Ignition Thresholds and Flame Propagation of Methane/Air Mixtures Ignited via Radiatively Heated Inert Particles
Abstract
1. Introduction
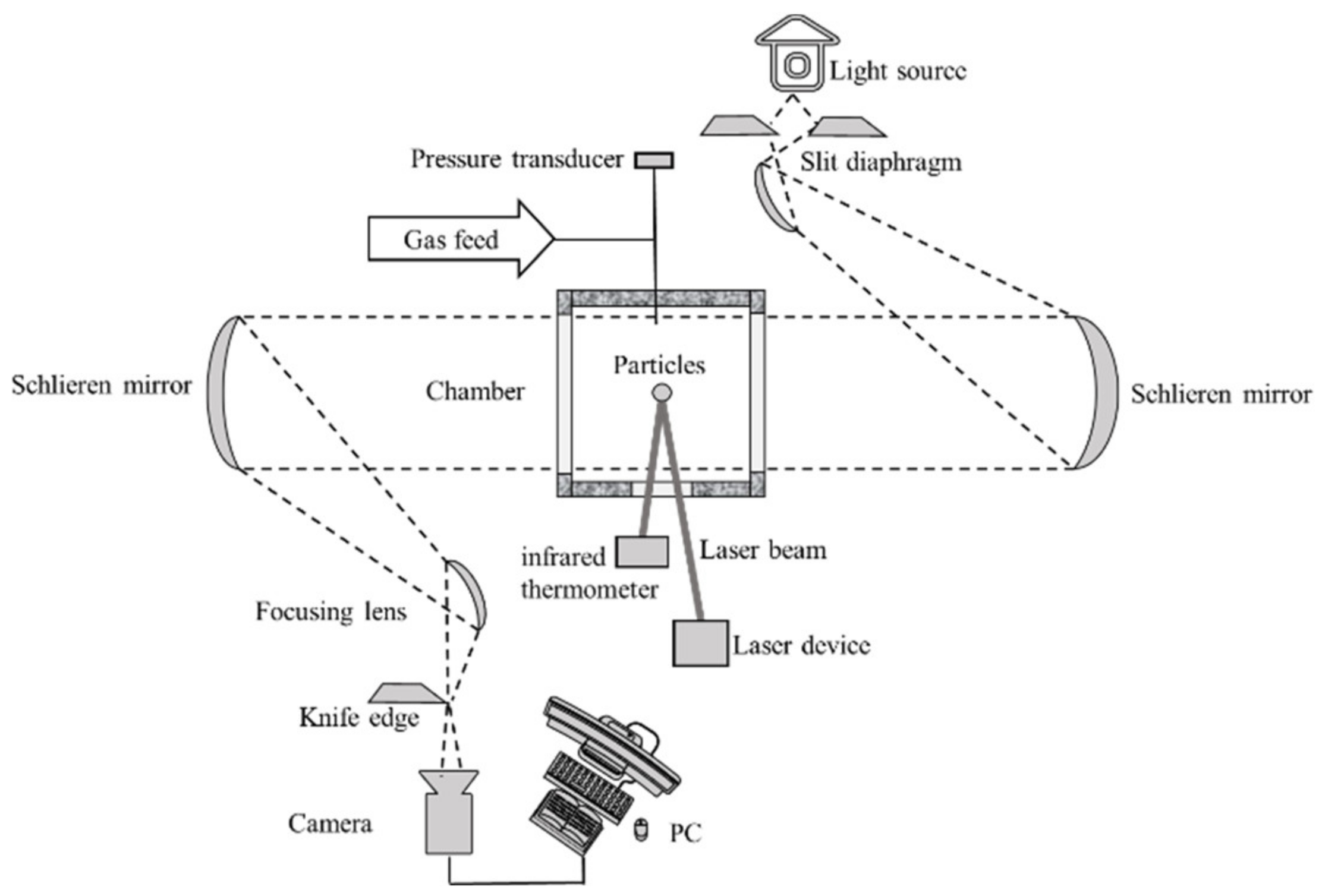
2. Experiment Methods
3. Results and Discussion
