Physicochemical Properties Enhancement of Biodiesel Synthesis from Various Feedstocks of Waste/Residential Vegetable Oils and Palm Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Pure Biodiesel Samples
2.2. Fuels
2.3. Evaluation of Kinematic Viscosity and Density
2.4. Evaluation of Oxidative Stability
2.5. Evaluation of Cloud Point and Pour Point
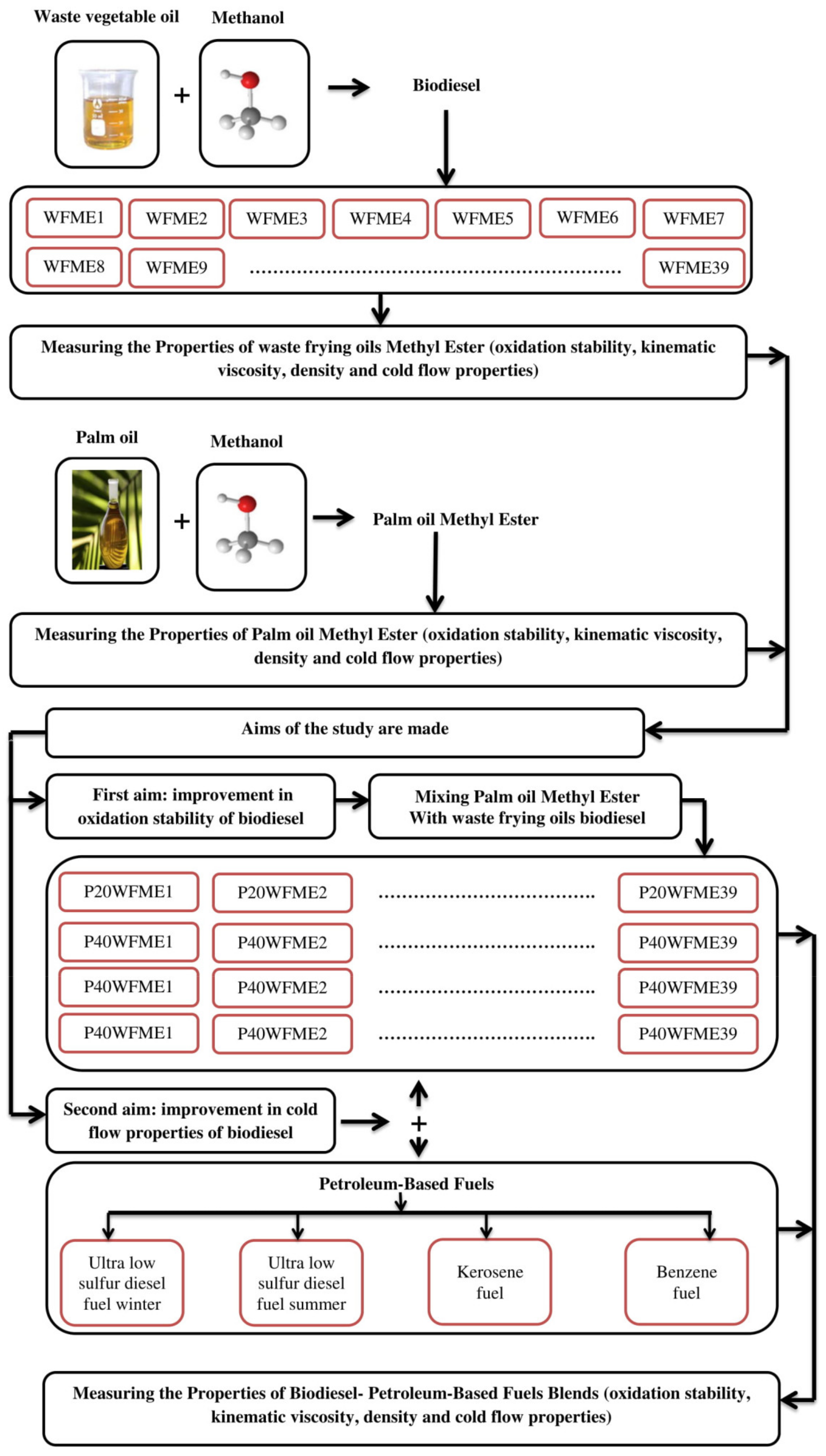
2.6. Preparation of Fuel Mixtures
