Branched Electron-Donor Core Effect in D-π-A Star-Shaped Small Molecules on Their Properties and Performance in Single-Component and Bulk-Heterojunction Organic Solar Cells † †
Abstract
1. Introduction
2. Results and Discussion
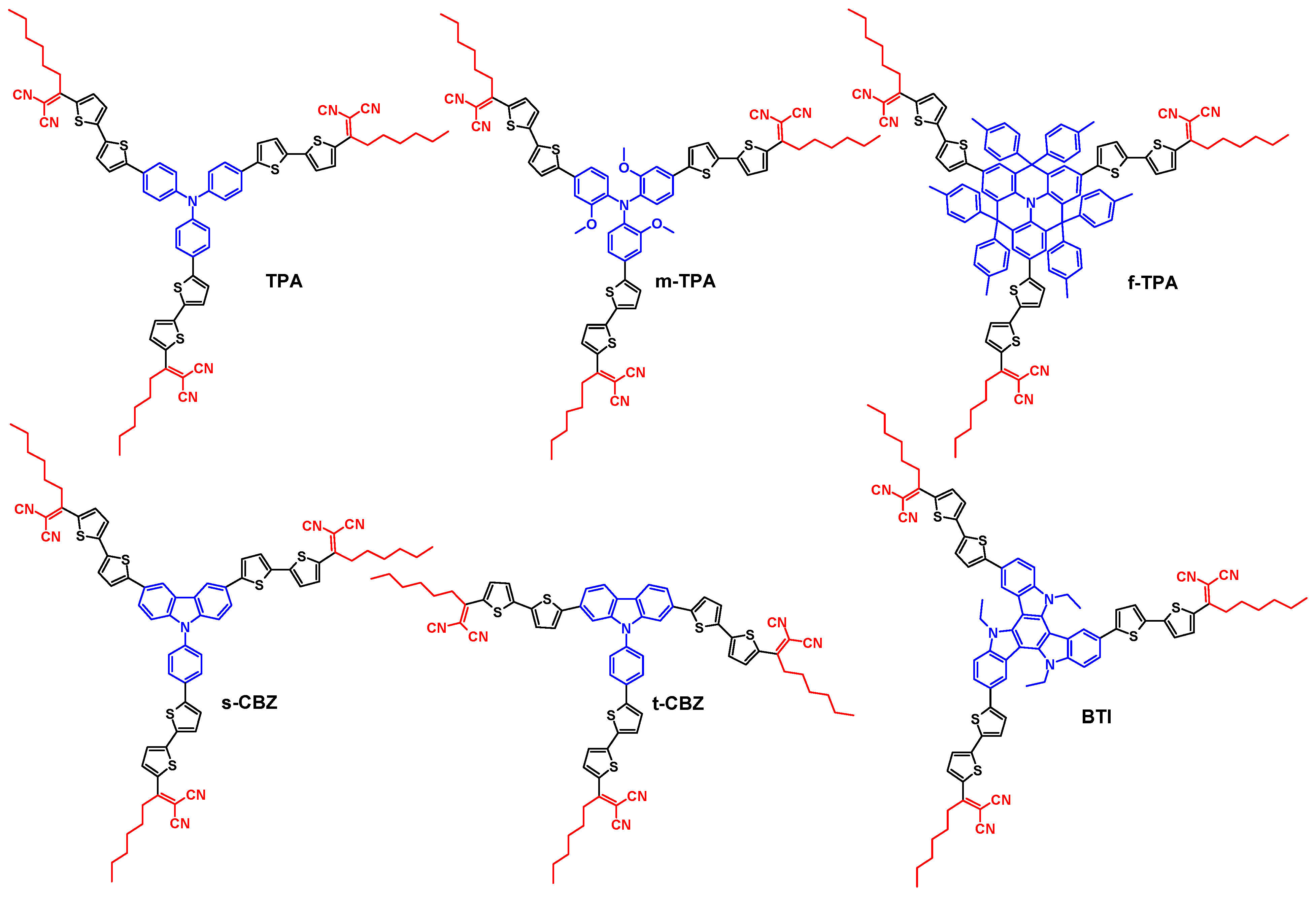
2.1. Synthesis
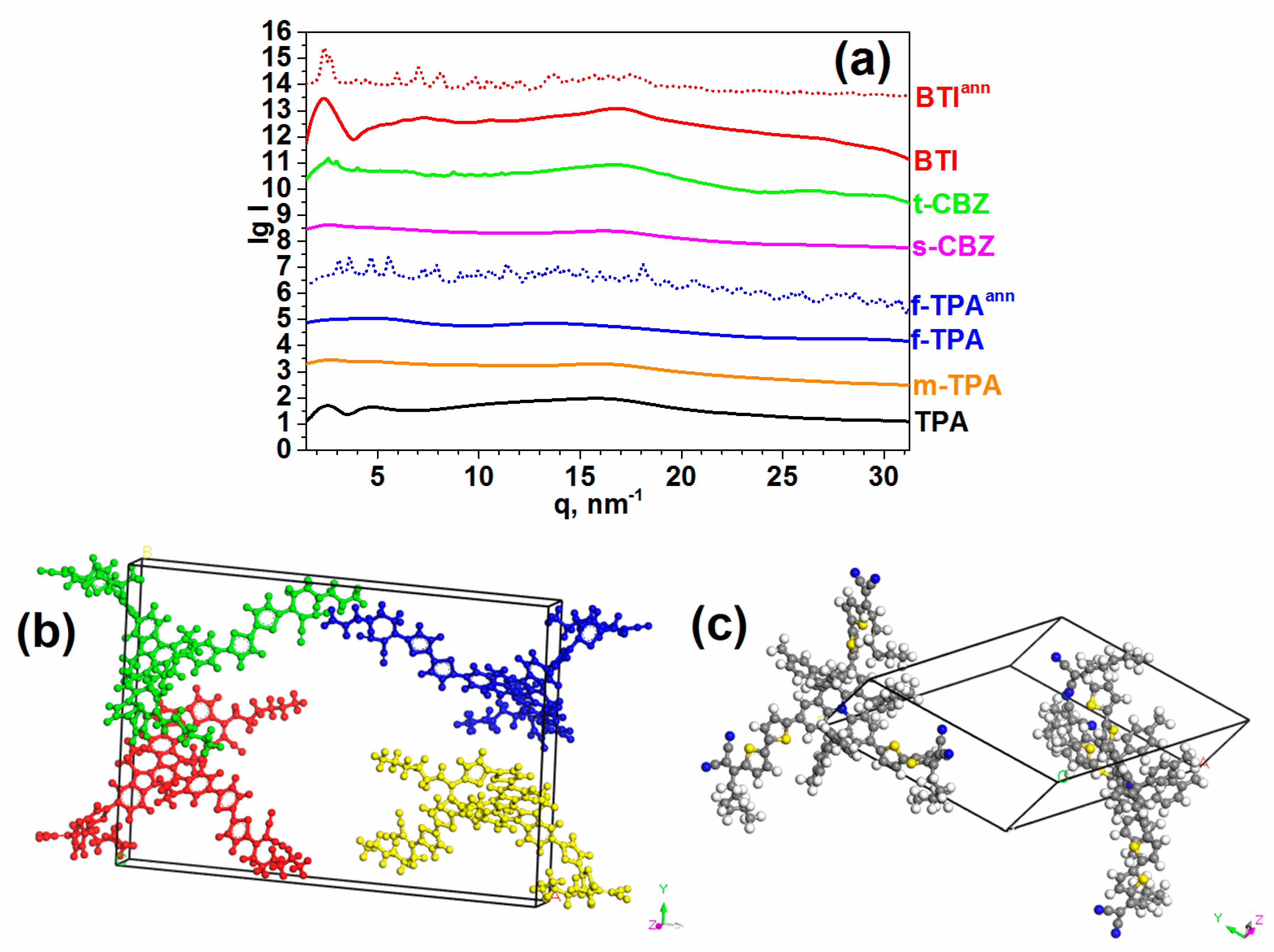
2.2. Solubility, Thermal and Structural Properties
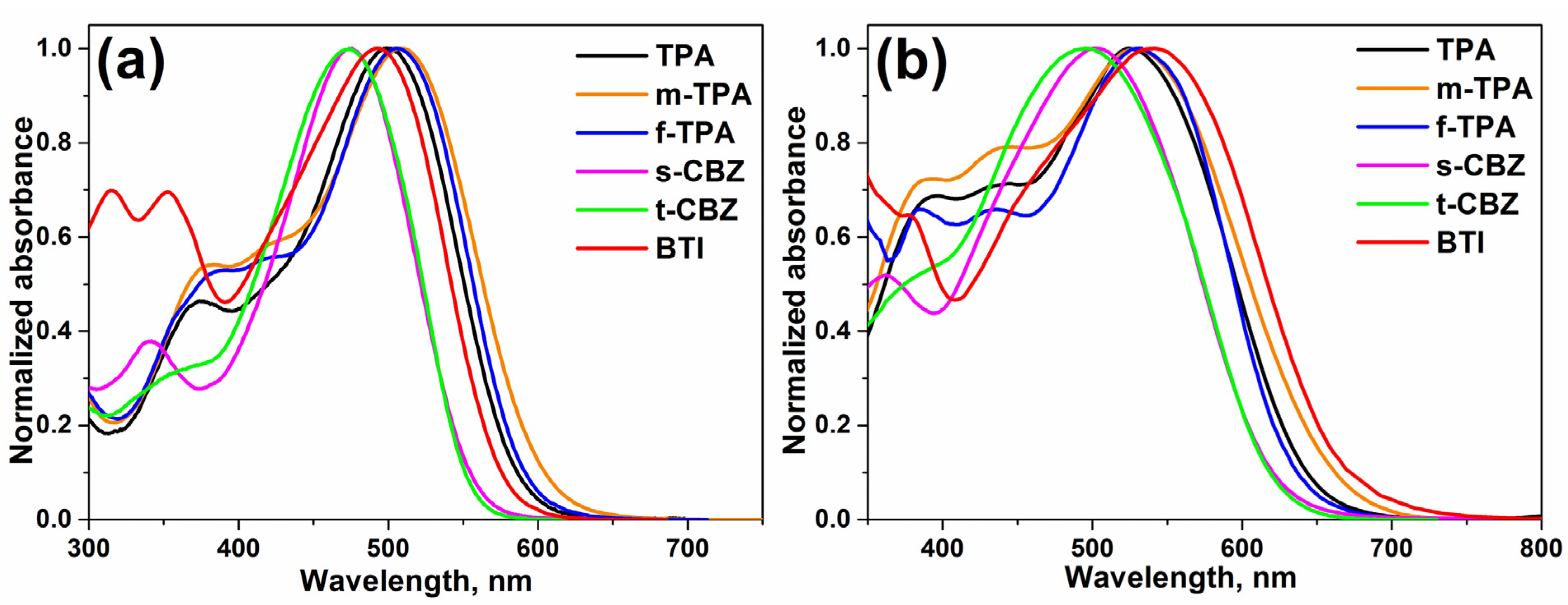
2.3. Optical and Electrochemical Properties
2.4. Charge Transport and Photovoltaic Properties
2.4.1. Single-Component Solar Cells
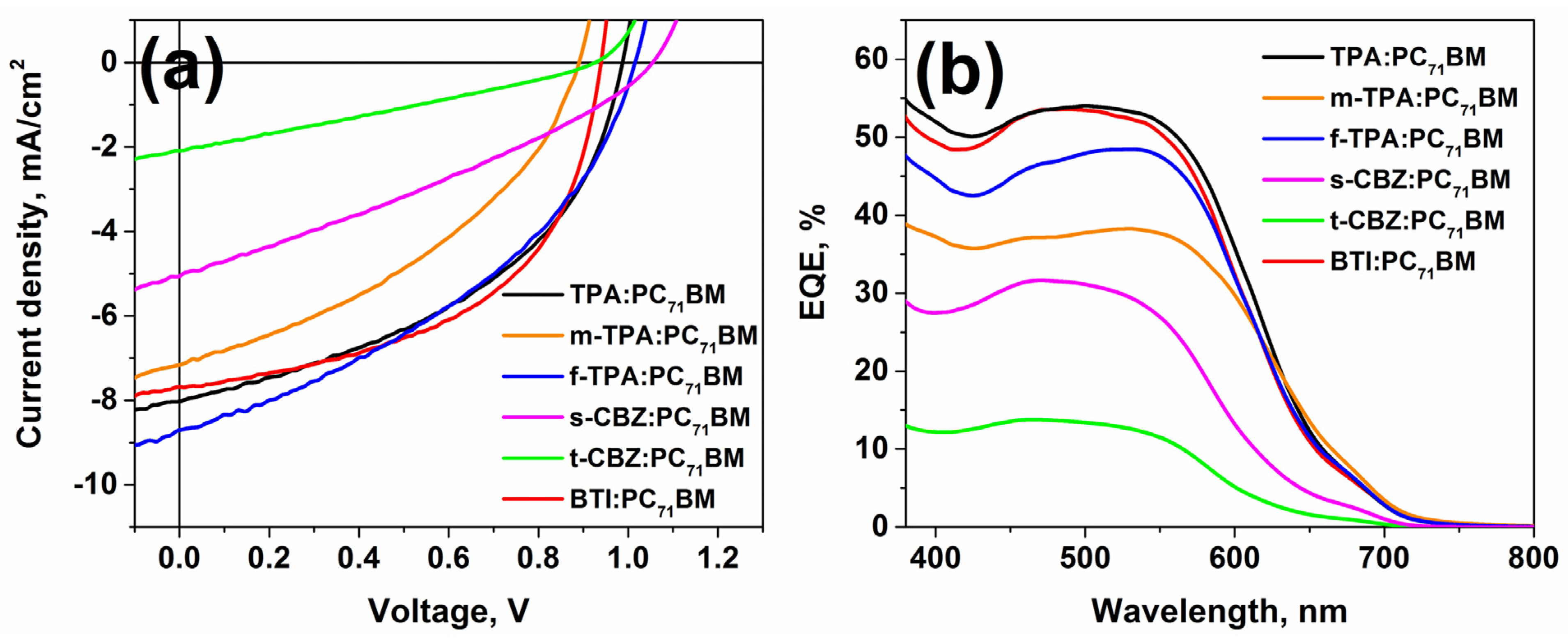
2.4.2. Bulk-Heterojunction Solar Cells
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Xu, G.; Cui, C.; Li, Y. Flexible and semitransparent organic solar cells. Adv. Energy Mater. 2018, 8, 1701791. [Google Scholar] [CrossRef]
- Wang, G.; Adil, M.A.; Zhang, J.; Wei, Z. Large-area organic solar cells: Material requirements, modular designs, and printing methods. Adv. Mater. 2019, 31, e1805089. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Mehta, S.; Gupta, D. Fully printed organic solar cells—A review of techniques, challenges and their solutions. Opto-Electron. Rev. 2019, 27, 298–320. [Google Scholar] [CrossRef]
- Wienhold, K.S.; Chen, W.; Yin, S.; Guo, R.; Schwartzkopf, M.; Roth, S.V.; Müller-Buschbaum, P. Following in operando the structure evolution-induced degradation in printed organic solar cells with nonfullerene small molecule acceptor. Sol. RRL 2020, 4, 2000251. [Google Scholar] [CrossRef]
- Kuznetsov, P.M.; Proshin, P.I.; Nikitenko, S.L.; Lolaeva, A.V.; Vasil’Ev, S.G.; Troshin, P.A.; Akkuratov, A.V. Design of novel thiazolothiazole-based conjugated polymer for efficient fullerene and non-fullerene organic solar cells. Synth. Met. 2020, 268, 116508. [Google Scholar] [CrossRef]
- Wienhold, K.S.; Körstgens, V.; Grott, S.; Jiang, X.; Schwartzkopf, M.; Roth, S.V.; Müller-Buschbaum, P. Effect of solvent additives on the morphology and device performance of printed nonfullerene acceptor based organic solar cells. ACS Appl. Mater. Interfaces 2019, 11, 42313–42321. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019, 10, 2515. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Feng, K.; Bi, Z.; Ma, W.; Zhang, G.; Peng, Q. Single-Junction Polymer Solar Cells with 16.35% Efficiency Enabled by a Platinum(II) Complexation Strategy. Adv. Mater. 2019, 31, e1901872. [Google Scholar] [CrossRef]
- Meier, H. Conjugated oligomers with terminal donor–Acceptor substitution. Angew. Chem. Int. Ed. 2005, 44, 2482–2506. [Google Scholar] [CrossRef]
