Integration of Safety Aspects in Modeling of Superheated Steam Flash Drying of Tobacco
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
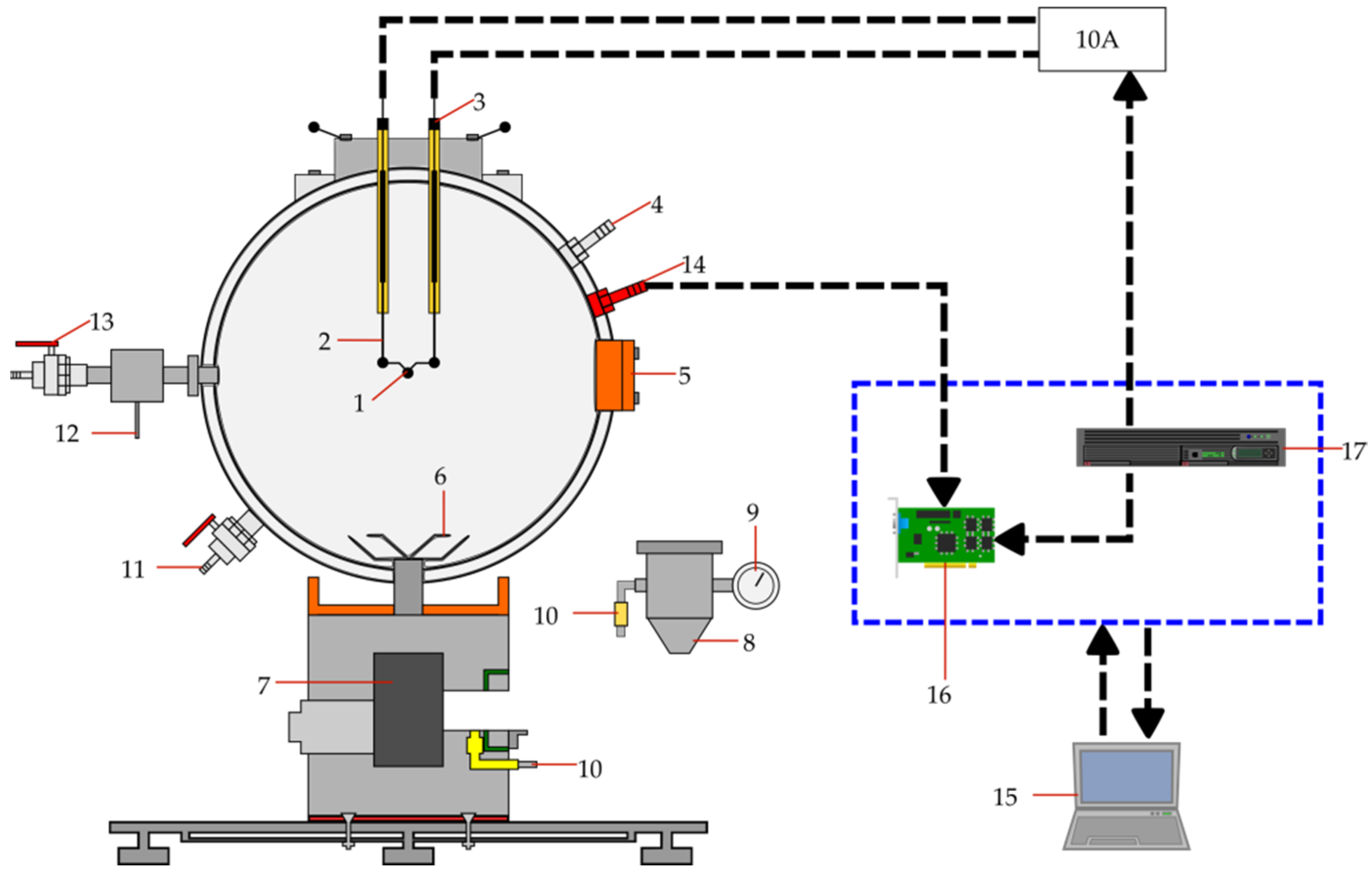
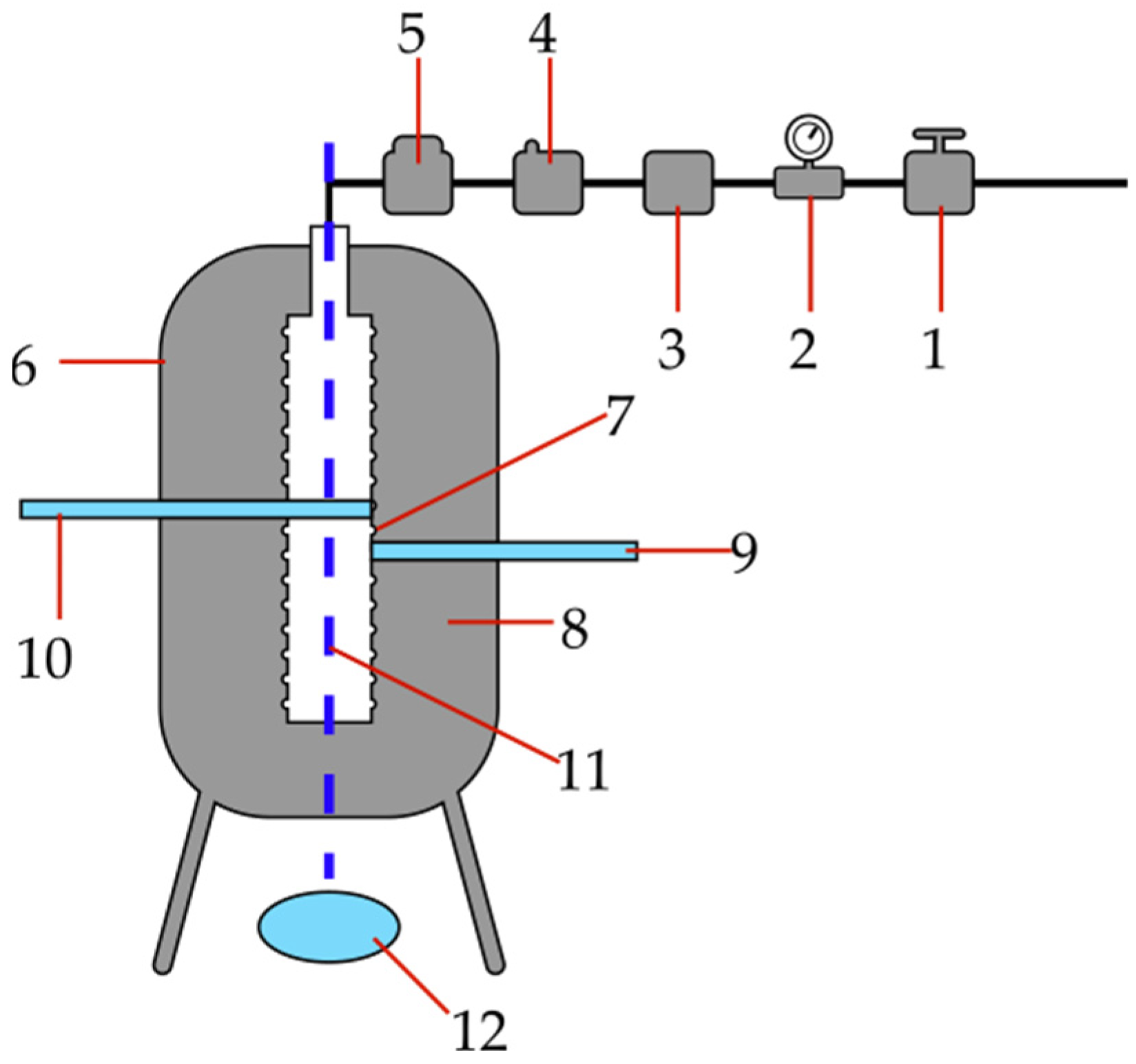
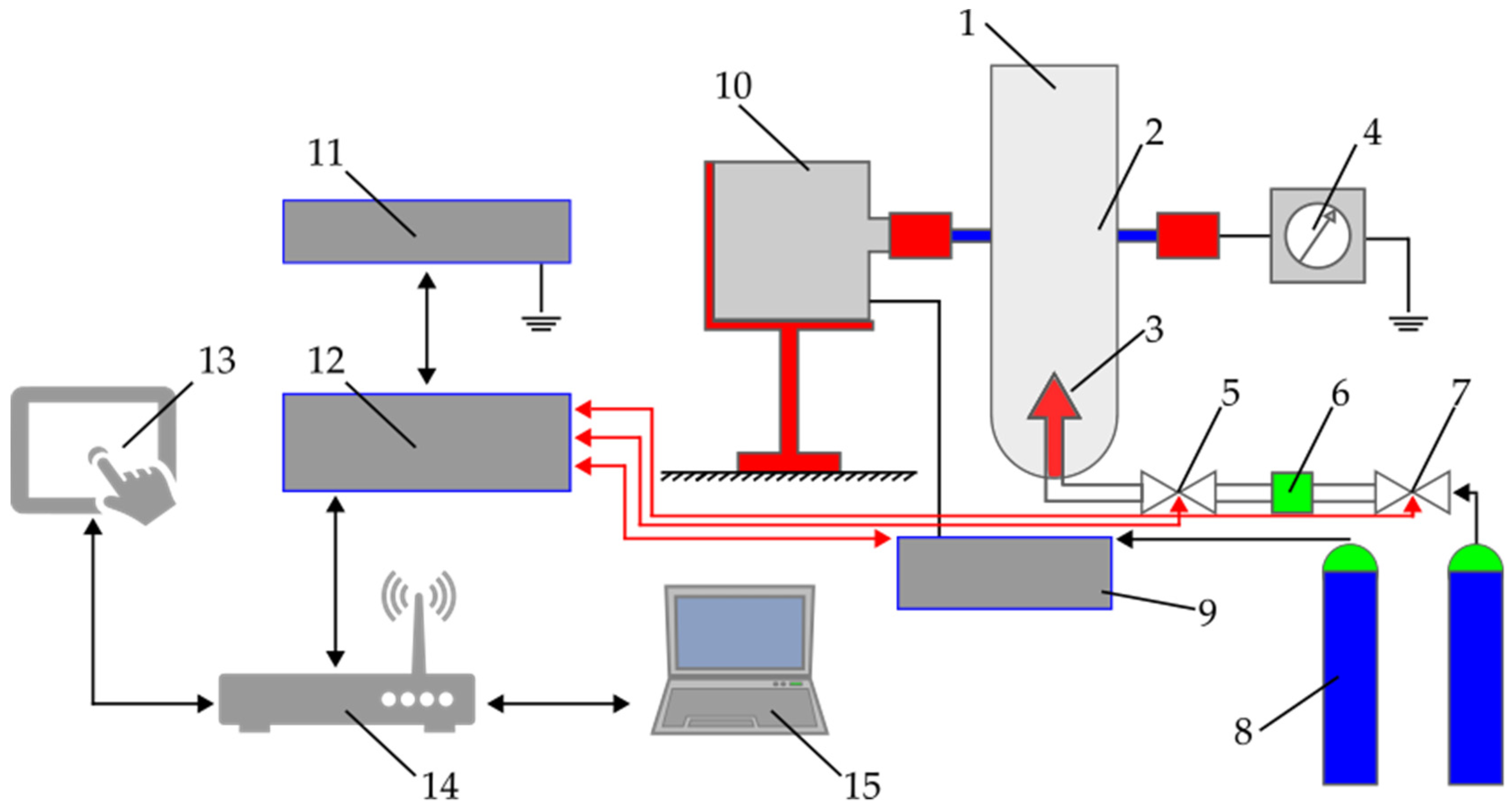
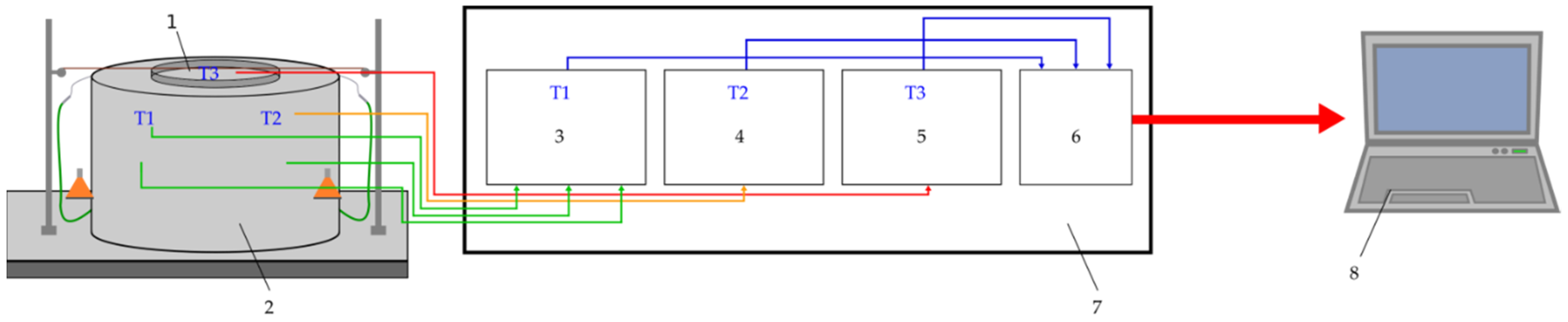