3. Results
3.1. Characterization of Pure Biodiesel
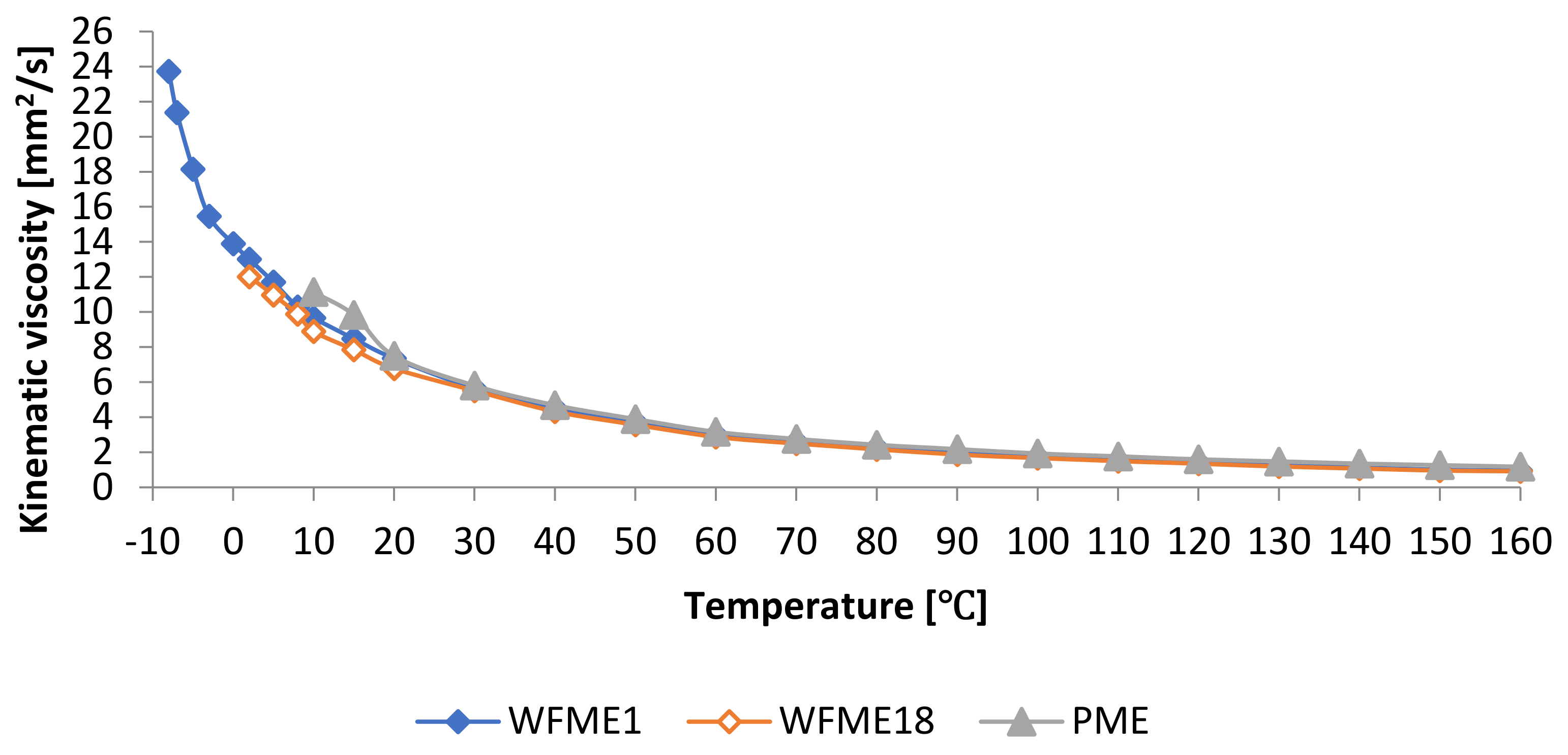
