Power Enhancement of a PV Module Using Different Types of Phase Change Materials
Abstract
:1. Introduction
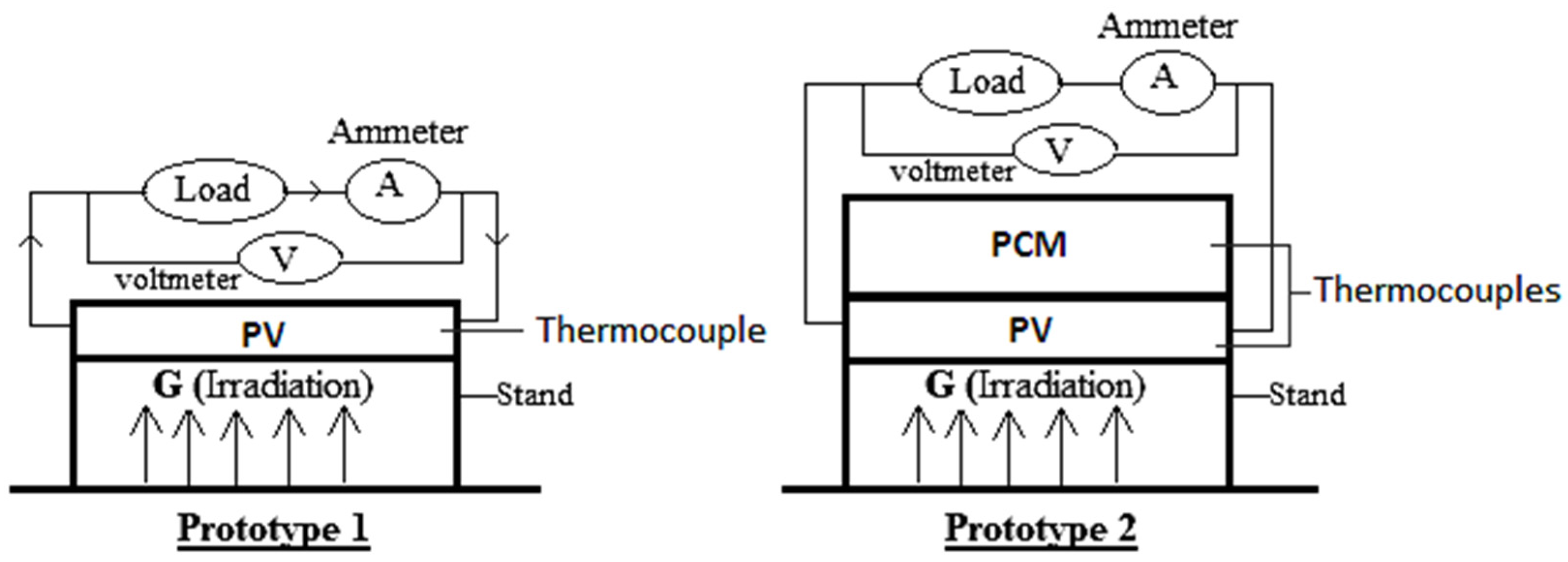
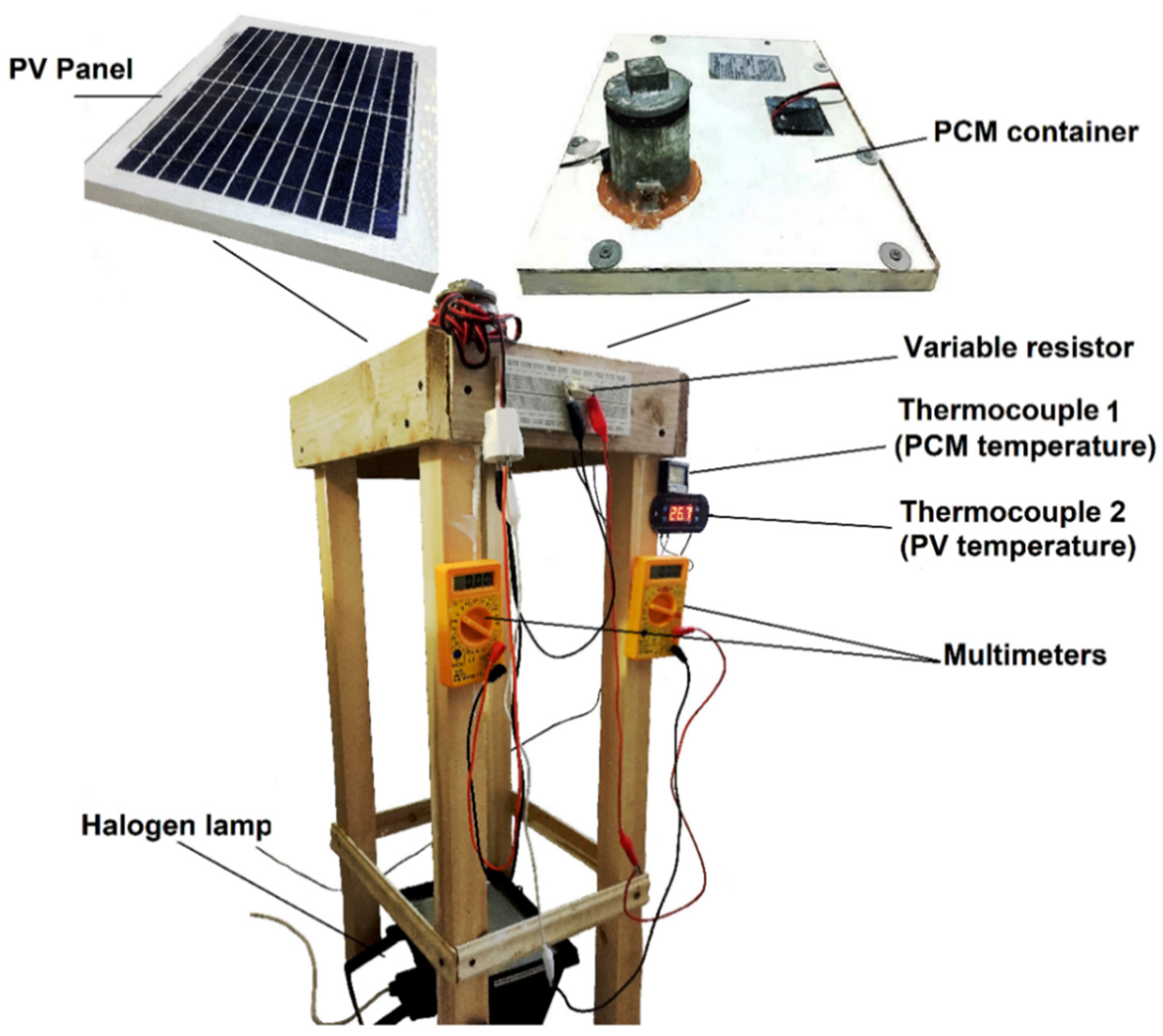
2. Experimental Setup
2.1. Setup
2.2. PCMs Used
2.3. Experimental Procedure
- The five prototypes were fixed perpendicular to the light direction in an ambient temperature of 20 °C.
- Using the Lux meter, the solar simulator (halogen lamp) was placed at a defined distance away from the prototype in order to ensure an irradiance of 1000 W/m2.
- The measuring tools were connected to the PV system.
- The experiment was conducted at maximum power transmission for an appropriate amount of time.
- The output voltages and currents were recorded for different resistors. In addition, the temperatures of the PV panel during the experiment were recorded as a function of time. The sampling frequency was one reading every 20 s.
- Different graphs were drawn based on the obtained results including the (I–V) curve, (P–V) curve, efficiency versus time, temperature versus time, and PV panel power versus time.
3. Results
3.1. Uncertainty Analysis
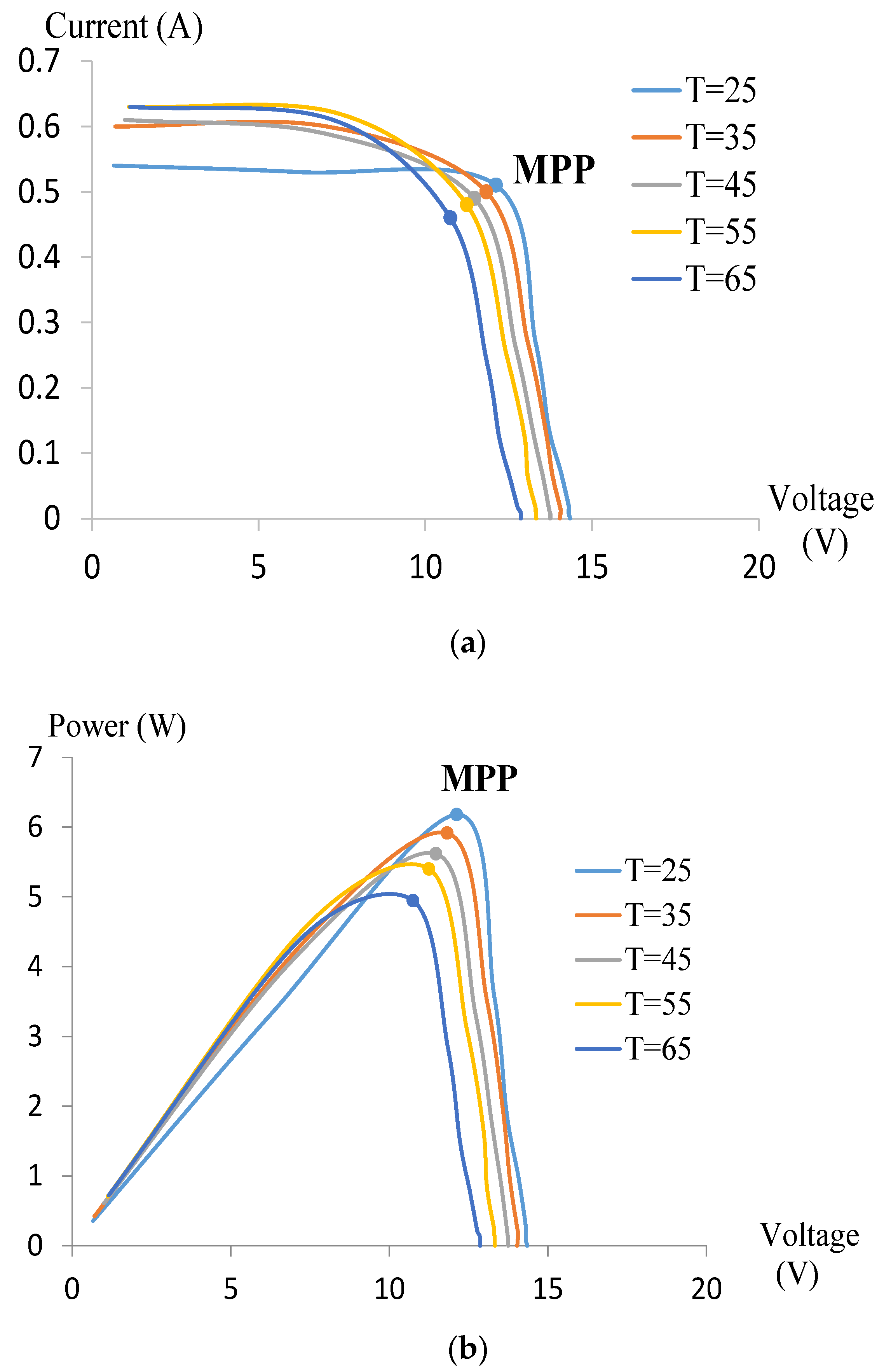
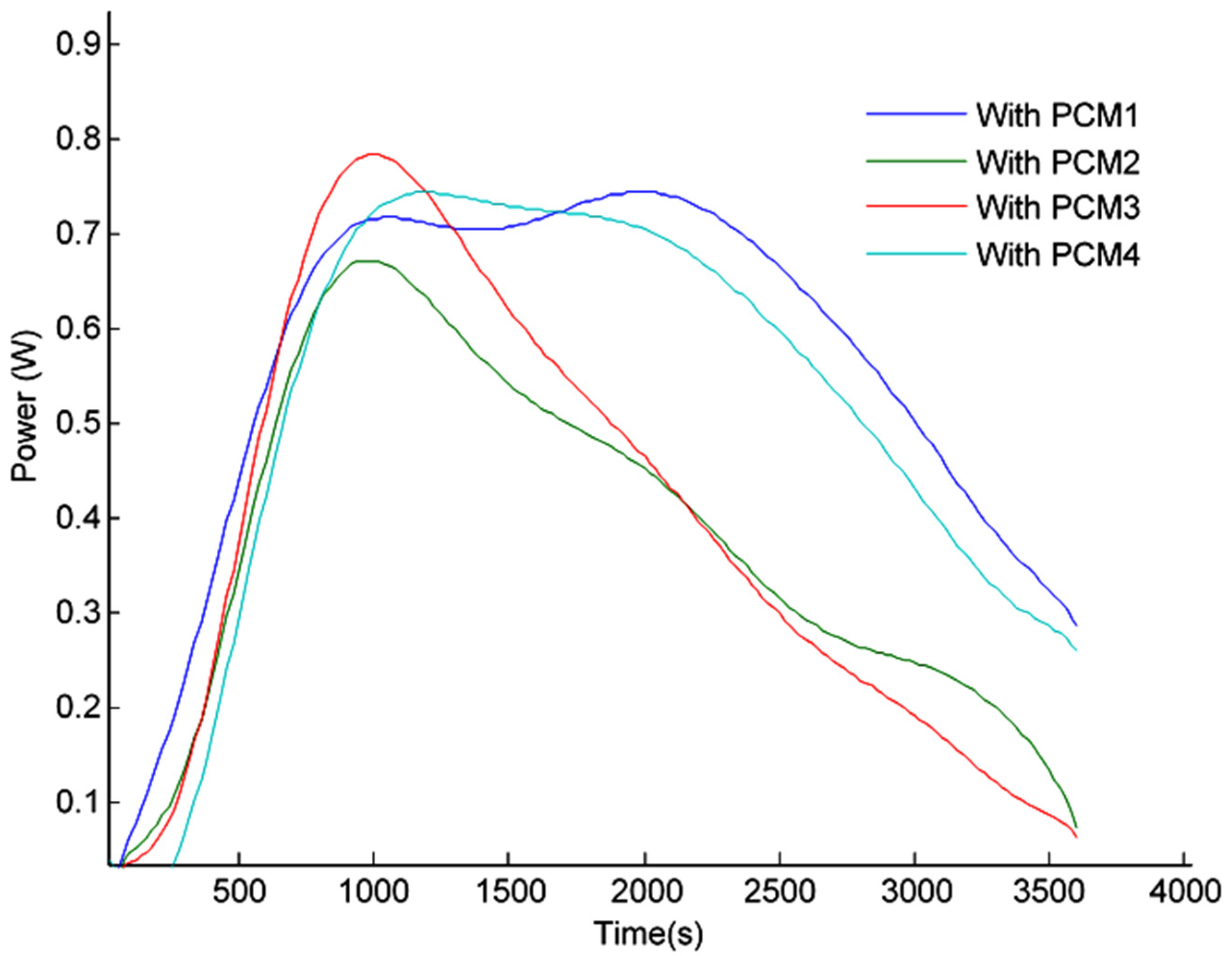
3.2. Maximum Power Point (MPP)