2.2. Methods and Experiments
2.2.1. Properties of Tobacco
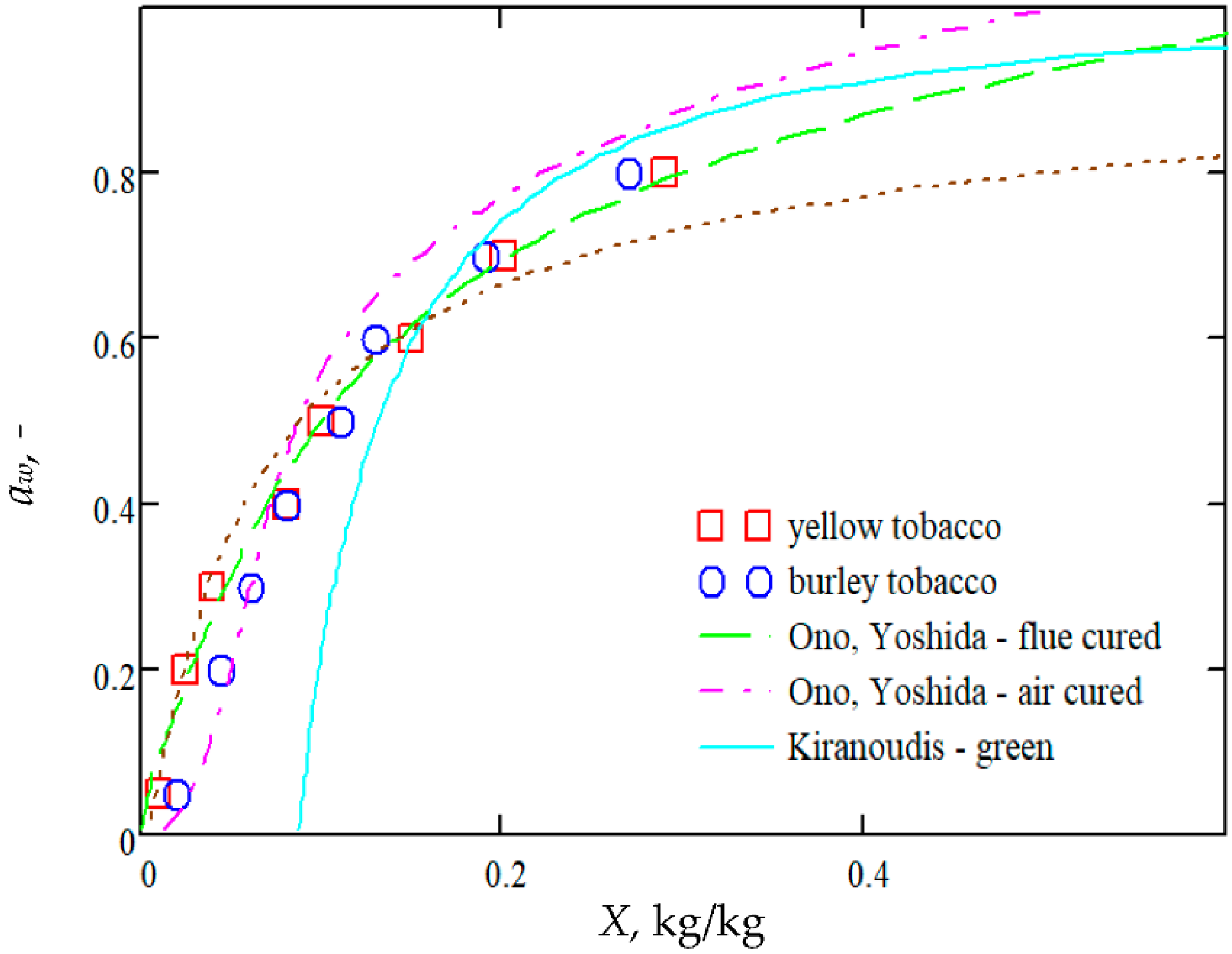
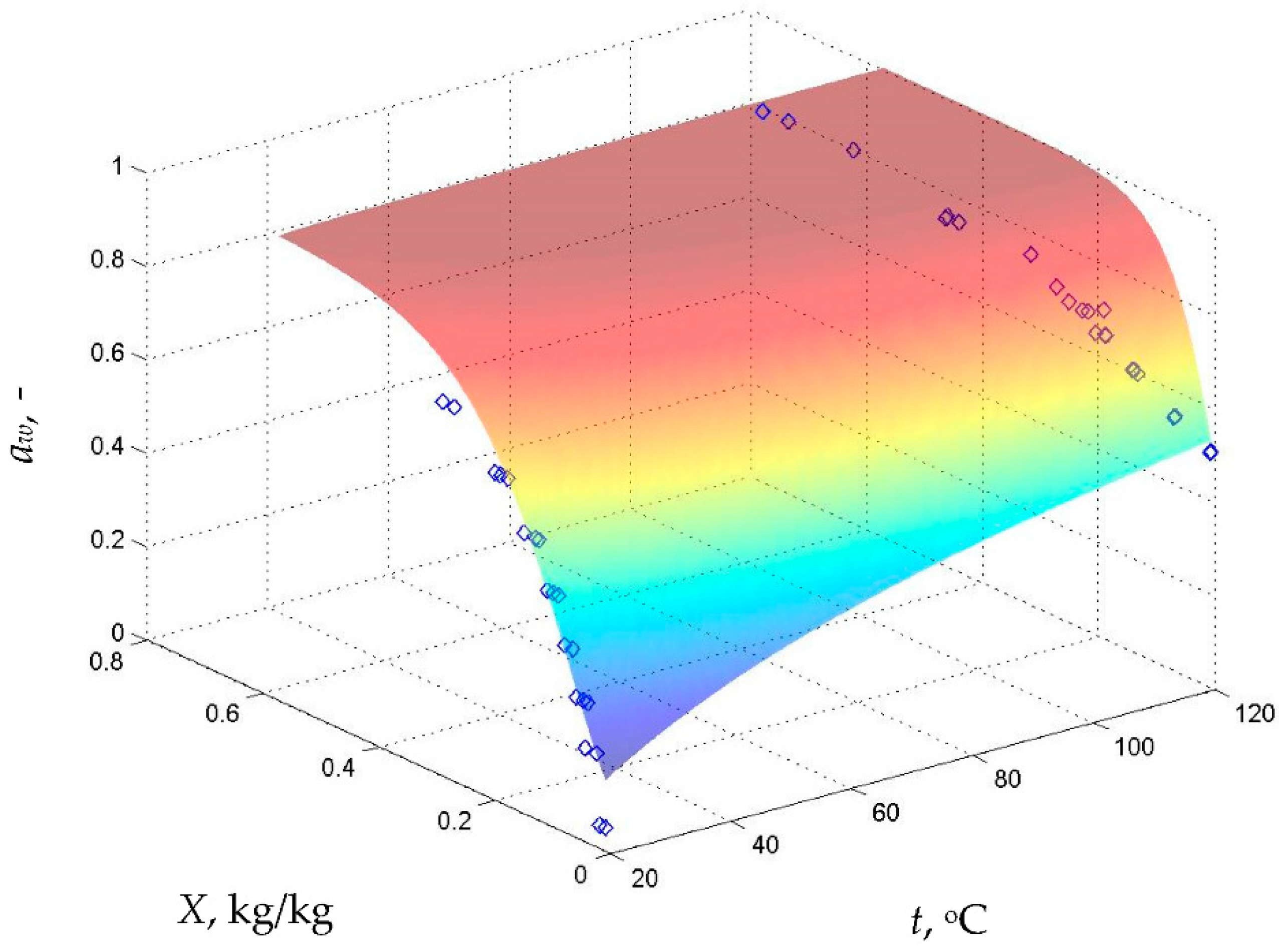
2.2.2. Sorption Isotherms and Isobars
- Heat capacity of tobacco:
- Particle morphology and drag coefficient:
- Explosion and fire properties of dust tobacco:
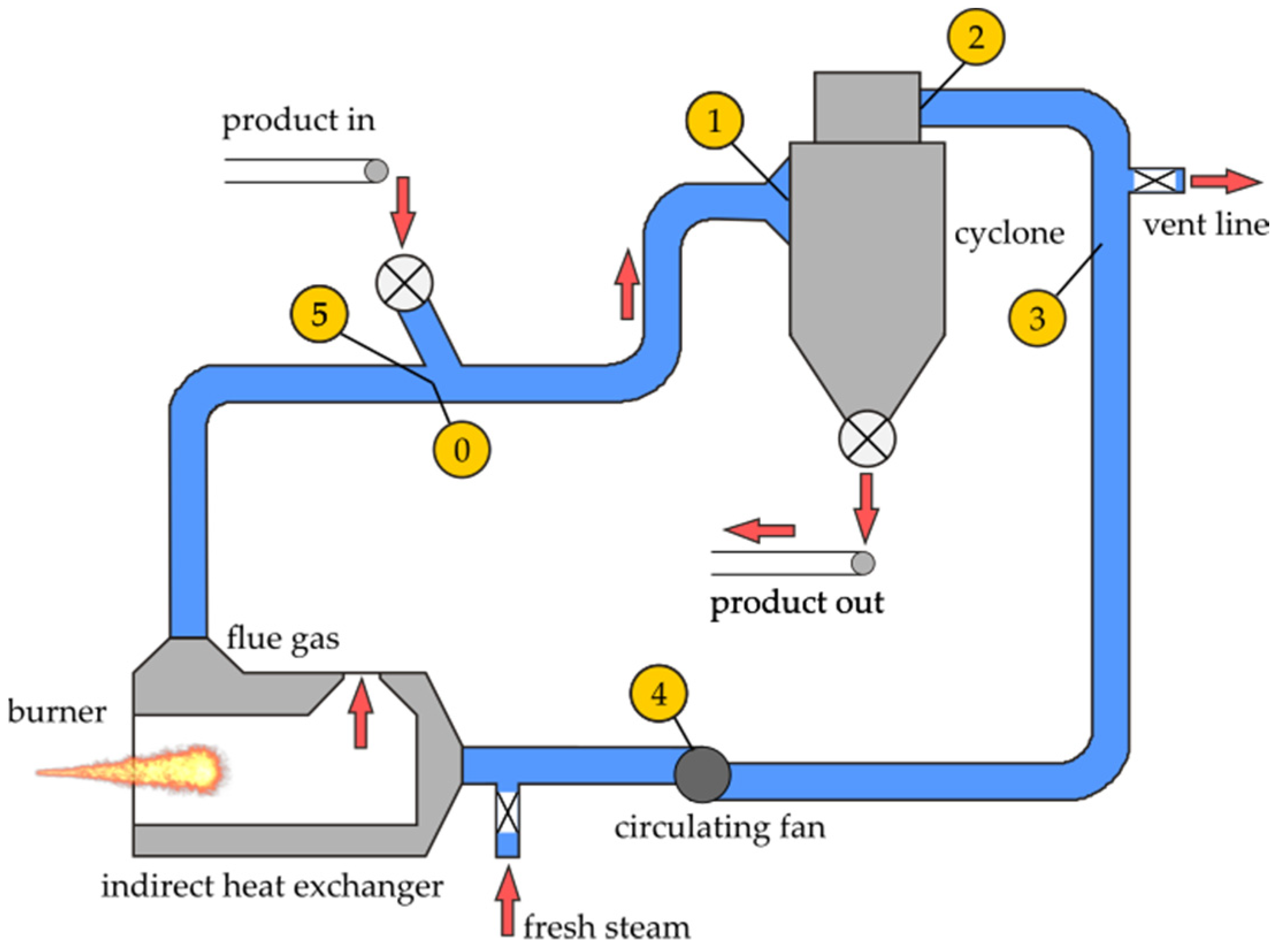
3. Principles of Superheated Steam-Drying
- Heat used for drying can be recovered thus lowering the net heat consumption even below the heat of vaporization.
- Solid temperature exceeds boiling point temperature: This effect causes the expansion of tobacco and sterilizes the product biologically.