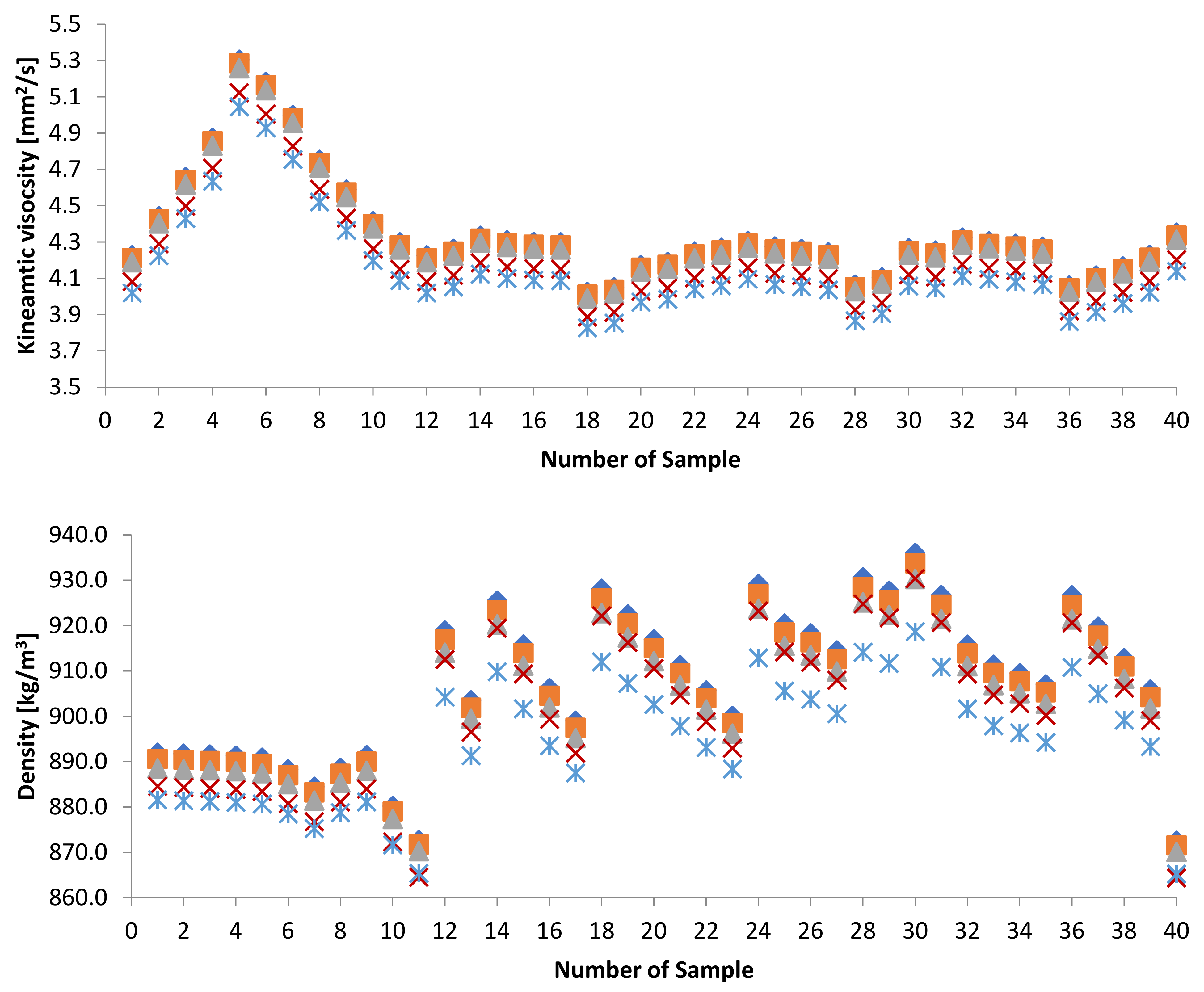
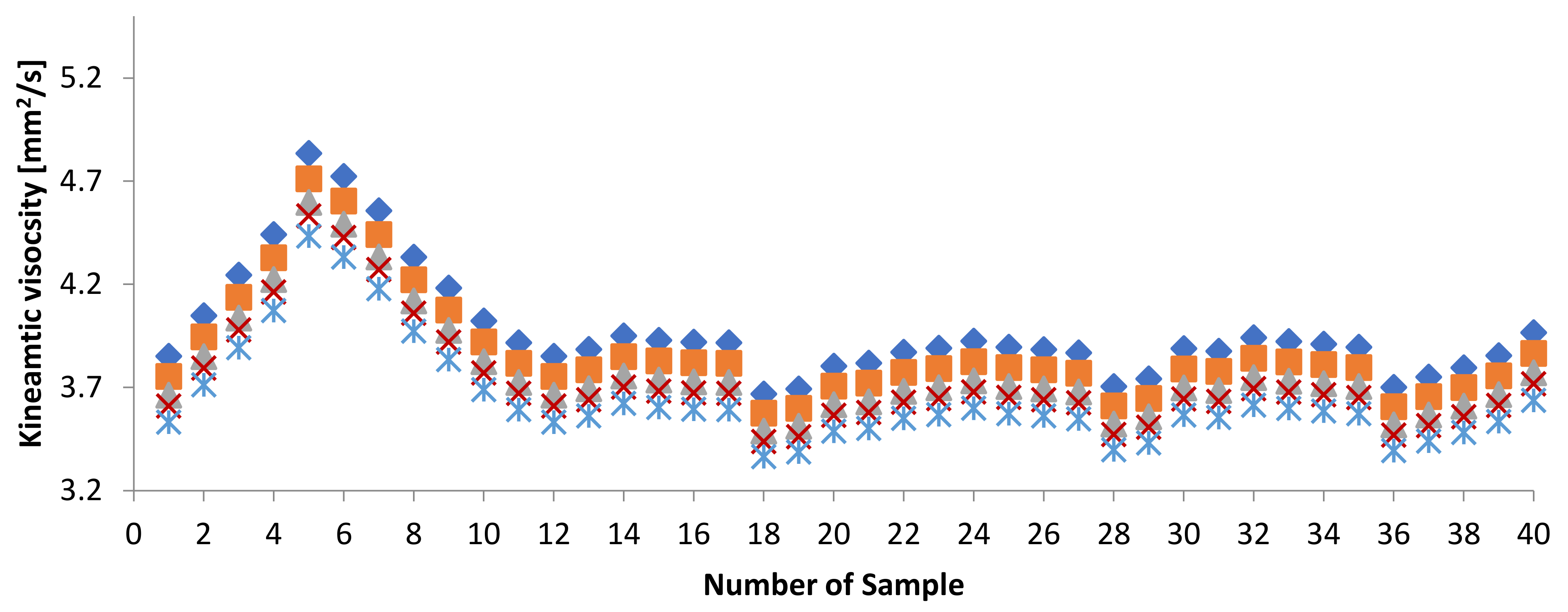
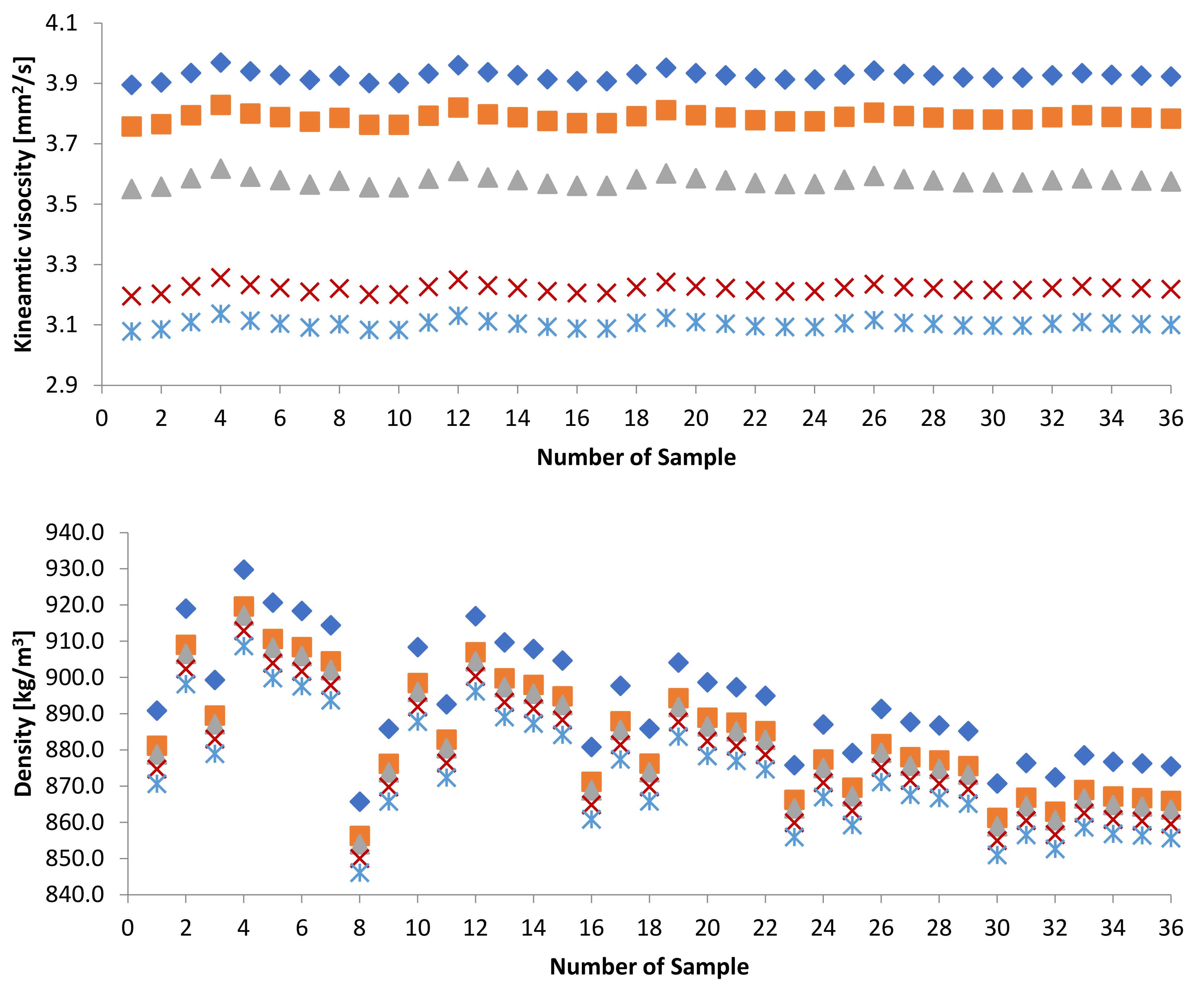
