Bioenergy Production through Mono and Co-Digestion of Tomato Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
2.2. Residues Characterization
2.3. Design of Experiments for the Anaerobic Digestion
2.3.1. Optimization of Operating Conditions
2.3.2. Optimization of Co-Digestion
2.4. Anaerobic Digestion Procedure
2.5. Statistical Analysis
3. Results and Discussion
3.1. Residues Characteristics
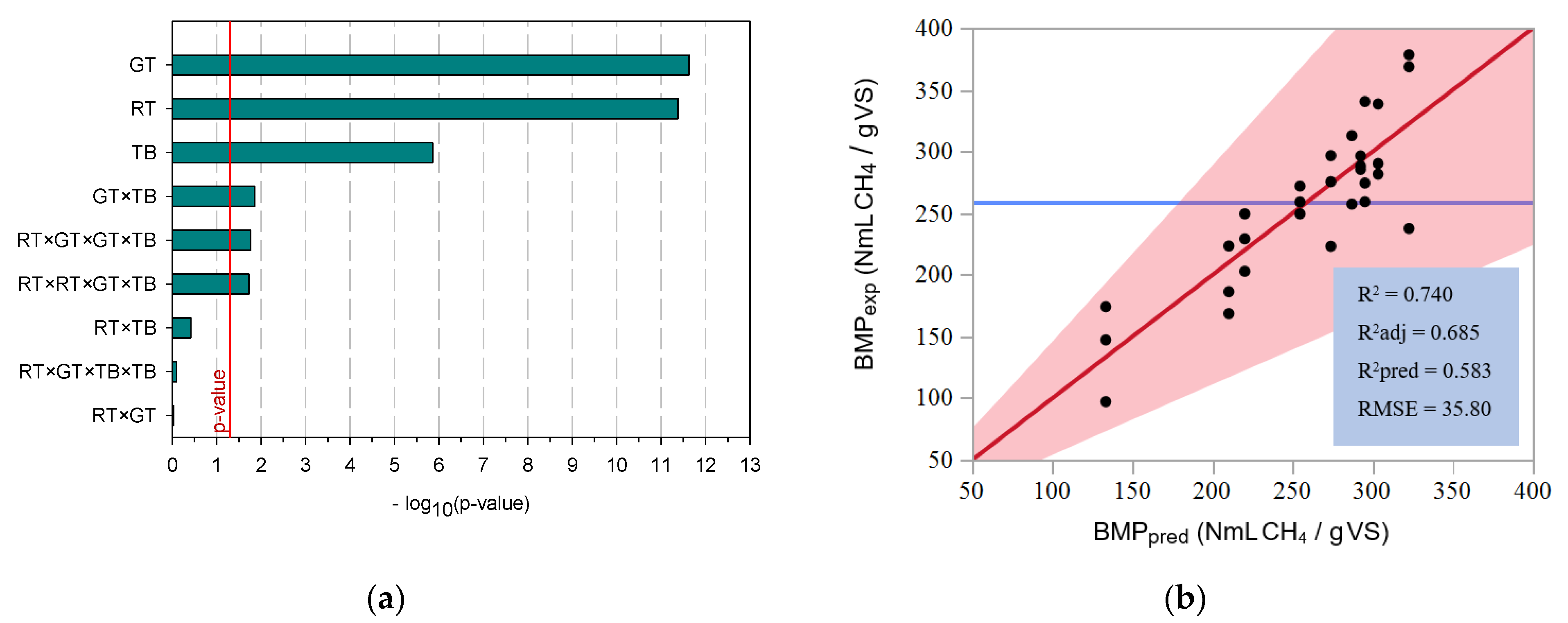
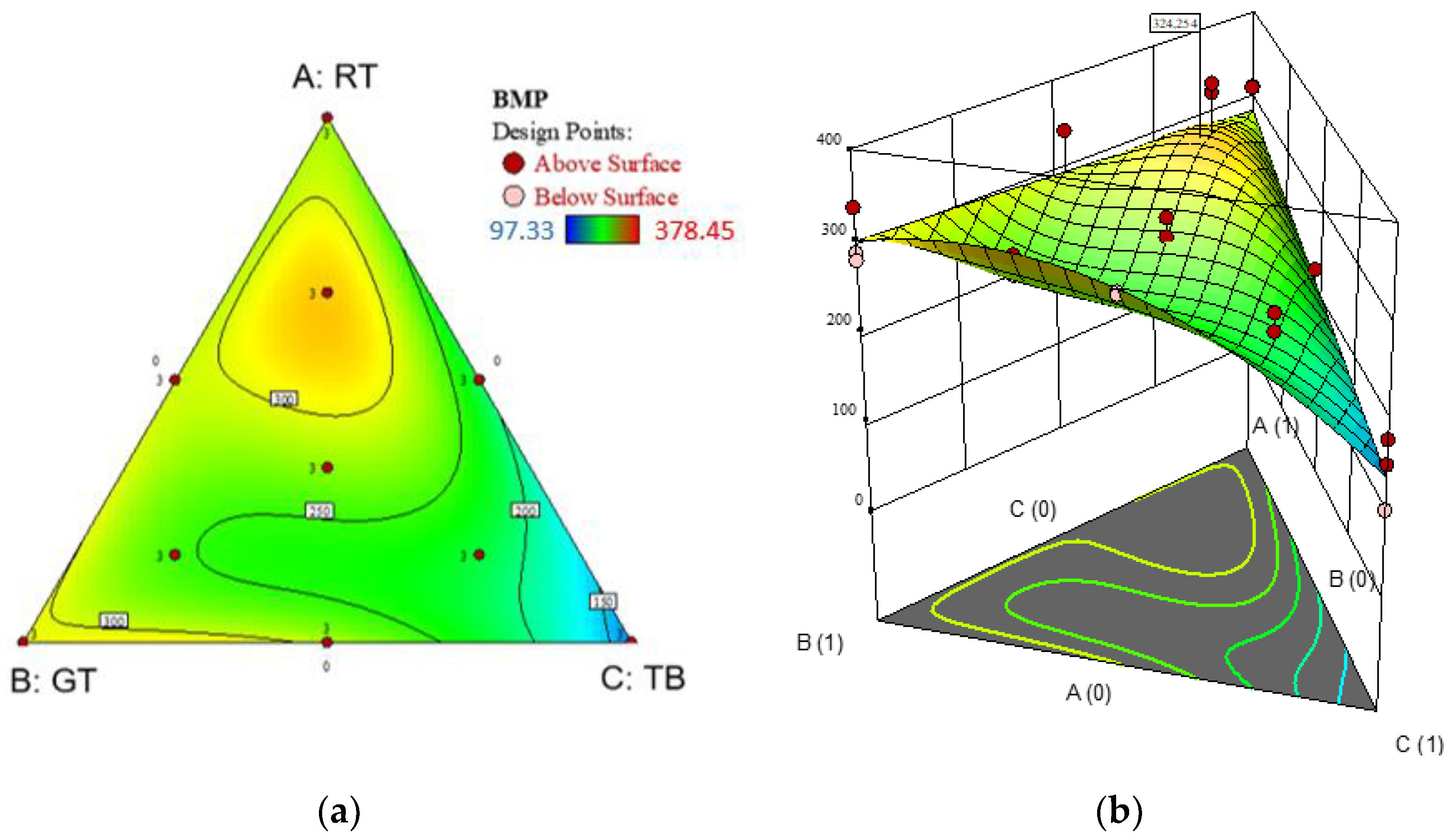
3.2. Optimization of the Anaerobic Digestion
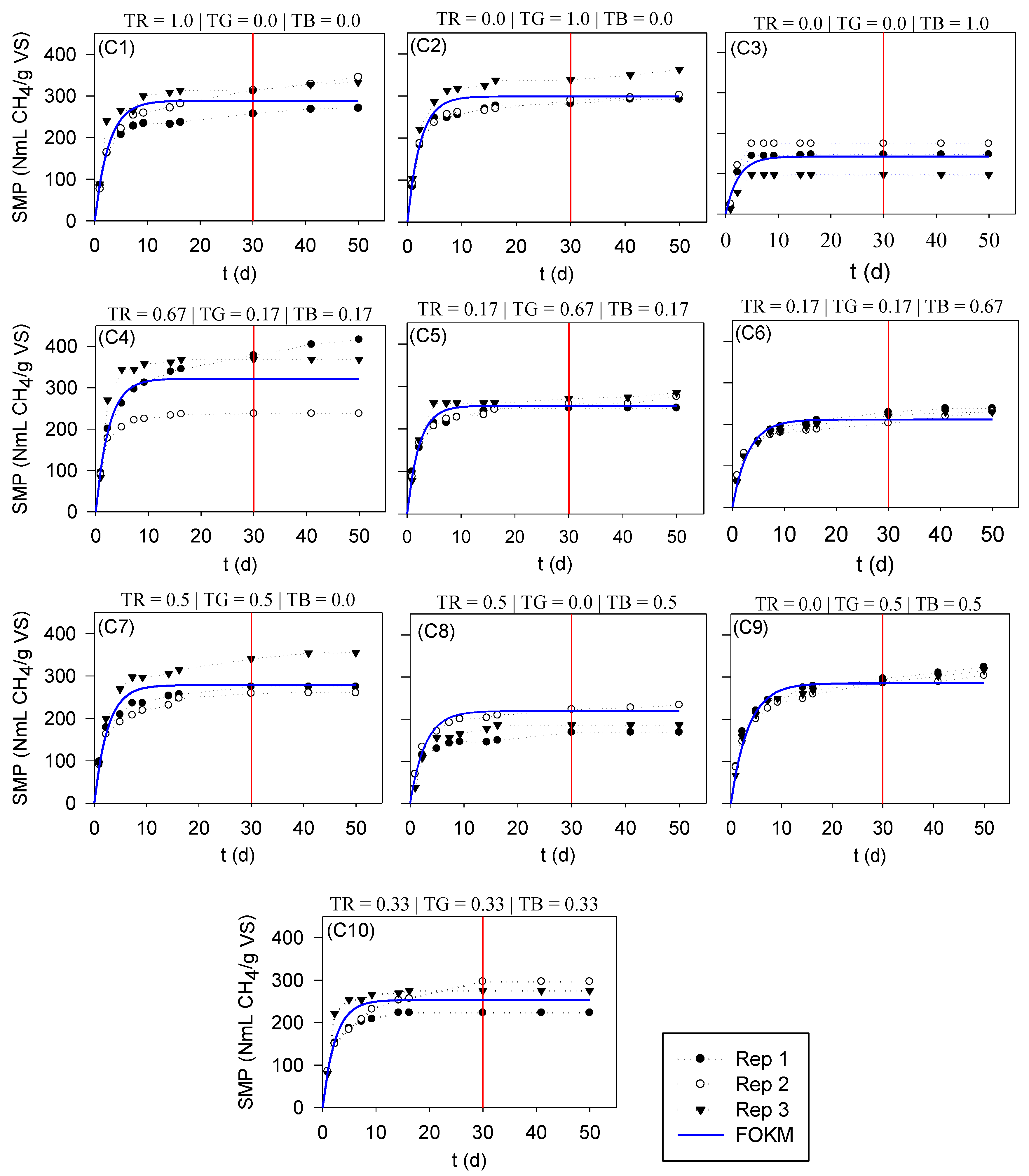
3.3. Co-Digestion of Tomato Residues