- The atmosphere is virtually oxygen-free and thus oxidation reactions and danger of ignition are eliminated.
- Steam-drying is more effective than air-drying, provided that steam temperature is higher than the so-called inversion temperature (at atmospheric pressure the inversion temperature is ca. 175 °C).
4. Proposed Model of Superheated Steam Flash Drying of Cut Tobacco
4.1. Earlier Work
- The solid is represented by single isometric particles of the same size;
- The flow of the suspension is one-dimensional. That is to say, no radial distribution of velocity, temperature, and/or concentration in the pipe are allowed;
- A steady-state plant operation is assumed;
- Only the process of drying in the pipe is considered: drying in a cyclone would require another model.
4.2. Model Formulation
- Mass balance for the solid phase:
- Enthalpy balance for the solid phase:
- Mass balance for the gas phase:
- Enthalpy balance for gas phase:
- The continuity equation for the solid phase:
- Kinetic energy balance for the solid phase:
- Sensible heat flux:
- Drying rate:
- Transport velocity:
- Voidage:
- Friction factors:
- Specific area of contact:
5. Results
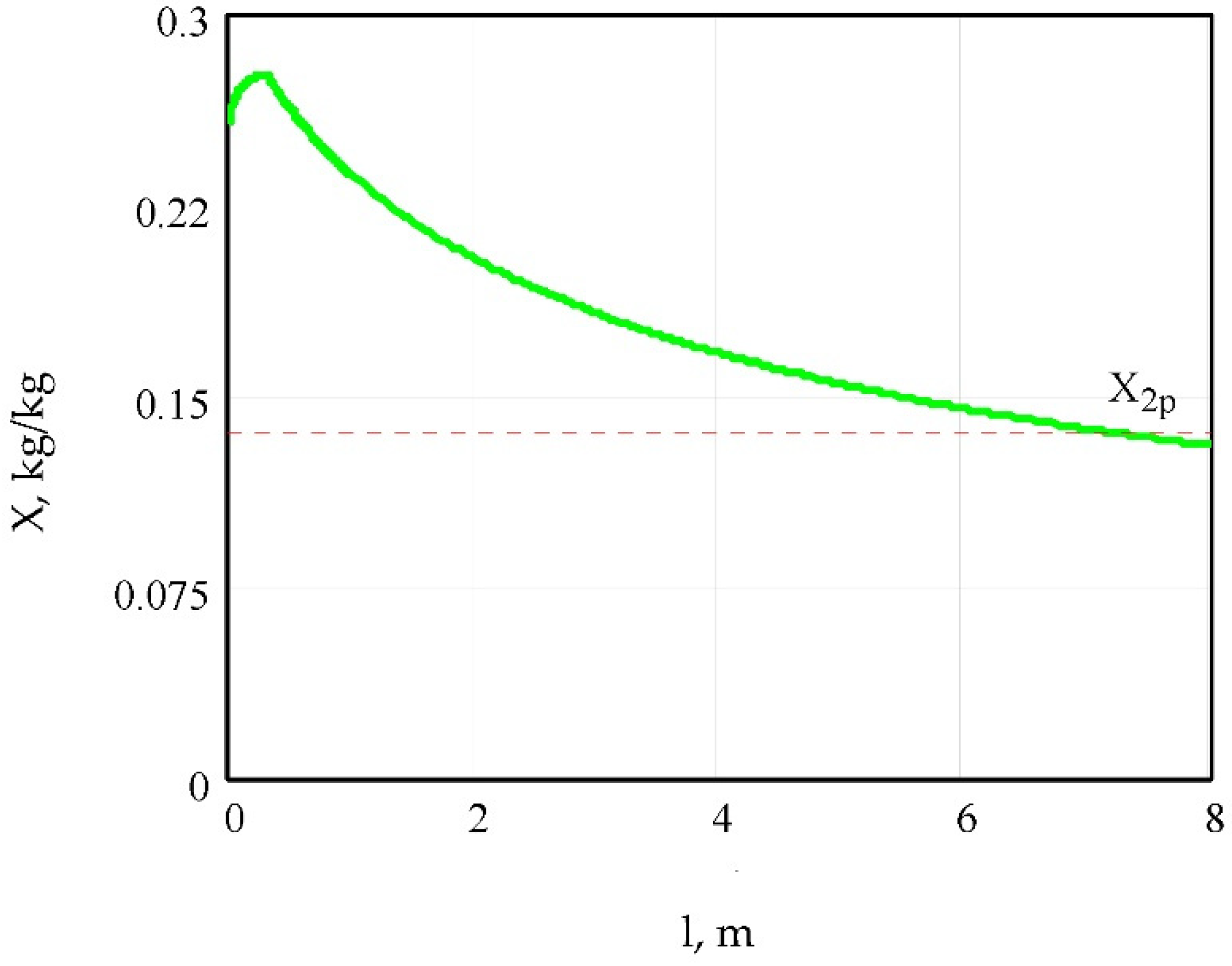
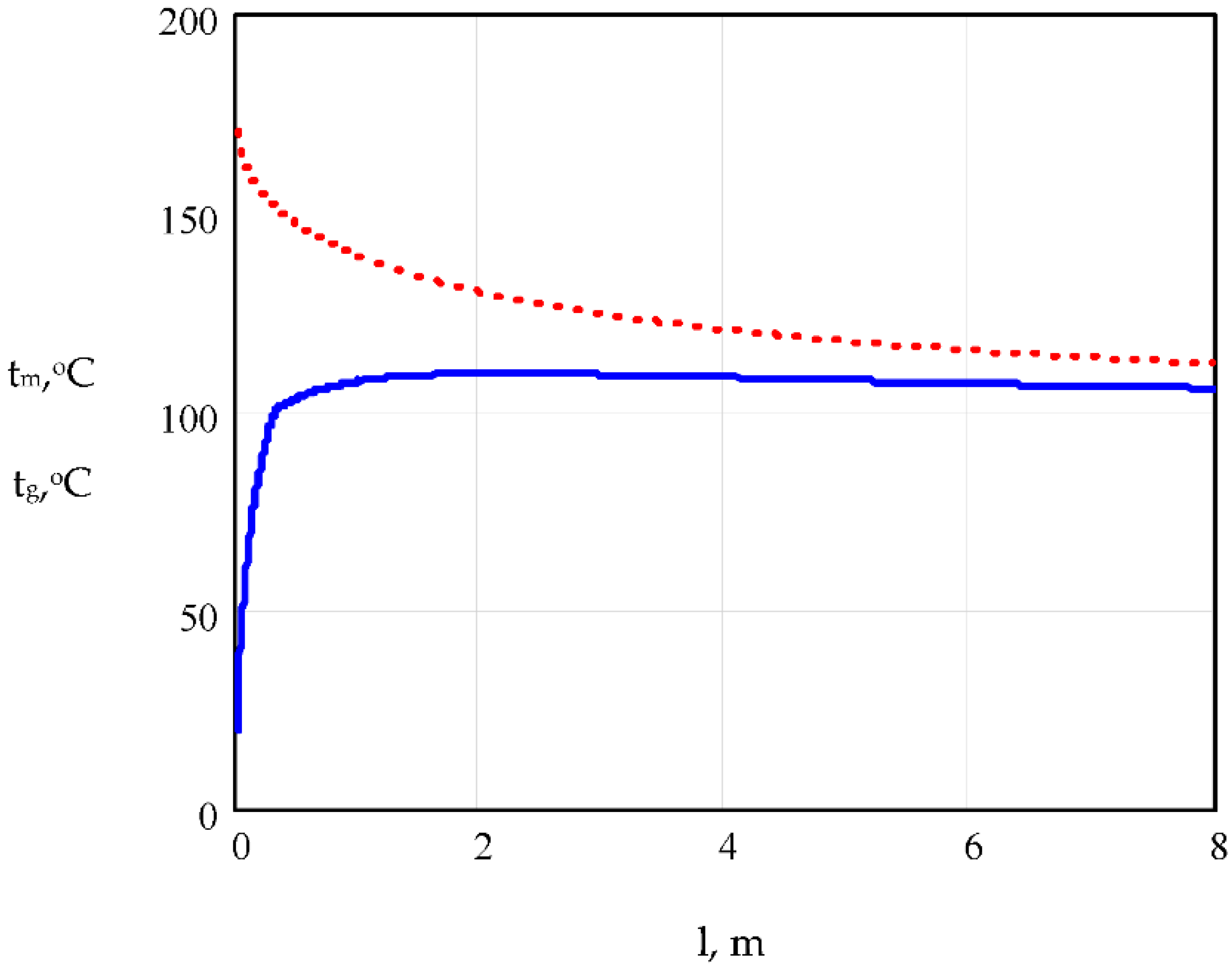
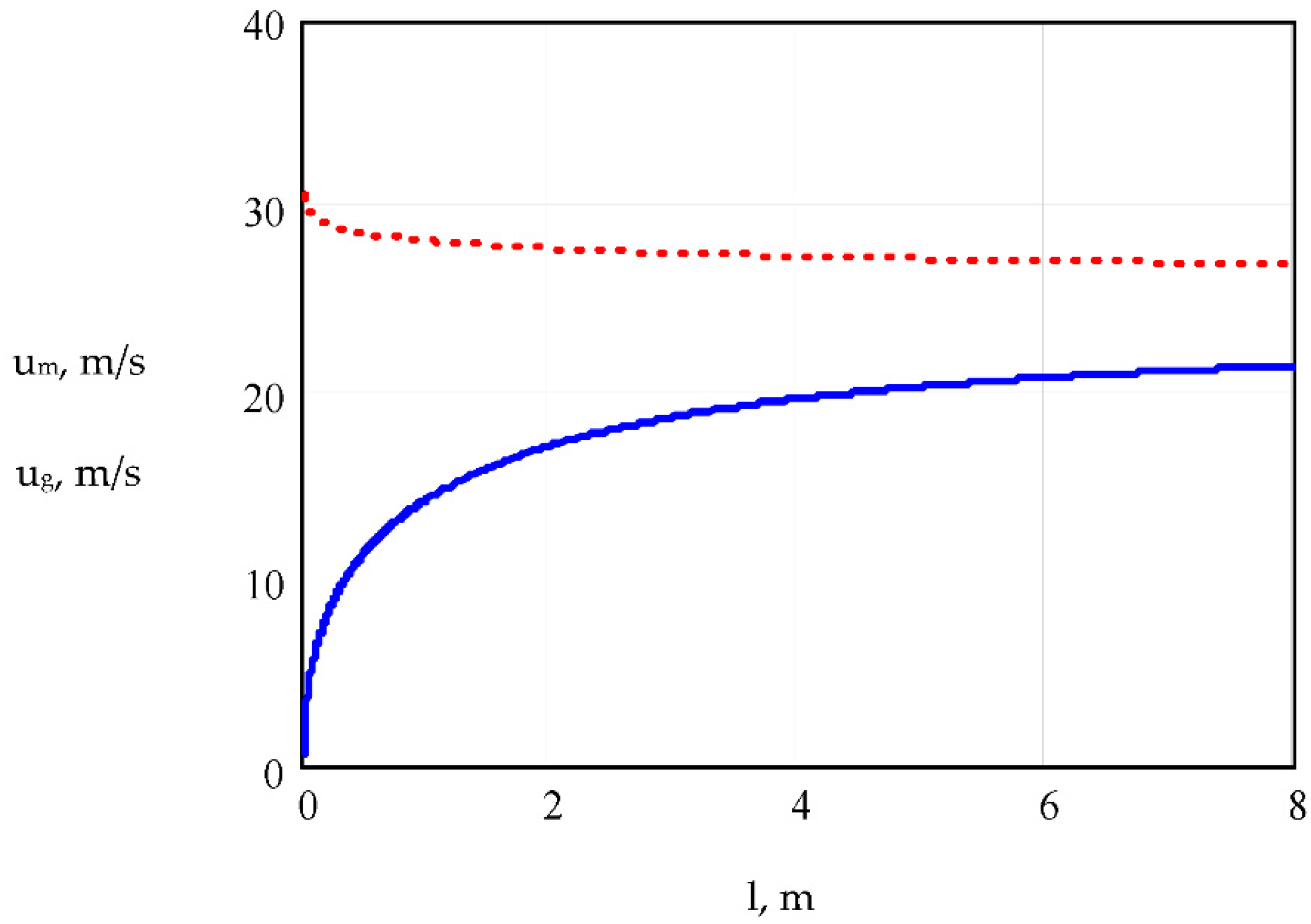
5.1. Model Simulation
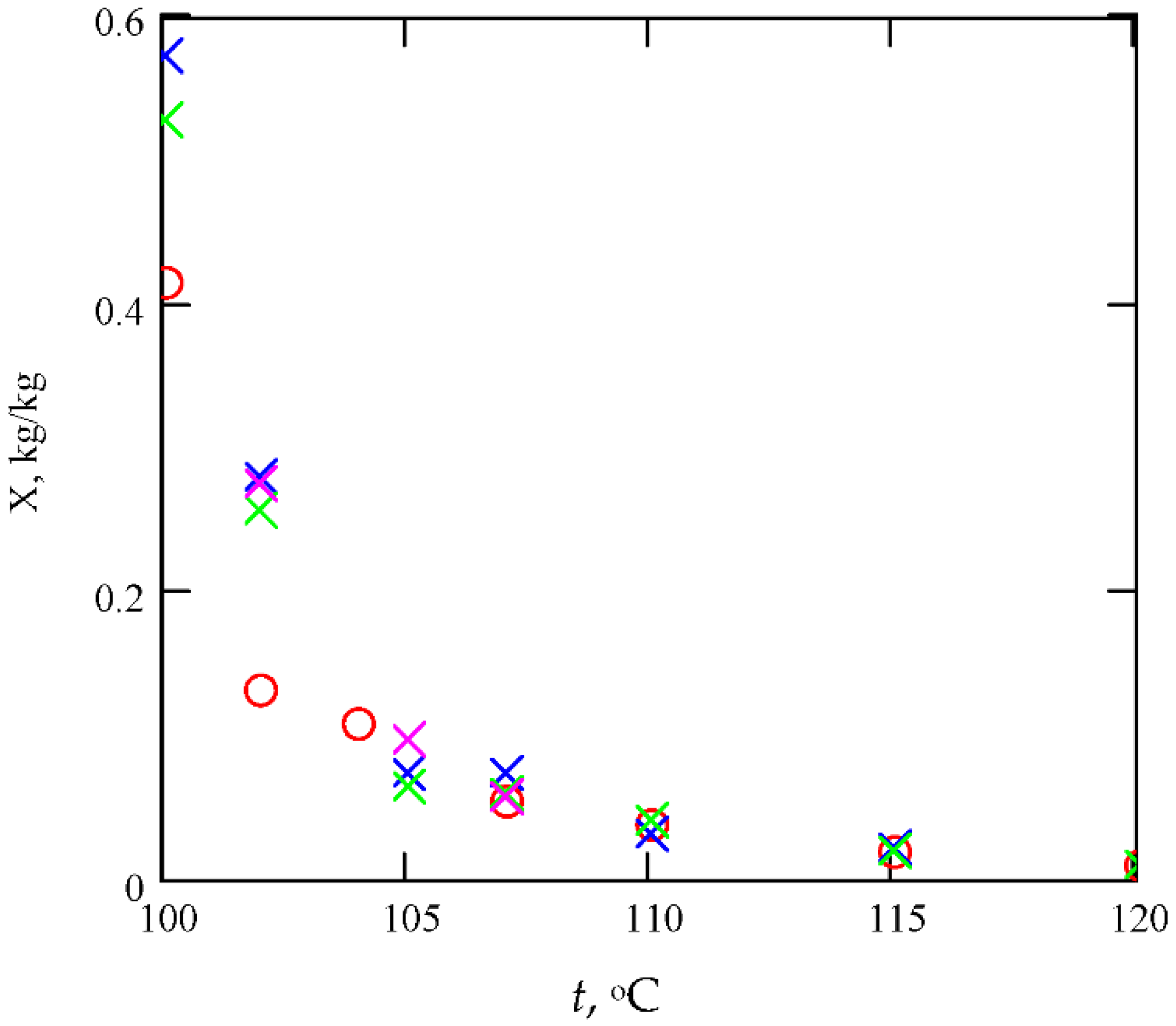
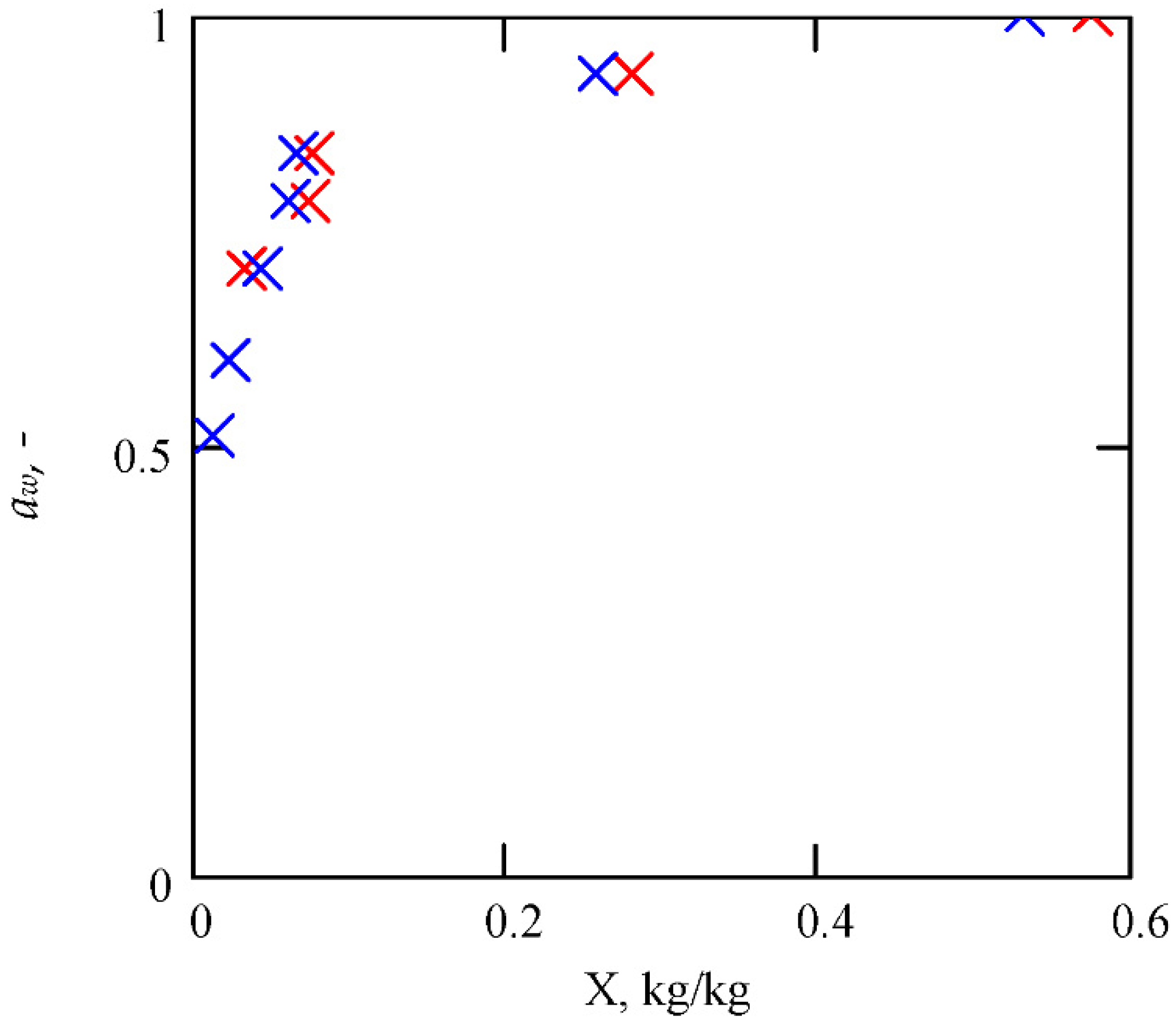
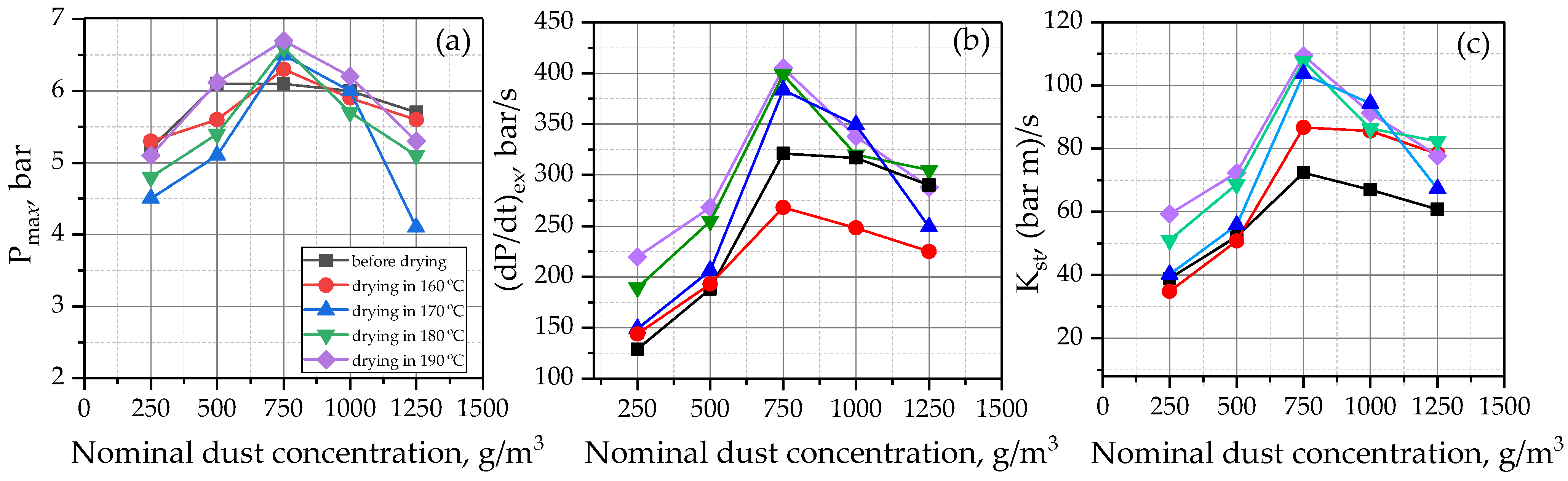
5.2. Fire and Explosion Properties of Tobacco
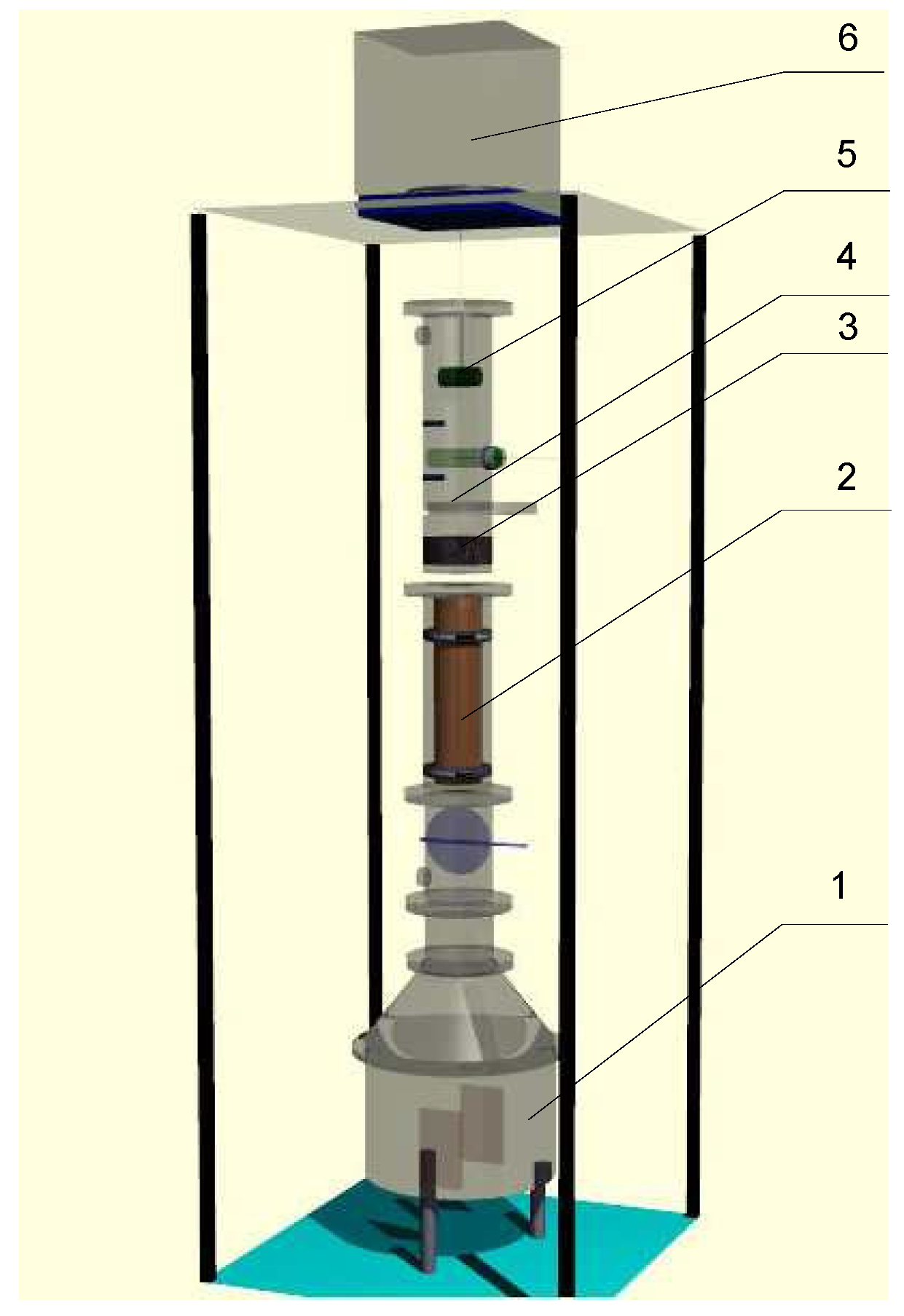
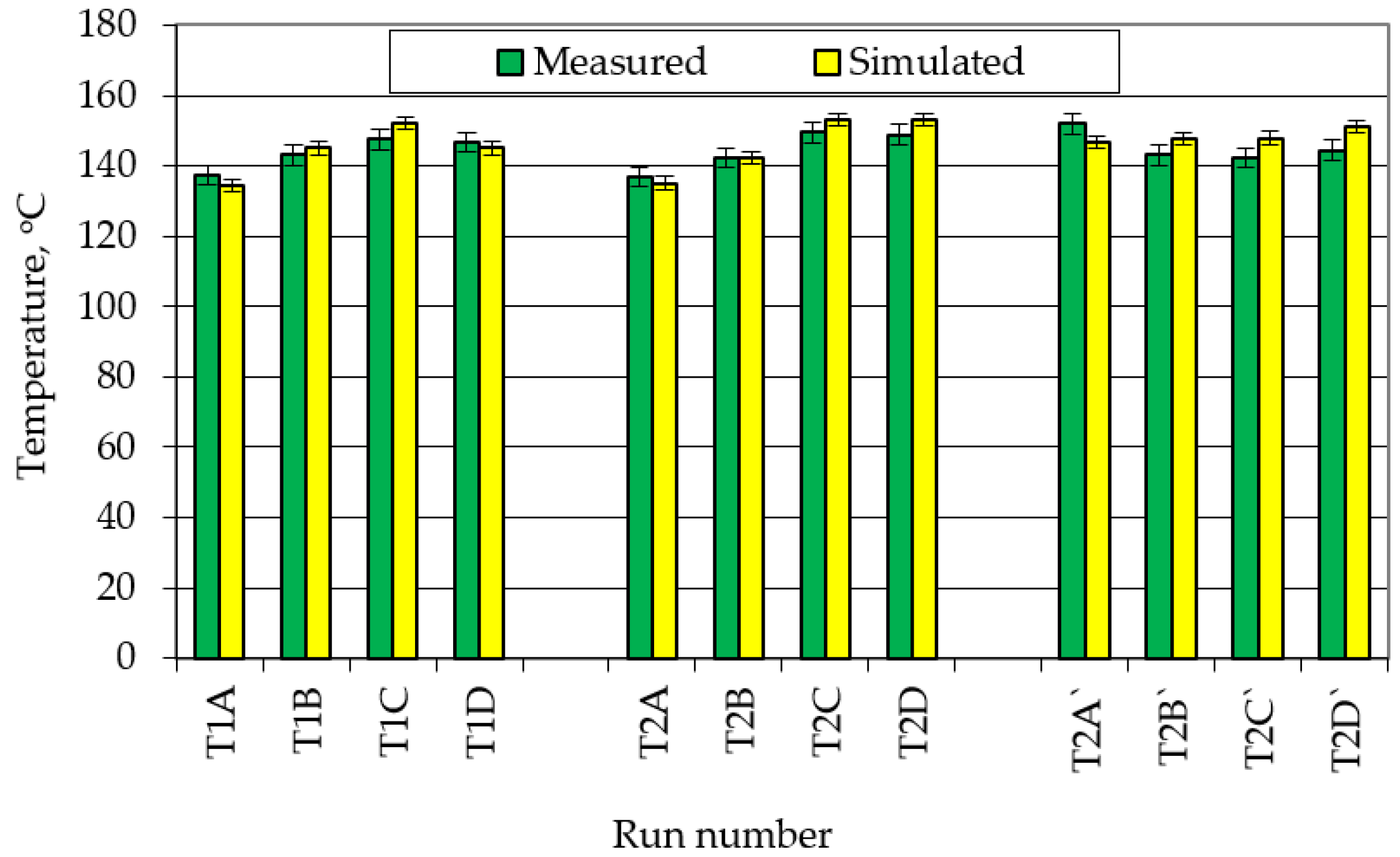
5.3. Validation of Results Obtained from the Proposed Model
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Liang, Q.-C.; Bennamoun, L. Superheated steam drying: Design aspects, energetic performances, and mathematical modeling. Renew. Sustain. Energy Rev. 2016, 60, 1562–1583. [Google Scholar] [CrossRef]
- Jewiarz, M.; Wróbel, M.; Mudryk, K.; Szufa, S. Impact of the drying temperature and grinding technique on biomass grindability. Energies 2020, 13, 3392. [Google Scholar] [CrossRef]