3.1.1. Kinematic Viscosity
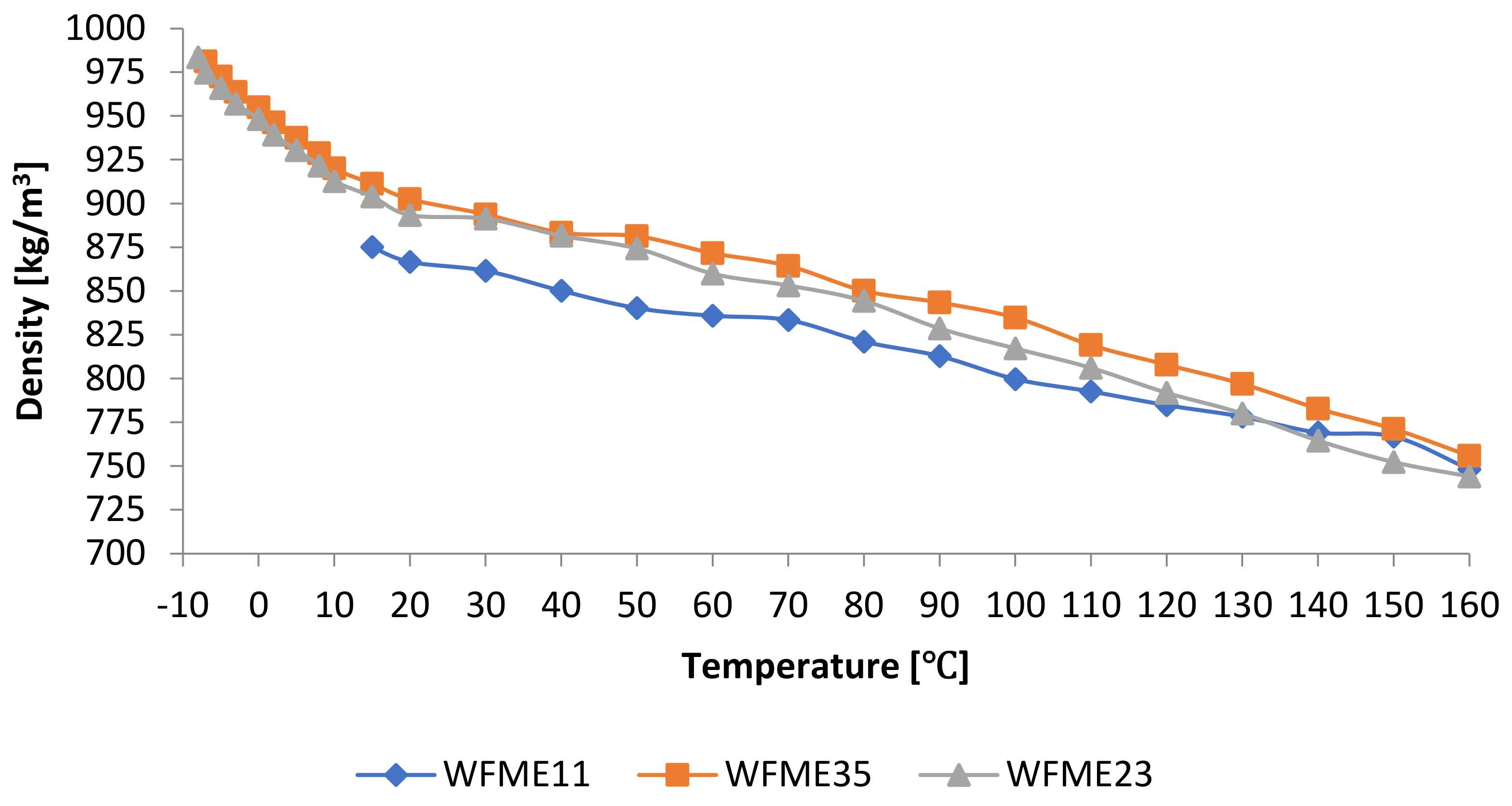
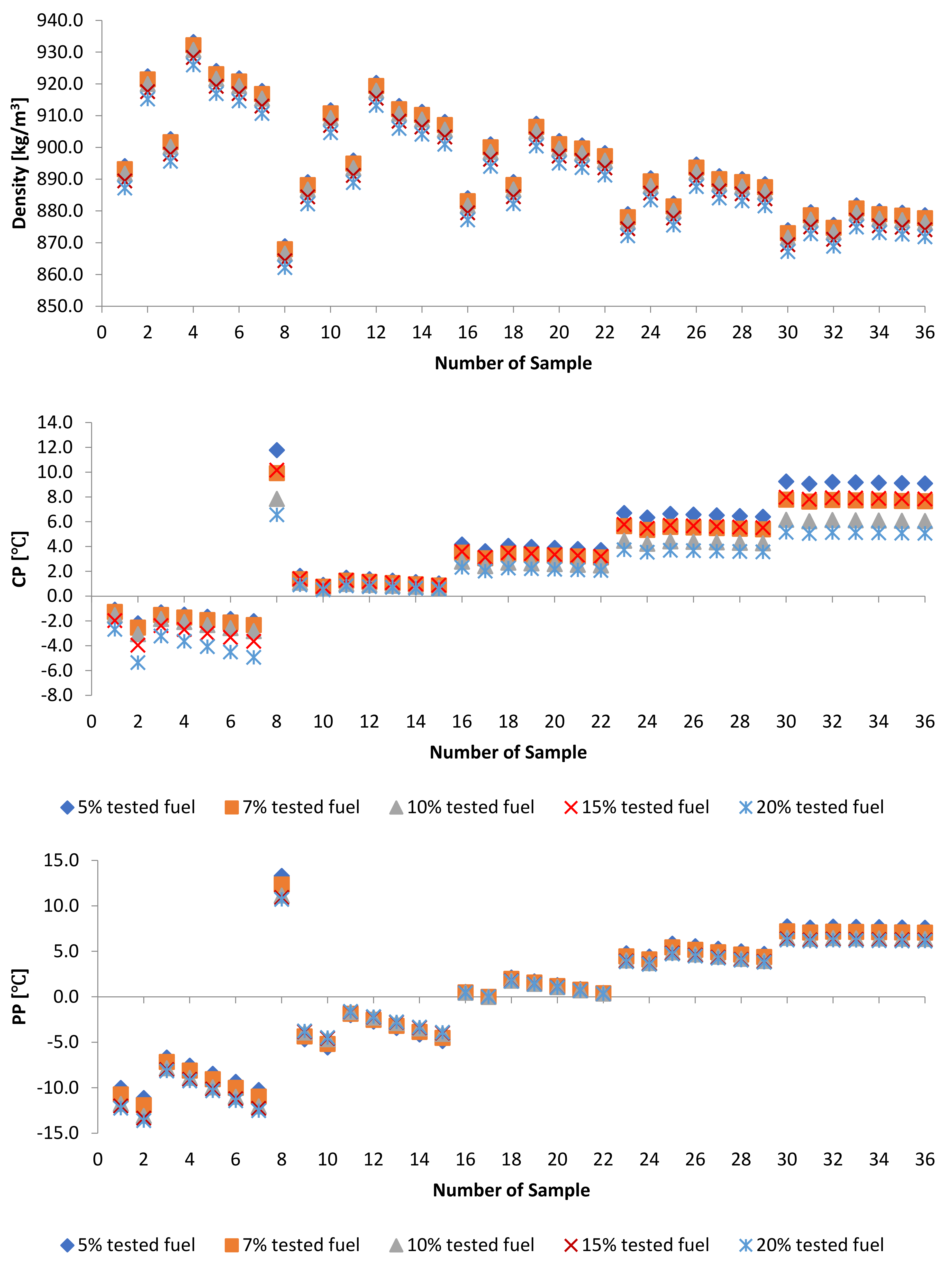
