Biomonitoring Studies and Preventing the Formation of Biogenic H2S in the Wierzchowice Underground Gas Storage Facility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formation Waters
Description of the PCR Method
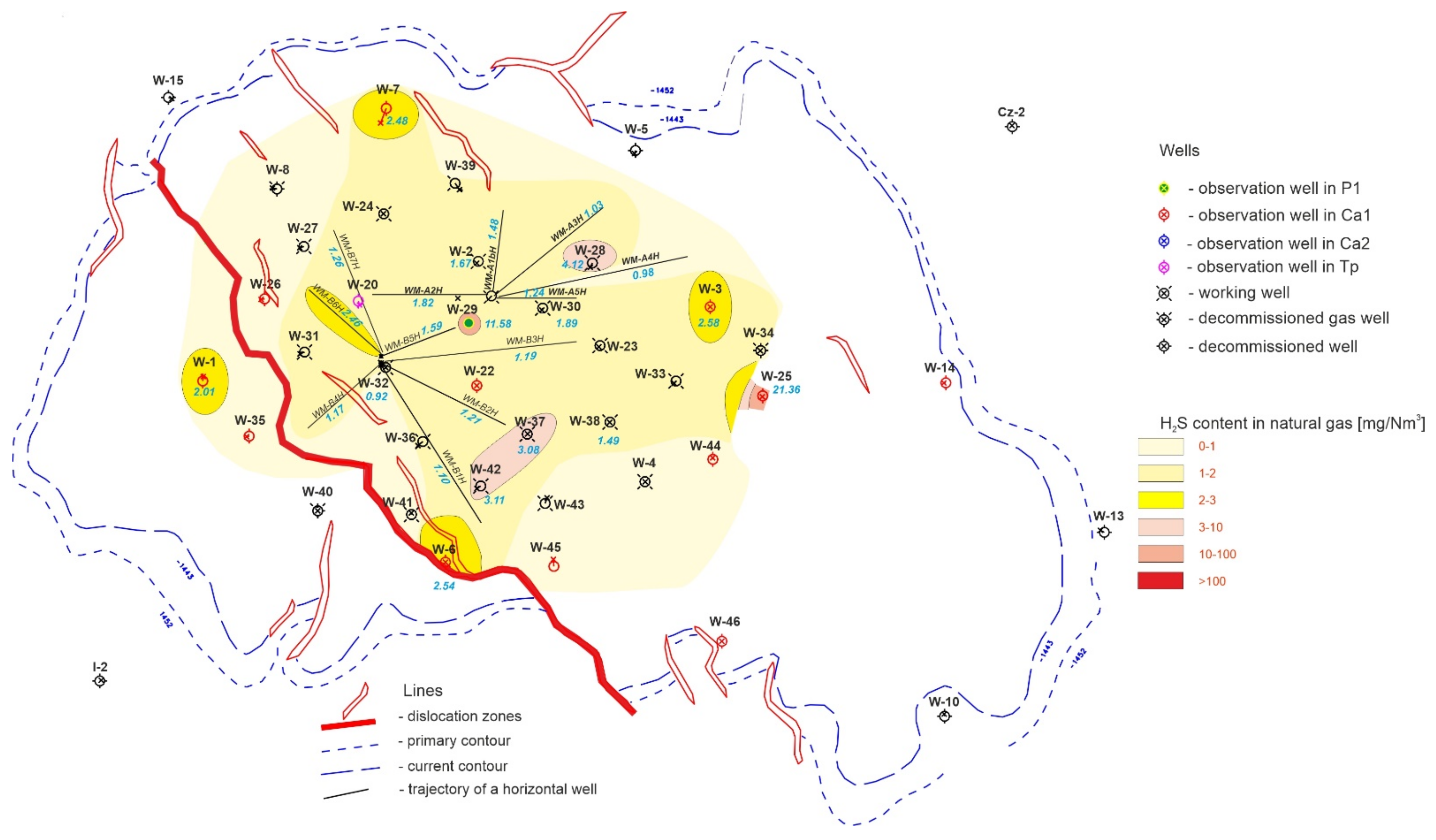
2.2. Natural Gas
2.3. Isotopic Composition of Hydrogen Sulphide
2.4. Isotopic Composition of Sulphates
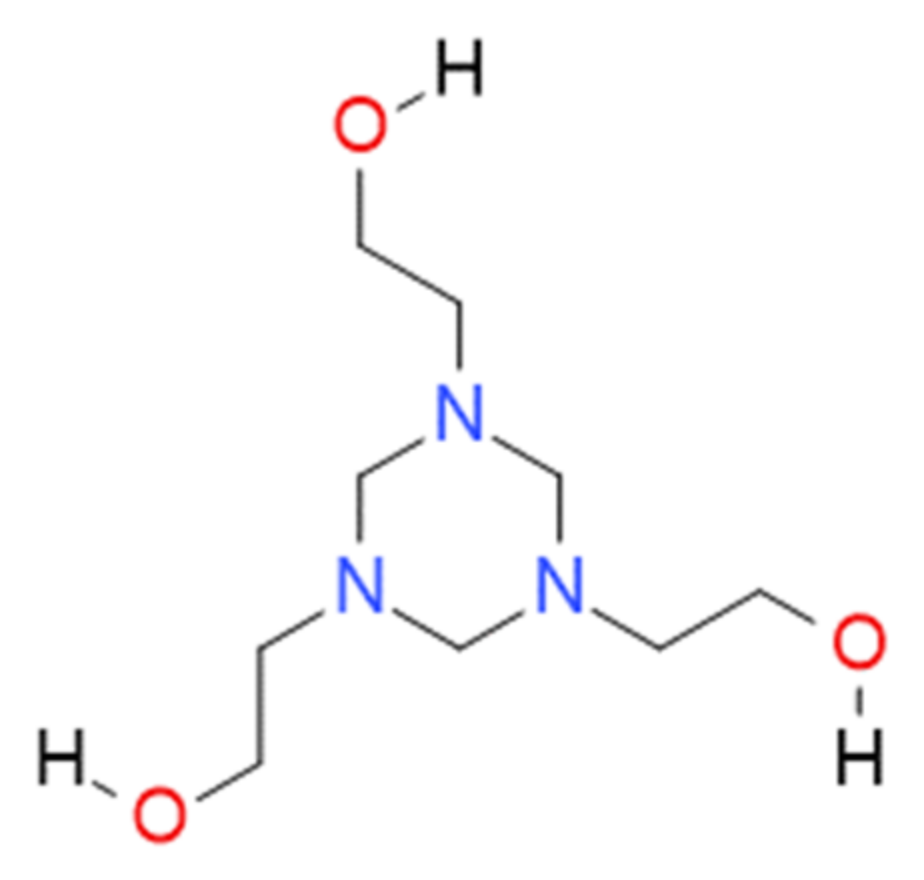
2.5. Biocide Characteristics
3. Results
3.1. Microbial Test on Formation Waters
3.2. Results of Natural Gas Research
3.3. The Hydrogen Sulphide Biocid and Scavengers Aplication Technologie UGS Wierzchowice
3.4. Results of Isotope Research
4. Discussion
5. Conclusions
- Biomonitoring of the formation waters and cyclical analyses of the composition of the stored gas at Wierzchowice UGS enable the assessment of the microbiological condition of the formation environment and individual storage wells of UGS Wierzchowice over the next years of operation.
