Technical and Economic Evaluation of CO2 Capture and Reinjection Process in the CO2 EOR and Storage Project of Xinjiang Oilfield
Abstract
1. Introduction
2. Potential CCRPs in the CO2 EOR and Storage Project in XinJiang Oilfield
3. Establishment of Technical and Economic Evaluation Model of CCRP
3.1. Calculation Method of Capital Cost
3.1.1. Gas–Liquid Separator and Molecular Sieve
3.1.2. Compressor
3.1.3. Booster Pump
3.1.4. Carbon Capture Module
3.2. Calculation Method of Running Cost
3.3. Calculation Method of CO2 Capture Parameters
3.4. Calculation Method of Unit Cost
4. Evaluation of the CCRPs in the CO2 EOR and Storage Project in XinJiang Oilfield
4.1. Evaluation of the CCRPs Based on the Assumed Gas Production and CO2 Purity
4.2. Evaluation of the CCRPs Based on the Designed CO2 Flooding Schemes
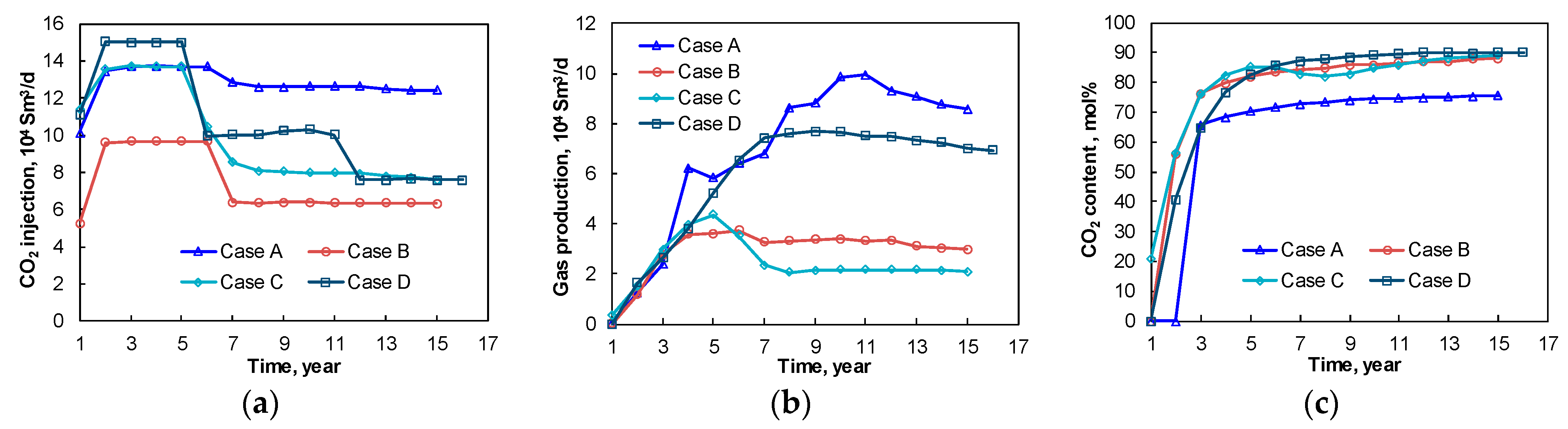
4.2.1. CO2 Injection Schemes and Predicted Gas Production
4.2.2. Comparison Analysis of the Different CCRPs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Nomenclature
| Abbreviations | |
| CA | Chemical absorption |
| CCRE | CO2 capture and reinjection efficiency |
| CCRP | CO2 capture and reinjection process |
| CCS | CO2 capture and storage |
| CCUS | CO2 capture, utilization, and storage |
| CFGR | combustion and flue gas reinjection |
| DRM | direct reinjection mixed |
| EOR | enhanced oil recovery |
| LTF | low-temperature fractionation |
| MDEA | methyldiethanolamine |
| MS | membrane separation |
| PSA | pressure swing adsorption |
| Nomenclature | |
| Csep | capital cost of gas–liquid separator (US$) |
| Mtrain | mass flow rate of disposal gas (t/d) |
| Cmol | capital cost of molecular sieve (US$) |
| Ccomp | total capital cost of compressor (US$) |
| mtrain | mass flow rate of CO2 gas in each compressor unit (kg/s) |
| Ntrain | number of parallel compressors (dimensionless) |
| mCO2 | CO2 mass flow rate (t/d) |
| Pin–comp | inlet pressure of compressor (MPa) |
| Pout–comp | outlet pressure of compressor (MPa) |
| Wcomp | compressor power (kW) |
| Zs | average compression factor of CO2 at each stage (dimensionless) |
| Tin–comp | inlet temperature of compressor (K) |
| MCO2gas | molar mass of CO2 gas (kg/kmol) |
| ηcomp | efficiency of compressor (dimensionless) |
| ks | average heat capacity ratio of CO2 at each stage of compressor (dimensionless) |
| CR | optimal compression ratio (dimensionless) |
| Nstage | number of compression stages (dimensionless) |
| Cpump | capital cost of booster pump (US$) |
| Wpump | booster pump power (kW) |
| Pout–pump | outlet pressure of booster pump (MPa) |
| Pin–pump | inlet pressure of booster pump (MPa) |
| ρl–CO2 | density of liquid CO2 (kg/m3) |
| ηpump | efficiency of booster pump (dimensionless) |
| WPSA–ad | mass of adsorbent in PSA module (kg) |
| QPSA–g | flow rate of the feed gas in the adsorption tower of PSA module (m3/s) |
| tPSA–ad | adsorption time of single bed operation of tower in PSA module (s) |
| yPSA–CO2 | CO2 mole fraction of the feed gas in PSA module (dimensionless) |
| ΔqPSA | adsorption capacity in PSA module (kg/kg) |
