An Investigation into CO2–Brine–Cement–Reservoir Rock Interactions for Wellbore Integrity in CO2 Geological Storage
Abstract
:1. Introduction
2. Methodology
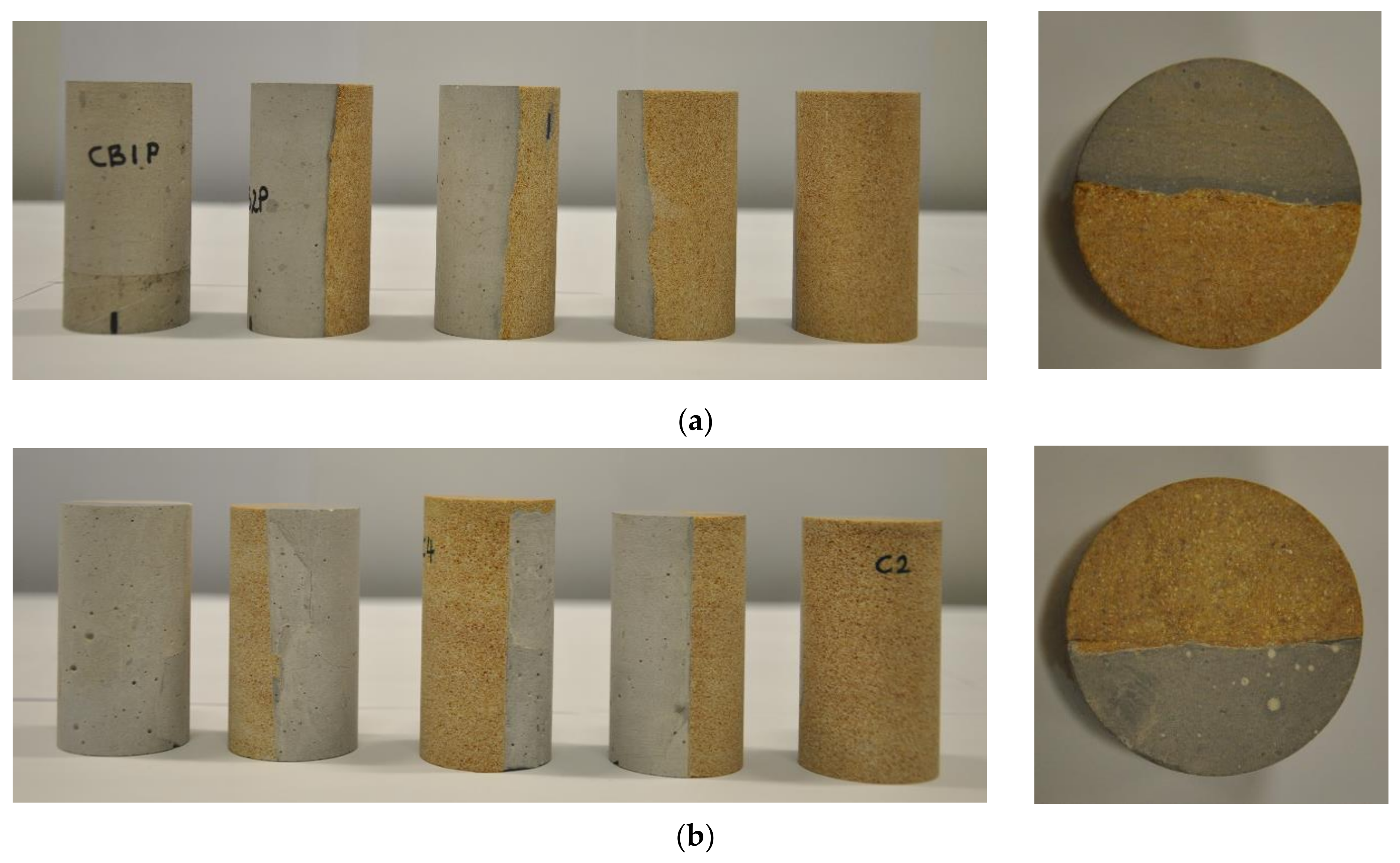
2.1. Cement–Reservoir Rock Composite Samples and Fluid Preparation
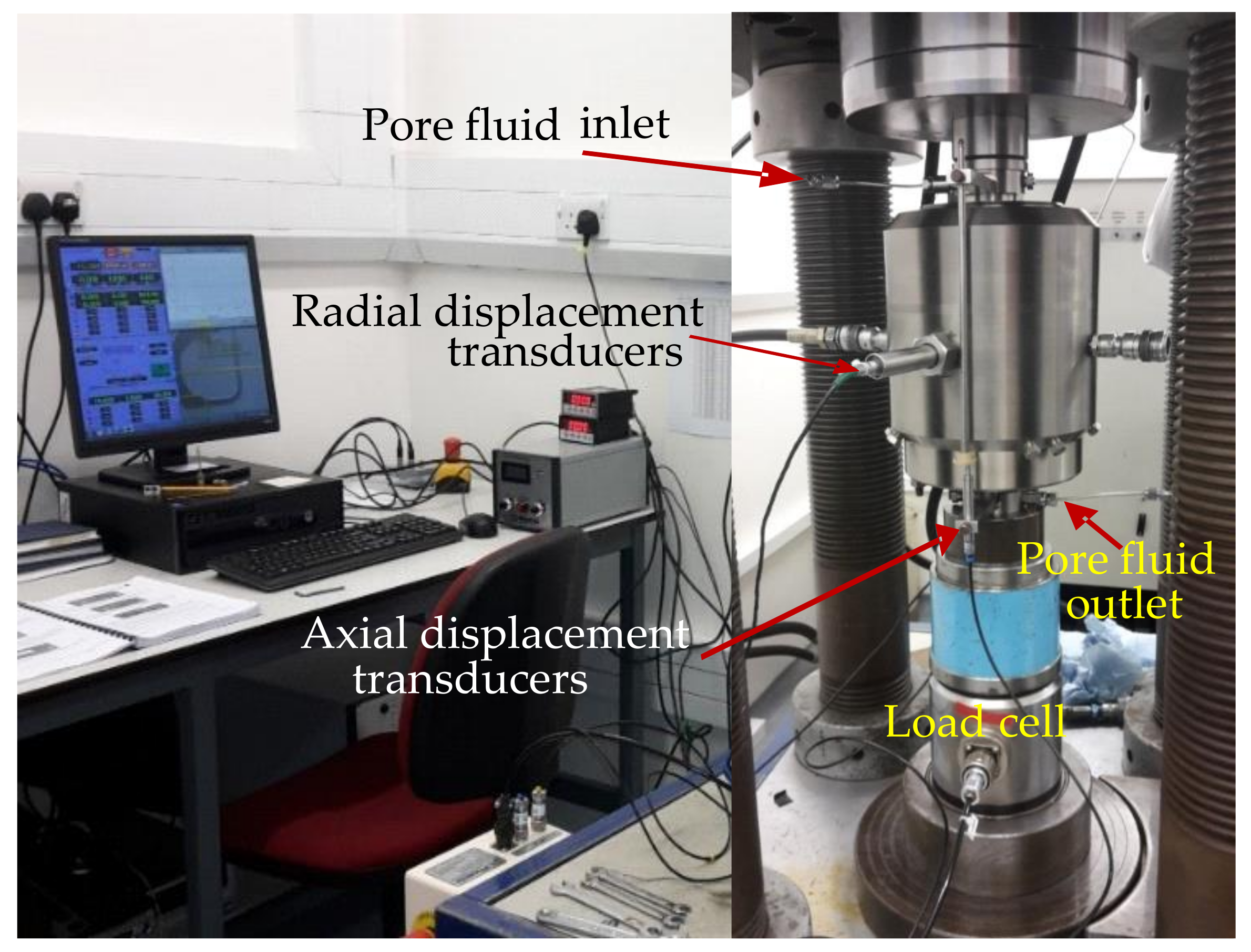
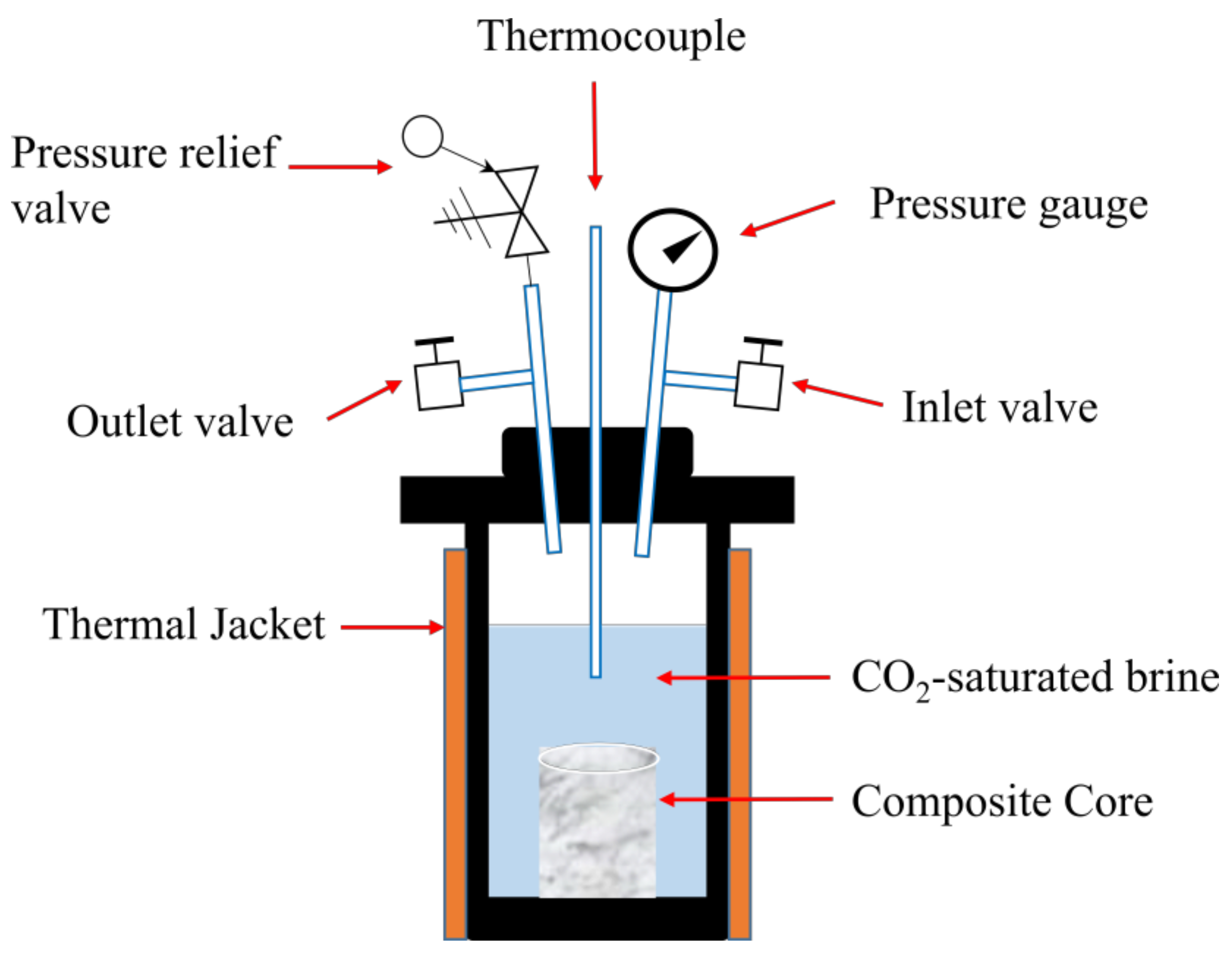
2.2. Hydrothermal Testing Experiments
3. Results and Discussion
3.1. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
3.2. XRD Analysis
3.2.1. Cement
3.2.2. Sandstone
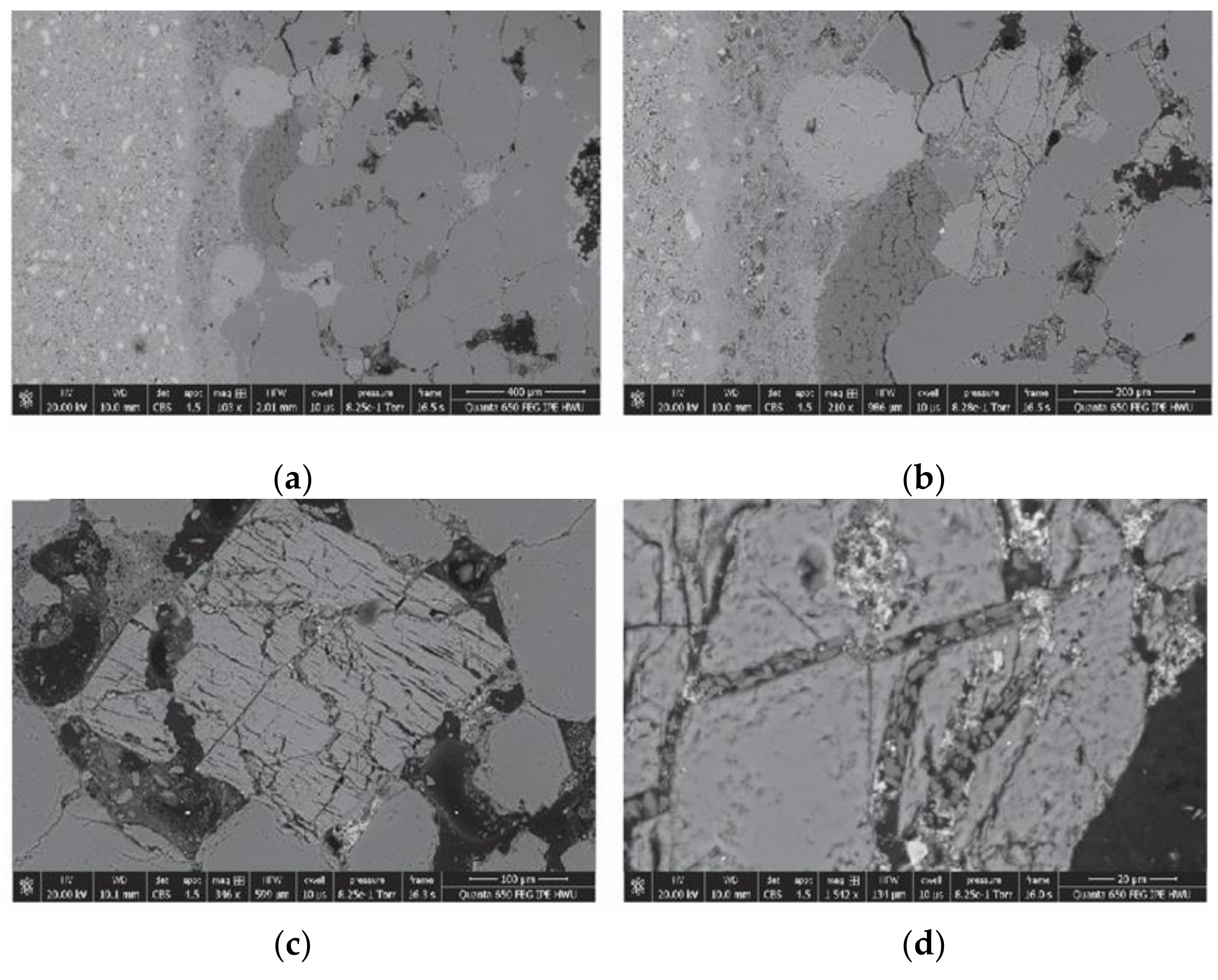
3.3. SEM–EDX Analysis
3.3.1. Cement
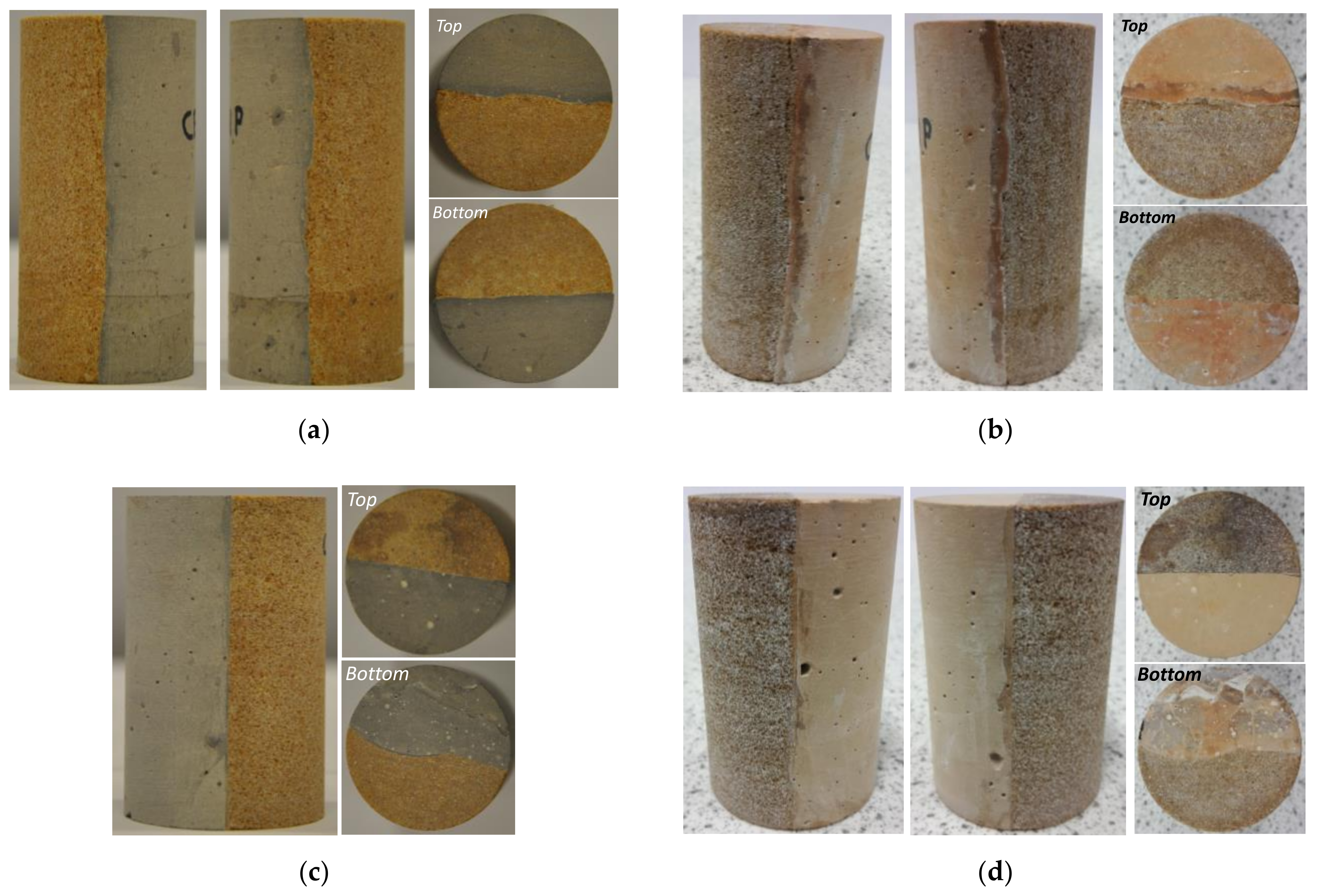
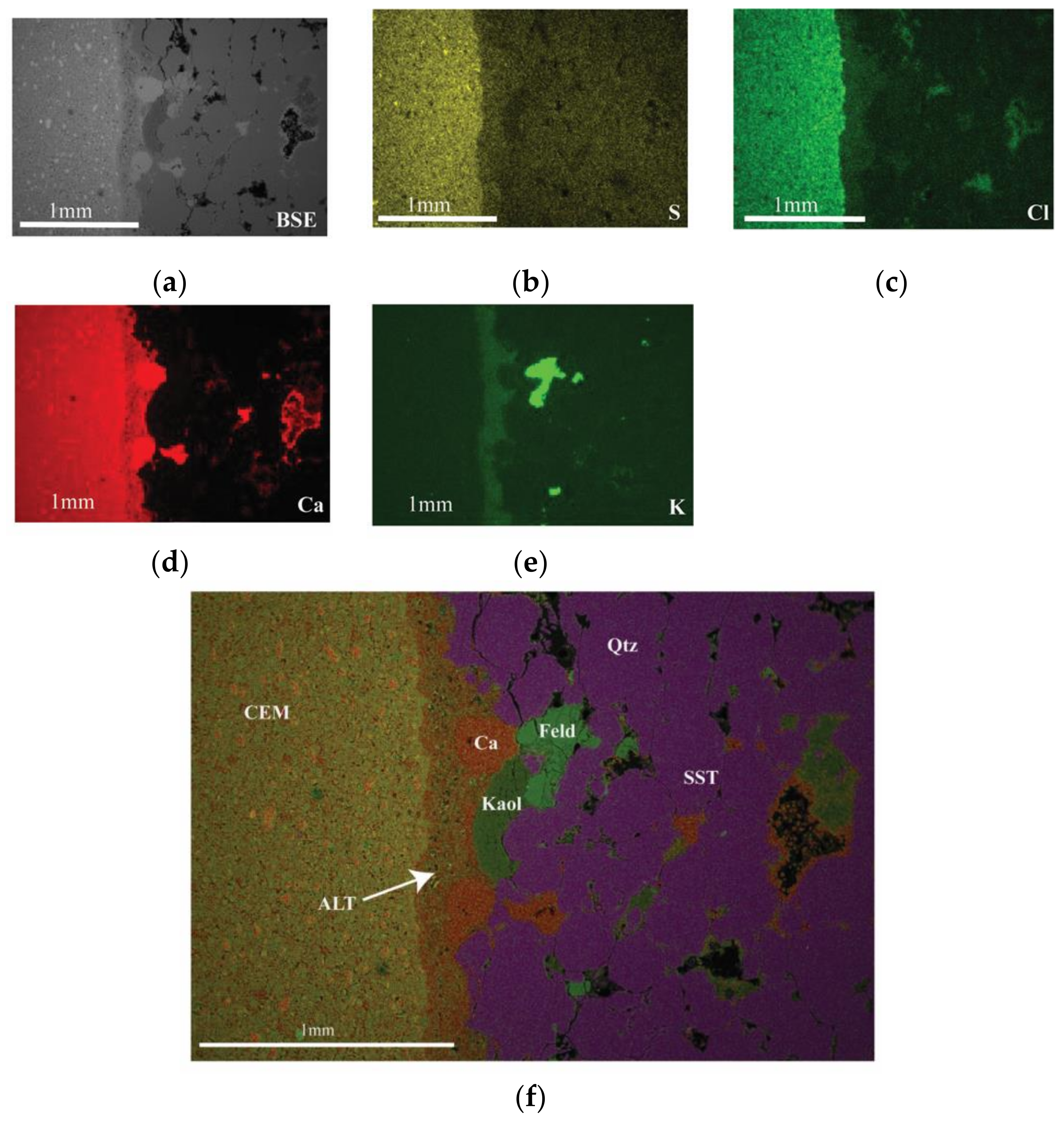
3.3.2. Cement–Sandstone Interface
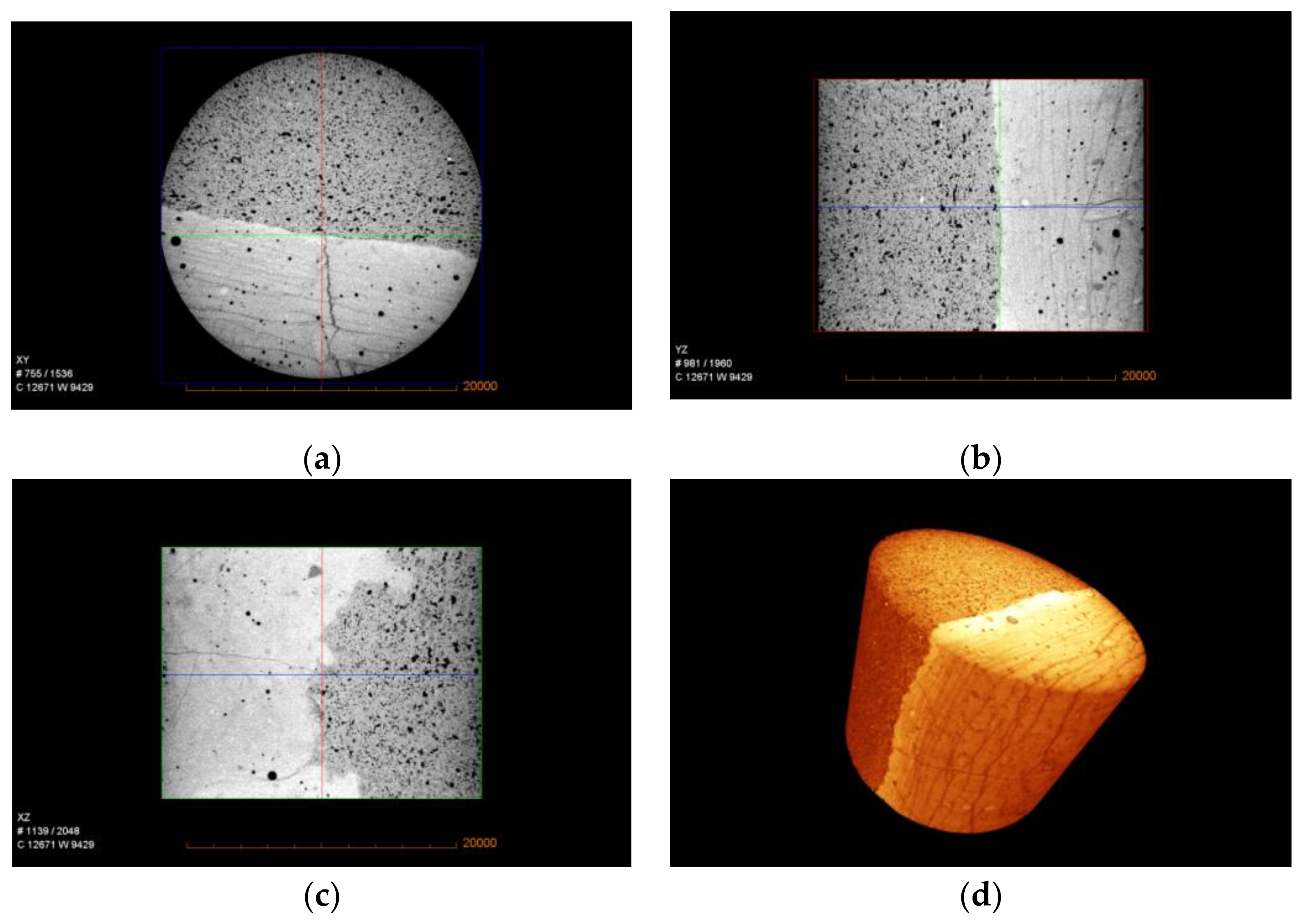
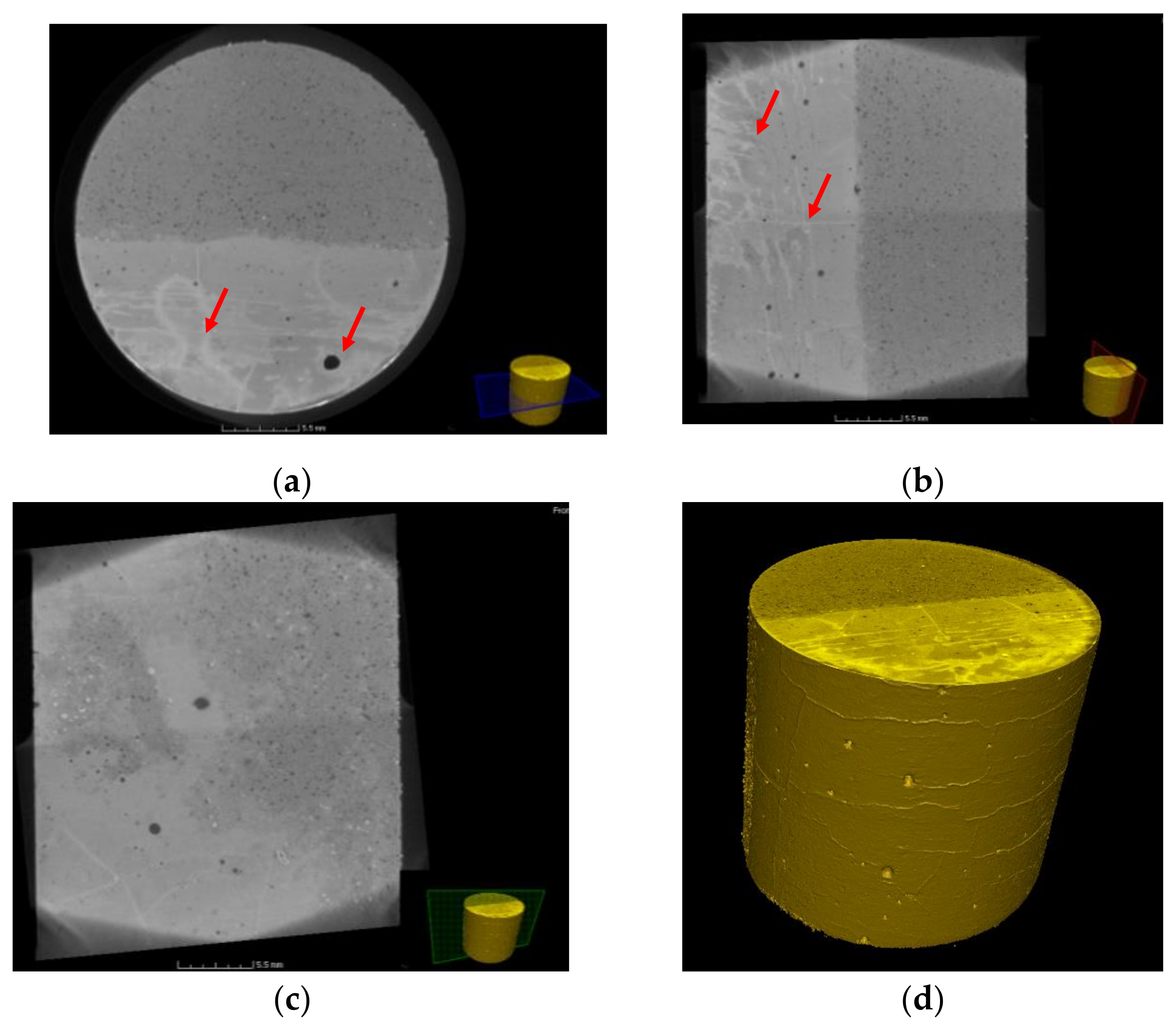
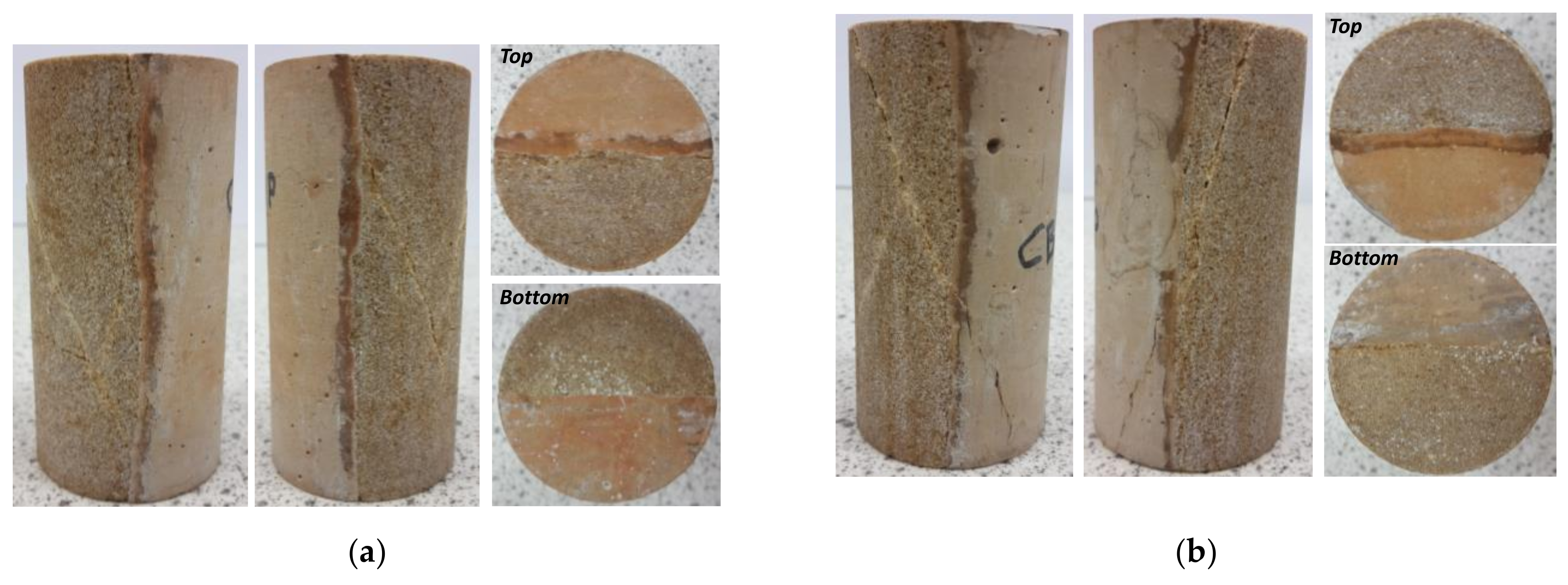
3.4. X-Ray µ-CT Image Analysis
3.5. Changes in Petrophysical and Geomechanical Properties of the Cement–Sandstone Composite Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-Dimensional |
| 3D | Three-Dimensional |
| CCUS | Carbon dioxide Capture, Utilisation, and Storage |
| X-ray µ-CT | X-ray Microcomputed Tomography |
| XRD | X-ray Diffraction |
| SEM–EDX | Scanning Electron Microscopy–Energy-Dispersive X-ray |
| ICP | Inductively Coupled Plasma |
| ICP-OES | Inductively Coupled Plasma-Optical Emission Spectrometry |
| HPHT | High-Pressure and High-Temperature |
| PRV | Pressure-Relief Valve |
| PTFE | Polytetrafluoroethylene |
| BSE | Backscattered Electron |
| CEM | Cement |
| SST | Sandstone |
| ALT | Altered cement zones |
| Qtz | Quartz |
| Feld | Feldspar |
| Kaol | Kaolinite |
References
- Zhang, M.; Bachu, S. Review of integrity of existing wells in relation to CO2 geological storage: What do we know? Int. J. Greenh. Gas Control 2011, 5, 826–840. [Google Scholar] [CrossRef]
- Iyer, J.; Walsh, S.D.C.; Hao, Y.; Carroll, S.A. Assessment of two-phase flow on the chemical alteration and sealing of leakage pathways in cemented wellbores. Int. J. Greenh. Gas Control 2018, 69, 72–80. [Google Scholar] [CrossRef]
- Onishi, T.; Nguyen, M.C.; Carey, J.W.; Will, B.; Zaluski, W.; Bowen, D.W.; Devault, B.C.; Duguid, A.; Zhou, Q.; Fairweather, S.H.; et al. Potential CO2 and brine leakage through wellbore pathways for geologic CO2 sequestration using the National Risk Assessment Partnership tools: Application to the Big Sky Regional Partnership. Int. J. Greenh. Gas Control 2019, 81, 44–65. [Google Scholar] [CrossRef]