5458.165 × (RT × GT2 × TB)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| BMP | biochemical methane potential (NmLCH4/gVS) |
| BMPexp | experimental biochemical methane potential (NmLCH4/gVS) |
| BMPpred | predicted biochemical methane potential (NmLCH4/gVS) |
| CL | cellulose (%TS) |
| COD | chemical oxygen demand (mg O2/g VS) |
| t | time (days) |
| HM | hemicellulose (%TS) |
| Inc | incubation |
| LG | lignin (%TS) |
| Nu | nutrients |
| R2 | R-squared |
| R2adj | adjusted R-squared |
| R2pred | predicted R-square |
| RSME | root mean square error (NmLCH4/gVS) |
| SMP | cumulative specific methane production (NmLCH4/gVS) |
| SMP∞ | maximum methane production (NmLCH4/gVS) |
| TS | total solids (%) |
| VS | volatile solids (%TS) |
| VSt | concentration of total volatile solids (g/L) |
| WV | working volume (%) |
| Acronyms | |
| AcoD | anaerobic co-digestion |
| AD | anaerobic digestion |
| CPI | co-digestion performance index |
| DoE | design of experiments |
| FOKM | first order kinetic model |
| GT | green tomato |
| RT | rotten tomato |
| SIR | substrate to inoculum ratio |
| TB | tomato plant |
References
- Directive (EU) 2018/2001. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources. European Parliament, Council of the European Union. Available online: http://data.europa.eu/eli/dir/2018/2001/oj (accessed on 11 March 2021).