| nPSA–bed | number of beds for continuous adsorption in a single tower in PSA module (dimensionless) |
| HPSA | height of the tower in PSA module (m) |
| vPSA–g | gas flow speed in adsorption tower of PSA module (m/s) |
| ρPSA–ad | adsorbent density in PSA module (kg/m3) |
| DPSA | diameter of the tower in PSA module (m) |
| nPSA–tower | number of towers in PSA module (dimensionless) |
| CPSA–tower | capital cost of towers in PSA module (US$) |
| CPSA–pc | unit height capital cost of the tower in PSA module (US$/m) |
| CPSA–ad | purchase cost of adsorbent in PSA module (US$) |
| PPSA–ad | unit cost of adsorbent in PSA module (US$/kg) |
| CPSA | capital cost of the PSA module (US$) |
| WPSA | power of the PSA module (kW) |
| Am | film area in MS module (m2) |
| YMS–F | mole fraction of high-speed group (CO2) in feed gas in MS module (dimensionless) |
| YMS–R | mole fraction of the high-speed group in the nonpenetrating gas in MS module (dimensionless) |
| YMS–1 | mole fraction of the high-speed group in the permeation gas in MS module (dimensionless) |
| QMS–P | flow rate of permeation gas in MS module (kmol/s) |
| RMS–f | weighted average permeation velocity of the high-speed group in MS module (m/s) |
| PMS–1 | total pressure on the low-pressure side of the membrane in MS module (bar) |
| PMS–2 | total pressure on the high-pressure side of the membrane in MS module (bar) |
| CM | capital cost of MS device (US$) |
| Im | cost of membrane material in MS device (US$) |
| Imf | cost of membrane frame in MS device (US$) |
| Km | membrane material cost of unit film area (US$/m2) |
| Kmf | membrane frame cost of unit film area (US$/m2) |
| CMS | capital cost of MS module (US$) |
| WMS | power of MS module (kW) |
| Chx | capital cost of heat exchanger (US$) |
| Ahx–p | actual heat exchange area in heat exchanger (m2) |
| Qhx | heat flow in heat exchanger (kJ/h) |
| mhf | mass flow rate of hot fluid in heat exchanger (kg/h) |
| Cp | specific heat capacity of fluid in heat exchanger in heat exchanger (kJ·kg−1·°C−1) |
| Δthx | temperature change of hot fluid in heat exchanger (°C) |
| Khc | heat transfer coefficient between the hot fluid and the cold fluid in heat exchanger (W·m−2·°C−1) |
| ΔTm | logarithmic mean temperature changes of heat exchanger (°C) |
| THI | hot fluid temperature at the inlet of the heat exchanger (°C) |
| THO | hot fluid temperature at the outlet of the heat exchanger (°C) |
| TCI | cold fluid temperature at the inlet of the heat exchanger (°C) |
| TCO | cold fluid temperature at the outlet of the heat exchanger (°C) |
| Whx | power of heat exchanger (kW) |
| CLTF | capital cost of LTF module (US$) |
| WLTF | power of LTF module (kW) |
| DCA–ab | diameter of absorption tower in CA module (m) |
| VCA–ab | flow rate of feed gas in the absorption tower in CA module (m3/h) |
| vCA–ab | gas flow velocity in the adsorption tower in CA module (m/s) |
| HCA–ab | cumulative height of absorption towers in CA module (m) |
| mCA–CO2 | mass flow rate of CO2 gas in CA module (kg/h) |
| KGa | mass transfer coefficient in CA module (kmol·m−3·h−1·atm−1) |
