Plasma Technology for Phosphogypsum Treatment
Abstract
:1. Introduction
2. Materials and Methods
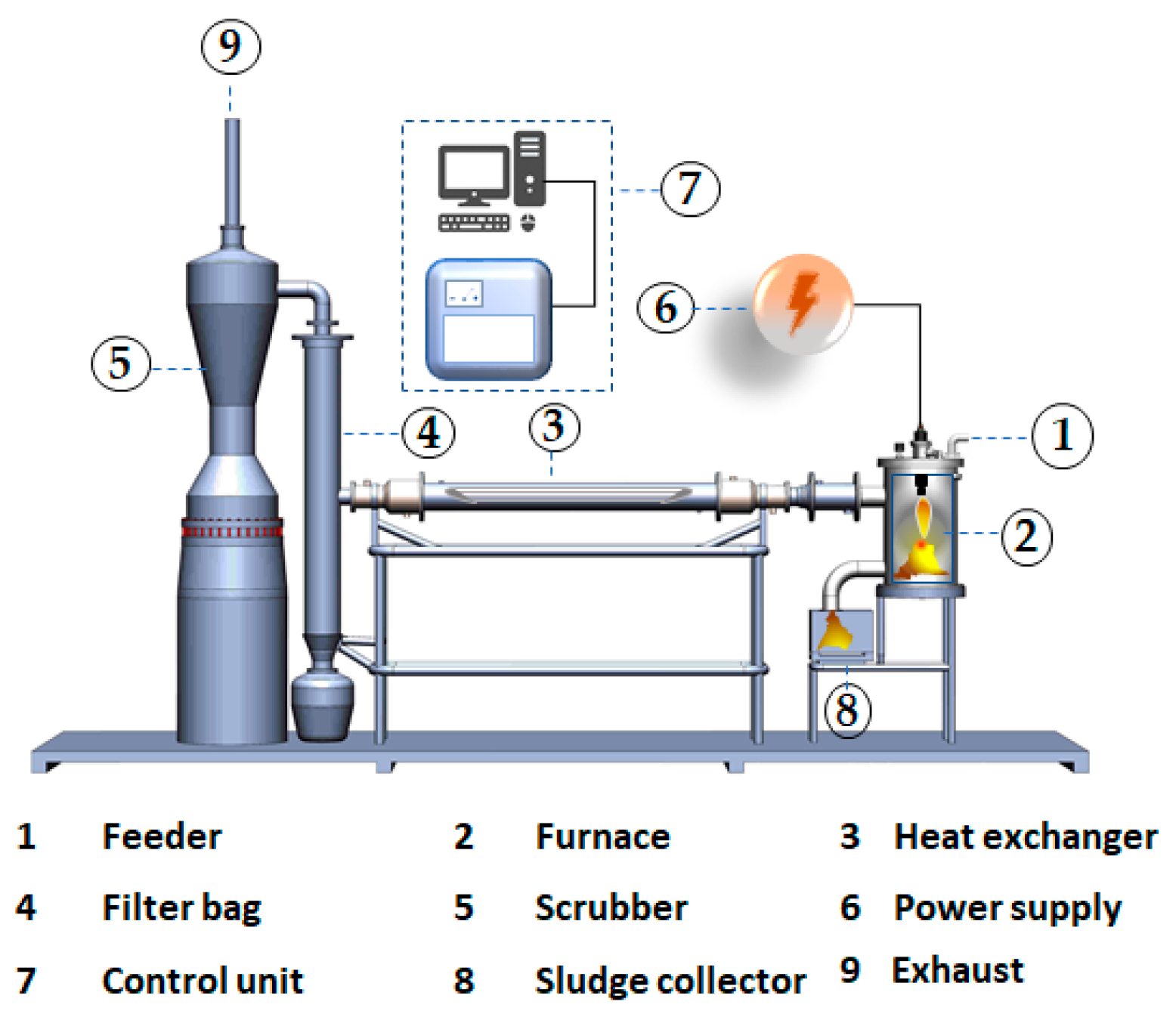
2.1. Experimental Setup
2.2. Chemical Qualitative Methods
3. Results
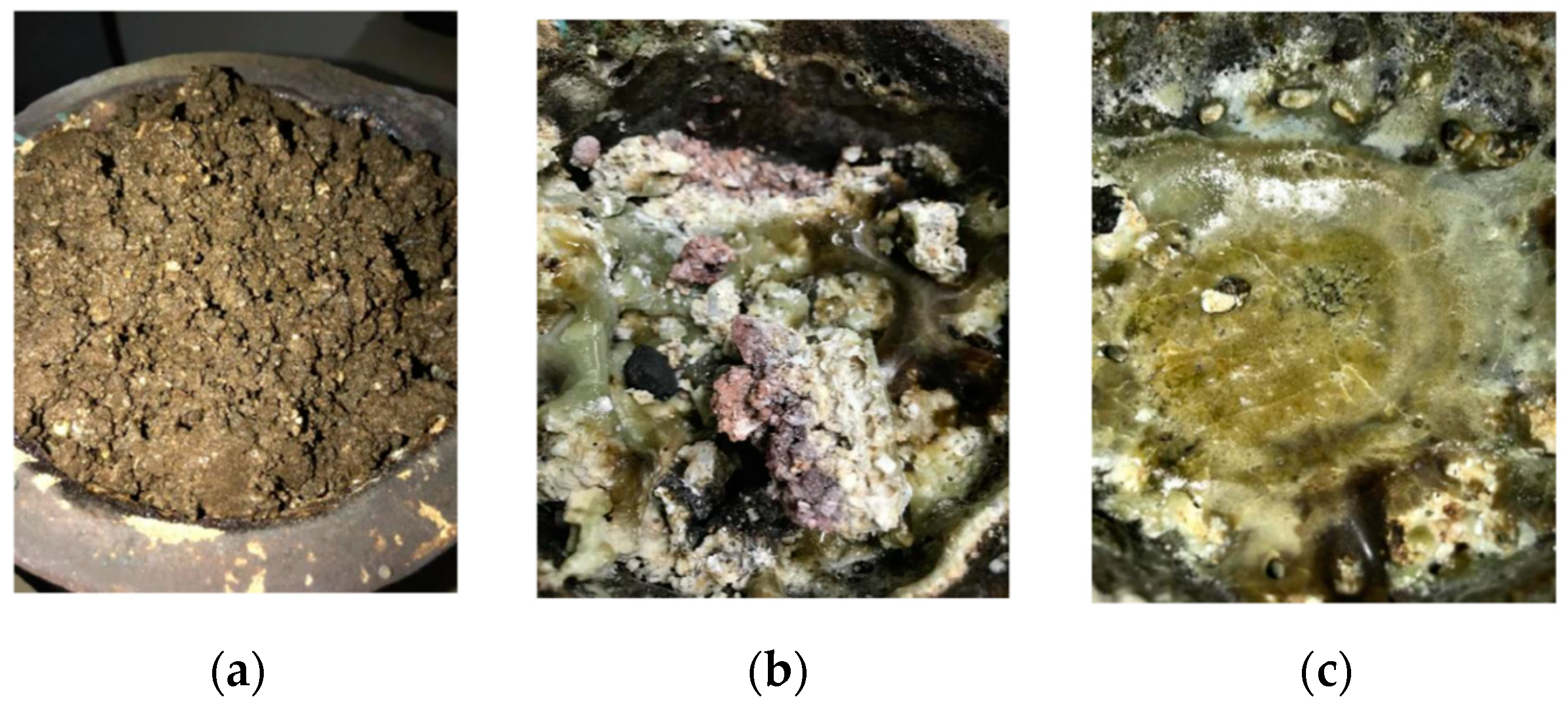
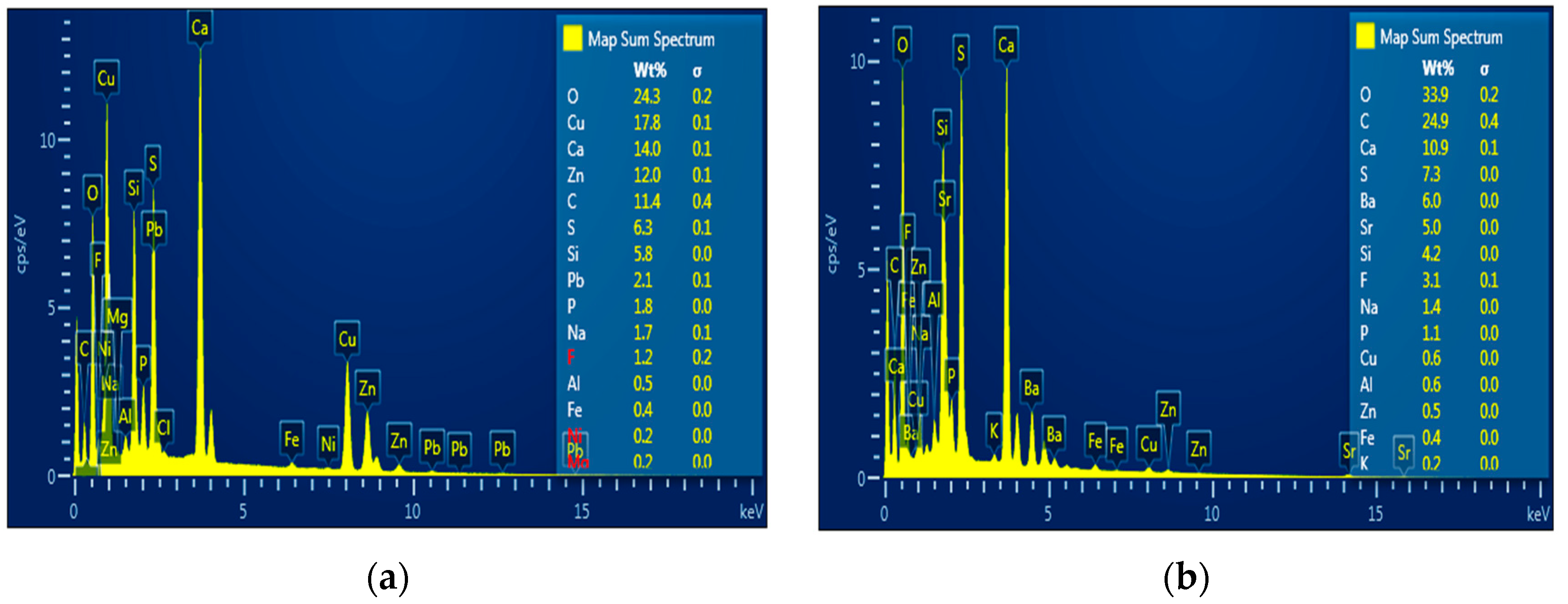
3.1. Characterization of Phosphate Waste before Treatment
3.2. Treatment of Waste Using Thermal Plasma
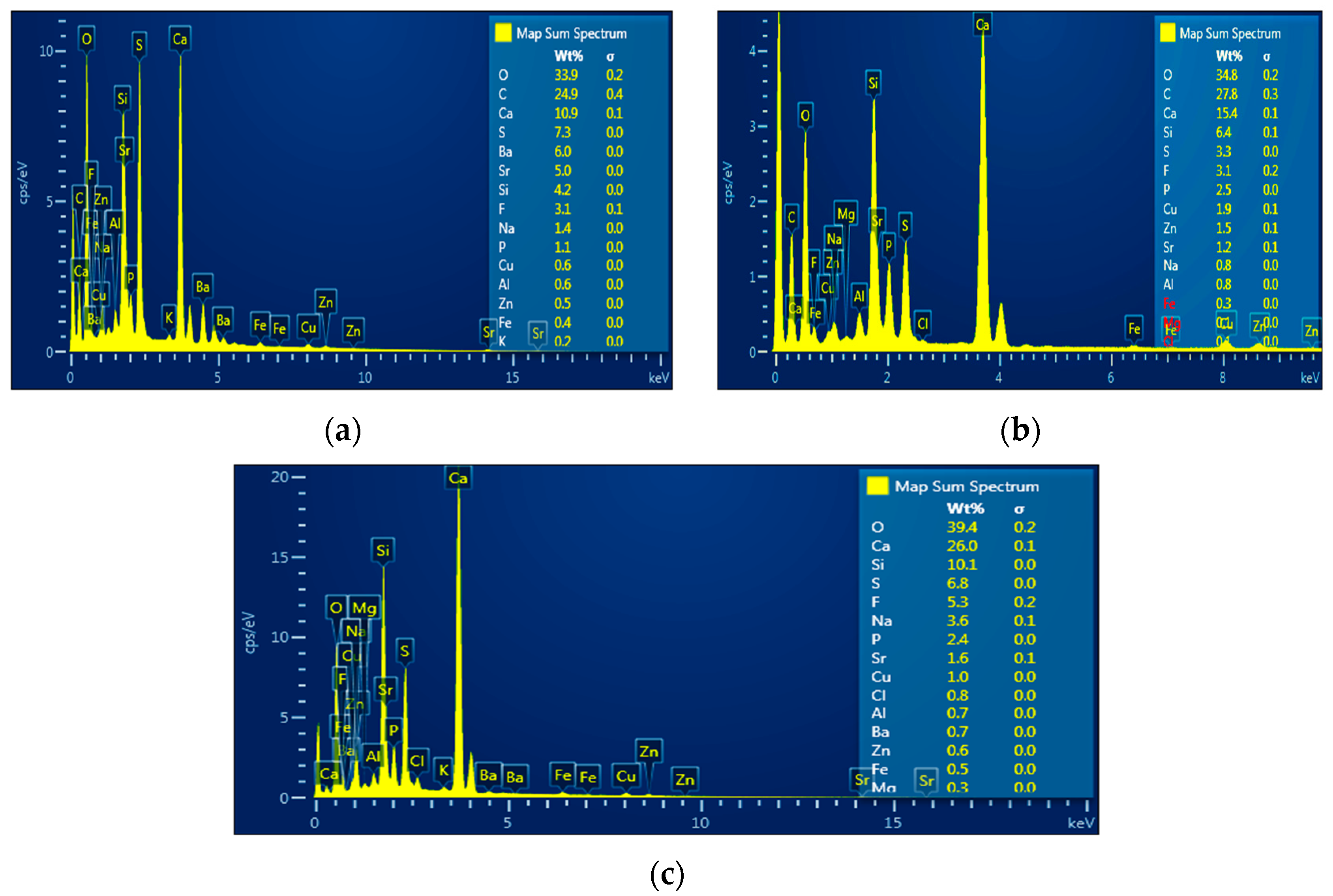
3.2.1. Influence of Time of Treatment
3.2.2. Influence of Plasma Current
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruttenberg, K.C. Reassessment of the oceanic residence time of phosphorus. Chem. Geol. 1993, 107, 405–409. [Google Scholar] [CrossRef]
- FoÈ llmi, K.B. The phosphorus cycle, phosphogenesis and marine phosphate-rich deposits. Earth-Sci. Rev. 1996, 40, 55–124. [Google Scholar] [CrossRef]
- Ruttenberg, K.C.; Dyhrman, S.T. Dissolved organic phosphorus production during simulated phytoplankton blooms in a coastal upwelling system. Front. Microbiol. 2012, 3, 274. [Google Scholar] [CrossRef] [Green Version]
- Cisse, L.; Mrabet, T. World Phosphate Production: Overview and Prospects. Phosphorus Res. Bull. 2004, 15, 21–25. [Google Scholar] [CrossRef] [Green Version]
