco, M. Microaerobic control of biogas sulphide content during sewage sludge digestion by using biogas production and hydrogen sulphide concentration. Chem. Eng. J. 2014, 250, 303–311. [Google Scholar] [CrossRef]
- Nemati, M.; Harrison, S.T.L.; Hansford, G.S.; Webb, C. Biological oxidation of ferrous sulphate by Thiobacillus ferrooxidans: A review on the kinetic aspects. Biochem. Eng. J. 1998, 1, 171–190. [Google Scholar] [CrossRef]
- Ramos, I.; Pérez, R.; Reinoso, M.; Torio, R.; Fdz-Polanco, M. Microaerobic digestion of sewage sludge on an industrial-pilot scale: The efficiency of biogas desulphurisation under different configurations and the impact of O2 on the microbial communities. Bioresour. Technol. 2014, 164, 338–346. [Google Scholar] [CrossRef]
- Dudek, J.; Klimek, P.; Kołodziejak, G.; Niemczewska, J.; Zalewska-Bartosz, J. Technologies for Energetic Use of Landfill Gas. USA EPA, Oil and Gas Institute, Kraków. Available online: http://www.metmarkt.com/project/2/dws/out/LFG-podrecznik.pdf (accessed on 22 February 2021).
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for transformation of biogas to biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Allegue, L.B.; Hinge, J. Biogas Upgrading Evaluation of Methods for H2S Removal; Danish Technology Institute: Taastrup, Denmark, 2014; pp. 1–31. Available online: https://www.teknologisk.dk/_/media/60599_Biogas%20upgrading.%20Evaluation%20of%20methods%20for%20H2S%20removal.pdf (accessed on 17 September 2021).
- Kujawski, J. Kujawski, O. Overview of biogas production technology (part three). Czysta Energ. Abrys 2010, 2, 28–30. [Google Scholar]
- Gattermann, H.; Kaltschmitt, M.; Niebaum, A.; Schattauer, A.; Scholwin, F.; Weiland, P. Produkcja i Wykorzystanie Biogazu. Institut für Energetik und Umwelt GmbH. Available online: http://www.ieo.pl/pl/aktualnosci/183-produkcjabiogazu.html (accessed on 10 December 2014).
- Cebula, J. Biogaz purification by sorption techniques. Archit. Civil. Eng. Environ. 2009, 2, 95–103. [Google Scholar]
- Brudniak, A.; Dębowski, M.; Zieliński, M. Purification and enrichment of biogas in ash-water suspension. Ecol. Eng. 2013, 32, 7–16. [Google Scholar]