| YCO2–inab | CO2 content of inlet gas in absorption tower (g/m3) |
| YCO2–outab | CO2 content of outlet gas in absorption tower (g/m3) |
| ACA–t | cross–section area of absorption tower in CA module (m2) |
| ∆PCA–m | driving pressure difference in the absorption tower of CA module (atm) |
| CCA–ab | cost of unit height tower in CA module (US$/m) |
| CCA–abt | capital cost of absorption tower in CA module (US$) |
| DCA–de | diameter of the desorption tower in CA module (m) |
| VCA–de | flow rate of feed gas in the desorption tower in CA module (m3/h) |
| vCA–de | gas flow velocity in the desorption tower in CA module (m/s) |
| NCA–t | total number of theoretical plates in desorption tower in CA module (dimensionless) |
| CCA–de | tower cost of a single plate of desorption towers in CA module (US$) |
| CCA–det | capital cost of desorption tower in CA module (US$) |
| MMDEA | required circulation amount of MDEA solution (t) |
| Cs | purchase cost of MEDA solution (US$) |
| Cus | unit cost of MEDA solution (US$/t) |
| CCA | capital cost of CA module (US$) |
| WCA | power of CA module (kW) |
| O&Mannual | annual running cost of CCRP (US$) |
| Cunit | capital cost of equipment unit (US$) |
| Mfactor | ratio of annual maintenance cost to total infrastructure cost (dimensionless) |
| Wunit | power of equipment unit (kW) |
| Felec | electricity price (US$/kWh) |
| Qin–gas | gas flow rate at the inlet of equipment unit (Sm3/d) |
| xin–CO2 | CO2 content at the inlet of equipment unit (dimensionless) |
| Qin–CO2 | pure CO2 gas flow rate at the inlet of equipment unit (Sm3/d) |
| Qout–gas | gas flow rate at the outlet of equipment unit (Sm3/d) |
| Qout–CO2gas | CO2 gas flow rate at the outlet of equipment unit (Sm3/d) |
| Qout–CH4gas | CH4 gas flow rate at the outlet of equipment unit (Sm3/d) |
| xout–CO2 | CO2 purity of CO2 gas flow at the outlet of equipment unit (dimensionless) |
| yout–CH4 | CH4 purity of CH4 gas flow at the outlet of equipment unit (dimensionless) |
| Qout–CO2 | pure CO2 gas flow rate at the outlet of equipment unit (Sm3/d) |
| Qpower–CO2 | energy consumption equivalent CO2 emission of equipment unit (Sm3/d) |
| η | CO2 capture efficiency of the capture module (dimensionless) |
| Mcoal | coal consumption required for unit power generation (kg/kWh) |
| ECO2 | CO2 emissions per unit coal by burning (kg CO2/kg coal) |
| tu | unit time (h) |
| ρCO2 | density of CO2 gas (kg/m3) |
| Clev | CO2 capture and reinjection cost per 500 Sm3 CO2 gas (US$/500Sm3) |
| Ctca | total annual cost of CCRP (US$) |
| Cannual | annual capital cost by dividing the total capital cost equally over each year of the project duration (US$) |
| CRF | the discount factor (dimensionless) |
References
- Ren, S.R.; Li, D.Y.; Zhang, L.; Huang, H. Leakage Pathways and Risk Analysis of Carbon Dioxide in Geological Storage. Acta Pet. Sin. 2014, 35, 591–601, (In Chinese with English abstract). [Google Scholar]
- Qin, J.X.; Han, H.S.; Liu, X.L. Application and enlightenment of carbon dioxide flooding in the United States of America. Pet. Explor. Dev. 2015, 42, 209–216. [Google Scholar] [CrossRef]