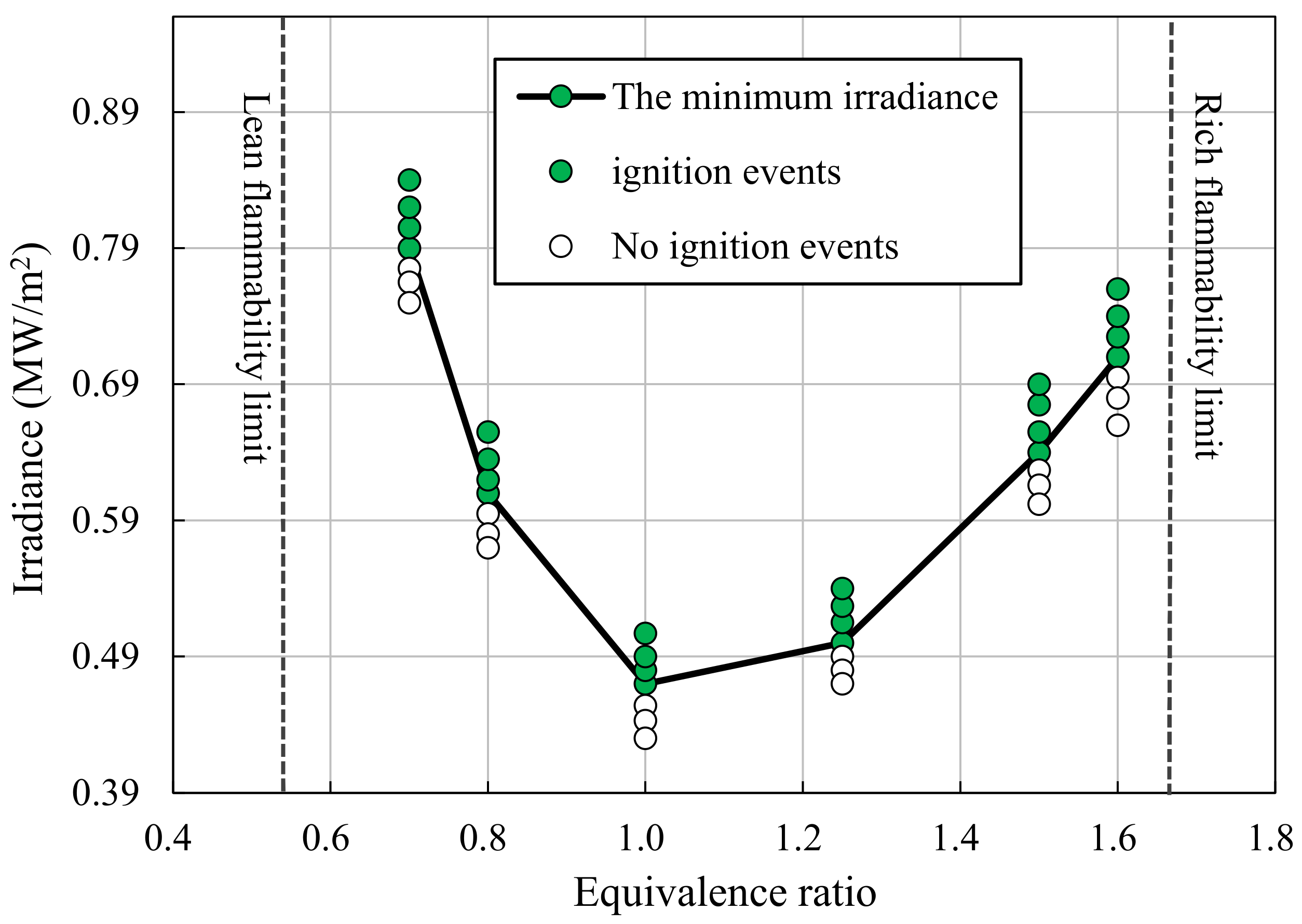
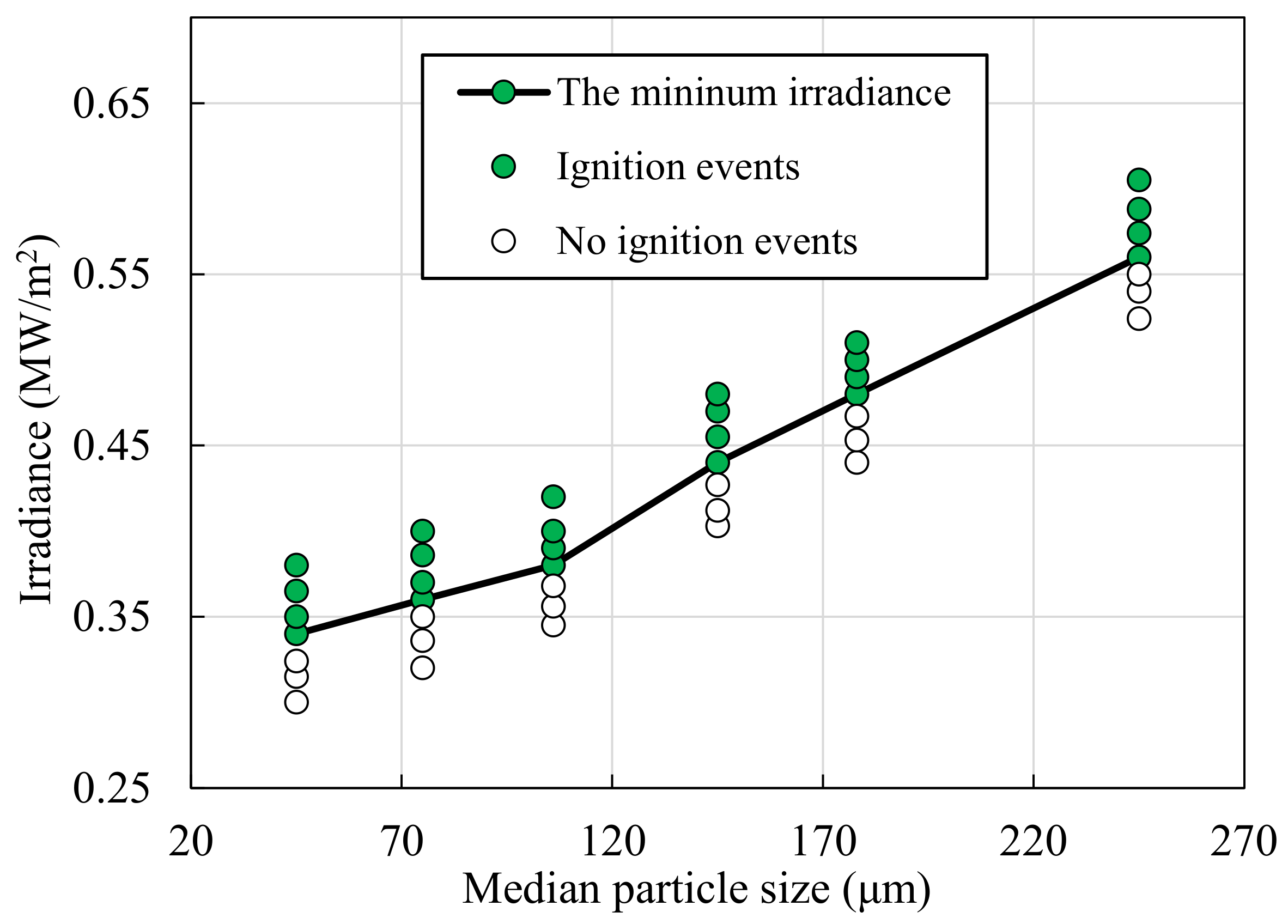
3.1. Irradiance Threshold of Ignition
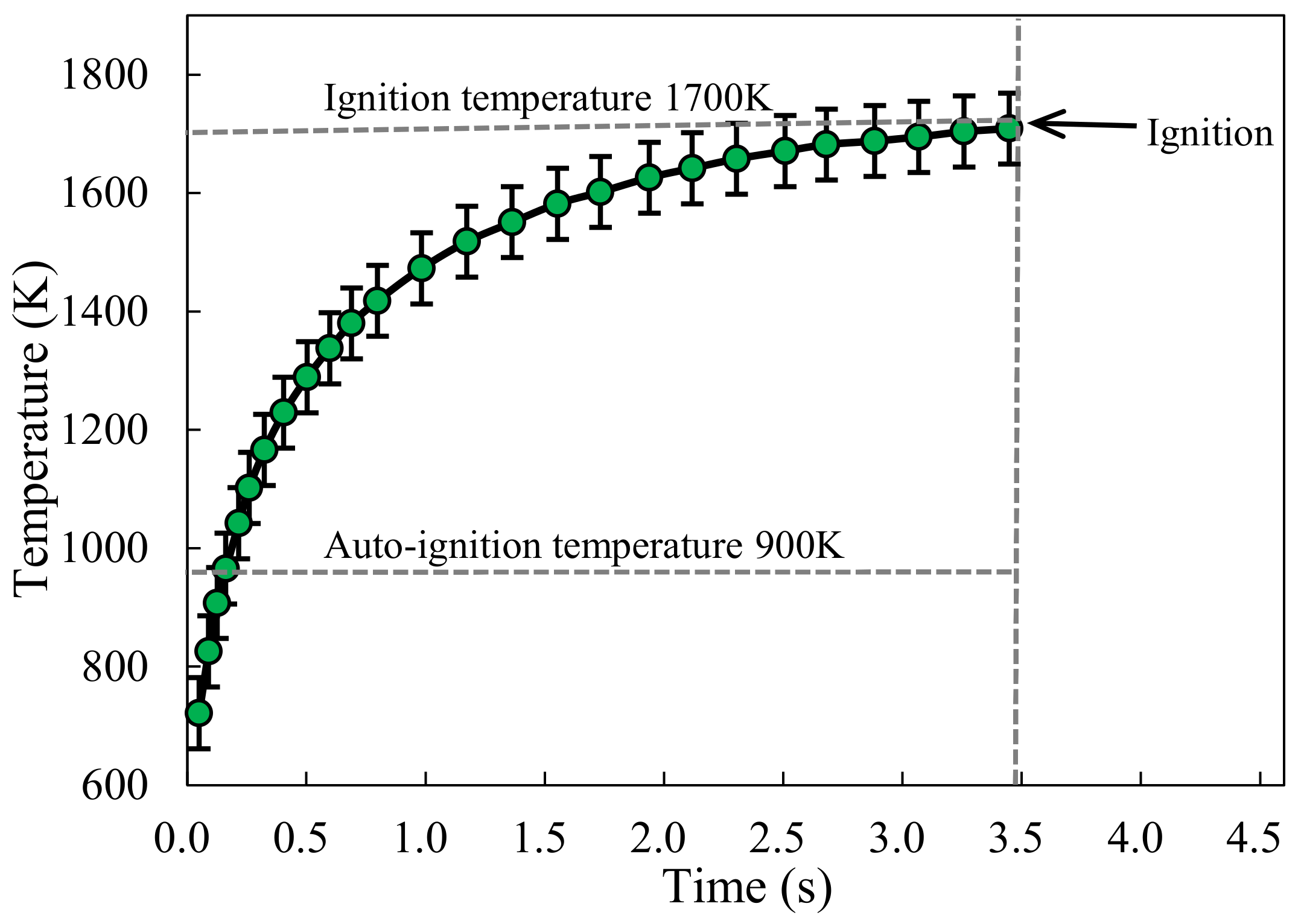
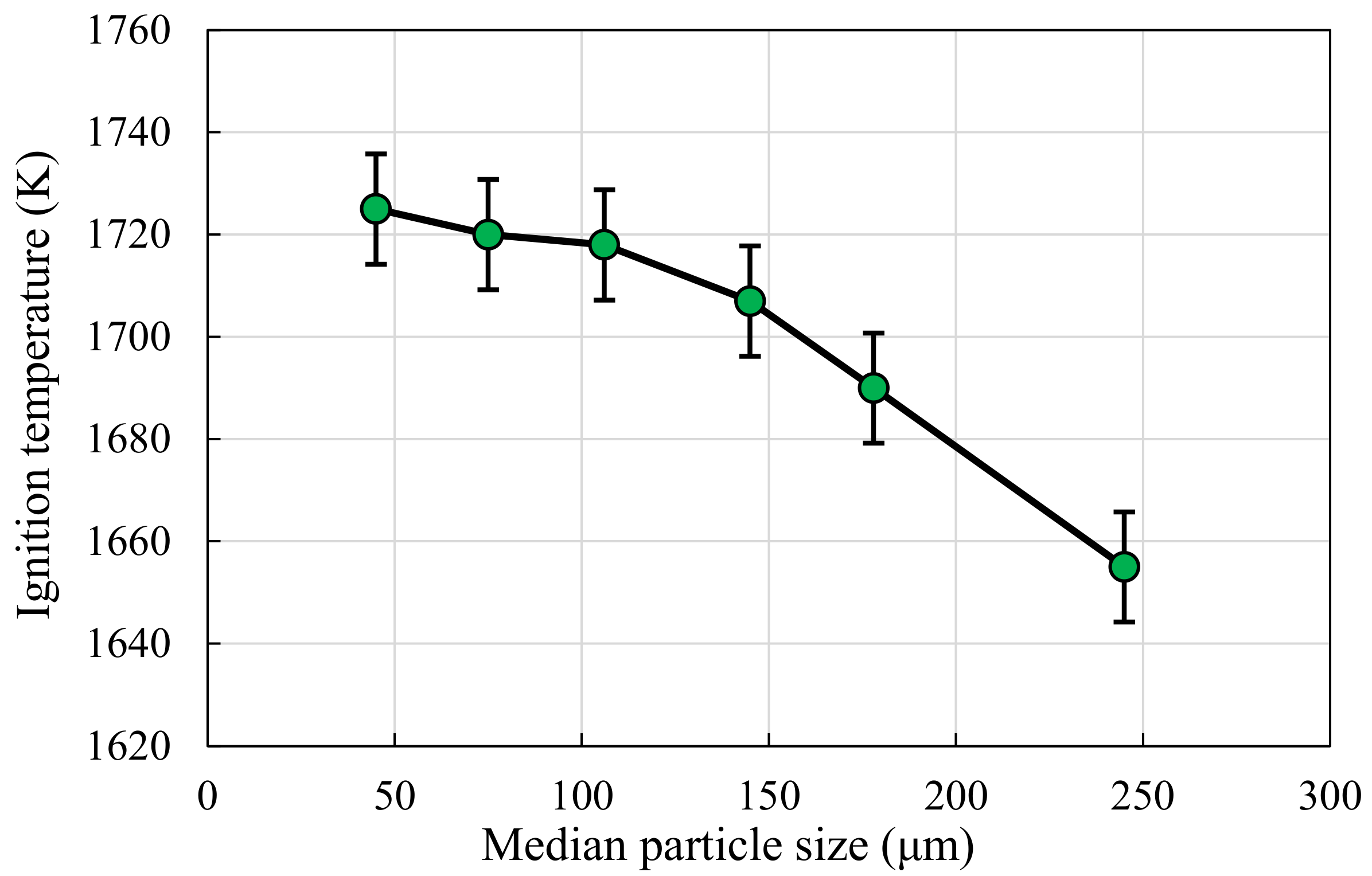
3.2. Temperature Threshold of Ignition