- El Kateb, A.; Stalder, C.; RuÈggeberg, A.; Neururer, C.; Spangenberg, J.E.; Spezzaferri, S. Impact of industrial phosphate waste discharge on the marine environment in the Gulf of Gabes (Tunisia). PLoS ONE 2018, 13, e0197731. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P. Comprehensive recovery and sustainable development of phosphate resources. Procedia Eng. 2014, 83, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Pérez-López, R.; Alvarez-Valero, A.M.; Nieto, J.M. Changes in mobility of toxic elements during the production of phosphoric acid in the fertilizer industry of Huelva (SW Spain) and environmental impact of phosphogypsum wastes. J. Hazard. Mater. 2007, 1487, 745–750. [Google Scholar] [CrossRef]
- Reta, G.; Dong, X.; Zhonghua, L. Environmental impact of phosphate mining and beneficiation. Rev. Int. J. Hydrol. 2018, 2, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, P.M.; Dudas, M.J.; Samek, R.A. Environmental impacts of phosphogypsum. Sci. Total Environ. 1994, 149, 1–38. [Google Scholar] [CrossRef]
- El Afifi, E.M.; Hilal, M.A.; Attallah, M.F.; El-Reefy, S.A. Characterization of phosphogypsum wastes associated with phosphoric acid and fertilizers production. J. Environ. Radioact. 2009, 100, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Rosales, J.; Pérez, S.M.; Cabrera, M.; Gázquez, M.J.; Bolivar, J.P.; de Brito, J.; Agrela, F. Treated phosphogypsum as an alternative set regulator and mineral addition in cement production. J. Clean. Prod. 2020, 244, 118752. [Google Scholar] [CrossRef]
- Zairi, M.; Rouis, M.J. Impacts environnementaux du stockage du phosphogypse à Sfax (Tunisie). Bull. Lab. Ponts Chaussées 1999, 219, 29–40. [Google Scholar]