- Roncali, J.; Leriche, P.; Blanchard, P. Molecular materials for organic photovoltaics: Small is beautiful. Adv. Mater. 2014, 26, 3821–3838. [Google Scholar] [CrossRef]
- Schulze, K.; Uhrich, C.; Schüppel, R.; Leo, K.; Pfeiffer, M.; Brier, E.; Reinold, E.; Bäuerle, P. Efficient vacuum-deposited organic solar cells based on a new low-bandgap oligothiophene and fullerene C60. Adv. Mater. 2006, 18, 2872–2875. [Google Scholar] [CrossRef]
- Patrizi, B.; Cozza, C.; Pietropaolo, A.; Foggi, P.; De Cumis, M.S. Synergistic approach of ultrafast spectroscopy and molecular simulations in the characterization of intramolecular charge transfer in push-pull molecules. Molecules 2020, 25, 430. [Google Scholar] [CrossRef] [PubMed]
- Bureš, F. Fundamental aspects of property tuning in push–pull molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar] [CrossRef]
- Ledwon, P. Recent advances of donor-acceptor type carbazole-based molecules for light emitting applications. Org. Electron. 2019, 75, 105422. [Google Scholar] [CrossRef]
- Feng, H.-T.; Zeng, J.; Yin, P.-A.; Wang, X.-D.; Peng, Q.; Zhao, Z.; Lam, J.W.Y.; Tang, B.Z. Tuning molecular emission of organic emitters from fluorescence to phosphorescence through push-pull electronic effects. Nat. Commun. 2020, 11, 2617. [Google Scholar] [CrossRef]
- Roncali, J.; Leriche, P.; Cravino, A. From one- to three-dimensional organic semiconductors: In search of the organic silicon? Adv. Mater. 2007, 19, 2045–2060. [Google Scholar] [CrossRef]
- Ripaud, E.; Olivier, Y.; Leriche, P.; Cornil, J.; Roncali, J. Polarizability and internal charge transfer in thiophene–triphenylamine hybrid π-conjugated systems. J. Phys. Chem. B 2011, 115, 9379–9386. [Google Scholar] [CrossRef][Green Version]
- Wu, H.-C.; Zhang, J.; Bo, Z.; Chen, W.-C. Well-defined star-shaped donor–acceptor conjugated molecules for organic resistive memory devices. Chem. Commun. 2015, 51, 14179–14182. [Google Scholar] [CrossRef]
- Solodukhin, A.N.; Luponosov, Y.; Mannanov, A.; Dmitryakov, P.V.; Peregudova, S.M.; Chvalun, S.N.; Parashchuk, D.Y.; Ponomarenko, S.A. Effect of branching on the physical and photovoltaic properties of donor–acceptor oligomers based on triphenylamine. Mendeleev Commun. 2019, 29, 385–387. [Google Scholar] [CrossRef]
- Popli, C.; Jang, Y.; Patil, Y.; Misra, R.; D’Souza, F. Formation of highly efficient, long-lived charge separated states in star-shaped ferrocene-diketopyrrolopyrrole-triphenylamine donor–acceptor–donor conjugates. Chem. A Eur. J. 2020, 26, 15109–15115. [Google Scholar] [CrossRef] [PubMed]
- Kanibolotsky, A.L.; Perepichka, I.; Skabara, P.J. Star-shaped π-conjugated oligomers and their applications in organic electronics and photonics. Chem. Soc. Rev. 2010, 39, 2695–2728. [Google Scholar] [CrossRef]