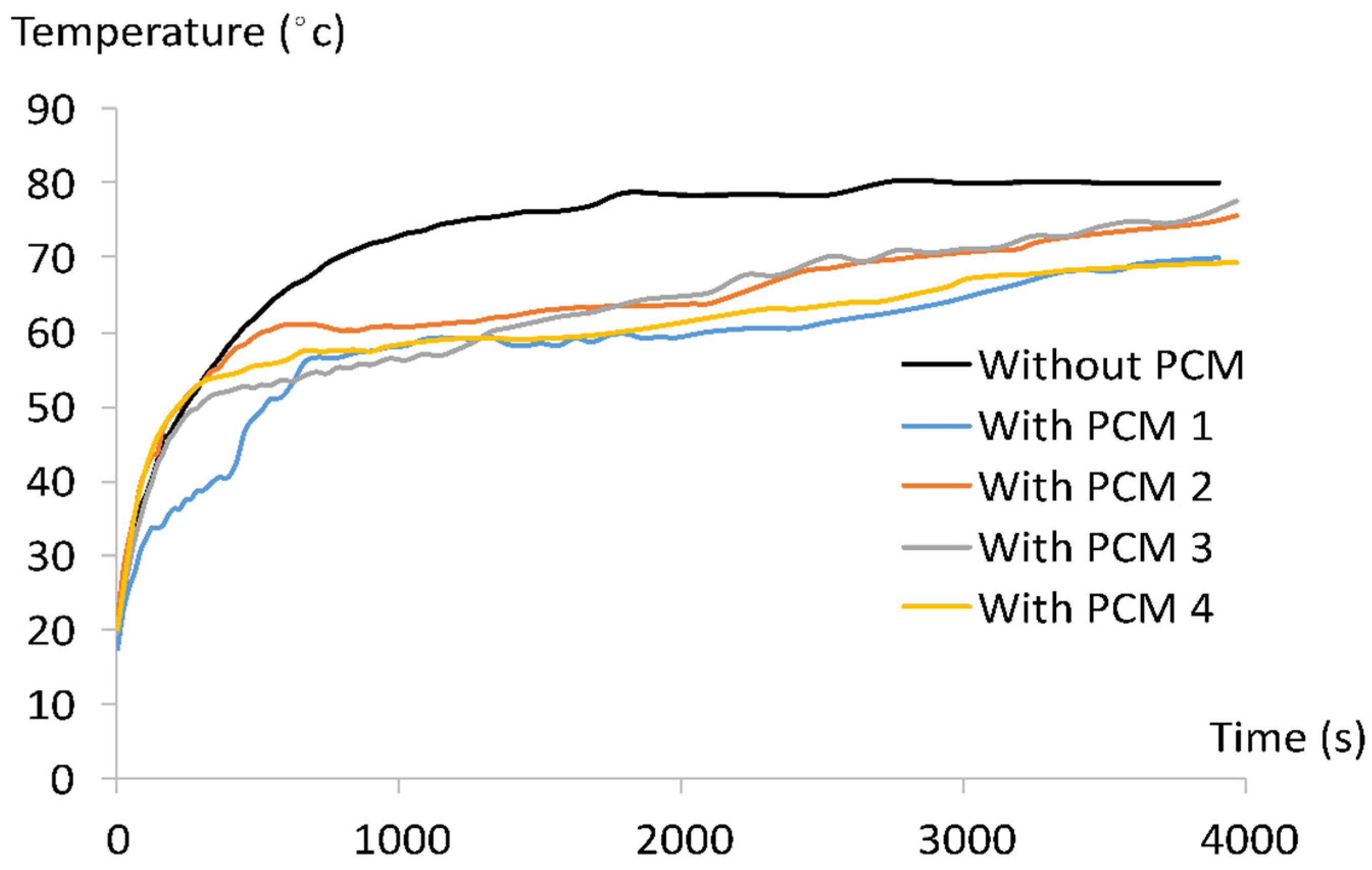
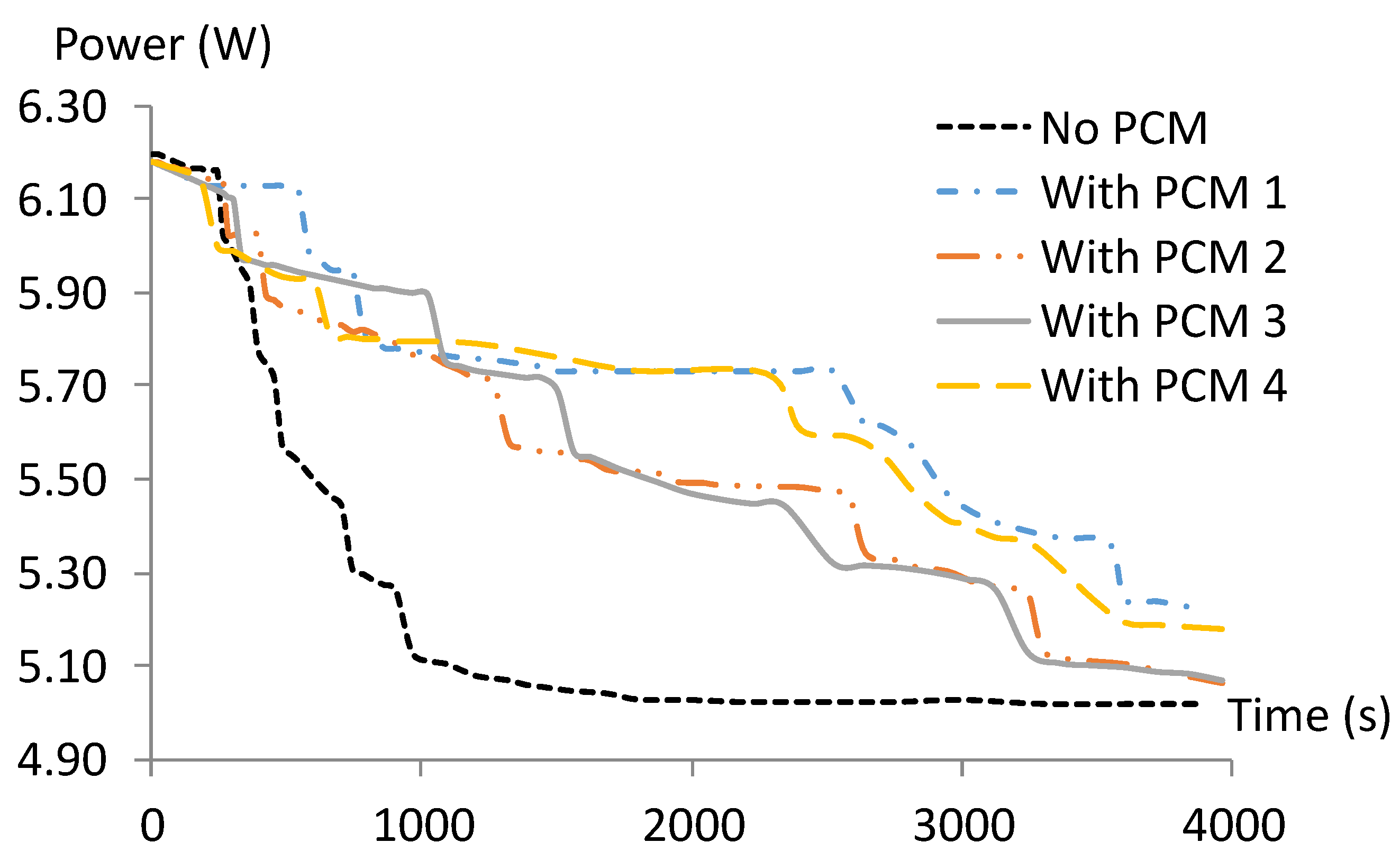
3.3. PCM and PV Temperature Variations
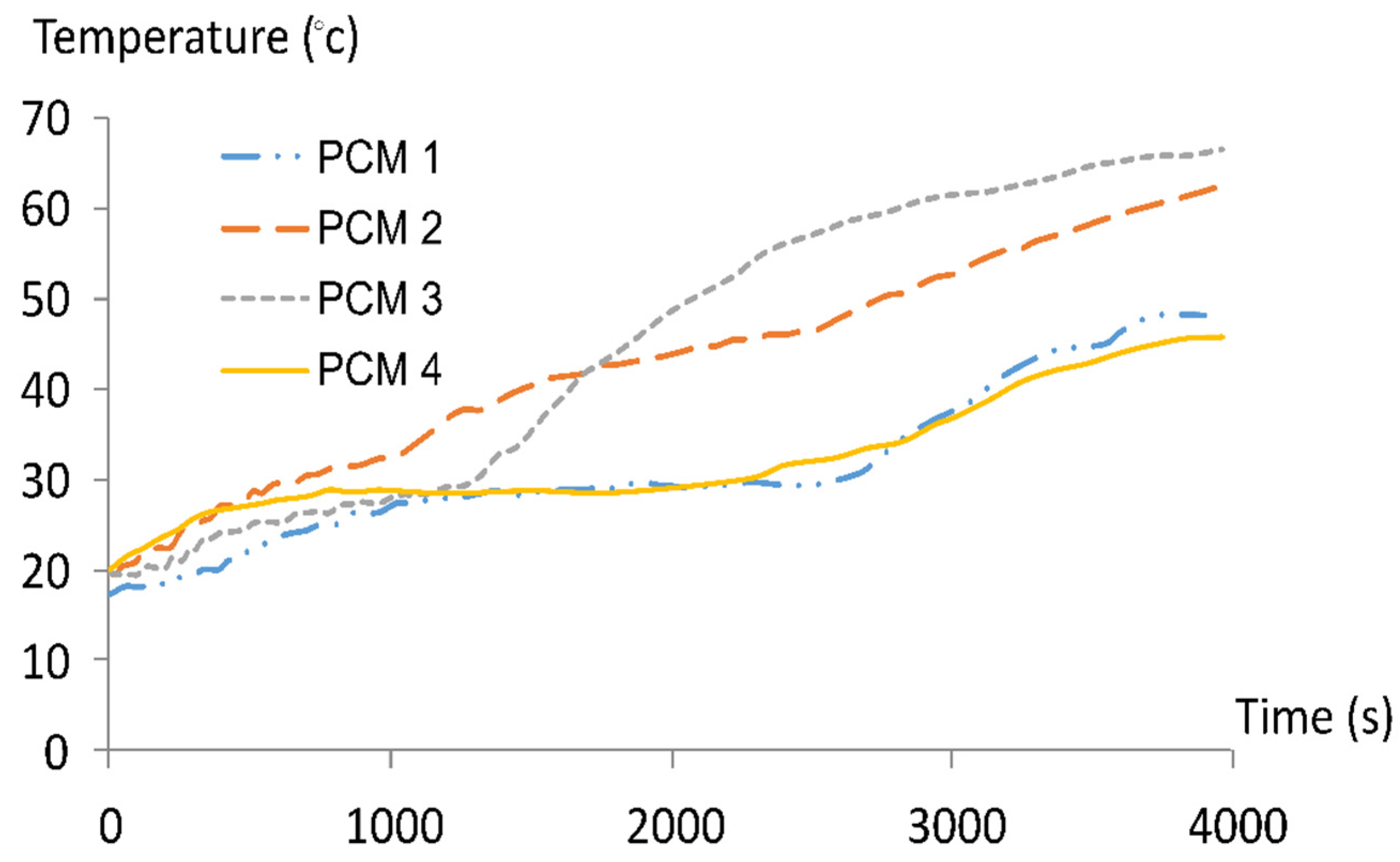
3.3.1. PCMs Temperature Variation
3.3.2. PV Temperature Variation with and without PCM
3.3.3. PV Panel Power Output
3.3.4. Power and Energy Gained
4. Discussion
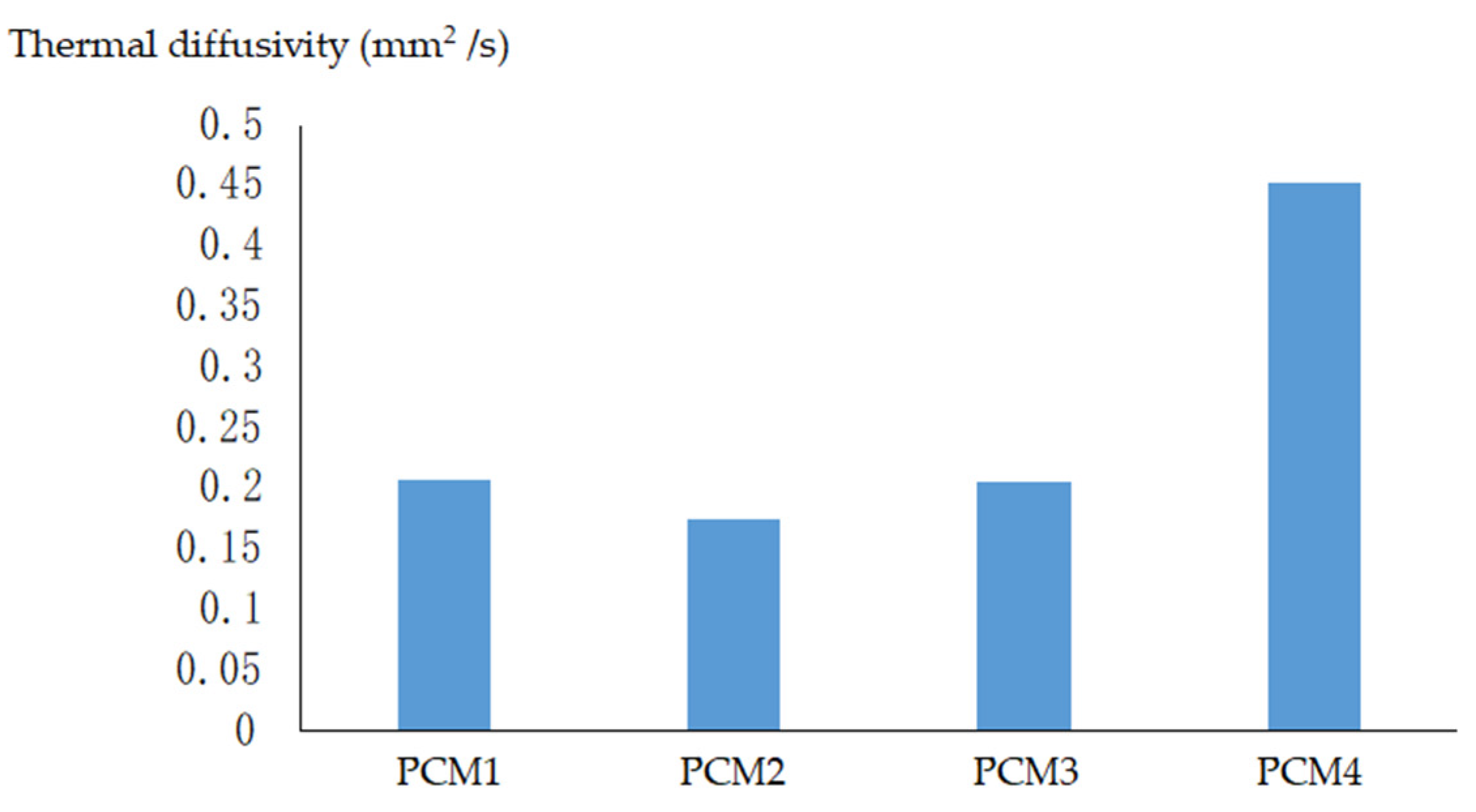
4.1. Thermal Diffusivity
4.2. Economic Analysis: A Case Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brinkworth, B.J. Estimation of flow and heat transfer for the design of PV cooling duct. Sol. Energy 2020, 69, 413–420. [Google Scholar] [CrossRef]
- Kawtharani, F.; Kawtharani, A.; Hammoud, M.; Hallal, A.; Shaito, A.; Assi, A.; Assi, I. Cooling PV modules using phase change material. In Proceedings of the 29th International Conference on Microelectronics, Beirut, Lebanon, 10–13 December 2017; pp. 347–351. [Google Scholar]
- Fayaz, H.; Rahim, N.A.; Hasanuzzaman, M.; Rivai, A.; Nasrin, R. Numerical and outdoor real time experimental investigation of performance of PCM based PVT system. Sol. Energy 2019, 179, 135–150. [Google Scholar] [CrossRef]
- Wongwuttanasatian, T.; Sarikarin, T.; Suksri, A. Performance enhancement of a photovoltaic module by passive cooling using phase change material in a finned container heat sink. Sol. Energy 2020, 195, 47–53. [Google Scholar] [CrossRef]