- Ajam, L.; Ben Ouezdou, M.; Sfar Felfoul, H.; El Mensi, R. Characterization of the Tunisian phosphogypsum and its valorization in clay bricks. Constr. Build. Mater. 2009, 23, 3240–3247. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; LoÂpez, F.A.; Alguacil, F.J.; LoÂpez-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajmal, P.Y.; Bhangare, R.C.; Tiwari, M.; Sahu, S.K.; Pandit, G.G. External gamma radiation levels and natural radioactivity in soil around a phosphate fertilizer plant at Mumbai. J. Radioanal. Nucl. Chem. 2014, 300, 23–27. [Google Scholar] [CrossRef]
- Darmoul, B.; Hadj Ali Salem, M.; Vitiello, P. Effets des rejets industriels de la reÂgion de Gabès (Tunisie) sur le milieu marin reÂcepteur. Bull. L’institut Natl. Sci. Tech. D’oceâanographie Pêche Salammbo 1980, 7, 5–61. [Google Scholar]
- Nafeh Kassir, L.; Lartiges, B.; Ouaini, N. Effects of fertilizer industry emissions on local soil contamination: A case study of a phosphate plant on the east Mediterranean coast. Environ. Technol. 2012, 33, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Ennaciri, Y.; Bettach, M.; El Alaoui-Belghiti, H. Phosphogypsum Conversion into Calcium Fluoride and Sodium Sulfate. Ann. Chim. Sci. Matériaux 2020, 44, 407–412. [Google Scholar] [CrossRef]
- Singh, M.; Garg, M. Making of anhydrite cement from waste gypsum. Cem. Concr. Res. 2000, 30, 571–577. [Google Scholar] [CrossRef]