- Jarosz, T.; Lapkowski, M.; Ledwon, P. Advances in star-shaped π-conjugated systems: Properties and applications. Macromol. Rapid Commun. 2014, 35, 1006–1032. [Google Scholar] [CrossRef]
- Lin, K.; Wang, S.; Wang, Z.; Yin, Q.; Liu, X.; Jia, J.; Jia, X.; Luo, P.; Jiang, X.; Duan, C.; et al. Electron acceptors with a truxene core and perylene diimide branches for organic solar cells: The effect of ring-fusion. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sun, G. Star-shaped non-fullerene small acceptors for organic solar cells. ChemSusChem 2019, 12, 4570–4600. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wu, G.; He, C.; Bai, F.-Q.; Xi, H.; Zhang, H.-X.; Li, Y. Solution-processable multiarmed organic molecules containing triphenylamine and DCM Moieties: Synthesis and photovoltaic properties. J. Phys. Chem. C 2009, 113, 2636–2642. [Google Scholar] [CrossRef]
- Singh, S.P.; Roy, M.S.; Thomas, K.R.J.; Balaiah, S.; Bhanuprakash, K.; Sharma, G.D. New triphenylamine-based organic dyes with different numbers of anchoring groups for dye-sensitized solar cells. J. Phys. Chem. C 2012, 116, 5941–5950. [Google Scholar] [CrossRef]
- Metri, N.; Sallenave, X.; Plesse, C.; Beouch, L.; Aubert, P.-H.; Goubard, F.; Chevrot, C.; Sini, G. Processable star-shaped molecules with triphenylamine core as hole-transporting materials: Experimental and theoretical approach. J. Phys. Chem. C 2012, 116, 3765–3772. [Google Scholar] [CrossRef]
- Luponosov, Y.N.; Solodukhin, A.N.; Balakirev, D.O.; Surin, N.M.; Svidchenko, E.A.; Pisarev, S.; Fedorov, Y.V.; Ponomarenko, S.A. Triphenylamine-based luminophores with different side and central aromatic blocks: Synthesis, thermal, photophysical and photochemical properties. Dye. Pigment. 2020, 56, 104–134. [Google Scholar] [CrossRef]
- Zhou, P.; Dang, D.; Wang, Q.; Duan, X.; Xiao, M.; Tao, Q.; Tan, H.; Yang, R.; Zhu, W. Enhancing the photovoltaic performance of triphenylamine based star-shaped molecules by tuning the moiety sequence of their arms in organic solar cells. J. Mater. Chem. A 2015, 3, 13568–13576. [Google Scholar] [CrossRef]
- Lian, X.; Zhao, Z.; Cheng, D. Recent progress on triphenylamine materials: Synthesis, properties, and applications. Mol. Cryst. Liq. Cryst. 2017, 648, 223–235. [Google Scholar] [CrossRef]
- Cabanetos, C.; Blanchard, P.; Roncali, J. Arylamine based photoactive push-pull molecular systems: A brief overview of the chemistry “Made in Angers”. Chem. Rec. 2019, 19, 1123–1130. [Google Scholar] [CrossRef]