- Bagheri, M.; Shariatipour, S.M.; Ganjian, E. A Review of oil well cement alteration in CO2-rich environments. Constr. Build. Mater. 2018, 186, 946–968. [Google Scholar] [CrossRef]
- Watson, T.L.; Bachu, S. Evaluation of the potential for gas and CO2 leakage along wellbores. SPE Drill. Complet. 2009, 24, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Gasda, S.E.; Bachu, S.; Celia, M.A. Spatial characterization of the location of potentially leaky wells penetrating a deep saline aquifer in a mature sedimentary basin. Environ. Geol. 2004, 46, 707–720. [Google Scholar] [CrossRef]
- Celia, M.A.; Bachu, S.; Nordbotten, J.M.; Gasda, S.E.; Dahle, H.K. Quantitative estimation of CO2 leakage from geological storage: Analytical models, numerical models, and data needs. In Proceedings of the 7th International Conference Greenhouse Gas Control Technologies, Vancouver, BC, Canada, 5–9 September 2004; pp. 663–671. [Google Scholar]
- Dalla Vecchia, F.; dos Santos, V.H.J.M.; Schütz, M.K.; Ponzi, G.G.D.; de Guimarães e Stepanha, A.S.; de Fraga Malfatti, C.; da Costa, E.M. Wellbore integrity in a saline aquifer: Experimental steel-cement interface degradation under supercritical CO2 conditions representative of Brazil’s Parana basin. Int. J. Greenh. Gas Control 2020, 98, 103077. [Google Scholar] [CrossRef]
- Sweatman, R.E.; Marsic, S.D.; McColpin, G.R. Advancements in technology and process approach reduce cost and increase performance of CO2-flow monitoring and remediation. In Proceedings of the SPE International Conference CO2 Capture, Storage and Utilization, Society of Petroleum Engineers, New Orleans, LA, USA, 10–12 November 2010; Volume 20. [Google Scholar] [CrossRef]
- Druckenmiller, M.L.; Maroto-Valer, M.M. Carbon sequestration using brine of adjusted pH to form mineral carbonates. Fuel Process. Technol. 2005, 86, 1599–1614. [Google Scholar] [CrossRef]
- Brunet, J.-P.L.; Li, L.; Karpyn, Z.T.; Kutchko, B.G.; Strazisar, B.; Bromhal, G. Dynamic evolution of cement composition and transport properties under conditions relevant to geological carbon sequestration. Energy Fuels 2013, 27, 4208–4220. [Google Scholar] [CrossRef]