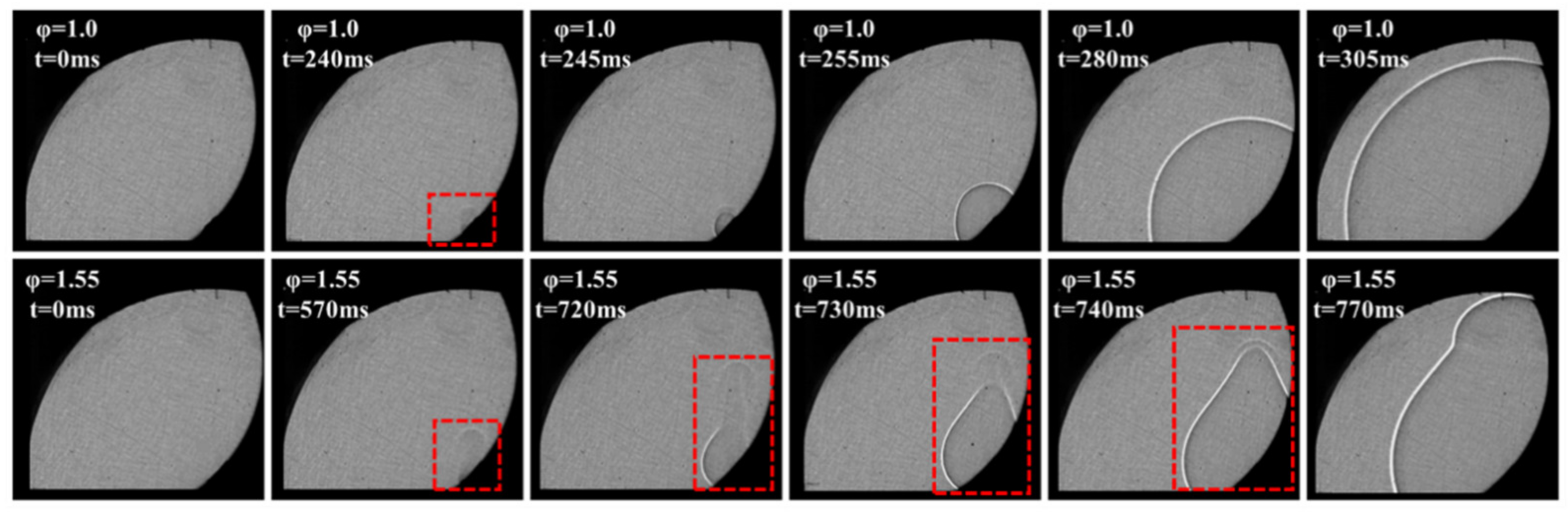
3.3. Formation and Propagation of the Flame
4. Conclusions
- 1.
- As the equivalence ratio approaches the flammability limits, the ignition threshold of irradiances increases, due to the decreases in the reactivity of the fuel mixture. Furthermore, the ignition threshold of irradiance increases as the particle size increases, due to increased heat capacities and decreased surface area-volume ratios at larger particle sizes.
- 2.
- The ignition threshold of temperature of the particles decreases with the increase of the particle size. Within the flammability limits, the trends of the temperature and irradiance ignition thresholds are similar; however, changes in the equivalence ratio affect irradiance more significantly than temperature. Due to the energy dissipation, the particle-ignition temperatures are significantly higher than the fuel mixture auto-ignition temperatures.
- 3.