- Luponosov, Y.N.; Min, J.; Solodukhin, A.N.; Kozlov, O.V.; Obrezkova, M.A.; Peregudova, S.M.; Ameri, T.; Chvalun, S.N.; Pshenichnikov, M.S.; Brabec, C.J.; et al. Effects of electron-withdrawing group and electron-donating core combinations on physical properties and photovoltaic performance in D-π-A star-shaped small molecules. Org. Electron. 2016, 32, 157–168. [Google Scholar] [CrossRef]
- Huang, B.; Yin, Z.; Ban, X.; Ma, Z.; Jiang, W.; Tian, W.; Yang, M.; Ye, S.; Lin, B.; Sun, Y. Nondoped deep blue OLEDs based on Bis-(4-benzenesulfonyl-phenyl)-9-phenyl-9 H -carbazoles. J. Lumin. 2016, 172, 7–13. [Google Scholar] [CrossRef]
- Bakirov, A.V.; Solodukhin, A.N.; Luponosov, Y.N.; Svidchenko, E.A.; Obrezkova, M.A.; Peregudova, S.M.; Shcherbina, M.A.; Ponomarenko, S.A.; Chvalun, S.N. The effect of star-shaped oligothiophenes with a carbazole core on their structural and optical properties. Nanotechnol. Russ. 2017, 12, 385–394. [Google Scholar] [CrossRef]
- Dong, Q.; Lian, H.; Gao, Z.; Guo, Z.; Xiang, N.; Zhong, Z.; Guo, H.; Huang, J.; Wong, W.-Y. Novel spirofluorene/indole/carbazole-based hole transport materials with high triplet energy for efficient green phosphorescent organic light-emitting diodes. Dye. Pigment. 2017, 137, 84–90. [Google Scholar] [CrossRef]
- Grybauskaite-Kaminskiene, G.; Volyniuk, D.; Mimaite, V.; Bezvikonnyi, O.; Bucinskas, A.; Bagdziunas, G.; Grazulevicius, J.V. Aggregation-enhanced emission and thermally activated delayed fluorescence of derivatives of 9-phenyl-9h -carbazole: Effects of methoxy and tert -butyl substituents. Chem. A Eur. J. 2018, 24, 9581–9591. [Google Scholar] [CrossRef]
- Paek, S.; Cho, N.; Cho, S.; Lee, J.K.; Ko, J. Planar star-shaped organic semiconductor with fused triphenylamine core for solution-processed small-molecule organic solar cells and field-effect transistors. Org. Lett. 2012, 14, 6326–6329. [Google Scholar] [CrossRef]
- Jiang, Z.-Q.; Chen, Y.; Yang, C.; Cao, Y.; Tao, Y.; Qin, J.; Ma, D. A fully diarylmethylene-bridged triphenylamine derivative as novel host for highly efficient green phosphorescent OLEDs. Org. Lett. 2009, 11, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Ye, T.; Yang, C.; Yang, D.; Zhu, M.; Zhong, C.; Qin, J.; Ma, D. Star-shaped oligotriarylamines with planarized triphenylamine core: Solution-processable, high-tghole-injecting and hole-transporting materials for organic light-emitting devices. Chem. Mater. 2011, 23, 771–777. [Google Scholar] [CrossRef]
- Robertson, N.; Parsons, S.; MacLean, E.J.; Coxall, R.A.; Mount, A.R. Preparation, X-ray structure and properties of a hexabrominated, symmetric indole trimer and its TCNQ adduct: A new route to functional molecular systems. J. Mater. Chem. 2000, 10, 2043–2047. [Google Scholar] [CrossRef]