- The composition of the microflora isolated in the previous work cycles of UGS included sulphate reducing bacteria (SRB) from the Desulfovibrio and Desulfotomaculum genera and bacteria oxidizing sulphur compounds (including accumulated biogenic hydrogen sulphide). After the injection of biocide/H2S scavenger preparations was applied, the metabolic activity of SRB strains was significantly reduced, and in some wells, it was completely eliminated. There is a tendency towards the gradual disappearance or limitation of the microbial reduction of sulphates in the studied environment (after a series of treatments with the use of H2S scavengers on UGS wells). It should be mentioned that the active hydrogen sulphide producing bacteria isolated from the formation fluids of the UGS Wierzchowice facility constitute material for the further testing of H2S scavengers and which also have an antibacterial effect.
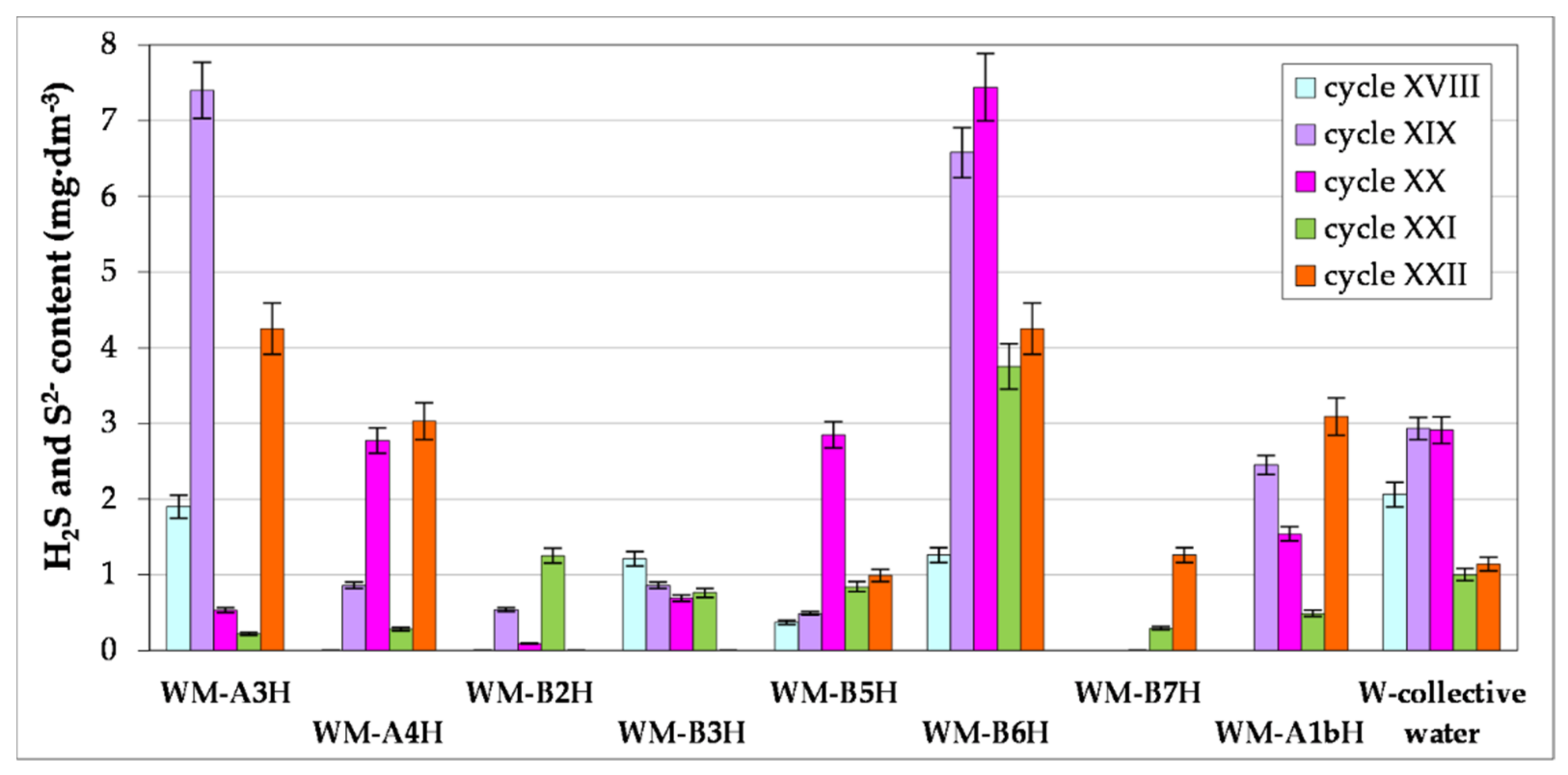
- The effects of the process of using H2S scavenger solutions in previous years can be considered satisfactory, as evidenced by the data contained in the article on the content of hydrogen sulphide in formation waters and recovered natural gas. In the course of the research, a number of changes in the technology of using preparations neutralizing hydrogen sulphide were introduced, including changing the type of carrier (solvent) or increasing the concentration of the active substance (aimed at combating contamination using the so-called “shock dose”, and then introducing a continuous dose, turned out to be effective especially in endangered wells—showing the highest biogenic hydrogen sulphide contamination.