- FAOSTAT, Food and Agricultural Organization of the United Nations. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 9 February 2021).
- Pinela, J.; Prieto, M.; Barreiro, M.F.; Carvalho, A.M.; Oliveira, M.B.P.; Curran, T.P.; Ferreira, I.C. Valorisation of tomato wastes for development of nutrient-rich antioxidant ingredients: A sustainable approach towards the needs of the today’s society. Innov. Food Sci. Emerg. Technol. 2017, 41, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Fritsch, C.; Staebler, A.; Happel, A.; Márquez, M.A.C.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.M.; Montanari, A.; López, M.J.; et al. Processing, Valorization and Application of Bio-Waste Derived Compounds from Potato, Tomato, Olive and Cereals: A Review. Sustainability 2017, 9, 1492. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Long, F.; Zhao, H.; Zhang, Y.; Liang, D.; Wang, L.; Lesnik, K.L.; Cao, H.; Zhang, Y.; Liu, H. Performance prediction of ZVI-based anaerobic digestion reactor using machine learning algorithms. Waste Manag. 2021, 121, 59–66. [Google Scholar] [CrossRef]
- Ometto, F.; Karlsson, A.; Ejlertsson, J.; Björn, A.V.; Shakeri, S.Y. Anaerobic digestion: An engineered biological process. In Substitute Natural Gas from Waste; Materazzi, M., Foscolo, P.U., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 63–74. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Raposo, F.; De La Rubia, M.A.; Fernández-Cegrí, V.; Borja, R. Anaerobic digestion of solid organic substrates in batch mode: An overview relating to methane yields and experimental procedures. Renew. Sustain. Energy Rev. 2012, 16, 861–877. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.; Fonoll, X.; Peces, M.; Astals, S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef] [Green Version]
- Karki, R.; Chuenchart, W.; Surendra, K.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Khanal, S.K. Anaerobic co-digestion: Current status and perspectives. Bioresour. Technol. 2021, 330, 125001. [Google Scholar] [CrossRef] [PubMed]
- Solé-Bundó, M.; Passos, F.; Romero-Güiza, M.S.; Ferrer, I.; Astals, S. Co-digestion strategies to enhance microalgae anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2019, 112, 471–482. [Google Scholar] [CrossRef]