- Yao, J.; Xu, H.; Dai, Y.; Huang, M. Performance analysis of solar assisted heat pump coupled with build-in PCM heat storage based on PV/T panel. Sol. Energy 2020, 197, 279–291. [Google Scholar] [CrossRef]
- Akshayveer; Kumar, A.; Singh, A.P.; Singh, O.P. Effect of novel PCM encapsulation designs on electrical and thermal performance of a hybrid photovoltaic solar panel. Sol. Energy 2020, 205, 320–333. [Google Scholar] [CrossRef]
- Krauter, S. Increased electrical yield via water flow over the front of photovoltaic panels. Sol. Energy Mat. Sol. C 2004, 82, 131–137. [Google Scholar] [CrossRef]
- Huang, M.J.; Eames, P.C.; Norton, B. Thermal regulation of building-integrated photovoltaics using phase change materials. Int. J. Heat Mass Tran. 2004, 47, 2715–2733. [Google Scholar] [CrossRef]
- Prieto, C.; Cabeza, L.F. Thermal energy storage (TES) with phase change materials (PCM) in solar power plants (CSP). Concept and plant performance. Appl. Energy 2019, 254, 113646. [Google Scholar] [CrossRef]
- Batrancea, L.; Rus, M.-I.; Masca, E.S.; Morar, I.D. Fiscal Pressure as a Trigger of Financial Performance for the Energy Industry: An Empirical Investigation across a 16-Year Period. Energies 2021, 14, 3769. [Google Scholar] [CrossRef]