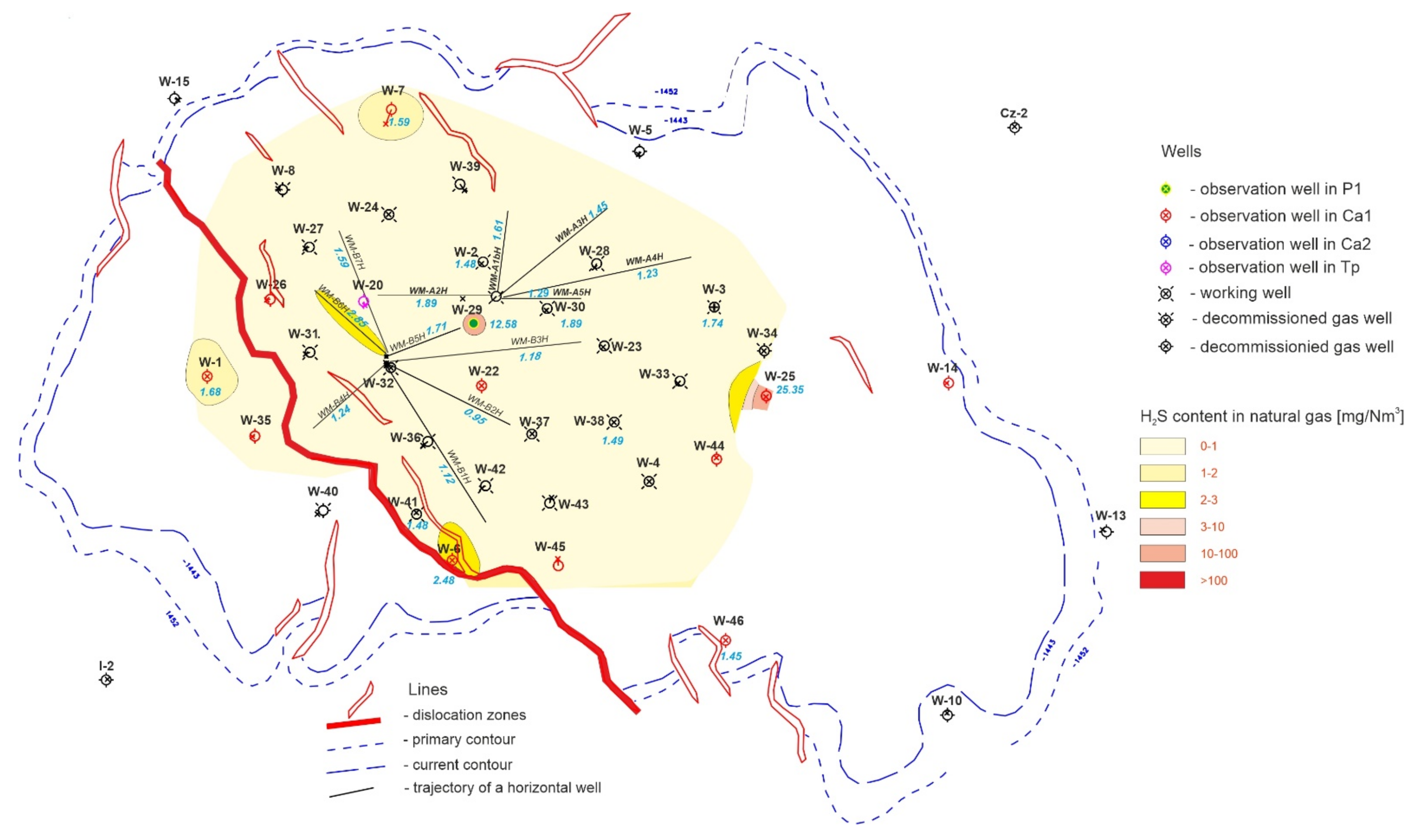
- The chromatographic analysis of the gas pumped into the UGS Wierzchowice facility ruled out the possibility of introducing contaminants in the form of sulphur compounds together with the pumped gas. This leads to the conclusion that the processes taking place in the formation result in the generation of sulphur