- Shao, J.; Guan, Z.; Yan, Y.; Jiao, C.; Xu, Q.-H.; Chi, C. Synthesis and characterizations of star-shaped octupolar triazatruxenes-based two-photon absorption chromophores. J. Org. Chem. 2011, 76, 780–790. [Google Scholar] [CrossRef]
- Connell, A.; Wang, Z.; Lin, Y.-H.; Greenwood, P.C.; Wiles, A.A.; Jones, E.W.; Furnell, L.; Anthony, R.; Kershaw, C.P.; Cooke, G.; et al. Low cost triazatruxene hole transporting material for >20% efficiency perovskite solar cells. J. Mater. Chem. C 2018, 7, 5235–5243. [Google Scholar] [CrossRef]
- Rakstys, K.; Abate, A.; Dar, M.I.; Gao, P.; Jankauskas, V.; Jacopin, G.; Kamarauskas, E.; Kazim, S.; Ahmad, S.; Grätzel, M.; et al. Triazatruxene-based hole transporting materials for highly efficient perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 16172–16178. [Google Scholar] [CrossRef]
- Li, X.-C.; Wang, C.-Y.; Lai, W.-Y.; Huang, W. Triazatruxene-based materials for organic electronics and optoelectronics. J. Mater. Chem. C 2016, 4, 10574–10587. [Google Scholar] [CrossRef]
- Cravino, A.; Leriche, P.; Aleveque, O.; Roquet, S.; Roncali, J. Light-emitting organic solar cells based on a 3d conjugated system with internal charge transfer. Adv. Mater. 2006, 18, 3033–3037. [Google Scholar] [CrossRef]
- Roncali, J. Single material solar cells: The next frontier for organic photovoltaics? Adv. Energy Mater. 2011, 1, 147–160. [Google Scholar] [CrossRef]
- Yu, P.; Feng, G.; Li, J.; Li, C.; Xu, Y.; Xiao, C.; Li, W. A selenophene substituted double-cable conjugated polymer enables efficient single-component organic solar cells. J. Mater. Chem. C 2020, 8, 2790–2797. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, J.; Karuthedath, S.; Li, J.; Lai, W.; Li, C.; Xiao, C.; Ye, L.; Ma, Z.; Tang, Z.; et al. Miscibility-controlled phase separation in double-cable conjugated polymers for single-component organic solar cells with efficiencies over 8%. Angew. Chem. 2020, 132, 21867–21876. [Google Scholar] [CrossRef]
- Marinelli, M.; Lanzi, M.; Liscio, A.; Zanelli, A.; Zangoli, M.; Di Maria, F.; Salatelli, E. Single-material organic solar cells with fully conjugated electron-donor alkoxy-substituted bithiophene units and electron-acceptor benzothiadiazole moieties alternating in the main chain. J. Mater. Chem. C 2020, 8, 4124–4132. [Google Scholar] [CrossRef]
- Nakayama, K.-I.; Okura, T.; Okuda, Y.; Matsui, J.; Masuhara, A.; Yoshida, T.; White, M.; Yumusak, C.; Stadler, P.; Scharber, M.; et al. Single-component organic solar cells based on intramolecular charge transfer photoabsorption. Materials 2021, 14, 1200. [Google Scholar] [CrossRef]