- Wałowski, G. Hydrodynamics of the biogas production process in a skeletal fermentation reactor. In Wacława Romaniuk: Innovative Technologies in Animal Production Taking into Account the Standards of the European Union, Environmental Protection and Renewable Energy; ITP Publishing House: Warsaw, Poland, 2018; pp. 199–209. ISBN 978-83-65426-34-5. [Google Scholar]
- Romaniuk, W.; Głaszczka, A.; Biskupska, K. Analysis of biogas installation solutions for family and farm farms. In Engineering in Agriculture; Monographs No. 9; ITP Publishing House: Warsaw, Poland, 2012. [Google Scholar]
- Staszewski, R.; Wagner-Staszewska, T. The Adsorbent Regeneration System in the Adsorption-Desorption Installation. Poland Patent Application P.165947, 9 December 1991. [Google Scholar]
- Hobler, A.; Stumpf, W.; Quasnitschka, H.; Kriebel, M. Method and Installation for the Treatment of Nitrogen-Containing Natural Gas. Poland Patent Application P.182645, 14 October 1996. [Google Scholar]
- Kumanowski, K.S.; Szewczyk, K.W.; Zamojska, A.M.; Kumanowska, E.J. The Method of Utilizing Liquid Manure by Methane Fermentation and the Installation for Utilizing Liquid Manure by Methane Fermentation. Poland Patent Application P.386459, 6 November 2008. [Google Scholar]
- Filanowski, P.; Pituła, M.; Rybicki, C. Biogas Purification Method for Transmission Gas Parameters and Biogas Purification Installation. Poland Patent Application P.403141, 13 March 2013. [Google Scholar]
- Urych, B.; Smoliński, A. Sewage sludge and phytomass co-pyrolysis and the gasification of its chars: A kinetics and reaction mechanism study. Fuel 2021, 285, 119186. [Google Scholar] [CrossRef]
- Smoliński, A.; Howaniec, N.; Bąk, A. Utilization of energy crops and sewage sludge in the process of co-gasification for sustainable hydrogen production. Energies 2018, 11, 809. [Google Scholar] [CrossRef]
- Smoliński, A.; Howaniec, N. Co-gasification of coal/sewage sludge blends to hydrogen-rich gas with the application of the simulated high temperature reactor excess heat. Int. J. Hydrog. Energy 2016, 41, 8154–8158. [Google Scholar] [CrossRef]
- Urych, B.; Smoliński, A. Kinetics of Sewage Sludge Pyrolysis and Air Gasification of Its Chars. Energy Fuels 2016, 30, 4869–4878. [Google Scholar] [CrossRef]
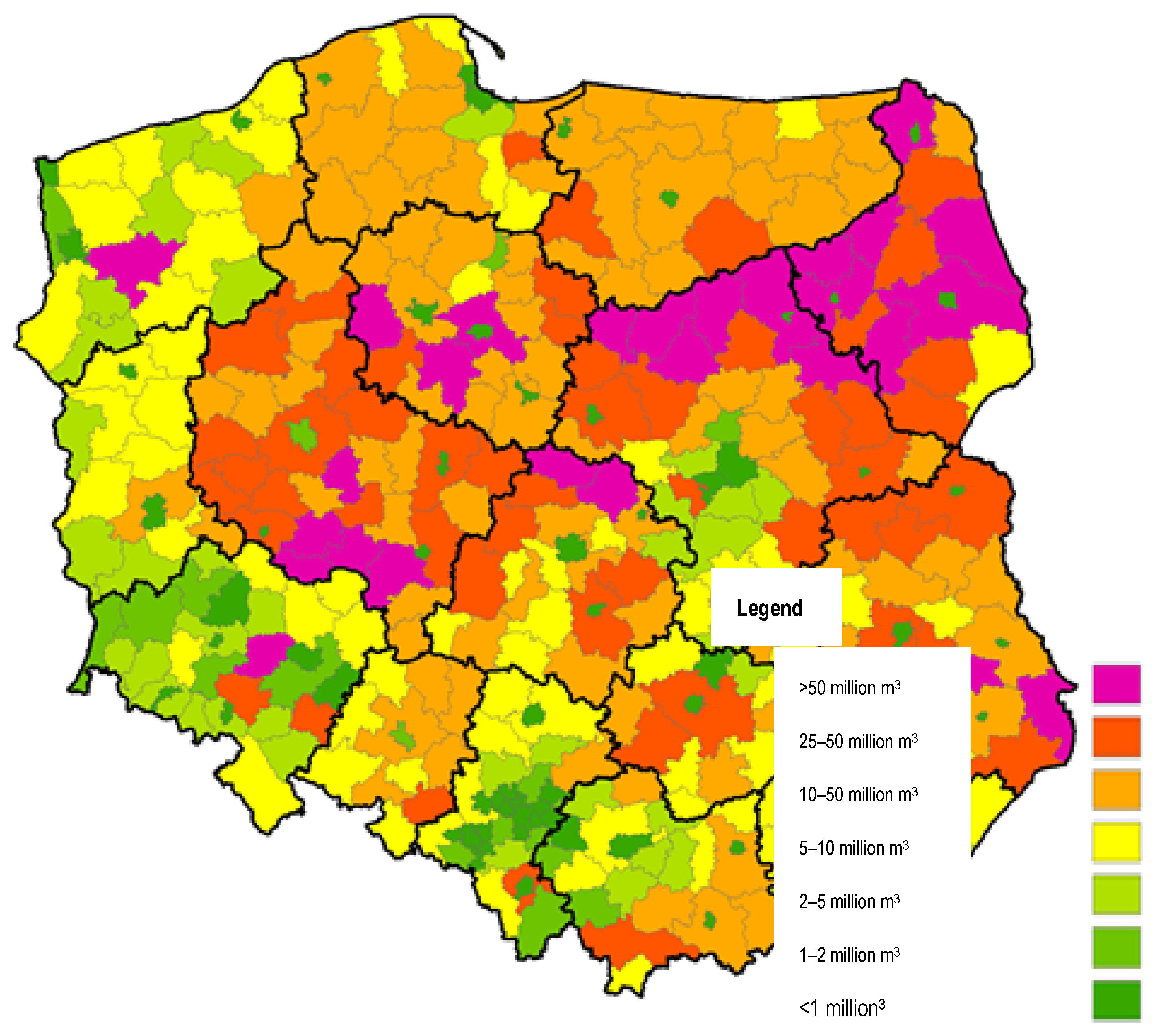