compounds causing contamination of the gas recovered from the Wierzchowice Underground Gas Storage facility.
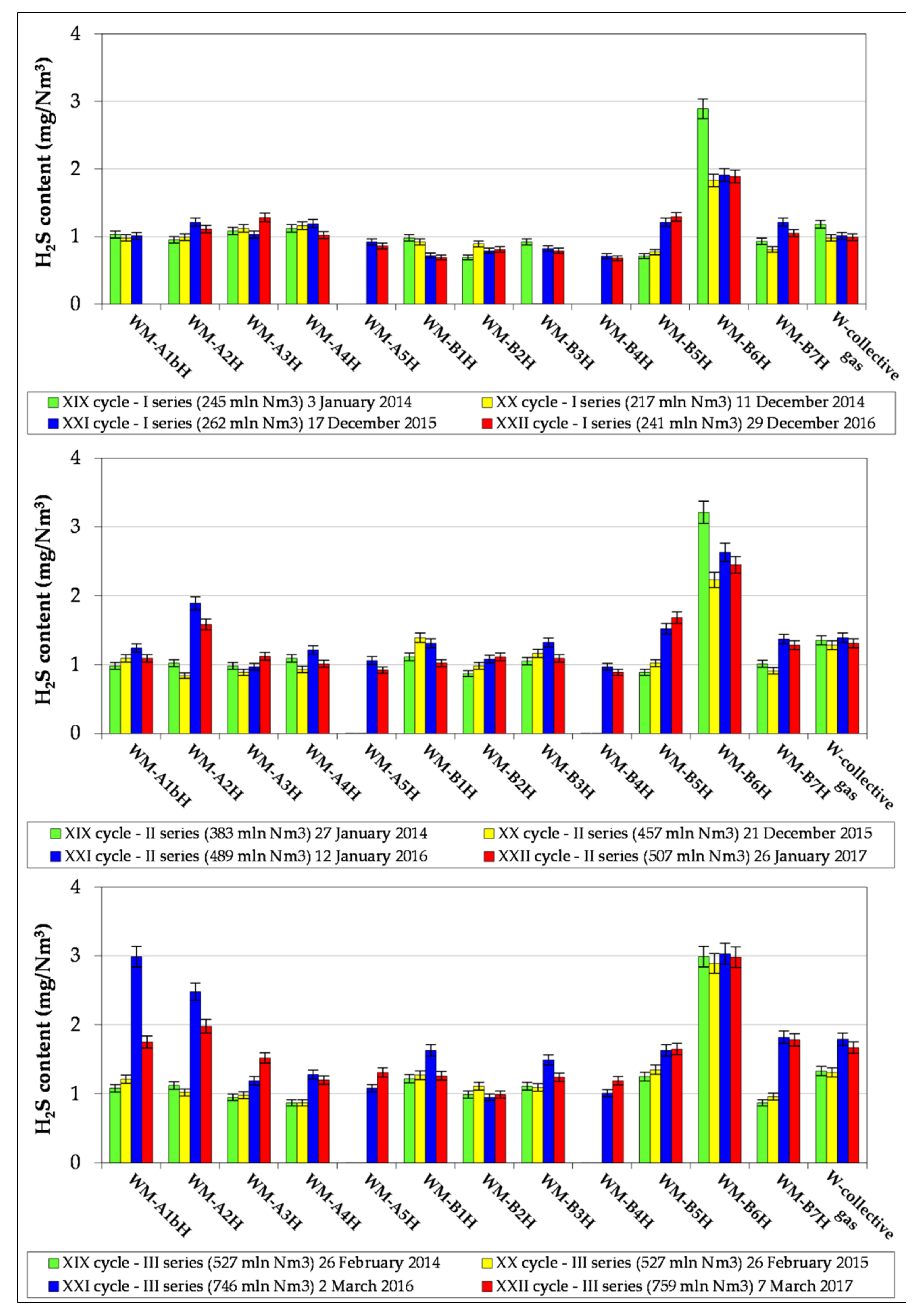
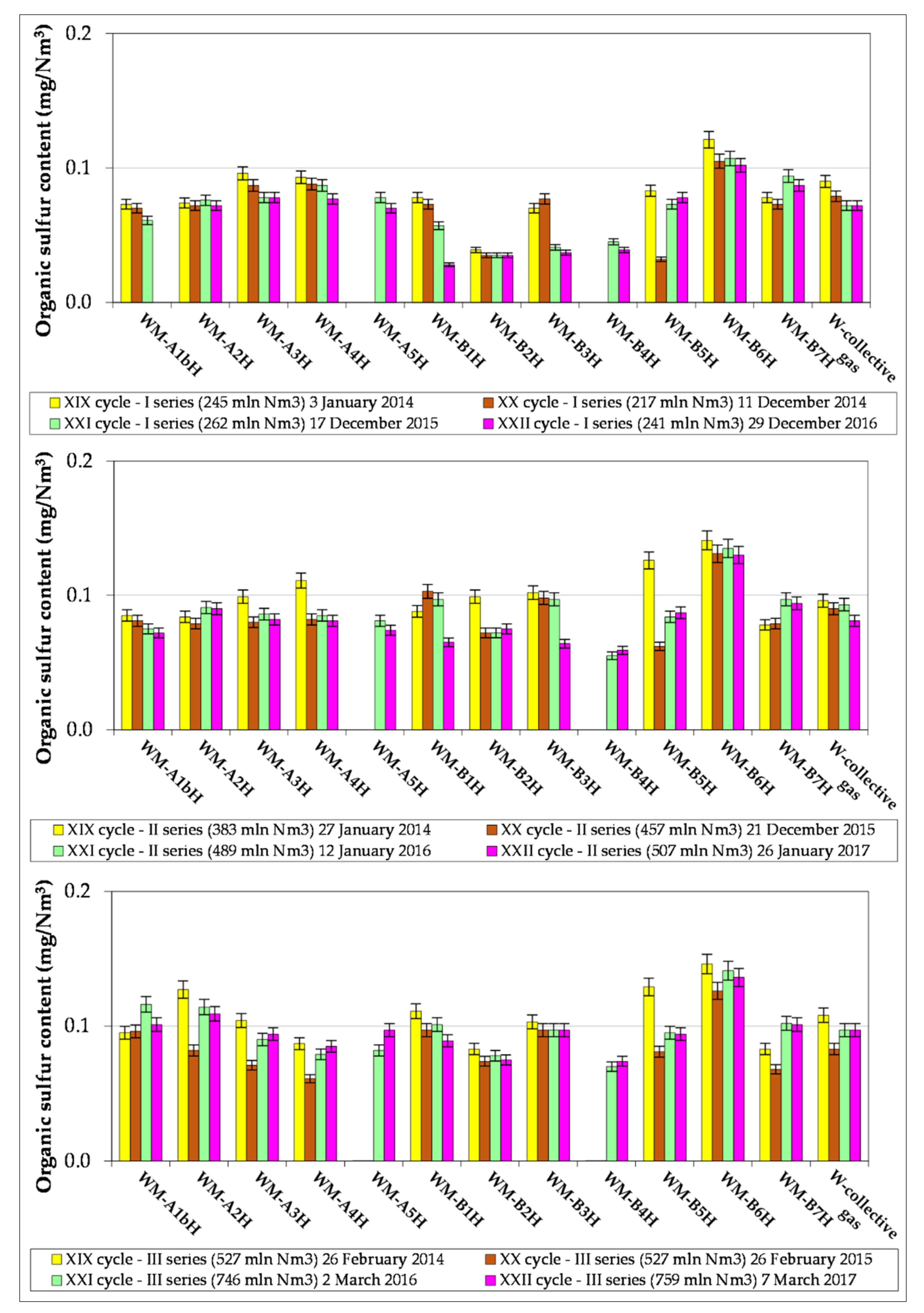
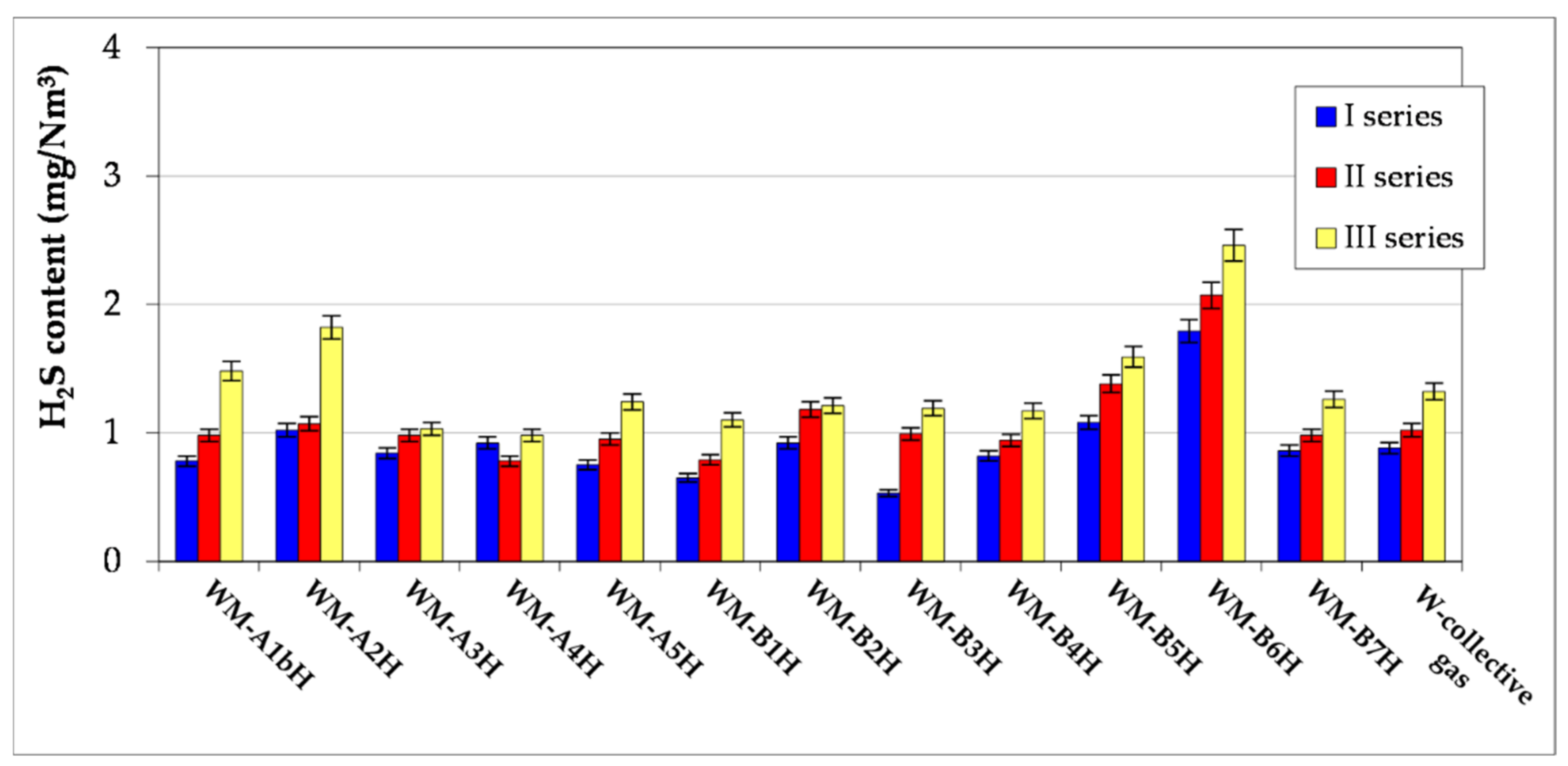
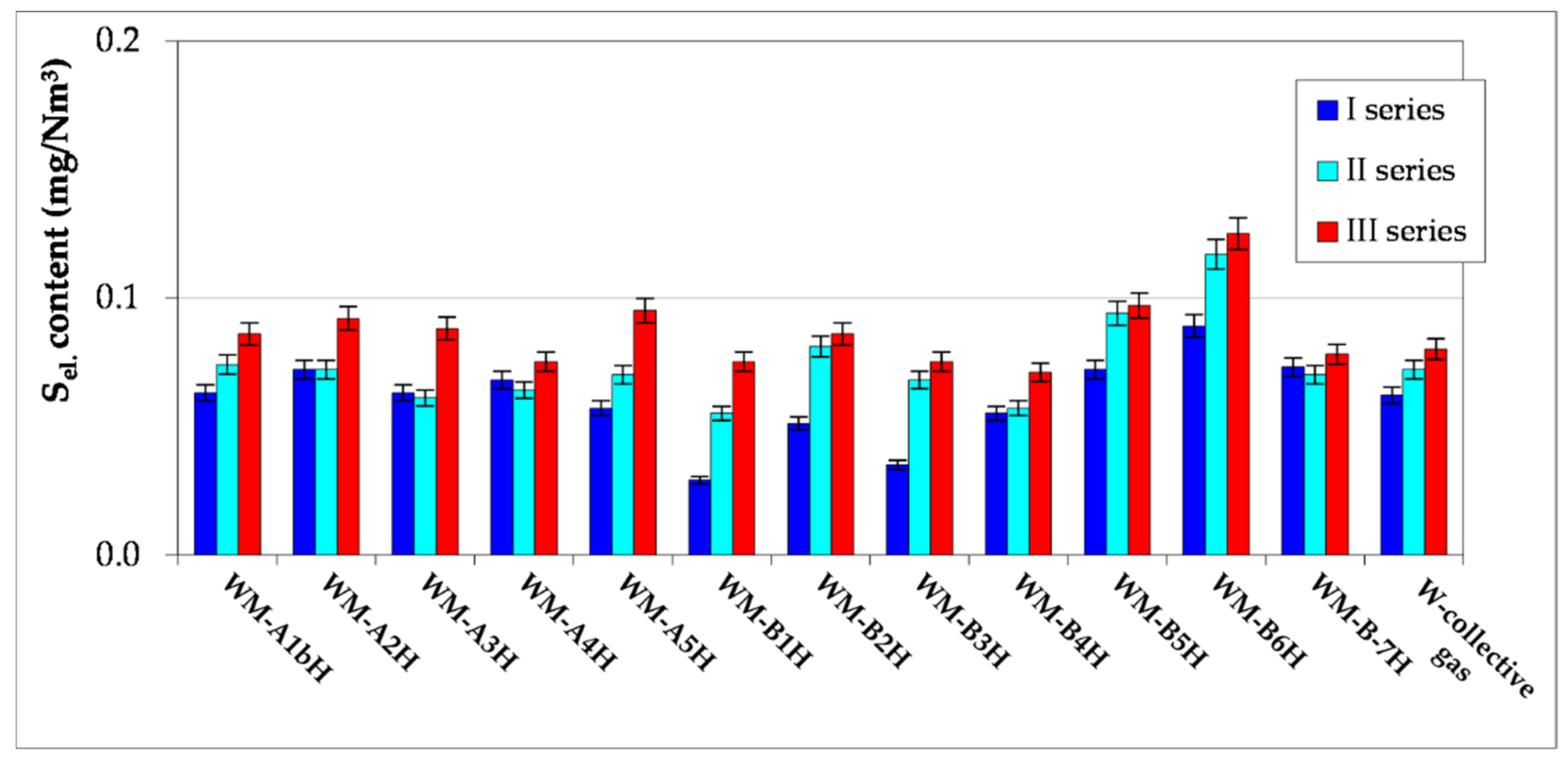
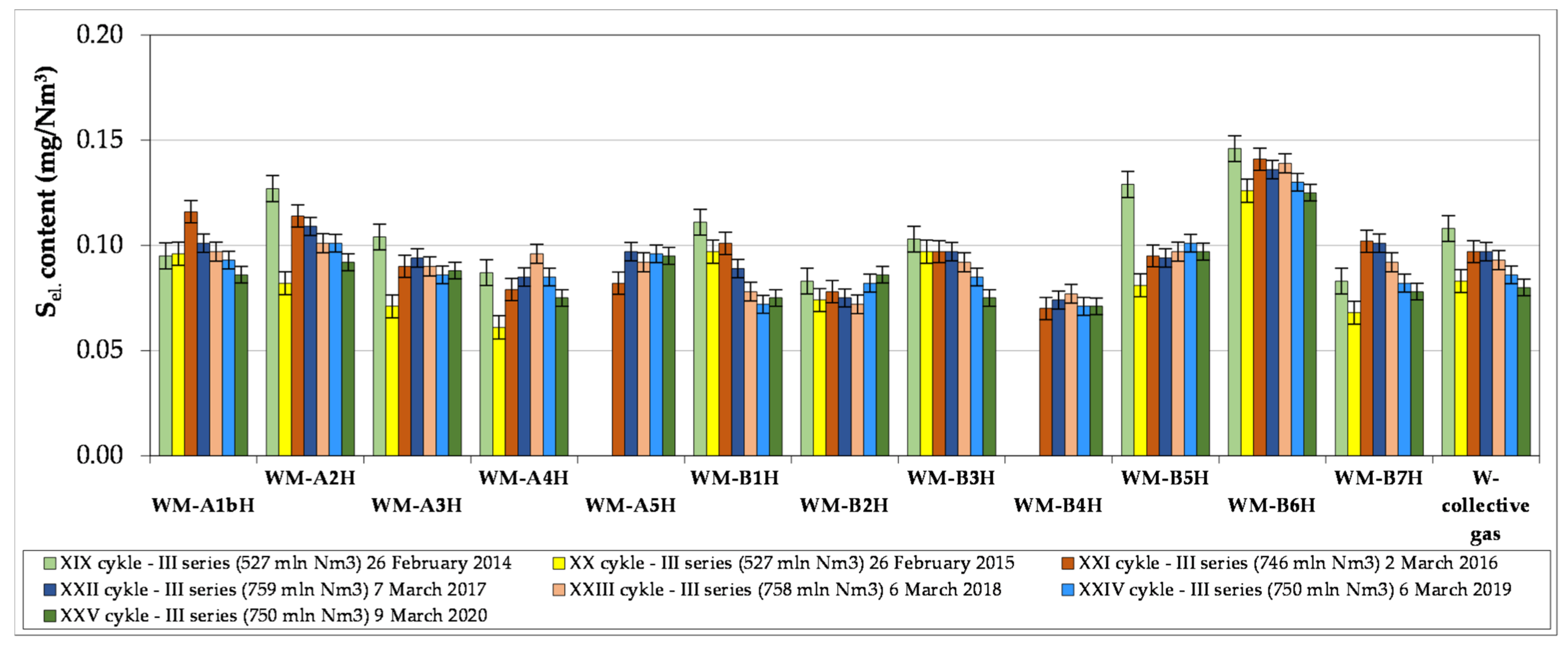
- The article contains detailed data from the chromatographic analyses of natural gas recovered from the Wierzchowice UGS facility (Table 5, Tables S1 and S3, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7) in the last cycles of exploitation. These data show the range of variability of the content of H2S and organic sulphur compounds in the gas over the subsequent stages of gas recovery from UGS.