- Prieto, C.; Lopez-Roman, A.; Martinez, N.; Morera, J.M.; Cabeza, L.F. Improvement of phase change materials (PCM) used for solar process heat applications. Molecules 2021, 26, 1260. [Google Scholar] [CrossRef] [PubMed]
- De Gracia, A.; Navarro, L.; Castell, A.; Boer, D.; Cabeza, L.F. Life cycle assessment of a ventilated facade with PCM in its air chamber. Sol. Energy 2014, 104, 115–123. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, J.; Zhou, Y.; Liu, Y. Preparation and Properties of Capric–Myristic Acid/Expanded Graphite Composite Phase Change Materials for Latent Heat Thermal Energy Storage. Energies 2020, 13, 2462. [Google Scholar] [CrossRef]
- Salameh, W.; Elabed, I.; Kaddoura, Z.; Assi, A.; El-Rab, M.G.; Hammoud, M. The use of phase change material in the design of heat recovery and energy storage system applied to diesel generators. In Proceedings of the International Conference on Renewable Energies for Developing Countries, Beirut, Lebanon, 26–27 November 2014; pp. 38–42. [Google Scholar]
- Sudhansu, S.D.; Pramod, K.; Sarbjot, S.S. Hybrid photovoltaic-thermal systems utilizing liquid-gas phase change material. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 43, 2896–2914. [Google Scholar]