- Sourisseau, K.; Ibrahim, I.; Al-Jabri, A.; Wetzels, G. Surface facilities considerations for the production of a large sour gas resource and the injection of sour and/or acid gas. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 13–15 October 2000. [Google Scholar]
- Zainal, Z.A.; Mahadzir, M.M. Experimental study of hydrodynamic characteristics and CO2 absorption in producer gas using CaO-sand mixture in a bubbling fluidized bed reactor. Int. J. Chem. React. Eng. 2011, 9, 154–156. [Google Scholar]
- Adewole, J.K.; Ahmad, A.L. Process modeling and optimization studies of high pressure membrane separation of CO2 from natural gas. Korean J. Chem. Eng. 2016, 33, 2998–3010. [Google Scholar] [CrossRef]
- Liu, B.L. Experimental Study of Low-Temperature and Pressure Swing Adsorption Removal Carbon Dioxide Gas from Natural Gas; Dalian University of Technology: Dalian, China, 2015; (In Chinese with English abstract). [Google Scholar]
- Torp, T.A.; Brown, K.R. CO2 underground storage costs as experienced at Sleipner and Weyburn. In Proceedings of the 7th International Conference on Greenhouse Gas Control Technologies, Vancouver, BC, Canada, 5 September 2004. [Google Scholar]
- Ma, P.F.; Han, B.; Zhang, L.; Xiong, X.Q.; Shen, X.X.; Zhang, X.; Ren, S.R. Disposal scheme of produced gas and CO2 capture for re-injection in CO2 EOR. Chem. Ind. Eng. Prog. 2017, 36, 533–539, (In Chinese with English abstract). [Google Scholar]
- Sun, R.Y.; Ma, X.H.; Wang, S.G. CO2 injection technology in Jilin Oilfield. Pet. Plan. Eng. 2013, 24, 1–6, (In Chinese with English abstract). [Google Scholar]
- Zhang, L.; Li, X.; Ren, B.; Cui, G.D.; Zhang, Y.; Ren, S.R.; Chen, G.L.; Zhang, H. CO2 storage potential and trapping mechanisms in the H-59 block of Jilin oilfield China. Int. J. Greenh. Gas Control 2016, 49, 267–280. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, B.; Huang, H.D.; Li, Y.Z.; Ren, S.R.; Chen, G.L.; Zhang, H. CO2 EOR and storage in Jilin Oilfield China: Monitoring program and preliminary Results. J. Pet. Sci. Eng. 2015, 125, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, C.H.; Niu, B.L.; Ren, S.R. EOR Principles and Application of CO2 Flooding; Press of China University of Petroleum: Qingdao, China, 2017. (In Chinese) [Google Scholar]
- Yu, B.Y.; Zhao, G.P.; An, R.D.; Chen, J.M.; Tan, J.X.; Li, X.Y. Study on China’s carbon emission path under the carbon neutral target. J. Beijing Inst. Technol. (Soc. Sci. Ed.) 2021, 23, 17–24, (In Chinese with English abstract). [Google Scholar]
- Kwak, D.H.; Yun, D.; Binns, M.; Yeo, Y.K.; Kin, J.K. Conceptual process design of CO2 recovery plants for enhanced oil recovery applications. Ind. Eng. Chem. Res. 2014, 53, 14385–14396. [Google Scholar] [CrossRef]
- Zhou, D.B. EOR Natural Gas CO2 Separation Process Simulation Study; Qingdao University of Science and Technology: Qingdao, China, 2014; (In Chinese with English abstract). [Google Scholar]
- Ciferno, J.P.; DiPietro, P.; Tarka, T. An Economic Scoping Study for CO2 Capture Using Aqueous Ammonia; Final Report; National Energy Technology Laboratory, US Department of Energy: Pittsburgh, PA, USA, 2005.