- Summing up, it should be stated that monitoring the content of sulphur compounds in natural gas from UGS Wierzchowice is extremely important, as it allows for controlling the effectiveness of actions taken to reduce the hydrogen sulphide content in gas recovered from UGS. In addition, chromatographic analyses of sulphur compounds in natural gas are helpful on an ongoing basis in correcting the project of pumping H2S scavenger solutions in next work cycles of the UGS Wierzchowice facility.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staniszewska, A.; Kunicka-Styczyńska, A.; Ziemiński, K. Microbiological contaminations of Underground Gas Storage facilities and natural gas pipelines. Adv. Microbiol. 2017, 56, 381–388. [Google Scholar] [CrossRef]
- Blanko, H.; Faaij, A. A review at the role of storage in energy systems with focus on Power to Gas and long-term storage. Renew. Sustain. Energy Rev. 2018, 8, 1049–1086. [Google Scholar] [CrossRef]
- UGS. Wierzchowcie Folder. Available online: https://pgnig.pl/documents/29748/961630/PMG+wierzchowice_folder.pdf/c601d1f3-7bfa-46e0-8eed-201926094ff3 (accessed on 16 July 2021).
- Cypionka, H. Oxygen respiration by Desulfovibrio species. Ann. Rev. Microbiol. 2000, 54, 827–848. [Google Scholar] [CrossRef] [PubMed]
- Prajapat, G.; Jain, S.; Agrawal, A. Microbial diversity and dynamics in hydrocarbon resource environments. In Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications; Satyanarayana, T., Johri, B., Das, S., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Xue, Y.; Voordouw, G. Control of microbial sulfide production with biocides and nitrate in oil reservoirs simulating bioreactors. Front. Microbiol. 2015, 6, 1387. [Google Scholar] [CrossRef]
- Tang, K.; Baskaran, V.; Nemati, M. Bacteria of the sulphur cycle: An overview of microbiology, biokinetics and their role in petroleum and mining industries. Biochem. Eng. J. 2009, 44, 73–94. [Google Scholar] [CrossRef]
- Tian, H.; Gao, P.; Chen, Z.; Li, Y.; Li, Y.; Wang, Y.; Zhou, J.; Li, G.; Ma, T. Compositions and abundances of sulfate-reducing and sulfur-oxidizing microorganisms in water-flooded petroleum reservoirs with different temperatures in China. Front. Microbiol. 2017, 8, 143. [Google Scholar] [CrossRef] [Green Version]
- Turkiewicz, A. Sulfur bacteria and their metabolic products in the reservoir environment of Wierzchowice UGS. Nafta-Gaz 2003, 3, 121–128. [Google Scholar]
- Wolicka, D.; Borkowski, A.; Dobrzyński, D. Potential dependencies in the system: Crude oil, reservoir waters and microorganisms. In Proceedings of the 3rd Scientific and Technical Conference Oil and Gas–Conventional and Unconventional Deposits, Czarna, Poland, 11–14 April 2010. [Google Scholar]
- Sen, K.; Ashbolt, N.J. Environmental Microbiology; Caister Academic Press: Norfolk, UK, 2011. [Google Scholar]
- Bombach, P.; Van Almisick, T.; Richnow, H.H.; Zenner, M.; Kruger, M. Microbial life in an underground gas storage reservoir. Geophys. Res. Abstr. 2015, 17, 15756. [Google Scholar]
- Hemme, C.; Van Berk, W. H2S Generation and release in salt cavern gas storage. In Proceedings of the AAPG Annual Convention Exhibition, Denver, CO, USA, 26 September–1 October 2021. [Google Scholar]
- Bergey, D.H.; Holt, J.G. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Williams & Wilkins: Baltimore, MA, USA, 1994. [Google Scholar]
- Atlas, R.M. Handbook of Microbiological Media, 3rd ed.; CRC Press Inc.: Boca Raton, FL, USA, 2006. [Google Scholar]
- Barton, L.L.; Fardeau, M.L.; Fauque, G.D. Hydrogen sulfide: A toxic gas produced by dissimilatory sulfate and sulfur reduction and consumed by microbial oxidation. Met. Ions Life Sci. 2014, 14, 237–277. [Google Scholar] [CrossRef]
- Liu, W.T.; Jansson, J.K. Environmental Molecular Microbiology; Caister Academic Press: Berkeley, CA, USA, 2010. [Google Scholar]
- Ivanowa, A.E.; Borzenkow, I.A.; Tarasow, A.L.; Milekhina, E.I.; Belyeav, S.S. A microbiological study of an underground gas storage in the process of gas extraction. Microbiology 2007, 76, 461–468. [Google Scholar] [CrossRef]
- Błaszczyk, M.K. Microorganisms in Environmental Protection; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2007. [Google Scholar]
- Khan, S.; Haq, F.; Hasan, F.; Saeed, K.; Ullah, R. Growth and biochemical activities of Acidithiobacillus thiooxidans collected from black shale. J. Microbiol. 2012, 2, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Boden, R.; Cleland, D.; Green, P.N.; Katayama, Y.; Uchino, Y.; Murrell, J.C.; Kelly, D.P. Phylogenetic assessment of culture collection strains of Thiobacillus thioparus, and definitive 16S rRNA gene sequences for T. thioparus, T. denitrificans, and Halothiobacillus neapolitanus. Arch. Microbiol. 2012, 194, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Steliga, T.; Jakubowicz, P. Sulphur content in the natural gas from Wierzchowice UGS. Prace INiG–PIB 2002, 11, 643–648. [Google Scholar]
- Steliga, T.; Jakubowicz, P.; Mularczyk, A. Characteristics of changes in the content of sulfur compounds in natural gas in individual work cycles of UGS Wierzchowice. In Proceedings of the XIV International Conference WWNIG AGH, Zakopane, Poland, 11–13 June 2003. [Google Scholar]