- Carey, J.W. Geochemistry of wellbore integrity in CO2 sequestration: Portland cement-steel-brine-CO2 interactions. Rev. Mineral. Geochem. 2013, 77, 505–539. [Google Scholar] [CrossRef]
- Mainguy, M.; Ulm, F.-J. Coupled diffusion-dissolution around a fracture channel: The solute congestion phenomenon. Transp. Porous Media 2001, 45, 479–495. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Strazisar, B.R.; Dzombak, D.A.; Lowry, G.V.; Thauiow, N. Degradation of well cement by CO2 under geologic sequestration conditions. Environ. Sci. Technol. 2007, 41, 4787–4792. [Google Scholar] [CrossRef] [PubMed]
- Duguid, A.; Scherer, G.W. Degradation of oilwell cement due to exposure to carbonated brine. Int. J. Greenh. Gas Control 2010, 4, 546–560. [Google Scholar] [CrossRef]
- Walsh, S.D.C.; Du Frane, W.L.; Mason, H.E.; Carroll, S.A. Permeability of wellbore-cement fractures following degradation, by carbonated brine. Rock Mech. Rock Eng. 2013, 46, 455–464. [Google Scholar] [CrossRef]
- Richardson, M.G. Carbonation of Reinforced Concrete: Its Causes and Management; Citis: New York, NY, USA, 1988; 205p. [Google Scholar]
- Thomas, M.D.A.; Matthews, J.D. Carbonation of flyash concrete. Mag. Concr. Res. 1992, 44, 217–228. [Google Scholar] [CrossRef]
- Loo, Y.H.; Chin, M.S.; Tam, C.T.; Ong, K.C. A carbonation prediction model for acclerated carbonation testing of concrete. Mag. Concr. Res. 1994, 46, 191–200. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Strazisar, B.R.; Lowry, G.V.; Dzombak, D.; Thaulow, N. Rate of CO2 attack on hydrated class H well cement under geologic sequestration conditions. Environ. Sci. Technol. 2008, 42, 6237–6242. [Google Scholar] [CrossRef]
- Strazisar, B.; Kutchko, B.; Huerta, N. Chemical reactions of wellbore cement under CO2 storage conditions: Effects of cement additives. Energy Procedia 2009, 1, 3603–3607. [Google Scholar] [CrossRef] [Green Version]
- Walsh, S.D.C.; Mason, H.E.; Du Frane, W.L.; Carroll, S.A. Experimental calibration of a numerical model describing the alteration of cement/caprock interfaces by carbonated brine. Int. J. Greenh. Gas Control 2014, 22, 176–188. [Google Scholar] [CrossRef]
- Carey, J.W.; Svec, R.; Grigg, R.; Lichtner, P.C.; Zhang, J.; Crow, W. Wellbore integrity and CO2-brine flow along the casing-cement microannulus. Energy Procedia 2009, 1, 3609–3615. [Google Scholar] [CrossRef] [Green Version]