- Cai, C.; Wang, L.; Wang, G.; Hao, J.; Bai, X.; Wang, Z.; Wang, D. Effects of dry explosion pretreatment on physicochemical and fuel properties of hybrid pennisetum (Pennisetum americanum × P. purpureum). Bioresour. Technol. 2020, 297, 122508. [Google Scholar] [CrossRef] [PubMed]
- Pakowski, Z.; Druzdzel, A.; Drwiega, J. Validation of a model of an expanding superheated steam flash dryer for cut tobacco based on processing data. Dry. Technol. 2004, 22, 45–57. [Google Scholar] [CrossRef]
- Kukfisz, B. The potential fire and explosion hazards in biomass co-firing with conventional fossil fuels based on data obtained during testing. E3S Web Conf. 2018, 45, 00039. [Google Scholar] [CrossRef]
- Kukfisz, B.; Siuta, D.; Szaferski, W. Analysis of Coal Dust Ignition Deposited in a Layer at Constant and Variable Temperature of Heating Panel—Practical Aspects of Chemical Engineering; Ochowiak, M., Woziwodzki, S., Mitkowski, P.T., Doligalski, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 209–215. [Google Scholar]
- Adamski, R.; Siuta, D.; Kukfisz, B.; Mitkowski, P.T.; Szaferski, W. Influence of process parameters in superheated steam drying on fire and explosion parameters of woody biomass. Fuel Process. Technol. 2021, 211, 106597. [Google Scholar] [CrossRef]
- Markowski, A.S.; Siuta, D. Fuzzy logic approach for identifying representative accident scenarios. J. Loss Prev. Process. Ind. 2018, 56, 414–423. [Google Scholar] [CrossRef]
- Le, K.H.; Tran, T.T.H.; Kharaghani, A.; Tsotsas, E. Modeling of superheated steam drying of wood particles. J. Mech. Eng. Res. Dev. 2020, 43, 160–170. [Google Scholar]
- Tran, T.T.H. Modelling of drying in packed bed by super heated steam. J. Mech. Eng. Res. Dev. 2020, 43, 135–142. [Google Scholar]
- Ono, T.; Yoshida, T. Designing of Dryer in Tobacco Manufacturing Process. Drying’86 1986, 2, 630–636. [Google Scholar]
- Kiranoudis, C.T.; Maroulis, Z.B.; Marinos-Kouris, D. Mass transfer modeling for virginia tobacco curing. Dry. Technol. 1990, 8, 351–366. [Google Scholar] [CrossRef]