- Kapusta, P.; Turkiewicz, A. Microbiological Processes in Oil and Gas. Engineering; Polish Mining Congress: Cracow, Poland, 2007. [Google Scholar]
- Raczkowski, J.; Turkiewicz, A.; Kapusta, P. Elimination of Biogenic Hydrogen Sulfide in Underground Gas. Storage: A Case Study; SPE ATCE: Houston, TX, USA, 2004; p. 89906. [Google Scholar]
- Turkiewicz, A. Microbiological threats in the environment of underground gas storage facilities. In Proceedings of the 3rd Scientific Conference of the Lodz University of Technology on Decomposition and Microbial Corrosion of Technical Materials, Lodz, Poland, 8–10 September 2003; pp. 85–89. [Google Scholar]
- Gerba, C.P. Quarternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 8, 464–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Md Zain, W.S.; Salleh, N.J.H.; Abdullah, A. Natural biocides for mitigation of sulphate reducing bacteria. Int. J. Corros. 2018, 2018, 7. [Google Scholar] [CrossRef]
- Pawlak, Z.; Pawlak, A.S. Modification of iodometric determination of total and reactive sulfide in environmental samples. Talanta 1999, 48, 347–353. [Google Scholar] [CrossRef]
- Chuwku, E.; Nwaokorie, F.O.; Coker, A.O.; Avila-Campos, M.J.; Ogunsola, F.T. 16S rRNA gene sequencing: A practical approach to confirm the identity of food borne bacteria. Ife J. Sci. 2019, 21, 14–22. [Google Scholar] [CrossRef]
- Wang, J.; Tao, D.; Wang, S.; Li, C.L.Y.; Zheng, F.; Wu, Z. Disifection of lettuce using organic acids: An ecological analysis using 16S rRNA sequencing. RSC Adv. 2019, 9, 17514–17520. [Google Scholar] [CrossRef] [Green Version]
- Robinson, B.; Kusakabe, M. Quantitative preparation of sulfur dioxide, for 34S/32S analysis, from sulfides by combustion with cuprous oxide. Anal. Chem. 1975, 47, 1179–1181. [Google Scholar] [CrossRef]
- Kotarba, M.J.; Bilkiewicz, E.; Hałas, S. Mechanisms of generation of hydrogen sulphide, carbon dioxide and hydrocarbon gases from selected petroleum fields of the Zechstein Main Dolomite carbonates of the western part of Polish Southern Permian Basin: Isotopic and geological approach. J. Pet. Sci. Eng. 2017, 157, 380–391. [Google Scholar] [CrossRef]
- Kusakabe, M. A closed pentane trap for separation of SO2 from CO2 for precise δ18O and δ34S measurements. Geochem. J. 2005, 39, 285–287. [Google Scholar] [CrossRef]
- Hałas, S.; Szafran, J. Improved thermal de com position of sulfates to SO2 and mass spectrometric determination of IAEA SO-5, IAEA SO-6 and NBS-127 sulfate standards. Rapid Commun. Mass Spectrom. 2001, 15, 1618–1620. [Google Scholar] [CrossRef]
- Hałas, S.; Szafran, J. Use of Cu2O–NaPO3 mixtures for SO2 extraction from BaSO4 for sulfur isotope analysis. Isotopes Environ. Health Stud. 2004, 40, 229–231. [Google Scholar] [CrossRef]
- Peryt, T.M.; Hałas, S.; Hryniv, S.P. Sulphur and oxygen isotope signatures of late Permian Zechstein anhydrites, West Poland: Seawater evolution and diagenetic constraints. Geol. Q. 2010, 54, 387–400. [Google Scholar]
- Mizutani, Y. An improvement in the carbon-reduction method for the isotopic analysis of sulfates. Geochem. J. 1971, 5, 69–77. [Google Scholar] [CrossRef]
- Hałas, S.; Szaran, J.; Czarnacki, M.; Tanweer, A. Refinements in BaSO4 to CO2 preparation and δ18O calibration of the sulfate reference materials NBS-127, IAEA SO-5 and IAEA SO-6. Geostand. Geoanal. Res. 2007, 31, 61–68. [Google Scholar] [CrossRef]
- Russell, H.A.; Hugo, W.B.; Fraise, A.P.; Ayliffe, G.A.; Lambert, P.A.; Maillard, J.-Y. Principles and Practice of Disinfection, Preservation and Sterilization; Willey-Blackwell: Hoboken, NJ, USA, 2004; pp. 212–213. [Google Scholar]
- Hałas, S.; Wójtowicz, A.; Nowak, J.; Durakiewicz, T. Temperature controller for thermal ionization mass spectrometry. Rapid Commun. Mas Spectrom. 2002, 16, 77–80. [Google Scholar] [CrossRef]
- Kovalevych, V.; Peryt, T.; Beer, W.; Geluk, M.; Hałas, S. Geochemistry of Early Tiassic seawater as indicated by study of the Rot halite in the Netherlands, Germany and Poland. Chem. Geol. 2002, 182, 549–563. [Google Scholar] [CrossRef]
- Pluta, I.; Hałas, S. Sulphur and oxygen isotopic composition in sulphates from mesozoic formations. Przegl. Geol. 2002, 50, 634–638. [Google Scholar]
- Krouse, H.R.; Viau, C.A.; Eliuk, A.L.; Ueda, S.; Halas, S. Chemical and isotopic evidence of thermochemical sulphate reduction by light hydrocarbon gases in deep carbonate reservoirs. Nature 1988, 333, 415–419. [Google Scholar] [CrossRef]
- Turkiewicz, A.; Kania, M.; Janiga, M. Microbiological tests and chemical analysis of contents of sulfur compounds in the samples collected from salt caverns of an underground gas storage. Nafta-Gaz 2013, 8, 588–598. [Google Scholar]
- Canfield, D.E.; Olesen, C.A.; Cox, R.P. Temperature and its control of isotope fractionation by a sulphate-reducing bacterium. Geochim. Cosmochim. Acta 2006, 70, 548–561. [Google Scholar] [CrossRef]
- Kerkar, S.; Bharathi, L.P.A. Stimulation of sulfate-reducing activity at salt-saturation in the salterns of Ribandar, Goa, India. Geomicrobiol. J. 2007, 24, 101–110. [Google Scholar] [CrossRef]
- Stam, M.C.; Mason, P.R.D.; Pallud, C.; Van Cappellen, P. Sulfate reducing activity and sulfur isotope fractionation by natural microbial communities in sediments of a hypersaline soda lake (Mono Lake, California). Chem. Geol. 2010, 278, 23–30. [Google Scholar] [CrossRef]