- Dong, Y.; Nikolis, V.C.; Talnack, F.; Chin, Y.-C.; Benduhn, J.; Londi, G.; Kublitski, J.; Zheng, X.; Mannsfeld, S.C.B.; Spoltore, D.; et al. Orientation dependent molecular electrostatics drives efficient charge generation in homojunction organic solar cells. Nat. Commun. 2020, 11, 4617. [Google Scholar] [CrossRef] [PubMed]
- Mannanov, A.L.; Savchenko, P.S.; Luponosov, Y.N.; Solodukhin, A.N.; Ponomarenko, S.A.; Paraschuk, D.Y. Charge photogeneration and recombination in single-material organic solar cells and photodetectors based on conjugated star-shaped donor-acceptor oligomers. Org. Electron. 2020, 78, 105588. [Google Scholar] [CrossRef]
- Solodukhin, A.N.; Luponosov, Y.N.; Mannanov, A.L.; Paraschuk, D.Y.; Ponomarenko, S.A. Novel star-shaped donor-acceptor molecules based on trihenylamine for organic solar cells and photodetectors. In Proceedings of the Online School on Hybrid, Organic and Perovskite Photovoltaics, Moscow, Russia, 3–5 November 2020; p. 24. [Google Scholar]
- Min, J.; Luponosov, Y.N.; Baran, D.; Chvalun, S.N.; Shcherbina, M.A.; Bakirov, A.V.; Dmitryakov, P.V.; Peregudova, S.M.; Kausch-Busies, N.; Ponomarenko, S.A.; et al. Effects of oligothiophene π-bridge length on physical and photovoltaic properties of star-shaped molecules for bulk heterojunction solar cells. J. Mater. Chem. A 2014, 2, 16135–16147. [Google Scholar] [CrossRef]
- Balakirev, D.O.; Luponosov, Y.N.; Mannanov, A.; Savchenko, P.S.; Minenkov, Y.; Paraschuk, D.Y.; Ponomarenko, S.A. Star-shaped benzotriindole-based donor-acceptor molecules: Synthesis, properties and application in bulk heterojunction and single-material organic solar cells. Dye. Pigment. 2020, 181, 108523. [Google Scholar] [CrossRef]
- Luponosov, Y.N.; Solodukhin, A.N.; Mannanov, A.; Savchenko, P.S.; Minenkov, Y.; Paraschuk, D.Y.; Ponomarenko, S.A. Effect of fused triphenylamine core in star-shaped donor-π-acceptor molecules on their physicochemical properties and performance in bulk heterojunction organic solar cells. Dye. Pigment. 2020, 177, 108260. [Google Scholar] [CrossRef]
- Luponosov, Y.N.; Min, J.; Solodukhin, A.N.; Bakirov, A.V.; Dmitryakov, P.V.; Shcherbina, M.A.; Peregudova, S.M.; Cherkaev, G.V.; Chvalun, S.N.; Brabec, C.J.; et al. Star-shaped D–π–A oligothiophenes with a tris(2-methoxyphenyl)amine core and alkyldicyanovinyl groups: Synthesis and physical and photovoltaic properties. J. Mater. Chem. C 2016, 4, 7061–7076. [Google Scholar] [CrossRef]
- Luponosov, Y.N.; Balakirev, D.O.; Dyadishchev, I.V.; Solodukhin, A.N.; Obrezkova, M.A.; Svidchenko, E.A.; Surin, N.M.; Ponomarenko, S.A. In search of efficient solubilizing groups for liquid and luminescent oligo(phenylene-thiophene) chromophores. J. Mater. Chem. C 2020, 8, 17074–17082. [Google Scholar] [CrossRef]