- Ebner, J.H.; Labatut, R.A.; Lodge, J.S.; Williamson, A.A.; Trabold, T.A. Anaerobic co-digestion of commercial food waste and dairy manure: Characterizing biochemical parameters and synergistic effects. Waste Manag. 2016, 52, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yuan, H.; Wachemo, A.C.; Li, X. Impacts of Modification of Corn Stover on the Synergistic Effect and Microbial Community Structure of Co-Digestion with Chicken Manure. Energy Fuels 2019, 34, 401–411. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Structural Carbohydrates and Lignin Inbiomass—Laboratory Analytical Procedure (LAP); Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Gaspar, M.C.; Mendes, C.V.T.; Pinela, S.; Moreira, R.; Carvalho, M.G.V.S.; Quina, M.J.; Braga, M.E.M.; Portugal, A. Assessment of Agroforestry Residues: Their Potential within the Biorefinery Context. ACS Sustain. Chem. Eng. 2019, 7, 17154–17165. [Google Scholar] [CrossRef]
- Almeida, P.; Rodrigues, R.; Mendes, C.; Szeląg, R.; Pietrzyk, D.; Klepacz-Smółka, A.; Quina, M. Assessment of NIR spectroscopy for predicting biochemical methane potential of agro-residues—A biorefinery approach. Biomass-Bioenergy 2021, 151, 106169. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.; Klepacz-Smolka, A.; Martins, R.; Quina, M. Comparative analysis of methods and models for predicting biochemical methane potential of various organic substrates. Sci. Total Environ. 2019, 649, 1599–1608. [Google Scholar] [CrossRef]
- Chandler, J.A.; Jewell, W.J.; Gossett, J.M.; Vansoest, P.; Robertson, J. Predicting methane fermentation biodegradability. Biotechnol. Bioeng. 1980, 22, 93–107. [Google Scholar]
- Şevik, F.; Tosun, I.; Ekinci, K. Composting of olive processing wastes and tomato stalks together with sewage sludge or dairy manure. Int. J. Environ. Sci. Technol. 2016, 13, 1207–1218. [Google Scholar] [CrossRef] [Green Version]
- Monterumici, C.M.; Rosso, D.; Montoneri, E.; Ginepro, M.; Baglieri, A.; Novotny, E.H.; Kwapinski, W.; Negre, M. Processed vs. Non-Processed Biowastes for Agriculture: Effects of Post-Harvest Tomato Plants and Biochar on Radish Growth, Chlorophyll Content and Protein Production. Int. J. Mol. Sci. 2015, 16, 8826–8843. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, P.; Cambra-Lopez, M.; Cerisuelo, A.; Peñaranda, D.S.; Moset, V. The use of agricultural substrates to improve methane yield in anaerobic co-digestion with pig slurry: Effect of substrate type and inclusion level. Waste Manag. 2014, 34, 196–203. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Xu, F.; Li, Y.; Li, D.; Wang, G.; Li, S.; Zhang, H.; Wu, Y.; Shah, A.; et al. Reactor performance and economic evaluation of anaerobic co-digestion of dairy manure with corn stover and tomato residues under liquid, hemi-solid, and solid state conditions. Bioresour. Technol. 2018, 270, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Katsoulas, N.; Barros, L.; Ferreira, I.C. The effect of covering material on the yield, quality and chemical composition of greenhouse-grown tomato fruit. J. Sci. Food Agric. 2019, 99, 3057–3068. [Google Scholar] [CrossRef]
- Acosta-Quezada, P.G.; Raigón, M.D.; Riofrío-Cuenca, T.; García-Martínez, M.D.; Plazas, M.; Burneo, J.I.; Figueroa, J.G.; Vilanova, S.; Prohens, J. Diversity for chemical composition in a collection of different varietal types of tree tomato (Solanum betaceum Cav.), an Andean exotic fruit. Food Chem. 2015, 169, 327–335. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Rincon, B.; Jiménez-Rodríguez, A.-M. Assessment of process control parameters in the biochemical methane potential of sunflower oil cake. Biomass-Bioenergy 2008, 32, 1235–1244. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, R.; El Mashad, H.; Dong, R. Effect of feed to inoculum ratios on biogas yields of food and green wastes. Bioresour. Technol. 2009, 100, 5103–5108. [Google Scholar] [CrossRef]
- Gil, A.; Siles, J.Á.; Serrano, A.; Martín, M.Á. Mixture optimization of anaerobic co-digestion of tomato and cucumber waste. Environ. Technol. 2015, 36, 2628–2636. [Google Scholar] [CrossRef] [PubMed]
- Gunaseelan, V. Biochemical methane potential of fruits and vegetable solid waste feedstocks. Biomass-Bioenergy 2004, 26, 389–399. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues. Energies 2018, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Andriamanohiarisoamanana, F.J.; Yasui, S.; Yamashiro, T.; Ramanoelina, V.; Ihara, I.; Umetsu, K. Anaerobic co-digestion: A sustainable approach to food processing organic waste management. J. Mater. Cycles Waste Manag. 2020, 22, 1501–1508. [Google Scholar] [CrossRef]
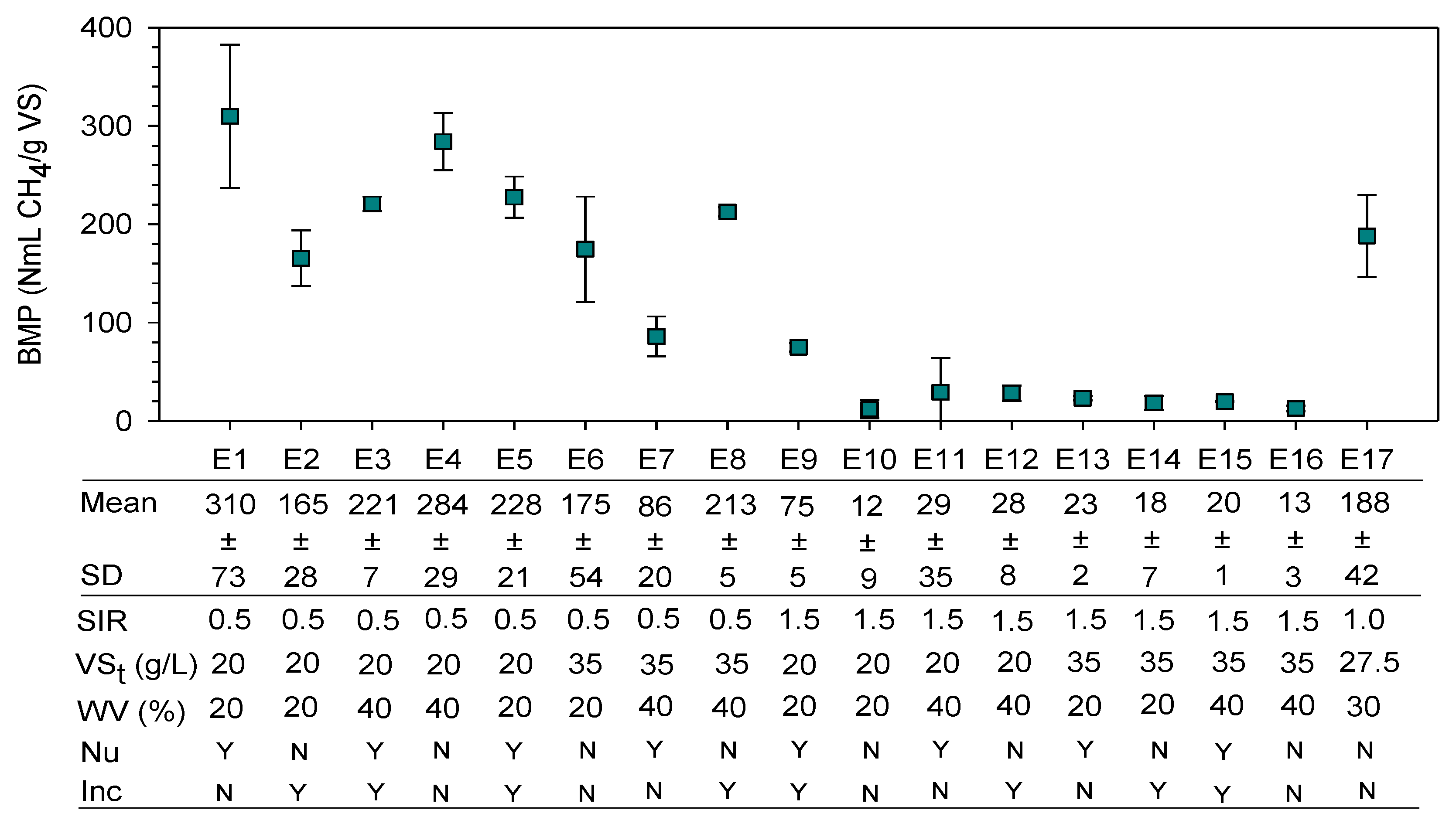

| Factorial Design 25−1 | ||||||
|---|---|---|---|---|---|---|
| Continuous | Nominal | |||||
| SIR | VSt (g/L) | WV (%) | Nu | Inc | ||
| Level | −1 | 0.5 | 20 | 20 | N | N |
| 0 | 1.0 | 27.5 | 30 | n.a. | n.a | |
| +1 | 1.5 | 35 | 40 | Y | Y | |
| Validation Experiments | ||||||
| V1 | 0.75 | 25 | 25 | Y | N | |
| V2 | 0.75 | 25 | 35 | Y | N | |
| V2 | 1.25 | 30 | 25 | Y | N | |
| V4 | 1.25 | 30 | 35 | Y | N | |
| RT | GT | TB | |