- Skoplaki, E.; Palyvos, P.A. On the temperature dependence of photo-voltaic module electrical performance: A review of efficiency/power correlations. Sol. Energy 2009, 83, 614–624. [Google Scholar] [CrossRef]
- Mahamudul, H.; Rahman, M.M.; Metselaar, H.S.C.; Mekhilef, S.; Shezan, S.A.; Sohel, R.; Abu Karim, S.B.; Badiuzaman, W.N.I. Temperature Regulation of Photovoltaic Module Using Phase Change Material: A Numerical Analysis and Experimental Investigation. Int. J. Photoenergy 2016, 2016, 5917028. [Google Scholar] [CrossRef]
- Browne, M.C.; Norton, B.; McCormack, S.J. Phase change materials for photovoltaic thermal management. Renew. Sust. Energy Rev. 2015, 47, 762–782. [Google Scholar] [CrossRef]
- Mjallal, I.; Farhat, H.; Hammoud, M.; Ali, S.; Assi, I. Improving the Cooling Efficiency of Heat Sinks through the Use of Different Types of Phase Change Materials. Technologies 2018, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Mjallal, I.; Farhat, H.; Hammoud, M.; Ali, S.; Shaer, A.A.L.; Assi, A. Cooling Performance of Heat Sinks Used in Electronic Devices. MATEC Web. Conf. 2018, 171, 02003. [Google Scholar] [CrossRef]
