Influence of Oxygen Ion Migration from Substrates on Photochemical Degradation of CH3NH3PbI3 Hybrid Perovskite
Abstract
:1. Introduction
2. Experimental
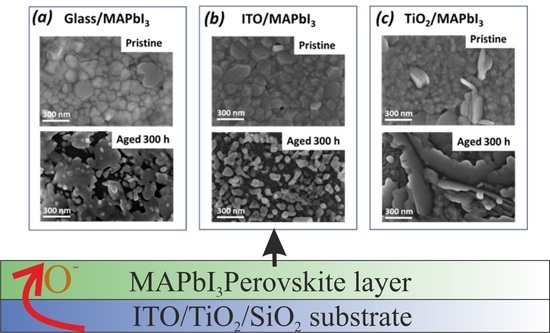
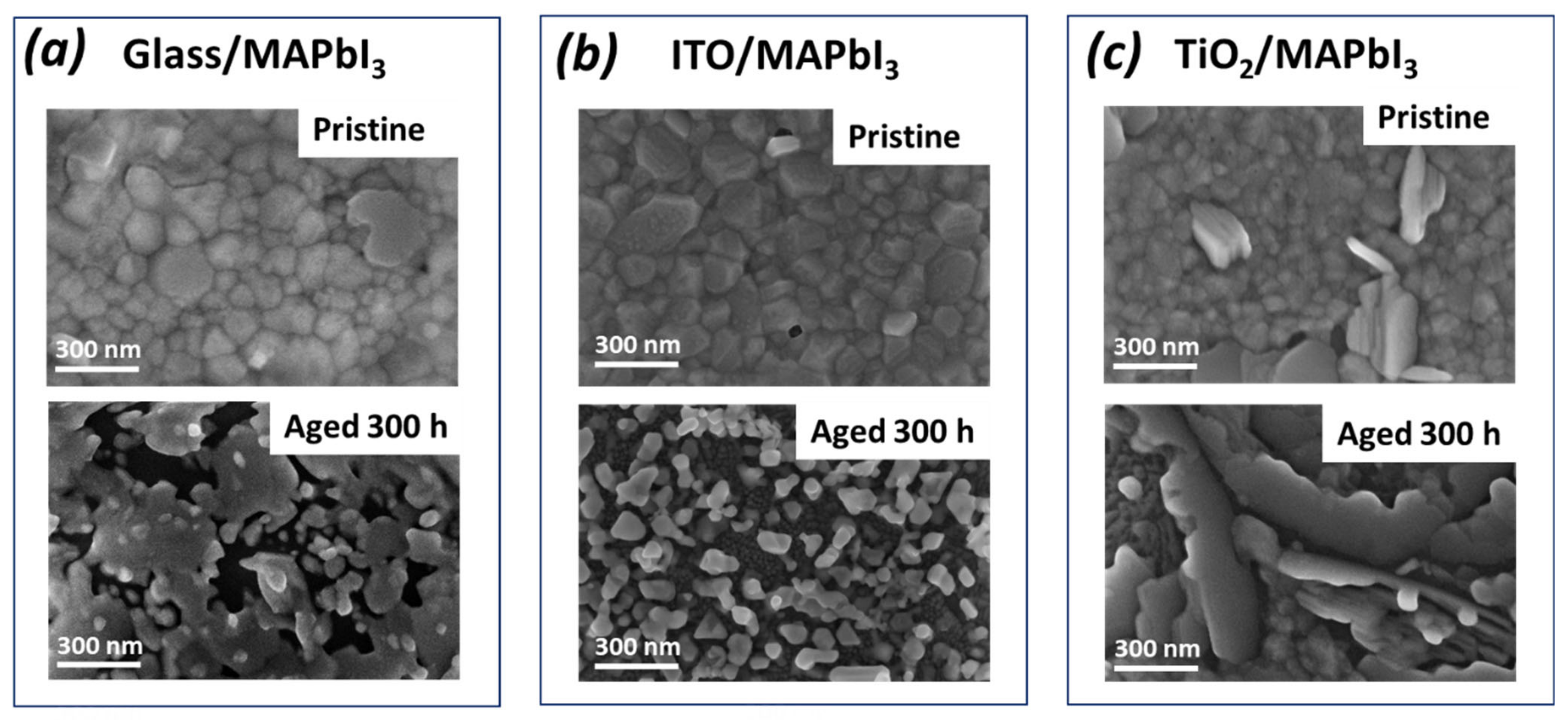
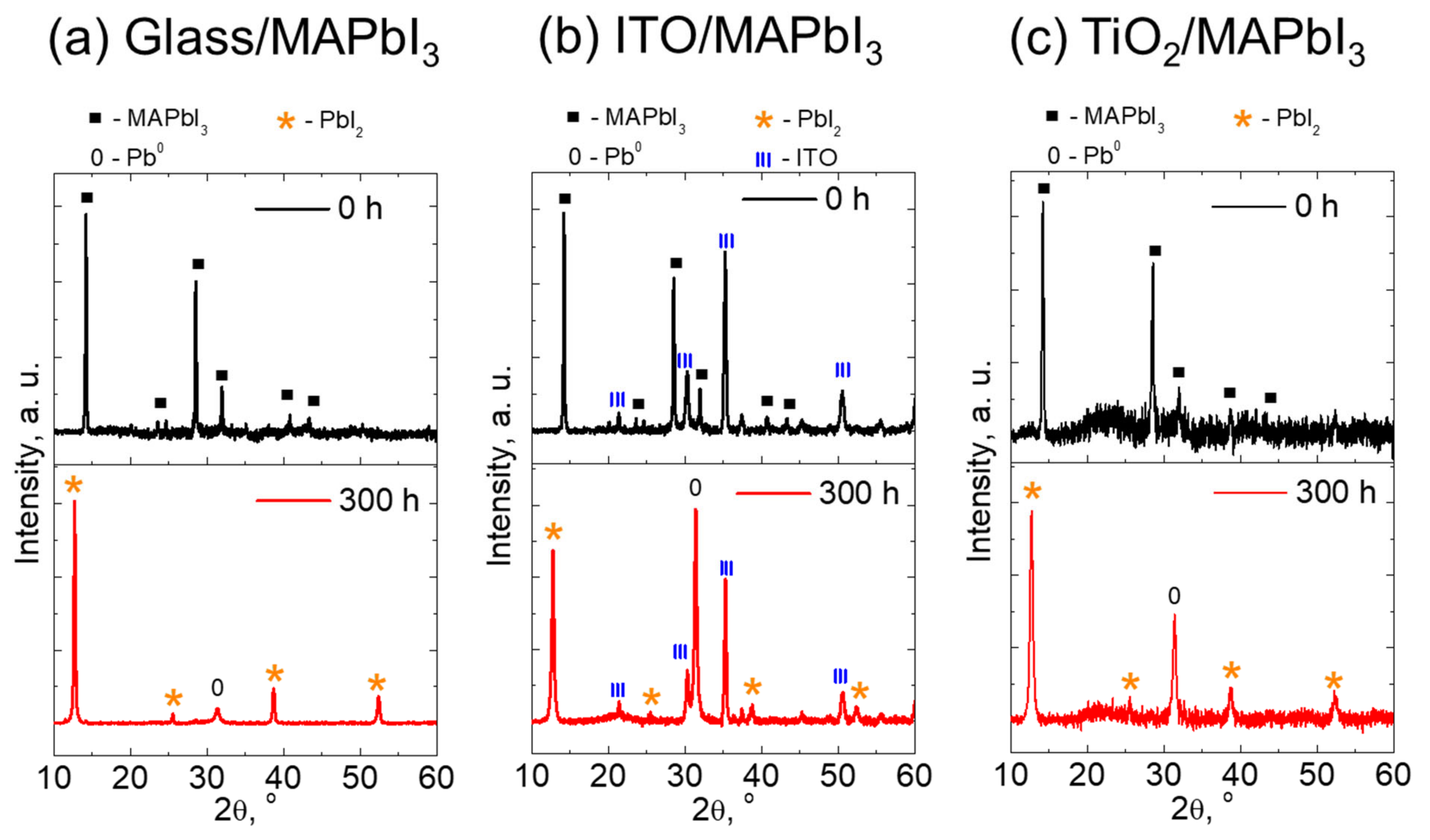
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838–842. [Google Scholar] [CrossRef]
- Antonietta Loi, M.; Hummelen, J.C. Perovskites under the Sun. Nat. Mater. 2013, 12, 1087–1089. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Jayatissa, A. Perovskites-Based Solar Cells: A Review of Recent Progress, Materials and Processing Methods. Materials 2018, 11, 729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grätzel, M. The Rise of Highly Efficient and Stable Perovskite Solar Cells. Acc. Chem. Res. 2017, 50, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; So, F.; Tsang, S.-W. Progress in air-processed perovskite solar cells: From crystallization to photovoltaic performance. Mater. Horiz. 2019, 6, 1611–1624. [Google Scholar] [CrossRef]
- Fu, Z.; Xu, M.; Sheng, Y.; Yan, Z.; Meng, J.; Tong, C.; Li, D.; Wan, Z.; Ming, Y.; Mei, A.; et al. Encapsulation of Printable Mesoscopic Perovskite Solar Cells Enables High Temperature and Long-Term Outdoor Stability. Adv. Funct. Mater. 2019, 29, 1809129. [Google Scholar] [CrossRef]
- Zhidkov, I.S.; Boukhvalov, D.W.; Akbulatov, A.F.; Frolova, L.A.; Finkelstein, L.D.; Kukharenko, A.I.; Cholakh, S.O.; Chueh, C.-C.; Troshin, P.A.; Kurmaev, E.Z. XPS spectra as a tool for studying photochemical and thermal degradation in APbX3 hybrid halide perovskites. Nano Energy 2020, 79, 105421. [Google Scholar] [CrossRef]