- When irradiance is lower than the irradiance threshold, the inability to achieve ignition can be explained by the attainment of thermal equilibrium in the particle-gas system. At thermal equilibrium, the particles are unable to transfer additional heat to the surrounding fuel mixture, meaning that the temperature of the fuel mixture cannot reach the required ignition temperature.
- 4.
- Ignition always initiates from the surface of the particle because the temperature of the gases surrounding the particle is the highest. Increased ignition times allow for the formation of heat convection currents above the particle due to the effects of buoyancy, which will cause the non-uniformity of the gas temperatures surrounding the particle and a velocity field to form. The non-uniformity of gas temperature and the velocity field will cause the flame to be deformed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, R.A.; Laguna, G.A. Laser initiated combustion of CH4 + O2 mixtures. Opt. Commun. 1980, 32, 435–439. [Google Scholar] [CrossRef]
- Raffel, B.; Warnatz, J.; Wolfrum, J. Experimental study of laser-induced thermal ignition in O2/O3 mixtures. Appl. Phys. A 1985, 37, 189–195. [Google Scholar] [CrossRef]
- Lavid, M.; Stevens, J.G. Photochemical ignition of premixed hydrogenoxidizer mixtures with excimer lasers. Combust. Flame 1985, 60, 195–202. [Google Scholar] [CrossRef]
- Starik, A.M.; Kuleshov, P.S.; Titova, N.S. Laser-initiated ignition of hydrogen-air mixtures. Technol. Phys. 2009, 54, 354–364. [Google Scholar] [CrossRef]
- Pickett, L.M.; Kook, S.; Persson, H.; Andersson, I. Diesel fuel jet lift-off stabilization in the presence of laser-induced plasma ignition. Proc. Combust. Inst. 2009, 32, 2793–2800. [Google Scholar] [CrossRef]
- Moore, S.R.; Weinberg, F.J. A study of the role of radiative ignition in the propagation of large explosions. Proc. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 1983, 385, 373–388. [Google Scholar]
- Liberman, A.M.; Ivanov, F.M.; Kiverin, D.A. Radiation heat transfer in particle-laden gaseous flame: Flame acceleration and triggering detonation. Acta Astronaut. 2015, 115, 82–93. [Google Scholar] [CrossRef]
- Dubaniewicz, T.H., Jr. Threshold powers and delays for igniting propane and butane-air mixtures by cw laser-heated small particles. J. Laser Appl. 2006, 18, 312–319. [Google Scholar] [CrossRef]
- Hills, P.C.; Zhang, D.K.; Samson, P.J.; Wall, T.F. Laser ignition of combustible gases by radiative heating of small particles. Combust. Flame 1992, 91, 399–412. [Google Scholar] [CrossRef]
- Welzel, M.M.; Schenk, S.; Hau, M.; Cammenga, H.K.; Bothe, H. Ignition of combustible/air mixtures by small radiatively heated surfaces. J. Hazard. Mater. 2000, 72, 1–9. [Google Scholar] [CrossRef]
- Dubaniewicz, T.H., Jr.; Cashdollar, K.L.; Green, G.M. Continuous wave laser ignition thresholds of coal dust clouds. J. Laser Appl. 2003, 15, 184–191. [Google Scholar] [CrossRef][Green Version]
- Beyer, M.; Markus, D. Ignition of explosive atmospheres by small hot particles: Comparison of experiments and simulations. Sci. Technol. Energetic Mater. J. Jpn. Explos. Soc. 2012, 73, 1–7. [Google Scholar]
- Silver, R.S. The ignition of gaseous mixtures by hot particles. Philos. Mag. J. Sci. 1937, 23, 633–657. [Google Scholar] [CrossRef]