- Farhat, H.; Mjallal, I.; Hammoud, M.; Ali, S.; Shaer, A.A.L.; Assi, A.; Assi, I. Investigating the effect of the density of phase change materials on cooling electronic packages. In Proceedings of the 29th International Conference on Microelectronics, Beirut, Lebanon, 10–13 December 2017; pp. 185–189. [Google Scholar]
- Assi, I.; Mjallal, I.; Farhat, H.; Hammoud, M.; Ali, S.; Shaer, A.A.L.; Assi, A. Using phase change material in heat sinks to cool electronics devices with intermittent usage. In Proceedings of the IEEE 7th International Conference on Power and Energy Systems ICPES, Toronto, ON, Canada, 1–3 November 2017; pp. 66–69. [Google Scholar]
- Cui, Y.; Xie, J.; Liu, J.; Wang, J.; Chen, S. A review on phase change material application in building. Adv. Mech. Eng. 2017, 9, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekar, M.; Rajkumar, S.; Valavan, D. A review on the thermal regulation techniques for non-integrated flat PV modules mounted on building top. Energy Build. 2015, 86, 692–697. [Google Scholar] [CrossRef]
- Biwole, P.H.; Eclache, P.; Kuznik, F. Phase-change materials to improve solar panel’s performance. Energy Build. 2013, 62, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Ibra Hadad, Sigma Aldrich Company LLC, Lebanon. Available online: https://www.sigmaaldrich.com/chemistry.html (accessed on 10 March 2020).