|---|---|---|---|
| TS (%) | 5.99 ± 0.46 | 7.76 ± 0.07 | 71.4 ± 1.14 |
| VS (%TS) | 86.0 ± 0.1 | 88.0 ± 0.3 | 80.0 ± 0.3 |
| pH | 4.75 ± 0.04 | 4.00 ± 0.04 | 6.82 ± 0.02 |
| COD (mg O2/g VS) | 1517 ± 19 | 1223 ± 44 | 1592 ± 73 |
| C (%TS) | 42.0 ± 0.1 | 38.4 ± 0.1 | 37.3 ± 0.1 |
| N (%TS) | 2.43 ± 0.04 | 2.01 ± 0.02 | 2.87 ± 0.02 |
| O (%TS) | 35.1 ± 0.2 | 41.7 ± 0.1 | 34.7 ± 0.1 |
| H (%TS) | 6.49 ± 0.12 | 5.98 ± 0.04 | 5.10 ± 0.03 |
| LG (%TS) | 7.87 ± 0.29 | 4.11 ± 0.85 | 20.27 ± 0.44 |
| CL (%TS) | 15.45 ± 0.06 | 23.15 ± 0.83 | 23.99 ± 0.19 |
| HM (%TS) | 16.33 ± 0.21 | 31.02 ± 0.99 | 17.57 ± 0.24 |
| BMP = +480.554 − 278.423 × SIR − 6.392 × VSt + 3.477 × SIR × VSt + a1 × (VSt − 27.5) + a2 × (WV − 30) + a3 × (VSt − 27.5) + a4 | (4) | ||||
| Coefficients | |||||
| (Nu,Inc) | a1 | a2 | a3 | a4 | |
| (Y,Y) | −1.726 | −2.795 | 2.731 | 12.951 | |
| (Y,N) | −1.726 | −2.795 | −2.731 | −12.951 | |
| (N,Y) | 1.726 | 2.795 | 2.731 | −12.951 | |
| (N,N) | 1.726 | 2.795 | −2.731 | 12.951 | |
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| BMPexp | 294 | 304 | 140 | 374 | 260 | 217 | 291 | 193 | 290 | 286 |
| BMP | 289 | 299 | 142 | 322 | 255 | 212 | 273 | 220 | 258 | 253 |
| µ | 0.371 | 0.417 | 0.410 | 0.399 | 0.445 | 0.321 | 0.388 | 0.358 | 0.288 | 0.418 |
| R2 | 0.796 | 0.841 | 0.751 | 0.597 | 0.913 | 0.897 | 0.728 | 0.715 | 0.923 | 0.764 |
| RMSE | 33.09 | 28.83 | 32.57 | 60.32 | 16.71 | 16.16 | 36.79 | 39.38 | 19.60 | 30.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, P.V.; Rodrigues, R.P.; Teixeira, L.M.; Santos, A.F.; Martins, R.C.; Quina, M.J. Bioenergy Production through Mono and Co-Digestion of Tomato Residues. Energies 2021, 14, 5563. https://doi.org/10.3390/en14175563
Almeida PV, Rodrigues RP, Teixeira LM, Santos AF, Martins RC, Quina MJ. Bioenergy Production through Mono and Co-Digestion of Tomato Residues. Energies. 2021; 14(17):5563. https://doi.org/10.3390/en14175563
Chicago/Turabian StyleAlmeida, Patrícia V., Rafaela P. Rodrigues, Leonor M. Teixeira, Andreia F. Santos, Rui C. Martins, and Margarida J. Quina. 2021. "Bioenergy Production through Mono and Co-Digestion of Tomato Residues" Energies 14, no. 17: 5563. https://doi.org/10.3390/en14175563
APA StyleAlmeida, P. V., Rodrigues, R. P., Teixeira, L. M., Santos, A. F., Martins, R. C., & Quina, M. J. (2021). Bioenergy Production through Mono and Co-Digestion of Tomato Residues. Energies, 14(17), 5563. https://doi.org/10.3390/en14175563