- Legros, R.; Alan Millington, C.; Clift, R. Drying of tobacco particles in a mobilised bed. Dry. Technol. 1994, 12, 517–543. [Google Scholar] [CrossRef]
- Miyauchi, M.; Miyake, A.; Nakanishi, Y.; Sagara, Y. Characteristics of water adsorption isotherms for materials contained in a box of a tobacco product. Dry. Technol. 1995, 13, 351–370. [Google Scholar] [CrossRef]
- Pakowski, Z.; Krupinska, B.; Adamski, R. Prediction of sorption equilibrium both in air and superheated steam drying of energetic variety of willow Salix viminalis in a wide temperature range. Fuel 2007, 86, 1749–1757. [Google Scholar] [CrossRef]
- Pakowski, Z.; Krupińska, B.; Adamski, R. Sorption isobars of tobacco materials required for the superheated steam drying processes. Chem. Process Eng. Inz. Chem. Proces. 2006, 27, 507–517. [Google Scholar]
- Bandrowski, J.; Kaczmarzyk, G. Gas-to-particle heat transfer in vertical pneumatic conveying of granular materials. Chem. Eng. Sci. 1978, 33, 1303. [Google Scholar] [CrossRef]
- Kerekes, B.; Lengyel, A.; Sikolya, L. Thermophysical and transport properties in curing of tobacco. Drying’98 1998, 98, 719–726. [Google Scholar]
- Haider, A.; Levenspiel, O. Drag coefficient and terminal velocity of spherical and nonspherical particles. Powder Technol. 1989, 58, 63–70. [Google Scholar] [CrossRef]
- Hausbrand, E. Das Trocken Mit Luft Und Dampf, 5th ed.; Springer: Berlin/Heidelberg, Germany, 1920. [Google Scholar]
- Pang, L.; Cao, J.; Ma, R.; Zhao, Y.; Yang, K. Risk assessment method of polyethylene dust explosion based on explosion parameters. J. Loss Prev. Process Ind. 2021, 69, 104397. [Google Scholar] [CrossRef]
- Eckhoff, R.K. Measurement of minimum ignition energies (MIEs) of dust clouds—History, present, future. J. Loss Prev. Process Ind. 2019, 61, 147–159. [Google Scholar] [CrossRef]
- Dyduch, Z.; Majcher, B. Ignition of a dust layer by a constant heat flux-heat transport in the layer. J. Loss Prev. Process Ind. 2006, 19, 233–237. [Google Scholar] [CrossRef]
- Meunier, J.; Munz, R.J. Flash drying with superheated steam—A mathematical model. Drying’86 1986, 2, 580–587. [Google Scholar]