- Kleme, J.; Bulatov, I.; Cockerill, T. Techno-economic modeling and cost functions of CO2 capture processes. Comput. Chem. Eng. 2007, 31, 445–455. [Google Scholar] [CrossRef]
- Tuinier, M.J.; Hammers, H.P.; van Sint Annaland, M. Techno-economic evaluation of cryogenic CO2 capture—A comparison with absorption and membrane technology. Int. J. Greenh. Gas Control 2011, 5, 1559–1565. [Google Scholar] [CrossRef]
- Huang, Y.P.; Rebennack, S.; Zheng, Q.P. Techno-economic analysis and optimization models for carbon capture and storage: A survey. Energy Syst. 2013, 4, 315–353. [Google Scholar] [CrossRef]
- Zhang, Z.H. Techno-economic assessment of carbon capture and storage facilities coupled to coal-fired power plants. Energy Environ. 2015, 26, 1069–1080. [Google Scholar] [CrossRef]
- Zohrabian, A.; Majoumerd, M.M.; Soltanieh, M.; Sourena, S. Techno-economic evaluation of an integrated hydrogen and power co-generation system with CO2 capture. Int. J. Greenh. Gas Control 2016, 44, 94–103. [Google Scholar] [CrossRef]
- Zhai, M.Y.; Lin, Q.G.; Zhong, L.F.; Pi, J.W.; Wang, W.S. Economic assessment of carbon capture and storage combined with utilization of deep saline water. Mod. Chem. Ind. 2016, 36, 8–12, (In Chinese with English abstract). [Google Scholar]
- Hu, B.; Zhai, H. The cost of carbon capture and storage for coal-fired power plants in China. Int. J. Greenh. Gas Control 2017, 65, 23–31. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhao, D.Y.; Tian, Q.H.; Li, Z.M. Modeling and optimization of the whole process of CO2 capture, transportation, oil displacement and storage. Oil Gas Field Surf. Eng. 2018, 37, 1–5, (In Chinese with English abstract). [Google Scholar]
- Decardi-Nelson, B.; Liu, S.; Liu, J.F. Improving flexibility and energy efficiency of post-combustion CO2 capture plants using economic model predictive control. Processes 2018, 6, 135. [Google Scholar] [CrossRef]
- Yun, S.; Oh, S.Y.; Kim, J.K. Techno-economic assessment of absorption-based CO2 capture process based on novel solvent for coal-fired power plant. Appl. Energy 2020, 268, 114933. [Google Scholar] [CrossRef]
- Gui, X.; Wang, C.W.; Yun, Z.; Zhang, L.; Tang, Z.G. Research progress of pre-combustion CO2 capture. Chem. Ind. Eng. Prog. 2014, 33, 1895–1901, (In Chinese with English abstract). [Google Scholar]
- Li, Q.F.; Lu, S.J.; Liu, X.D.; Zhang, J. Experimental research of absorbing carbon dioxide from flue gas by MEA-MDEA mixed amine solutions. Appl. Chem. Ind. 2010, 39, 1127–1131, (In Chinese with English abstract). [Google Scholar]
- Ahmad, A.L.; Adewole, J.K.; Leo, C.P.; Ismail, S.; Sultan, A.S.; Olatunji, S.O. Prediction of plasticization pressure of polymeric membranes for CO2 removal from natural gas. J. Membr. Sci. 2015, 480, 39–46. [Google Scholar] [CrossRef]
- Algharaib, M.; Al-Soof, N. Economical modeling of CO2 capturing and storage projects. SPE 120815-MS. In Proceedings of the SPE Saudi Arabia Section Technical Symposium, Al-Khobar, Saudi Arabia, 10–12 May 2008. [Google Scholar]