- Carey, J.W.; Wigand, M.; Chipera, S.J.; WoldeGabriel, G.; Pawar, R.; Lichtner, P.C.; Wehner, S.C.; Raines, M.A.; Guthrie, G.D., Jr. Analysis and performance of oil well cement with 30 years of CO2 exposure from the SACROC Unit, West Texas, USA. Int. J. Greenh. Gas Control 2007, 1, 75–85. [Google Scholar] [CrossRef]
- Crow, W.; Carey, J.W.; Gasda, S.; Williams, B.D.; Celia, M. Wellbore integrity analysis of a natural CO2 producer. Int. J. Greenh. Gas Control 2010, 4, 186–197. [Google Scholar] [CrossRef]
- Krilov, Z.; Loncaric, B.; Miksa, Z. Investigation of a long-term cement deterioration under a high-temperature, sour gas downhole environment. In Proceedings of the SPE International Symposium on Formation Damage Control, Society of Petroleum Engineers, Lafayette, LA, USA, 24–26 February 2000; Volume 9. [Google Scholar]
- Rochelle, C.A.; Milodowski, A.E. Carbonation of borehole seals: Comparing evidence from short-term laboratory experiments and long-term natural analogues. Appl. Geochem. 2013, 30, 161–177. [Google Scholar] [CrossRef] [Green Version]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Teldford: London, UK, 1997. [Google Scholar]
- Carroll, S.; Carey, J.W.; Dzombak, D.; Huerta, N.J.; Li, L.; Richard, T.; Um, W.; Walsh, S.D.C.; Zhang, L. Review: Role of chemistry, mechanics, and transport on well integrity in CO2 storage environments. Int. J. Greenh. Gas Control 2016, 49, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Carey, W.J.; Lichtner, P. Calcium Silicate Hydrate (C-S-H) Solid Solution Model Applied to Cement Degradation Using the Continuum Reactive Transport Model FLOTRAN. In Transport Properties and Concrete Quality: Materials Science of Concrete; Mobasher, B., Skalny, J., Eds.; American Ceramic Society: Columbus, OH, USA; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Special Volume, pp. 73–106. [Google Scholar]
- Mason, H.E.; Du Frane, W.L.; Walsh, S.D.C.; Dai, Z.; Charnvanichborikarn, S.; Carroll, S.A. Chemical and mechanical properties of wellbore cement altered by CO2-rich brine using a multianalytical approach. Environ. Sci. Technol. 2013, 47, 1745–1752. [Google Scholar] [CrossRef]