- Zhidkov, I.S.; Poteryaev, A.I.; Kukharenko, A.I.; Finkelstein, L.D.; Cholakh, S.O.; Akbulatov, A.F.; Troshin, P.A.; Chueh, C.-C.; Kurmaev, E.Z. XPS evidence of degradation mechanism in CH3NH3PbI3 hybrid perovskite. J. Phys. Condens. Matter 2020, 32, 095501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhidkov, I.S.; Boukhvalov, D.W.; Kukharenko, A.I.; Finkelstein, L.D.; Cholakh, S.O.; Akbulatov, A.F.; Juarez-Perez, E.J.; Troshin, P.A.; Kurmaev, E.Z. Influence of Ion Migration from ITO and SiO2 Substrates on Photo and Thermal Stability of CH3NH3SnI3 Hybrid Perovskite. J. Phys. Chem. C 2020, 12, 14928–14934. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Hawash, Z.; Raga, S.R.; Ono, L.K.; Qi, Y. Thermal degradation of CH3NH3PbI3 perovskite into NH3 and CH3I gases observed by coupled thermogravimetry–mass spectrometry analysis. Energy Environ. Sci. 2016, 9, 3406–3410. [Google Scholar] [CrossRef] [Green Version]
- Juarez-Perez, E.J.; Ono, L.K.; Uriarte, I.; Cocinero, E.J.; Qi, Y. Degradation Mechanism and Relative Stability of Methylammonium Halide Based Perovskites Analyzed on the Basis of Acid–Base Theory. ACS Appl. Mater. Interfaces 2019, 11, 12586–12593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, A.; Bongiorno, C.; Smecca, E.; Deretzis, I.; La Magna, A.; Spinella, C. Pb clustering and PbI2 nanofragmentation during methylammonium lead iodide perovskite degradation. Nat. Commun. 2019, 10, 2196. [Google Scholar] [CrossRef] [PubMed]