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers; McGraw-Hill: New York, NY, USA, 1968. [Google Scholar]
- Dong, X.; Han, P.; Yang, Z.; Xie, S.; Zhen, K. Pilot Field Test of Carbon Dioxide Flooding in Daqing Oilfield; Petroleum Industry Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Zhang, Y. Research on CO2-EOR and Geological Storage in Caoshe Oilfield, Jiangsu; China University of Petroluem: Qingdao, China, 2010; (In Chinese with English abstract). [Google Scholar]
- Hendriks, C.; Graus, W.; van Bergen, F. Global Carbon Dioxide Storage Potential and Costs; Ecofys: Utrecht, The Netherlands, 2004. [Google Scholar]
- McCollum, D.L. Techno-Economic Models for Carbon Dioxide Compression, Transport, and Storage; University of California: Davis, CA, USA, 2006. [Google Scholar]
- GHG IEA. Transmission of CO2 and Energy; IEA Greenhouse Gas R&D Programme, Report PH4/6; IEA: Paris, France, 2002. [Google Scholar]
- Han, Y.J.; Wang, S.L.; Zhang, P.Y.; Piao, P.Y. Status and progress of CO2 separation and capture technology. Nat. Gas Ind. 2009, 29, 79–82, (In Chinese with English abstract). [Google Scholar]
- Chen, D.Y. Remove Carbon Dioxide by PSA; Nanjing Tech University: Nanjing, China, 2003; (In Chinese with English abstract). [Google Scholar]
- Yang, H.Y. Research on Adsorbents for Separation of the CH4/CO2 Mixture; Northeast Agricultural University: Harbin, China, 2013; (In Chinese with English abstract). [Google Scholar]
- Meng, D. Structure Design and Stress Analysis of PSA Absorption Tower. Petro-Chem. Equip. 2010, 39, 33–36, (In Chinese with English abstract). [Google Scholar]
- Zhou, F. Design of CO2 absorption tower. Guangdong Chem. Ind. 2014, 41, 245–246, (In Chinese with English abstract). [Google Scholar]
- Zhao, L.; Menzer, R.; Riensche, E.; Blum, L.; Stolten, D. Concepts and investment cost analyses of multi-stage membrane systems used in post-combustion processes. Energy Procedia 2009, 1, 269–278. [Google Scholar] [CrossRef][Green Version]
- Su, Y.; Hu, L.; Liu, M.S. Gas membrane separation technology and application. Chem. Eng. Oil Gas 2001, 30, 113–116, (In Chinese with English abstract). [Google Scholar]
- Shi, M.Z.; Wang, Z.Z. Principle and Design of Heat Exchangers; Southeast University Press: Nanjing, China, 2009. (In Chinese) [Google Scholar]
- Peng, Y.X. Coupling of distillation and low temperature stripping—A new output gas recovery technology in CO2 flooding process in oilfields. Reserv. Eval. Dev. 2012, 2, 42–47, (In Chinese with English abstract). [Google Scholar]
- Zhang, X.F. Calculation of effective height of chemical absorption packed tower. Chem. Eng. Des. Commun. 1995, 21, 42–46, (In Chinese with English abstract). [Google Scholar]
- Liao, H.; Zhao, G.X.; Ma, B.Q. Design of HCl absorber for a 100 kt/a Deacon process. Chem. World 2015, 56, 653–657, (In Chinese with English abstract). [Google Scholar]
- Chen, J.; He, G.; Liu, J.; Liu, J. Measurement for the absorption rate of CO2 by aqueous MDEA solution. J. Tsinghua Univ. (Sci. Technol.) 2001, 41, 28–30, (In Chinese with English abstract). [Google Scholar]