- Paterson, S. The ignition of inflammable gases by hot moving particles. Philos. Mag. J. Sci. 1940, 30, 437–457. [Google Scholar] [CrossRef]
- Paterson, S.I. The ignition of inflammable Oases by hot moving particles. Philos. Mag. J. Sci. 1939, 28, 1–23. [Google Scholar] [CrossRef]
- Beyrau, F.; Hadjipanayis, M.A.; Lindstedt, R.P. Ignition of fuel/air mixtures by radiatively heated particles. Proc. Combust. Inst. 2013, 34, 2065–2072. [Google Scholar] [CrossRef]
- Beyrau, F.; Hadjipanayis, M.A.; Lindstedt, R.P. Time-resolved temperature measurements for inert and reactive particles in explosive atmospheres. Proc. Combust. Inst. 2015, 35, 2067–2074. [Google Scholar] [CrossRef]
- Melguizo-Gavilanes, J.; Mevel, R.; Coronel, S.; Shepherd, J. Effects of differential diffusion on ignition of stoichiometric hydrogen-air by moving hot spheres. Proc. Combust. Inst. 2017, 36, 1155–1163. [Google Scholar] [CrossRef]
- Häber, T.; Zirwes, T.; Roth, D.; Zhang, F.; Bockhorn, H.; Maas, U. Numerical Simulation of the Ignition of Fuel/Air Gas Mixtures around Small Hot Particles. Z. Phys. Chem. 2017, 231, 1625–1654. [Google Scholar] [CrossRef]
- Coronel, S.A.; Melguizo-Gavilanes, J.; Mével, R.; Shepherd, J.E. Experimental and numerical study on moving hot particle ignition. Combust. Flame 2018, 192, 495–506. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, C.; Wang, C.; Han, W. Ignition of methane/air mixtures with inert and reactive particles heated by radiation. Fuel 2020, 278, 118309. [Google Scholar] [CrossRef]
- Zhen, J.; Fang, C.X.; Gang, X.X.; Bo, H.X.; Na, N.L.; Min, W.Y. Optical Absorption Measurements on Nitrogen-doped 6H-SiC Single Crystals. Chin. J. Struct. Chem. 2007, 10, 1171–1174. [Google Scholar]
- Lamon, J. Properties and Characteristics of SiC and SiC/SiC Composites. Compr. Nucl. Mater. 2012, 15, 323–338. [Google Scholar] [CrossRef]
- Kondo, S.; Takahashi, A.; Tokuhashi, K. Calculation of minimum ignition energy of premixed gases. J. Hazard. Mater. 2003, 103, 11–23. [Google Scholar] [CrossRef]
- Holbrow, P.; Hawksworth, S.J.; Tyldesley, A. Thermal radiation from vented dust explosions. J. Loss Prev. Process. Ind. 2000, 13, 467–476. [Google Scholar] [CrossRef]
- Robinson, C.; Smith, D.B. The auto-ignition temperature of methane. J. Hazard. Mater. 1984, 8, 199–203. [Google Scholar] [CrossRef]
- Marín, E. Characteristic dimensions for heat transfer. Lat. Am. J. Phys. Educ. 2010, 4, 56–60. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Zhang, C. Ignition Thresholds and Flame Propagation of Methane/Air Mixtures Ignited via Radiatively Heated Inert Particles. Energies 2021, 14, 5173. https://doi.org/10.3390/en14165173
Ma J, Zhang C. Ignition Thresholds and Flame Propagation of Methane/Air Mixtures Ignited via Radiatively Heated Inert Particles. Energies. 2021; 14(16):5173. https://doi.org/10.3390/en14165173
Chicago/Turabian StyleMa, Junrong, and Changsuo Zhang. 2021. "Ignition Thresholds and Flame Propagation of Methane/Air Mixtures Ignited via Radiatively Heated Inert Particles" Energies 14, no. 16: 5173. https://doi.org/10.3390/en14165173
APA StyleMa, J., & Zhang, C. (2021). Ignition Thresholds and Flame Propagation of Methane/Air Mixtures Ignited via Radiatively Heated Inert Particles. Energies, 14(16), 5173. https://doi.org/10.3390/en14165173