- Carey, W.J.; Svec, R.; Grigg, R.; Zhang, J.; Crow, W. Experimental investigation of wellbore integrity and CO2–brine flow along the casing–cement microannulus. Int. J. Greenh. Gas Control 2010, 4, 272–282. [Google Scholar] [CrossRef]
- Wolterbeek, T.K.T.; Hangx, S.J.T.; Spiers, C.J. Effect of CO2-induced reactions on the mechanical behaviour of fractured wellbore cement. Geomech. Energy Environ. 2016, 7, 26–46. [Google Scholar] [CrossRef]
- Dusseault, M.B.; Gray, M.N.; Nawrocki, P.A. Why oilwells leak: Cement behaviour and long-term consequences. In Proceedings of the International Oil and Gas Conference and Exhibition in China, Society of Petroleum Engineers, Beijing, China, 7–10 November 2000. [Google Scholar]
- Ozyurtkan, M.H.; Radonjic, M. An experimental study of the effect of CO2 rich brine on artificially fractured well-cement. Cem. Concr. Compos. 2014, 45, 201–208. [Google Scholar] [CrossRef]
- Liteanu, E.; Spiers, C.J.; Peach, C.J. Failure behaviour wellbore cement in the presence of water and supercritical CO2. Energy Procedia 2009, 1, 3553–3560. [Google Scholar] [CrossRef] [Green Version]
- Onan, D.D. Effects of supercritical carbon dioxide on well cements. In Proceedings of the Permian Basin Oil and Gas Recovery Conference, Society of Petroleum Engineers, Midland, TX, USA, 8–9 March 1984; Volume 14. [Google Scholar]
- Šauman, Z. Effect of CO2 on porous concrete. Cem. Concr. Res. 1972, 2, 541–549. [Google Scholar] [CrossRef]
- Gervais, C.; Garrabrants, A.C.; Sanchez, F.; Barna, R.; Moszkowicz, P.; Kosson, D.S. The effects of carbonation and drying during intermittent leaching on the release of inorganic constituents from a cement-based matrix. Cem. Concr. Res. 2005, 34, 119–131. [Google Scholar] [CrossRef]
- Lea, F.M. The Chemistry of Cement and Concrete; Edward Arnold: London, UK, 1970. [Google Scholar]
- Robins, N.S.; Milodowski, A.E. Borehole cements and the downhole environment: A review. Q. J. Eng. Geol. Hydrogeol. 1986, 19, 175–181. [Google Scholar] [CrossRef]
- Claisse, P.A.; El-Sayad, H.I.; Shaaban, I.G. Permeability and pore volume of carbonated concrete. ACI Mater. J. 1999, 96, 378–381. [Google Scholar]
- Chi, J.M.; Huang, R.; Yang, C.C. Effects of carbonation on mechanical properties and durability of concrete using accelerated testing method. J. Mar. Sci. Technol. 2002, 10, 14–20. [Google Scholar]