- Marek, P. The amount of manure produced in pig production and the related potential of biogas production in Poland in 2018, broken down by municipalities. In Information Catalog. Developed as Part of: The Implementation of the Multiannual Program for 2016–2020 Entitled “Technological and Natural Projects for Innovative, Effective and Low-Emission Economy in Rural Areas”; Specified in the Contract No. GZ.me.032.1.9.2018 Concluded on 17 April 2018 between the Minister of Agriculture and Rural Development and the Institute of Technology and Life Sciences in Falenty;Unpublished Work.
- Decewicz, P. Spatial Model Based on Data from the Institute of Technology and Life Sciences in Falenty. Developed as Part of: The Implementation of the Multiannual Program for 2016–2020 Entitled "Technological and Natural Projects for Innovative, Effective and Low-Emission Economy in Rural Areas"; Specified in the Contract no. GZ.me.032.1.9.2018 Concluded on 17 April 2018 between the Minister of Agriculture and Rural Development and the Institute of Technology and Life Sciences in Falenty; Unpublished Work.
- Curkowski, A.; Onkisz-Popławska, A.; Mroczkowski, P.; Zowski, M.; Wiśniewski, G. A Guide for Investors Interested in Building Agricultural Biogas Plants; The Work Was Commis-Sioned by the Ministry of Economy at the Institute for Renewable Energy; Instytut Energetyki Odnawialnej: Warszawa, Poland, 2011; p. 126. [Google Scholar]
- Wałowski, G. Development of biogas and biorafinery systems in Polish rural communities. J. Water Land Dev. 2021, 49, 156–168. [Google Scholar] [CrossRef]
- Klimek, K.; Kapłan, M.; Syrotyuk, S.; Konieczny, R.; Anders, D.; Dybek, B.; Karwacka, A.; Wałowski, G. Production of Agricultural Biogas with the Use of a Hydrodynamic Mixing System of a Polydisperse Substrate in a Reactor with an Adhesive Bed. Energies 2021, 14, 3538. [Google Scholar] [CrossRef]
- Danbred Genes for Global Pig Production. Available online: www.danbred.com (accessed on 20 May 2021).
- Tuczniki. Available online: http://neorol.eu/tuczniki/html (accessed on 20 May 2021).
- Wałowski, G. Multi-phase flow assessment for the fermentation process in mono-substrate reactor with skeleton bed. J. Water Land Dev. 2019, 42, 150–156. [Google Scholar] [CrossRef][Green Version]
- Hagen, M.; Polman, E.; Jensen, J.; Myken, A.; Jönsson, O.; Dahl, A. Adding Gas from Biomass to the Gas Grid; Final Report 2001, Contract No: XVII/4.1030/Z/99-412. Available online: http://www.dgc.eu/pdf/altener.pdf (accessed on 17 September 2021).