- Ghiloufi, I.; Baronnet, J.M. Simulation of Heavy Metals Volatility during the Vitrification of Fly Ashes by Thermal Plasma. J. High Temp. Mater. Process 2006, 10, 117–139. [Google Scholar] [CrossRef]
- Ghiloufi, I. Modeling of Chemical System Vaporization at High Temperatures: Application to the Vitrification of Fly Ashes by Plasma. J. High Temp. Mater. Process 2008, 12, 1–10. [Google Scholar] [CrossRef]
- Al-Mayman, S.; AlShunaifi, I.; Albeladi, A.; Ghiloufi, I.; Binjuwair, S. Treatment of fly ash from power plants using thermal plasma. Beilstein J. Nanotechnol 2017, 8, 1043–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiloufi, I. Simulation of radioelement volatility during the vitrification of radioactive wastes by arc plasma. J. Hazard Mater 2009, 163, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ghiloufi, I. Study of 239Pu, 144Ce and 90Sr Behavior during Radioactive Wastes Treatment by Thermal Plasma Technology. J. Plasma Chem. Plasma Process. 2009, 29, 321–331. [Google Scholar] [CrossRef]
- Ghiloufi, I.; Girold, C. Optical Emission Spectroscopy Measurements and Simulation of Radioelement Volatility during Radioactive Waste Treatment by Plasma. J. Plasma Chem. Plasma Process. 2011, 31, 109–125. [Google Scholar] [CrossRef]
- Ghiloufi, I. Chapter’s Name: Electronic Waste Treatment by Thermal Plasma, Book’s Name: E-Waste: Management, Types and Challenges; Nova Science Publishers: Hauppauge, NY, USA, 2012. [Google Scholar]