- Blasco, R.; Vega, R.; Alvarez, P.I. Pneumatic drying with superheated steam: Bi-dimensional model for high solid concentration. Dry. Technol. 2001, 19, 2047–2061. [Google Scholar] [CrossRef]
- Goroshko, V.D.; Rozenbaum, R.B.; Todes, O.M. Hydrodynamics and Heat Transfer in Fluidized Beds; M.I.T. Press: Cambridge, MA, USA, 1966; p. 71. [Google Scholar]
- Reay, D.; Bahu, R. Particle velocities in pneumatic conveying dryers. Drying’84 1984, 1, 222–227. [Google Scholar]
- Nebra, S.; Silva, M.A.; Mujumdar, A.S. Drying in cyclones—A review. Dry. Technol. 2000, 18, 791–832. [Google Scholar] [CrossRef]
- Pakowski, Z.; Bartczak, Z.; Strumillo, C.; Stenström, S. Evaluation of equations approximating thermodynamic and transport properties of water, steam and air for use in cad of drying processes. Dry. Technol. 1991, 9, 753–773. [Google Scholar] [CrossRef]
- Markowski, A.S.; Mujumdar, A.S. Drying risk assessment strategies. Dry. Technol. 2004, 22, 395–412. [Google Scholar] [CrossRef]
- Markowski, A.S.; Siuta, D. Selection of representative accident scenarios for major industrial accidents. Process Saf. Environ. Prot. 2017, 111, 652–662. [Google Scholar] [CrossRef]


| Sample | C | H | N | S | O * |
|---|---|---|---|---|---|
| Tobacco | 36.69 ± 0.12 | 5.68 ± 0.04 | 2.90 ± 0.02 | 0.28 ± 0.02 | 54.45 |
| Total moisture | Volatiles | Fived carbon | Ash | HHV (MJ/kg) | |
| 20.10 ± 0.12 | 56.90 ± 0.35 | 11.90 ± 0.21 | 11.10 ± 0.11 | 14.30 |
| Parameter | Unit | Standard |
|---|---|---|
| Pmax | bar | EN 14034-1 |
| (dP/dt)max | bar/s | EN 14034-2 |
| Kstmax | ((bar·m)/s) | EN 14034-2 |
| MEC LOC | g/m3 volume % | EN 14034-3, ISO/IEC 80079-20-2 EN 14034-4 |
| MIT5mm MIT50mm | °C °C | EN 50281-2-1, ISO/IEC 80079-20-2 (method CTHP) EN 50281-2-1, ISO/IEC 80079-20-2 (method CTHP) |
| MIT5mm MIT50mm | °C °C | (non-standard method CRHG) (non-standard method CRHG) |
| Tcl | °C | EN 50281-2-1, ISO/IEC 80079-20-2 |
| MIE | mJ | EN 13821, ISO/IEC 80079-20-2 |
| Dimension and Parameters of Superheated Steam Pneumatic Dryer | Results |
|---|---|
| Length, m | 8.02 |
| Diameter, m | 0.5 |