- Zhang, T.W.; Ellis, G.S.; Wang, K.S.; Walters, C.C.; Kelemen, S.R.; Gillaizeau, B.; Tang, Y.C. Effect of hydrocarbon type on thermochemical sulfate reduction. Org. Geochem. 2007, 38, 897–910. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, S.; Liang, Y.; Dai, J.; Li, J. Isotopic evidence of TSR origin for natural gas bearing high H2S contents within the feixianguan formation of the Northeastern Sichuan Basin, Southwestern China. Sci. China Ser. D Earth Sci. 2005, 48, 1960–1971. [Google Scholar] [CrossRef]


| Exploitation Well | pH of Formation Water | Content of H2S and Sulphides (mg/L) | Sulphate Reducing Bacteria (CFU/100 mL) | Sulphur Compounds Oxidizing Bacteria | |
|---|---|---|---|---|---|
| Thiobacillus spp. (Growth of Bacteria on Neutrophilic Sulphur-oxidizing Bacteria Medium) | Acidithiobacillus spp. (Growth of Bacteria on Acidophilic Sulphur-Oxidizing Bacteria Medium) | ||||
| WM-B4H | 7.01 | 0.00 | - | - | - |
| WM-B7H | 5.22 | 0.00 | - | - | + |
| W-collective water (from all wells) | 5.10 | 0.48 | - | - | - |
| Exploitation Well | pH of Formation Water | Content of H2S and Sulphides (mg/L) | Sulphate Reducing Bacteria (CFU/100 mL) | Sulphur Compounds Oxidizing Bacteria | |
|---|---|---|---|---|---|
| Thiobacillus spp. (Growth of Bacteria on Neutrophilic Sulphur-Oxidizing Bacteria Medium) | Acidithiobacillus spp. (Growth of Bacteria on Acidophilic Sulphur-Oxidizing Bacteria Medium) | ||||
| WM-A5H | 4.06 | 1.49 | - | - | - |
| WM-B4H | 6.80 | 2.15 | - | + | - |
| WM-B5H | 5.04 | 1.97 | - | - | - |
| WM-B7H | 5.15 | 3.08 | - | - | - |
| W-collective water/I (from all wells) | 5.18 | 1.22 | - | - | - |
| W-collective water/II (from all wells) | 5.03 | 3.95 | - | - | + |
| W-collective water/III (from all wells) | 5.00 | 3.20 | - | - | + |
| W-collective water/IV (from all wells) | 5.09 | 2.97 | - | - | + |
| Exploitation Well | pH of Formation Water | Content of H2S and Sulphides (mg/L) | Sulphate Reducing Bacteria (CFU/100 mL) | Sulphur Compounds Oxidizing Bacteria | |
|---|---|---|---|---|---|
| Thiobacillus spp. (Growth of Bacteria on Neutrophilic Sulphur-oxidizing Bacteria Medium) | Acidithiobacillus spp. (Growth of Bacteria on Acidophilic Sulphur-Oxidizing Bacteria Medium) | ||||
| WM-A2H | 7.00 | 15.5 | 10 | + | - |
| WM-B3H | 4.97 | 0.00 | - | - | - |
| WM-B5H | 5.09 | 0.00 | - | - | - |
| W-33 | 4.92 | 2.72 | - | - | - |
| W-collective water (from all wells) | 5.16 | 3.27 | - | - | - |
| Exploitation Well | pH of Formation Water | Content of H2S and Sulphides (mg/L) | Sulphate Reducing Bacteria (CFU/100 mL) | Sulphur Compounds Oxidizing Bacteria | |
|---|---|---|---|---|---|
| Thiobacillus spp. (Growth of Bacteria on Neutrophilic Sulphur-Oxidizing Bacteria Medium) | Acidithiobacillus spp. (Growth of Bacteria on Acidophilic Sulphur-Oxidizing Bacteria Medium) | ||||
| W-28 | 5.24 | 1.31 | - | - | - |
| W-33 | 6.10 | 5.23 | - | - | - |
| W-36 | 5.30 | 0.00 | - | - | - |
| WM-A1bH | 5.29 | 1.86 | - | - | - |
| WM-A3H | 6.95 | 14.25 | 10 | + | - |
| WM-B2H | 5.21 | 0.00 | - | - | - |
| WM-B5H | 4.14 | 0.00 | - | - | + |
| WM-B6H | 5.71 | 5.07 | - | - | - |
| W-collective water (from all wells) | 5.02 | 2.30 | - | - | - |
| Strain Designation | Identification by Classical Methods | Identification by Sequencing | % Identity Most Similar Sequence in GenBank |
|---|---|---|---|
| W_1 | Desulfovibrio sp. | Desulfovibrio desulfuricans | 99%/DSM 642 |
| W_2 | Desulfovibrio sp. | Desulfovibrio vulgaris | 98%/DSM 644 |
| W_3 | Desulfotomaculum sp. | Desulfotomaculum nigrificans | 99%/DSM 574 |
| W_4 | Desulfotomaculum sp. | Desulfohalotomaculum halophilum | 99%/DSM 11559 |
| W_5 | Acidithiobacillus sp. | Acidithiobacillus ferrooxidans | 99%/DSM 14882 |
| W_6 | Acidithiobacillus sp. | Acidithiobacillus thiooxidans | 97%/DSM 14887 |
| W_7 | Thiobacillus sp. | Thiobacillus thioparus | 96%/DSM 505 |
| Sample | Well | δ34SH2S | |
|---|---|---|---|
| 1st Series | 2nd Series | ||
| Ag2S | W-7 | 9.97 ± 0.5 | 12.05 ± 0.3 |
| W-25 | 10.54 ± 0.5 | 12.89 ± 0.2 | |
| W-29 | 9.85 ± 0.5 | 12.78 ± 0.3 | |
| W-33 | 10.68 ± 0.5 | 12.01 ± 0.2 | |
| WMB-6H | 10.99 ± 0.5 | 12.25 ± 0.3 | |
| BaSO4 | W-46 | δ34SSO4 = 9.84 ± 0.3 δ18OSO4 = 11.35 ± 0.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turkiewicz, A.; Steliga, T.; Kluk, D.; Gminski, Z. Biomonitoring Studies and Preventing the Formation of Biogenic H2S in the Wierzchowice Underground Gas Storage Facility. Energies 2021, 14, 5463. https://doi.org/10.3390/en14175463
Turkiewicz A, Steliga T, Kluk D, Gminski Z. Biomonitoring Studies and Preventing the Formation of Biogenic H2S in the Wierzchowice Underground Gas Storage Facility. Energies. 2021; 14(17):5463. https://doi.org/10.3390/en14175463
Chicago/Turabian StyleTurkiewicz, Anna, Teresa Steliga, Dorota Kluk, and Zbigniew Gminski. 2021. "Biomonitoring Studies and Preventing the Formation of Biogenic H2S in the Wierzchowice Underground Gas Storage Facility" Energies 14, no. 17: 5463. https://doi.org/10.3390/en14175463
APA StyleTurkiewicz, A., Steliga, T., Kluk, D., & Gminski, Z. (2021). Biomonitoring Studies and Preventing the Formation of Biogenic H2S in the Wierzchowice Underground Gas Storage Facility. Energies, 14(17), 5463. https://doi.org/10.3390/en14175463