- Casalongue, H.S.; Kaya, S.; Viswanathan, V.; Miller, D.J.; Friebel, D.; Hansen, H.A.; Nørskov, J.K.; Nilsson, A.; Ogasawara, H. Direct observation of the oxygenated species during oxygen reduction on a platinum fuel cell cathode. Nat. Commun. 2013, 4, 2817. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., King, R.C.J., Eds.; ULVAK-PHI Inc.: Chanhassen, MN, USA, 1995. [Google Scholar]
- Payne, D.J.; Egdell, R.G.; Law, D.S.L.; Glans, P.-A.; Learmonth, T.; Smith, K.E.; Guo, J.; Walsh, A.; Watson, G.W. Experimental and theoretical study of the electronic structures of α-PbO and β-PbO2. J. Mater. Chem. 2007, 17, 267–277. [Google Scholar] [CrossRef]







| Sample | C | O | N | I | Pb | Sn | In | Na | Si | Ti | I:Pb | N:Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glass/MAPbI3 | 39.3 | 3.5 | 9.1 | 35.3 | 12.8 | - | - | - | - | - | 2.75 | 0.71 |
| Photo, 25 h | 41 | 4 | 7 | 34.1 | 13.9 | - | - | - | - | - | 2.45 | 0.50 |
| Photo, 50 h | 38.8 | 5.2 | 6.1 | 33.6 | 13.9 | - | - | 2.4 | - | - | 2.41 | 0.43 |
| Photo, 100 h | 45.7 | 9 | 4 | 25 | 10.9 | - | - | 3.6 | 1.8 | - | 2.29 | 0.36 |
| Photo, 200 h | 37.5 | 15.5 | - | 25.3 | 13 | - | - | 5.2 | 3.5 | - | 1.94 | - |
| ITO/MAPbI3 | 44.9 | 2.3 | 8.7 | 32.2 | 11.9 | - | - | - | - | - | 2.70. | 0.73 |
| Photo, 25 h | 39.3 | 17.2 | 1.9 | 21.1 | 10 | 1.1 | 9.4 | - | - | - | 2.11 | 0.19 |
| Photo, 50 h | 46.2 | 16 | - | 19.3 | 11.6 | 0.9 | 6 | -. | - | - | 1.66 | - |
| Photo, 100 h | 48.1 | 21.5 | 0.8 | 9.9 | 6 | 1 | 10.8 | - | 1.9 | - | 1.65 | 0.13 |
| Photo, 200 h | 49.9 | 20.5 | - | 10.2 | 6.5 | 1.1 | 11.8 | - | - | - | 1.56 | - |
| TiO2/MAPbI3 | 43.5 | 2.3 | 10.2 | 32.7 | 11.3 | - | - | - | - | - | 2.89 | 0.90 |
| Photo, 25 h | 32.7 | 25.8 | 1.6 | 20 | 10.8 | 0.5 | 0.1 | - | - | 8.5 | 1.85 | 0.14 |