- Samal, S. Thermal plasma technology: The prospective future in material processing. J. Clean. Prod. 2017, 142, 3131–3150. [Google Scholar] [CrossRef]
- Heberlein, J.; Murphy, A.B. Thermal plasma waste treatment. J. Phys. D Appl. Phys. 2008, 41, 053001. [Google Scholar] [CrossRef]
- Gagnon, J.; Carabin, P. A torch to light the way: Plasma gasification technology in waste treatment. Waste Manag. World 2006, 1, 65–68. [Google Scholar]
- Van Oost, G.; Hrabovsky, M.; Kopecky, V.; Konrad, M.; Hlina, M.; Kavka, T.; Chumak, A.; Beeckman, E.; Verstraeten, J. Pyrolysis of waste using a hybrid argon-water stabilized torch. Vacuum 2006, 80, 1132–1137. [Google Scholar] [CrossRef]
- Carpinlioglu, M.O.; Sanlisoy, A. Performance assessment of plasma gasification for waste to energy conversión, a methodology for thermodynamic analysis. Int. J. Hydrog. Energy 2018, 43, 11493–11504. [Google Scholar] [CrossRef]






| Parameter | Value |
|---|---|
| Power (max.) | 100 kW |
| Voltage (max.) | 400 V |
| Current (max.) | 400 A |
| Type of plasma gas | Air |
| Air consumption | 5–15 g/s |
| Arc-stabilizing method | Vertical gas |
| Temperature of plasma jet | 2000–4000 °C |
| Water consumption (max.) | 700 g/s |
| Air supply | 4.2 g/s |
| Element | Al | As | Be | Cd | Cr | Fe | Mg | Pb | V | Zn |
| C (mg/kg) | 60.1 | 5.4 | 16.5 | 4.8 | 5.1 | 100 | 17.5 | 64.8 | 1.9 | 17 |