| Dryer capacity, tons/h | 3 |
| Inlet steam temperature, °C | 300 |
| Steam velocity, m/s | 30 |
| Coefficient of heating medium excess, kg/kg | 9 |
| Material dwell time, s | 0.4 |
| Parameter | Unit | Before Drying | After Drying at Temperatures of | |||
|---|---|---|---|---|---|---|
| 160 °C | 170 °C | 180 °C | 190 °C | |||
| Pmax | bar | 6.1 ± 0.3 | 6.3 ± 0.3 | 6.5 ± 0.3 | 6.6 ± 0.3 | 6.7 ± 0.3 |
| (dP/dt)max | bar/s | 268 ± 22 | 321 ± 34 | 384 ± 38 | 399 ± 36 | 405 ± 32 |
| Kst max | (m·bar)/s | 72 ± 12 | 87 ± 10 | 104 ± 11 | 108 ± 18 | 109 ± 14 |
| MEC | g/m3 | 125 ± 24. 2 | 60 ± 11.2 | 60 ± 9.5 | 60 ± 9.1 | 60 ± 9.3 |
| LOC | %O2 | 21 | 20 | 20 | 20 | 20 |
| MIT5mm (CTHP) | °C | 320 ± 2.9 | 310 ± 2.6 | 300 ± 2.9 | 290 ± 2.8 | 290 ± 2.9 |
| MIT50mm (CTHP) | °C | 300 ± 2.7 | 280 ± 2.9 | 280 ± 2.8 | 270 ± 2.9 | 260 ± 2.8 |
| MIT5mm (CRHG) | °C | 280 ± 2.9 | 270 ± 2.8 | 270 ± 3.6 | 270 ± 3.2 | 260 ± 3.2 |
| MIT50mm (CRHG) | °C | 290 ± 4.3 | 280 ± 4.1 | 270 ± 3.8 | 260 ± 2.6 | 250 ± 3.8 |
| Tcl | C | 510 ± 3.8 | 440 ± 2.6 | 440 ± 3.6 | 440 ± 2.6 | 420 ± 3.6 |
| MIE | mJ | MIE > 1000 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamski, R.; Siuta, D.; Kukfisz, B.; Frydrysiak, M.; Prochoń, M. Integration of Safety Aspects in Modeling of Superheated Steam Flash Drying of Tobacco. Energies 2021, 14, 5927. https://doi.org/10.3390/en14185927
Adamski R, Siuta D, Kukfisz B, Frydrysiak M, Prochoń M. Integration of Safety Aspects in Modeling of Superheated Steam Flash Drying of Tobacco. Energies. 2021; 14(18):5927. https://doi.org/10.3390/en14185927
Chicago/Turabian StyleAdamski, Robert, Dorota Siuta, Bożena Kukfisz, Michał Frydrysiak, and Mirosława Prochoń. 2021. "Integration of Safety Aspects in Modeling of Superheated Steam Flash Drying of Tobacco" Energies 14, no. 18: 5927. https://doi.org/10.3390/en14185927
APA StyleAdamski, R., Siuta, D., Kukfisz, B., Frydrysiak, M., & Prochoń, M. (2021). Integration of Safety Aspects in Modeling of Superheated Steam Flash Drying of Tobacco. Energies, 14(18), 5927. https://doi.org/10.3390/en14185927