- Liu, Y.Z.; Shen, H.Y. Carbon Dioxide Emission Reduction Process and Technology--Solvent Absorption Method; Chemical Industry Press: Beijing, China, 2013. (In Chinese) [Google Scholar]
- Li, T. Study on Energy-Saving and Optimization Technology of Carbon Dioxide Captured System of Flue Gas in Coal-Fired Power; Qingdao University of Science and Technology: Qingdao, China, 2010; (In Chinese with English abstract). [Google Scholar]










| The Types of CCRPs | The 6 Main Equipment Units |
|---|---|
| SPA | Produced gas (0.5 MPa, 20 1 °C) → gas–liquid separator → low-pressure compressor → molecular sieve → SPA system → high-pressure compressor →reinjected gas (20 MPa, 40 °C) |
| MS | Produced gas (0.5 MPa, 20 °C) → gas–liquid separator → low-pressure compressor → molecular sieve → MS system → high-pressure compressor →reinjected gas (20 MPa, 40 °C) |
| LTF | Produced gas (0.5 MPa, 20 °C) → gas–liquid separator → low-pressure compressor → molecular sieve → LTF system → liquid CO2 storage tank → boost pump→ reinjected gas (20 MPa, −20 °C) |
| CA-MDEA | Produced gas (0.5 MPa, 20 °C) → gas–liquid separator → low-pressure compressor → molecular sieve → CA-MDEA → high-pressure compressor →reinjected gas (20 MPa, 40 °C) |
| DRM | Produced gas (0.5 MPa, 20 °C) → gas–liquid separator → low-pressure compressor → molecular sieve → high-pressure compressor →reinjected gas (20 MPa, 40 °C) |
| Equipment Units | Gas Flow at the Outlet, m3/d | CO2 Purity at the Outlet, Fraction | CO2 Flow at the Outlet, m3/d | Additional CO2 Emission, m3 | CO2 Capture Efficiency, Fraction |
|---|---|---|---|---|---|
| Compressor/Pump | Qout-gas = Qin-gas | xout-CO2 = xin-CO2 | Qout-CO2 = Qout-gas × xout-CO2 | Qpower-CO2 | H = (Qout-CO2−Qpower-CO2)/Qin-CO2 |
| Carbon Capture Module | Qout-CO2gas Qout-CH4gas Qin-gas = Qout-CO2gas + Qout-CH4gas | xout-CO2 yout-CH4 Qin-gas × xin-CO2 = Qout-CO2gas × xout-CO2 + Qout-CH4gas × (1 − yout-CH4) | Qout-CO2 = Qout-CO2gas × xout-CO2 | Qpower-CO2 | H = (Qout-CO2 − Qpower-CO2)/Qin-CO2 |
| Gas Purity | Capture Type | Regression Formula | Correlation Coefficient R2 |
|---|---|---|---|
| CO2 purity of captured CO2 gas | PSA | xout-CO2 = 0.036lnxin-CO2 + 0.8002 | R2 = 0.9952 |
| MS | xout-CO2 = 0.094lnxin-CO2 + 0.5420 | R2 = 0.9734 | |
| LTF | xout-CO2 = 0.265lnxin-CO2 − 0.2061 | R2 = 0.9934 | |
| MDEA | xout-CO2 = 0.9997 | R2 = 0 | |
| Hydrocarbon purity of captured natural gas | PSA | yout-CH4 = −0.060lnxin-CO2 + 1.1594 | R2 = 0.9615 |
| MS | yout-CH4 = −0.072lnxin-CO2 + 1.1573 | R2 = 0.8890 | |
| LTF | yout-CH4 = −0.025lnxin-CO2 + 1.0597 | R2 = 1 | |
| MDEA | y = 0.9725 | R2 = 0 |
| Scheme | Case A | Case B | Case C | Case D |
|---|---|---|---|---|
| Total CO2 injection rate, 104 Sm3/d | 10–14 | 5–10 | 8–14 | 8–15 |
| Total gas production rate, 104 Sm3/d | 0–10 | 0–3.7 | 0–4.5 | 0–7.8 |
| CO2 content in produced gas, % | 65–76 | 56–88 | 20–90 | 40–90 |