- Boukhelifa, L.; Moroni, N.; James, S.G.; Le Roy-Delage, S.; Thiercelin, M.J.; Lemaire, G. Evaluation of cement systems for oil- and gas-well zonal isolation in a full-scale annular geometry. SPE Drill. Complet. 2005, 20, Paper SPE-87195-PA. [Google Scholar] [CrossRef]
- Syed, A.; Shi, J.-Q.; Durucan, S.; Korre, A.; Nash, G. Experimental and numerical investigations into CO2 interactions with well infrastructure and its impact on long term well integrity. Energy Procedia 2014, 63, 5707–5714. [Google Scholar] [CrossRef]
- van Eijden, J.; Cornelissen, E.; Ruckert, F.; Wolterbeek, T. Development of experimental equipment and procedures to evaluate zonal isolation and well abandonment materials. In Proceedings of the SPE/IADC Drilling Conference and Exhibition, Society of Petroleum Engineers, The Hague, The Netherlands, 14–16 March 2017. SME/IADC-184640-MS. [Google Scholar] [CrossRef]
- Wolterbeek, T.K.T.; Ruckert, F.; van Moorsel, S.G.; Cornelissen, E.K. Reactive transport and permeability evolution in wellbore defects exposed to periodic pulses of CO2-rich water. Int. J. Greenh. Gas Control 2019, 91, 102835. [Google Scholar] [CrossRef]
- Rhino, K.; Iyer, J.; Walsh, S.D.C.; Carroll, S.A.; Smith, M.M. Influence of effective stress and transport on mechanical and chemical alteration processes at the Cement-Caprock interface. Int. J. Greenh. Gas Control 2021, 109, 103340. [Google Scholar] [CrossRef]
- Jung, H.B.; Kabilan, S.; Carson, J.P.; Kuprat, A.P.; Um, W.; Martin, P.; Michael, D.; Kafentzis, T.; Varga, T.; Stephens, S.; et al. Wellbore cement fracture evolution at the cement–basalt caprock interface during geologic carbon sequestration. Appl. Geochem. 2014, 47, 1–16. [Google Scholar] [CrossRef]
- American Petroleum Institute. Specification for Cements and Materials for Well Cementing, 25th ed.; API Spec 10A; American Petroleum Institute (API): Washington, DC, USA, 2019. [Google Scholar]
- Kovari, K.; Tisa, A.; Einstein, H.H.; Franklin, J.A. Suggested methods for determining the strength of rock materials in triaxial compression: Revised version. Int. J. Rock Mech. Min. Sci. Geomech. Abstr. 1983, 20, 285–290. [Google Scholar]
- Brown, E.T. Rock Characterization, Testing & Monitoring: ISRM Suggested Methods; Pergamon Press: Oxford, UK, 1981. [Google Scholar]