- Ripaud, E.; Rousseau, T.; Leriche, P.; Roncali, J. Unsymmetrical triphenylamine-oligothiophene hybrid conjugated systems as donor materials for high-voltage solution-processed organic solar cells. Adv. Energy Mater. 2011, 1, 540–545. [Google Scholar] [CrossRef]
- Kozlov, O.V.; Luponosov, Y.N.; Ponomarenko, S.A.; Kausch-Busies, N.; Paraschuk, D.Y.; Olivier, Y.; Beljonne, D.; Cornil, J.; Pshenichnikov, M.S. Ultrafast charge generation pathways in photovoltaic blends based on novel star-shaped conjugated molecules. Adv. Energy Mater. 2014, 5, 1401657. [Google Scholar] [CrossRef]
- Roncali, J.; Grosu, I. The dawn of single material organic solar cells. Adv. Sci. 2019, 6, 1801026. [Google Scholar] [CrossRef] [PubMed]




| Compounds | Solubility a, gL−1 | Tg, °C | ∆Cp, J(gK)−1 | Tm1, °C | ∆Hm1, Jg−1 | Tm2, °C | ∆Hm2, Jg−1 | Td (Air/N2), °C |
|---|---|---|---|---|---|---|---|---|
| TPA | 20 | 64 | 0.3 | – | – | – | – | 383/390 |
| m-TPA | 43 | 71 | 0.3 | – | – | – | – | 384/389 |
| f-TPA | 49 | 137 | 0.2 | 300 | 34 | – | – | 400/406 |
| s-CBZ | 24 | 66 | 0.4 | – | – | – | – | 379/397 |
| t-CBZ | 30 | 64 | 0.2 | 120 | 7 | 120 | 5 | 379/389 |
| BTI | 5 | – | – | 231 | 42 | 223 | 24 | 365/380 |
| Sample | System | a, Å | b, Å | c, Å | αº | βº | γº | V, Å3 |
|---|---|---|---|---|---|---|---|---|
| t-CBZ | Monoclinic | 18.5 | 24.5 | 9.4 | 90 | 95.7 | 90 | 4240 |
| f-TPA | Triclinic | 25.9 | 21.6 | 11.1 | 93.7 | 91.9 | 129 | 4780 |
| BTI | Orthorombic | 46.1 | 31.7 | 4.3 | 90 | 90 | 90 | 6299 |
| Compound | λmax a, nm | λmax b, nm | λonset c, nm | Egopt, eV | HOMO d, eV | LUMO d, eV | Egd eV |
|---|---|---|---|---|---|---|---|
| TPA | 500 | 525 | 637 | 1.95 | –5.34 | –3.41 | 1.93 |
| m-TPA | 509 | 529 | 662 | 1.87 | –5.20 | -3.39 | 1.81 |
| f-TPA | 506 | 533 | 636 | 1.95 | –5.30 | –3.38 | 1.92 |
| s-CBZ | 475 | 502 | 620 | 2.00 | –5.45 | –3.35 | 2.10 |
| t-CBZ | 476 | 496 | 618 | 2.01 | –5.44 | –3.34 | 2.10 |
| BTI | 493 | 542 | 663 | 1.87 | –5.36 | –3.32 | 2.04 |
| Material | µh, cm2/Vs | µe, cm2/Vs | µh/µe |
|---|---|---|---|
| TPA | (1.5 ± 0.4)·10−4 | (1.15 ± 0.12)·10−5 | 13.0 |
| m-TPA | (1.36 ± 0.15)·10−4 | (1.24 ± 0.20)·10−5 | 11.0 |
| f-TPA | (7.7 ± 1.3)·10−5 | (5.8 ± 1.3)·10−6 | 13.3 |
| s-CBZ | (4.8 ± 1.0)·10−5 | (3.8 ± 0.7)·10−6 | 12.6 |
| t-CBZ | (3.4 ± 0.9)·10−5 | (4.2 ± 0.8)·10−6 | 8.1 |
| BTI | (2.6 ± 0.7)·10−4 | (8.3 ± 1.6)·10−6 | 31.3 |
| Active Layer Material | JSC, mA/cm2 | JSC EQE a, mA/cm2 | VOC, V | FF, % | PCE, % | EQE, % |
|---|---|---|---|---|---|---|
| TPA | 1.70 | 1.55 | 1.13 | 24.3 | 0.47 | 11.8 |