| Cumulative CO2 injection, 104 t | 139.93 | 80.83 | 108.22 | 123.93 |
| Cumulative CO2 production, 104 t | 54.04 | 26.93 | 21.81 | 54.70 |
| Primary CO2 storage efficiency, % | 61.38 | 66.68 | 79.85 | 55.86 |
| Cumulative oil production, 104 t | 41.60 | 26.04 | 33.17 | 49.49 |
| Average CO2-oil ratio, t CO2/t oil | 3.36 | 3.10 | 3.26 | 2.50 |
| EOR, % | 29.50 | 18.47 | 23.26 | 25.75 |
| No of well group | 9 injection wells | 9 injection wells | 15 injection wells | 15 injection wells |
| Project period, year | 15 | 15 | 15 | 15 |
| CO2 Flooding Scheme | Type of CCRP | Unit Cost of Capture, US$/500 Sm3CO2 | Unit Cost of Capture and Reinjection, US$/500 Sm3CO2 | Unit Energy Consumption, MJ/500 Sm3CO2 | CO2 Capture and Reinjection Efficiency, % | CO2 Capture Purity, % |
|---|---|---|---|---|---|---|
| Case A | SPA | 21–25 | 30–36 | 358–386 | 87–89 | 95 |
| MDEA | 31–41 | 39–51 | 1148–1154 | 74 | 99.97 | |
| MS | 23–35 | 31–45 | 375–409 | 84–87 | 93–95 | |
| LTF | 26–39 | 29–46 | 843–939 | 75–79 | 90–94 | |
| DRM | 7–9 * | 13–15 | 253–260 | 91–93 | 80–94 ** | |
| Case B | SPA | 21–34 | 31–46 | 332–422 | 84–91 | 94–96 |
| MDEA | 33–55 | 43–67 | 1143–1161 | 73–75 | 99.97 | |
| MS | 24–48 | 34–60 | 345–454 | 79–91 | 92–96 | |
| LTF | 21–55 | 26–68 | 735–1049 | 71–83 | 86–98 | |
| DRM | 9–10 * | 15–16 | 245–266 | 90–94 | 92–95 ** | |
| Case C | SPA | 20–95 | 31–109 | 330–830 | 71–91 | 91–96 |
| MDEA | 35–238 | 45–94 | 1142–1254 | 65–75 | 99.97 | |
| MS | 23–166 | 36–180 | 344–884 | 63–91 | 83–96 | |
| LTF | 21–182 | 28–243 | 727–1803 | 31–83 | 60–98 | |
| DRM | 8–14 * | 15–20 | 244–284 | 70–94 | 95–97 ** | |
| Case D | SPA | 19–40 | 28–51 | 328–513 | 80–91 | 93–96 |
| MDEA | 28–58 | 37–69 | 1142–1180 | 71–75 | 99.97 | |
| MS | 19–65 | 28–77 | 341–575 | 69–91 | 89–96 | |
| LTF | 18–69 | 21–81 | 719–1276 | 61–83 | 78–99 | |
| DRM | 8–10 * | 14–16 | 244–274 | 85–94 | 91–95 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Geng, S.; Yang, L.; Hao, Y.; Yang, H.; Dong, Z.; Shi, X. Technical and Economic Evaluation of CO2 Capture and Reinjection Process in the CO2 EOR and Storage Project of Xinjiang Oilfield. Energies 2021, 14, 5076. https://doi.org/10.3390/en14165076
Zhang L, Geng S, Yang L, Hao Y, Yang H, Dong Z, Shi X. Technical and Economic Evaluation of CO2 Capture and Reinjection Process in the CO2 EOR and Storage Project of Xinjiang Oilfield. Energies. 2021; 14(16):5076. https://doi.org/10.3390/en14165076
Chicago/Turabian StyleZhang, Liang, Songhe Geng, Linchao Yang, Yongmao Hao, Hongbin Yang, Zhengmiao Dong, and Xian Shi. 2021. "Technical and Economic Evaluation of CO2 Capture and Reinjection Process in the CO2 EOR and Storage Project of Xinjiang Oilfield" Energies 14, no. 16: 5076. https://doi.org/10.3390/en14165076
APA StyleZhang, L., Geng, S., Yang, L., Hao, Y., Yang, H., Dong, Z., & Shi, X. (2021). Technical and Economic Evaluation of CO2 Capture and Reinjection Process in the CO2 EOR and Storage Project of Xinjiang Oilfield. Energies, 14(16), 5076. https://doi.org/10.3390/en14165076