- Total Air Pollution Control. Website: Gas Scrubbing—Activated Carbon Adsorbers. Available online: https://tapc.com.au/our-products/dry-scrubbers/ (accessed on 17 September 2021).
- Siefers, A.M. A Novel and Cost-Effective Hydrogen Sulfide Removal Technology Using Tire Derived Rubber Particles. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2010; p. 11281. [Google Scholar]
- Wellinger, A.; Linberg, A. Biogas Upgrading and Utilization—IEA Bioenergy Task 24: Energy from Biological Conversion of Organic Wastes; International Energy Association: Paris, France, 2005; Available online: http://www.iea-biogas.net/_download/publi-task37/Biogas%20upgrading.pdf (accessed on 17 September 2021).
- Abatzoglou, N.; Boivin, S. A review of biogas purification processes. Biofuels Bioprod. Bioref. 2009, 3, 42–71. [Google Scholar] [CrossRef]
- Skerman, A.G. Practical Options for Cleaning Biogas Prior to on-Farm Use at Piggeries; A Thesis Submitted in School of Chemical Engineering, Engineering Work; The University of Queensland: Brisbane, Australia, 2016. [Google Scholar]
- Pourzolfaghar, H.; Ismail, M.; Izhar, S.; MagharehEsfahan, Z. Review of H2S Sorbents at Low-Temperature Desulfurization of Biogas. Int. J. Chem. Environ. Eng. 2004, 5, 22–28. [Google Scholar]
- Okoro, O.V.; Sun, Z. Desulphurisation of Biogas: A Systematic Qualitative and Economic-Based Quantitative Review of Alternative Strategies. ChemEngineering 2019, 3, 76. [Google Scholar] [CrossRef]
- TVT. Biogas to Biomethane Technology Review; Vienna University of Technology: Vienna, Austria, 2012. [Google Scholar]
- Huynh, Q.; Thieu, V.Q.Q.; Dinh, T.P.; Akiyoshi, S. Removal of hydrogen sulfide (H2S) from biogas by adsorption method. In Proceedings of the 8th Biomas, Asia Workshop, Hanoi, Vietnam, 2 November–1 December 2011; Available online: http://www.biomass-asia-workshop.jp/biomassws/08workshop/files/20Fulltext%20-%20H2S.pdf (accessed on 17 September 2021).
- Pham, C.H.; Saggar, S.; Berben, P.; Palmada, T.; Ross, C. Removing Hydrogen Sulphide Contamination in Biogas Produced from Animal Wastes. In Farm Environmental Planning—Science, Policy and Practice; Occasional Report No. 31; Currie, L.D., Christensen, L.C., Eds.; Fertilizer and Lime Research Centre, Massey University: Palmerston North, New Zealand, 2018; 10p, Available online: http://flrc.massey.ac.nz/publications.html (accessed on 17 September 2021).
- Zicari, S.M. Removal of Hydrogen Sulphide from Biogas Using Cow-Manure Compost. Master’s Thesis, Cornell University in Partial Fulfilment, Ithaca, NY, USA. Available online: http://www.green-trust.org/ (accessed on 20 May 2021).
- Cherosky, P.; Li, Y. Hydrogen sulfide removal from biogas by bio-based iron sponge. Biosyst. Eng. 2013, 114, 55–59. [Google Scholar] [CrossRef]
- Krich, K.; Augenstein, D.; Batmale, J.P.; Benemann, J.; Rutledge, B.; Salour, D. Biomethane from Dairy Waste—A Sourcebook for the Production and Use of Renewable Natural Gas in California, Prepared for Western United Dairymen, Funded in Part through USDA Rural Development. 2005. Available online: http://www.calstart.org/Libraries/Publications/Biomethane_from_Dairy_Waste_Full_Report.sflb.ashx (accessed on 20 May 2021).