| Element | Al | As | Be | Cd | Cr | Fe | Mg | Pb | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| C (mg/kg) t = 30 min | 114.8 | 0 | 17.8 | 2.7 | 0.1 | 35.07 | 29.6 | 0 | 3.6 | 14.2 |
| C (mg/kg) t = 40 min | 7.6 | 0 | 11.7 | 3.9 | 3.4 | 18.21 | 10.8 | 0 | 3.3 | 4.2 |
| Element | Al | As | Be | Cd | Cr | Fe | Mg | Pb | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| C (mg/kg) I = 100 A | 40.5 | 0 | 11.7 | 3.9 | 3.4 | 36.3 | 36.3 | 0 | 3.3 | 4.2 |
| C (mg/kg) I = 130 A | 34.77 | 0 | 24.7 | 0.3 | 1.6 | 9.1 | 16.8 | 0 | 0.3 | 10.9 |
| C (mg/kg) I = 160 A | 13.6 | 0 | 1.2 | 0.8 | 0.9 | 4.5 | 17.2 | 0 | 1.2 | 6.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghiloufi, I.; Albishi, M.S.; Alharbi, A.A.; AlShunaifi, I.A. Plasma Technology for Phosphogypsum Treatment. Energies 2021, 14, 5813. https://doi.org/10.3390/en14185813
Ghiloufi I, Albishi MS, Alharbi AA, AlShunaifi IA. Plasma Technology for Phosphogypsum Treatment. Energies. 2021; 14(18):5813. https://doi.org/10.3390/en14185813
Chicago/Turabian StyleGhiloufi, Imed, Miqad S. Albishi, Ahmed A. Alharbi, and Ibrahim A. AlShunaifi. 2021. "Plasma Technology for Phosphogypsum Treatment" Energies 14, no. 18: 5813. https://doi.org/10.3390/en14185813
APA StyleGhiloufi, I., Albishi, M. S., Alharbi, A. A., & AlShunaifi, I. A. (2021). Plasma Technology for Phosphogypsum Treatment. Energies, 14(18), 5813. https://doi.org/10.3390/en14185813