| m-TPA | 1.61 | 1.52 | 1.17 | 23.2 | 0.44 | 10.7 |
| f-TPA | 0.52 | 0.37 | 0.74 | 25.7 | 0.10 | 3.0 |
| s-CBZ | 1.31 | 1.22 | 1.05 | 24.6 | 0.34 | 9.4 |
| t-CBZ | 0.45 | 0.44 | 1.15 | 21.0 | 0.11 | 3.5 |
| BTI b | 3.98 | 3.86 | 0.76 | 31.1 | 0.94 | 25.6 |
| Active Layer Material | JSC, mA/cm2 | JSC EQE a, mA/cm2 | VOC, V | FF, % | PCE, % | EQE, % |
|---|---|---|---|---|---|---|
| TPA:PC71BM | 8.03 | 7.68 | 0.99 | 45.1 | 3.59 | 54.7 |
| m-TPA:PC71BM | 7.16 | 5.84 | 0.89 | 39.2 | 2.50 | 38.8 |
| f-TPA:PC71BM b | 8.70 | 6.85 | 1.02 | 39.7 | 3.52 | 48.5 |
| s-CBZ:PC71BM | 5.07 | 3.81 | 1.05 | 31.0 | 1.65 | 31.6 |
| t-CBZ:PC71BM b | 2.09 | 1.62 | 0.93 | 27.8 | 0.54 | 13.8 |
| BTI:PC71BM b | 7.67 | 7.42 | 0.94 | 52.9 | 3.81 | 53.6 |
| Material | µh, cm2/Vs |
|---|---|
| TPA:PC71BM | (9.8 ± 1.1)·10−4 |
| m-TPA:PC71BM | (1.4 ± 0.3)·10−3 |
| f-TPA:PC71BM | (9.6 ± 1.7)·10−5 |
| s-CBZ:PC71BM | (5.1 ± 1.0)·10−4 |
| t-CBZ:PC71BM | (4.5 ± 0.7)·10−4 |
| BTI:PC71BM | (6.1 ± 1.1)·10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solodukhin, A.N.; Luponosov, Y.N.; Mannanov, A.L.; Savchenko, P.S.; Bakirov, A.V.; Shcherbina, M.A.; Chvalun, S.N.; Paraschuk, D.Y.; Ponomarenko, S.A. Branched Electron-Donor Core Effect in D-π-A Star-Shaped Small Molecules on Their Properties and Performance in Single-Component and Bulk-Heterojunction Organic Solar Cells †. Energies 2021, 14, 3596. https://doi.org/10.3390/en14123596
Solodukhin AN, Luponosov YN, Mannanov AL, Savchenko PS, Bakirov AV, Shcherbina MA, Chvalun SN, Paraschuk DY, Ponomarenko SA. Branched Electron-Donor Core Effect in D-π-A Star-Shaped Small Molecules on Their Properties and Performance in Single-Component and Bulk-Heterojunction Organic Solar Cells †. Energies. 2021; 14(12):3596. https://doi.org/10.3390/en14123596
Chicago/Turabian StyleSolodukhin, Alexander N., Yuriy N. Luponosov, Artur L. Mannanov, Petr S. Savchenko, Artem V. Bakirov, Maxim A. Shcherbina, Sergei N. Chvalun, Dmitry Yu. Paraschuk, and Sergey A. Ponomarenko. 2021. "Branched Electron-Donor Core Effect in D-π-A Star-Shaped Small Molecules on Their Properties and Performance in Single-Component and Bulk-Heterojunction Organic Solar Cells †" Energies 14, no. 12: 3596. https://doi.org/10.3390/en14123596
APA StyleSolodukhin, A. N., Luponosov, Y. N., Mannanov, A. L., Savchenko, P. S., Bakirov, A. V., Shcherbina, M. A., Chvalun, S. N., Paraschuk, D. Y., & Ponomarenko, S. A. (2021). Branched Electron-Donor Core Effect in D-π-A Star-Shaped Small Molecules on Their Properties and Performance in Single-Component and Bulk-Heterojunction Organic Solar Cells †. Energies, 14(12), 3596. https://doi.org/10.3390/en14123596