- Gróf, N.; Barbušová, J.; Hencelová, K.; Hutňan, M. Absorption removal of hydrogen sulfide from recirculated biogas. Acta Chim. Slovaca 2020, 13, 13–18. [Google Scholar] [CrossRef]
- Kupryś-Caruk, M. The agri-food industry as a source of substrates for biogas production. Adv. Sci. Technol. Agric. Food Ind. 2017, 2, 69–85. [Google Scholar]
- Marszałek, M.; Banach, M.; Kowalski, Z. Manure utilization by methane and aerobic fermentation—Biogas and compost production. Tech. J. 2011, 10, 143–158. [Google Scholar]
- Myczko, A.; Myczko, R.; Szulc, R.; Tupalski, L. Reactor for Methane Slurry. Fermentation. Patent Application P.220074, 4 July 2012. [Google Scholar]





| Method | Process or Technique | Application | Range of Biogas Desulphurisation Efficiency |
|---|---|---|---|
| Physical | Absorption (wet) |
| >94% [5,12] |
| Absorption (dry) |
| up to 95% [9,15] | |
| Absorption (wet) |
| >96% [5,12] | |
| Cryogenic separation |
| up to 99% [36] | |
| Chemical | Absorption |
| up to 90% [6,9,14,15,37] |
| Membrane (porous or diffusive) |
| up to 99% [15,38] | |
| Absorption (wet) |
| (95 ÷ 99)% [10,39] | |
| Biological | Oxidation |
| over 95% [6,15] |
| Microbiological reaction |
| (80 ÷ 99)% [40] |
| Desulphurisation | Course of Process | Application | Range of Biogas Desulphurisation Efficiency |
|---|---|---|---|
| chemical | in fermenter |
| (80 ÷ 99)% [40] |
| outside fermenter |
| up to 94% [6] | |
| biological | in fermenter |
| (80 ÷ 99)% [40] |
| outside fermenter |
| (70 ÷ 98)% [61] up to 95% [62] up to 95% [62] |
| Fattening Pig | Volume of Substrate | Cycle |
|---|---|---|
| quantity | time | |
| 3500 | 1400 m3 | 1 year |
| 1 | 0.4 m3 | 90 days |
| 1 | 0.4 L | 1 day |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapłan, M.; Klimek, K.; Syrotyuk, S.; Konieczny, R.; Jura, B.; Smoliński, A.; Szymenderski, J.; Budnik, K.; Anders, D.; Dybek, B.; et al. Raw Biogas Desulphurization Using the Adsorption-Absorption Technique for a Pilot Production of Agricultural Biogas from Pig Slurry in Poland. Energies 2021, 14, 5929. https://doi.org/10.3390/en14185929
Kapłan M, Klimek K, Syrotyuk S, Konieczny R, Jura B, Smoliński A, Szymenderski J, Budnik K, Anders D, Dybek B, et al. Raw Biogas Desulphurization Using the Adsorption-Absorption Technique for a Pilot Production of Agricultural Biogas from Pig Slurry in Poland. Energies. 2021; 14(18):5929. https://doi.org/10.3390/en14185929
Chicago/Turabian StyleKapłan, Magdalena, Kamila Klimek, Serhiy Syrotyuk, Ryszard Konieczny, Bartłomiej Jura, Adam Smoliński, Jan Szymenderski, Krzysztof Budnik, Dorota Anders, Barbara Dybek, and et al. 2021. "Raw Biogas Desulphurization Using the Adsorption-Absorption Technique for a Pilot Production of Agricultural Biogas from Pig Slurry in Poland" Energies 14, no. 18: 5929. https://doi.org/10.3390/en14185929
APA StyleKapłan, M., Klimek, K., Syrotyuk, S., Konieczny, R., Jura, B., Smoliński, A., Szymenderski, J., Budnik, K., Anders, D., Dybek, B., Karwacka, A., & Wałowski, G. (2021). Raw Biogas Desulphurization Using the Adsorption-Absorption Technique for a Pilot Production of Agricultural Biogas from Pig Slurry in Poland. Energies, 14(18), 5929. https://doi.org/10.3390/en14185929