- Steal, L. An Investigation of CO2-Brine Interactions Using North Sea Oil Well Data with Respect to Geological Storage of Carbon Dioxide and Enhanced Oil Recovery. Ph.D. Thesis, Heriot-Watt University, Edinburgh, UK, 2017. [Google Scholar]
- Farooqui, N.M.; Liu, Q.; Maroto-Valer, M.M.; Hadi Mosleh, M.; Korre, A.; Durucan, S. Understanding CO2-brine-wellbore cement-rock interactions for CO2 storage. Energy Procedia 2017, 114, 5206–5211. [Google Scholar] [CrossRef]
- Mito, S.; Xue, Z.; Sato, H. Experimental assessment of well integrity for CO2 geological storage: Batch experimental results on geochemical interactions between a CO2–brine mixture and a sandstone–cement–steel sample. Int. J. Greenh. Gas Control 2015, 39, 420–431. [Google Scholar] [CrossRef]
- Carroll, S.A.; McNab, W.W.; Torres, S.C. Experimental study of cement-sandstone/shale-brine-CO2 interactions. Geochem. Trans. 2011, 12, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkowitz, B. Characterizing flow and transport in fractured geological media: A review. Adv. Water Resour. 2002, 25, 861–884. [Google Scholar] [CrossRef]


| Cement Phase Dissolution | Reaction |
|---|---|
| Portlandite | Ca(OH)2 + 2H+ ↔ Ca2+ + 2H2O (Ca(OH)2(s) + 2H+ + CO3−2 CaCO3 (s) + 2H2O) |
| Calcium silicate hydrate | Ca1.2SiO3.2·2.06H2O + 2.4H+ ↔ 1.2Ca2+ + SiO2+3.26H2O |
| Monosulfate | Ca4Al2O6(SO4)·12H2O + 12H+ ↔ 4Ca2+ + 2Al3+ + SO42− + 18H2O |
| Trisulfate | Ca6Al2(SO4)3(OH)12·26H2O + 12H+ ↔ 6Ca2+ + 2Al3+ + 3SO42− + 38H2O |
| Calcium aluminate hydrate | Ca3Al2(OH)12 + 12H+ ↔ 3Ca2+ + 2Al3+ + 12H2O |
| Sample Type | He Porosity (−) | N2 Permeability (×10−15 m2) | Young’s Modulus (GPa) | Poisson’s Ratio |
|---|---|---|---|---|
| Cement–sandstone composites | 0.12 | 4.12/0.92 * | 20.47 | 0.31 |
| Sandstone | 0.08 | 10.32 | 18.96 | 0.24 |
| Portland cement | 0.03 | 0.15 | 12.13 | 0.36 |
| Species | Ca | K | Na | Mg | Ba | Sr |
|---|---|---|---|---|---|---|
| Concentration (ppm) | 612 | 303 | 5433 | 63 | 281 | 54 |
| Composite Sample | Ba | Ca | K | Mg | Na | S | Si | Sr | Fe |
|---|---|---|---|---|---|---|---|---|---|
| (ppm) | |||||||||
| Unreacted brine | 281 | 612 | 303 | 63 | 5433 | Not detected | Not detected | 54.0 | Not detected |
| CB3P (3 months) | 1 | 80 | 2362 | 24 | 2994 | 0 | 0 | 1 | 0 |
| CB4P (3 months) | 2 | 57 | 2605 | 28 | 3110 | 0 | 0 | 2 | 0 |
| CB1P (11 months) | 4 | 365 | 1689 | 19 | 6178 | 305 | 0 | 12 | 47 |