- Mjallal, I.; Hammoud, M.; Habchi, C.; Lemenand, T. Cost Effective Device to Characterize Phase Change Materials. Meas. Sci. Technol. 2020, 31, 025903. [Google Scholar] [CrossRef]
- Berjawi, A.H.; Najem, S.; Faour, G.; Abdallah, C.; Ahmad, A. Assessing Solar PV’s Potential in Lebanon. National Center for Remote Sensing; CNRS: Beirut, Lebanon, 2017. [Google Scholar]
- Gao, E.; Zhang, B.; Stephenson, L.D.; Boddu, B.; Trovillion, J. Prediction of phase change material (pcm) degradation. In Proceedings of the Thermal Performance of the Exterior Envelopes of Whole Buildings XII, Clearwater, FL, USA, 1–5 December 2013. [Google Scholar]
| PCM 1 | PCM 2 | PCM 3 | PCM 4 | |
|---|---|---|---|---|
| Name | Sodium Sulfate Decahydrate | Sodium Phosphate Dibasic Dodecahydrate | Decanoic Acid | Calcium Chloride Hexahydrate |
| Density (kg/m3) | 1485 | 1552 | 1004 | 1710 |
| Melting point (°C) | 32 | 40 | 30 | 29 |
| Latent heat (kJ/kg) | 252 | 270 | 153 | 180 |
| Specific heat (kJ/kg·K) | 1.8 | 1.9 | 1.81 | 1.4 |
| Thermal conductivity (W/m·K) | 0.554 | 0.514 | 0.372 | 1.08 |
| Equipment | Experiment Error |
|---|---|
| Thermocouple | 0.5 °C |