| Photo, 50 h | 32.9 | 27.2 | 2.1 | 17.7 | 9.8 | 0.7 | 0.2 | - | - | 9.4 | 1.80 | 0.21 |
| Photo, 100 h | 41.6 | 28.2 | 1.5 | 11.2 | 6.5 | 1 | 0.2 | - | - | 9.8 | 1.72 | 0.23 |
| Photo, 200 h | 45.8 | 28.9 | 1.4 | 7.1 | 5.7 | 0.4 | 0.2 | - | - | 10.5 | 1.24 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhidkov, I.S.; Akbulatov, A.F.; Inasaridze, L.N.; Kukharenko, A.I.; Frolova, L.A.; Cholakh, S.O.; Chueh, C.-C.; Troshin, P.A.; Kurmaev, E.Z. Influence of Oxygen Ion Migration from Substrates on Photochemical Degradation of CH3NH3PbI3 Hybrid Perovskite. Energies 2021, 14, 5062. https://doi.org/10.3390/en14165062
Zhidkov IS, Akbulatov AF, Inasaridze LN, Kukharenko AI, Frolova LA, Cholakh SO, Chueh C-C, Troshin PA, Kurmaev EZ. Influence of Oxygen Ion Migration from Substrates on Photochemical Degradation of CH3NH3PbI3 Hybrid Perovskite. Energies. 2021; 14(16):5062. https://doi.org/10.3390/en14165062
Chicago/Turabian StyleZhidkov, Ivan S., Azat F. Akbulatov, Liana N. Inasaridze, Andrey I. Kukharenko, Lyubov A. Frolova, Seif O. Cholakh, Chu-Chen Chueh, Pavel A. Troshin, and Ernst Z. Kurmaev. 2021. "Influence of Oxygen Ion Migration from Substrates on Photochemical Degradation of CH3NH3PbI3 Hybrid Perovskite" Energies 14, no. 16: 5062. https://doi.org/10.3390/en14165062
APA StyleZhidkov, I. S., Akbulatov, A. F., Inasaridze, L. N., Kukharenko, A. I., Frolova, L. A., Cholakh, S. O., Chueh, C.-C., Troshin, P. A., & Kurmaev, E. Z. (2021). Influence of Oxygen Ion Migration from Substrates on Photochemical Degradation of CH3NH3PbI3 Hybrid Perovskite. Energies, 14(16), 5062. https://doi.org/10.3390/en14165062