| CB2P (11 months) | 7 | 330 | 1700 | 18 | 6494 | 256 | 0 | 9 | 66 |
| C3 (3 months) | 3 | 68 | 1848 | 45 | 4416 | 0 | 0 | 1 | 0 |
| C1 (11 months) | 6 | 274 | 1332 | 11 | 6699 | 97 | 0 | 10 | 26 |
| C2 (11 months) | 1 | 80 | 1288 | 21 | 8214 | 141 | 0 | 12 | 29 |
| Sample No. | He Porosity (%) | N2 Permeability (×10−15 m2) | Young’s Modulus (GPa) | Poisson’s Ratio | ||
|---|---|---|---|---|---|---|
| Before HPHT Experiment | After HPHT Experiment | Before HPHT Experiment | After HPHT Experiment | After HPHT Experiment | After HPHT Experiment | |
| CB1P | 0.10 | 0.12 | 6.67 | 92.08 | 15.18 | 0.29 |
| CB2P | 0.09 | 0.10 | 5.17 | 44.23 | 14.62 | 0.20 |
| C1 | 0.12 | 0.11 | 0.68 | 3.33 | 14.47 | 0.33 |
| C2 | 0.14 | 0.11 | 0.93 | 7.30 | 16.82 | 0.27 |
| Mean baseline mechanical properties before treatment with CO2-rich brine in HPHT reactors (from Table 2) | ||||||
| 20.47 | 0.31 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahanbakhsh, A.; Liu, Q.; Hadi Mosleh, M.; Agrawal, H.; Farooqui, N.M.; Buckman, J.; Recasens, M.; Maroto-Valer, M.; Korre, A.; Durucan, S. An Investigation into CO2–Brine–Cement–Reservoir Rock Interactions for Wellbore Integrity in CO2 Geological Storage. Energies 2021, 14, 5033. https://doi.org/10.3390/en14165033
Jahanbakhsh A, Liu Q, Hadi Mosleh M, Agrawal H, Farooqui NM, Buckman J, Recasens M, Maroto-Valer M, Korre A, Durucan S. An Investigation into CO2–Brine–Cement–Reservoir Rock Interactions for Wellbore Integrity in CO2 Geological Storage. Energies. 2021; 14(16):5033. https://doi.org/10.3390/en14165033
Chicago/Turabian StyleJahanbakhsh, Amir, Qi Liu, Mojgan Hadi Mosleh, Harshit Agrawal, Nazia Mubeen Farooqui, Jim Buckman, Montserrat Recasens, Mercedes Maroto-Valer, Anna Korre, and Sevket Durucan. 2021. "An Investigation into CO2–Brine–Cement–Reservoir Rock Interactions for Wellbore Integrity in CO2 Geological Storage" Energies 14, no. 16: 5033. https://doi.org/10.3390/en14165033
APA StyleJahanbakhsh, A., Liu, Q., Hadi Mosleh, M., Agrawal, H., Farooqui, N. M., Buckman, J., Recasens, M., Maroto-Valer, M., Korre, A., & Durucan, S. (2021). An Investigation into CO2–Brine–Cement–Reservoir Rock Interactions for Wellbore Integrity in CO2 Geological Storage. Energies, 14(16), 5033. https://doi.org/10.3390/en14165033