| Lux meter | 1.5% |
| Voltmeter | 1% |
| Ammeter | 1% |
| Energy Generated (kJ) | Energy Gained (kJ) | PEG (%) | |
|---|---|---|---|
| Without PCM | 18.74 | 0 | 0 |
| With PCM 1 | 21.06 | 2.31 | 12.37 |
| With PCM 2 | 20.09 | 1.34 | 7.20 |
| With PCM 3 | 20.15 | 1.40 | 7.50 |
| With PCM 4 | 21.38 | 2.64 | 14.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaito, A.; Hammoud, M.; Kawtharani, F.; Kawtharani, A.; Reda, H. Power Enhancement of a PV Module Using Different Types of Phase Change Materials. Energies 2021, 14, 5195. https://doi.org/10.3390/en14165195
Shaito A, Hammoud M, Kawtharani F, Kawtharani A, Reda H. Power Enhancement of a PV Module Using Different Types of Phase Change Materials. Energies. 2021; 14(16):5195. https://doi.org/10.3390/en14165195
Chicago/Turabian StyleShaito, Ali, Mohammad Hammoud, Fadel Kawtharani, Ali Kawtharani, and Hilal Reda. 2021. "Power Enhancement of a PV Module Using Different Types of Phase Change Materials" Energies 14, no. 16: 5195. https://doi.org/10.3390/en14165195
APA StyleShaito, A., Hammoud, M., Kawtharani, F., Kawtharani, A., & Reda, H. (2021). Power Enhancement of a PV Module Using Different Types of Phase Change Materials. Energies, 14(16), 5195. https://doi.org/10.3390/en14165195