3.1.2. Density
3.1.3. Oxidation Stability
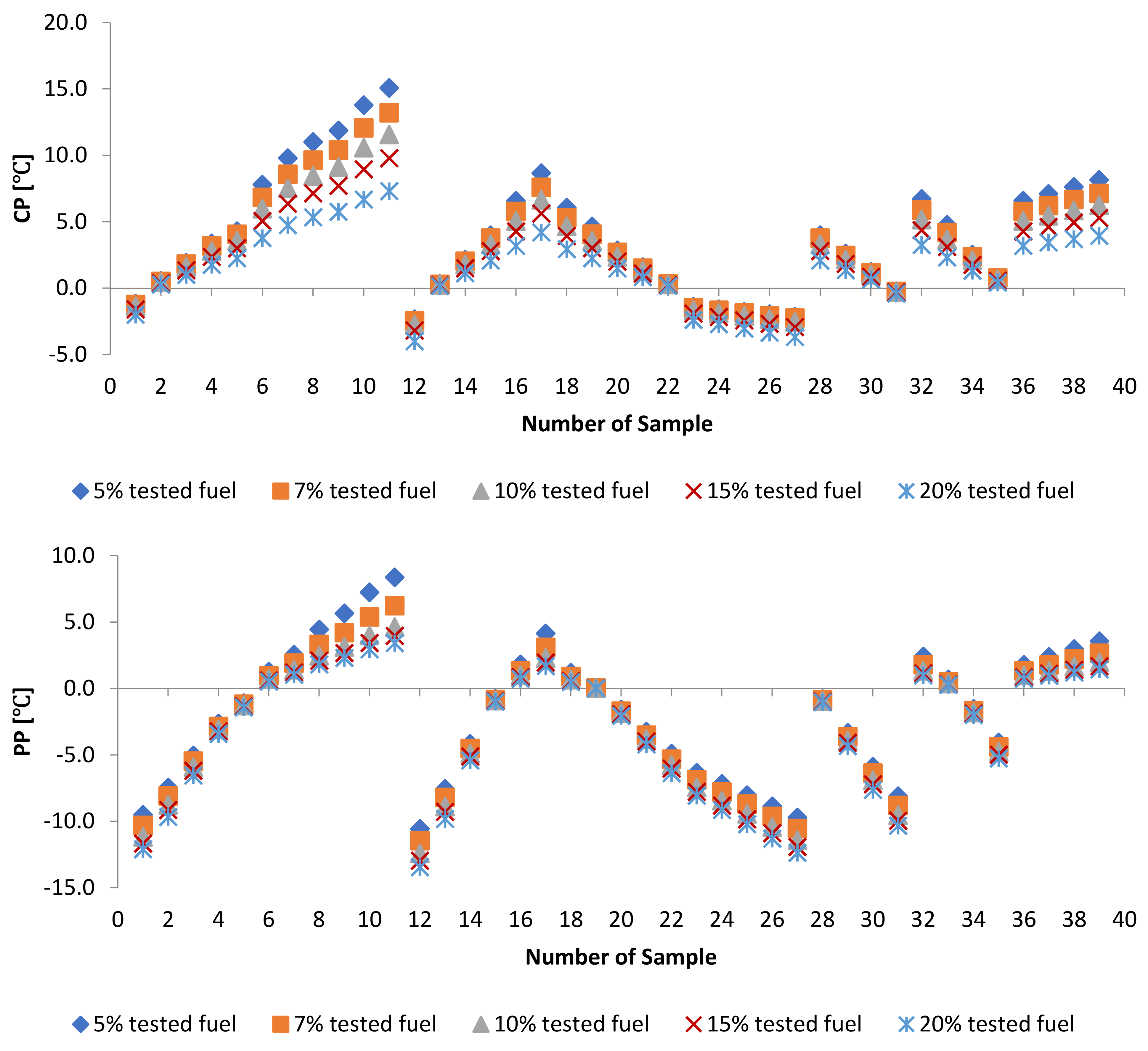
3.1.4. Cold Flow Properties
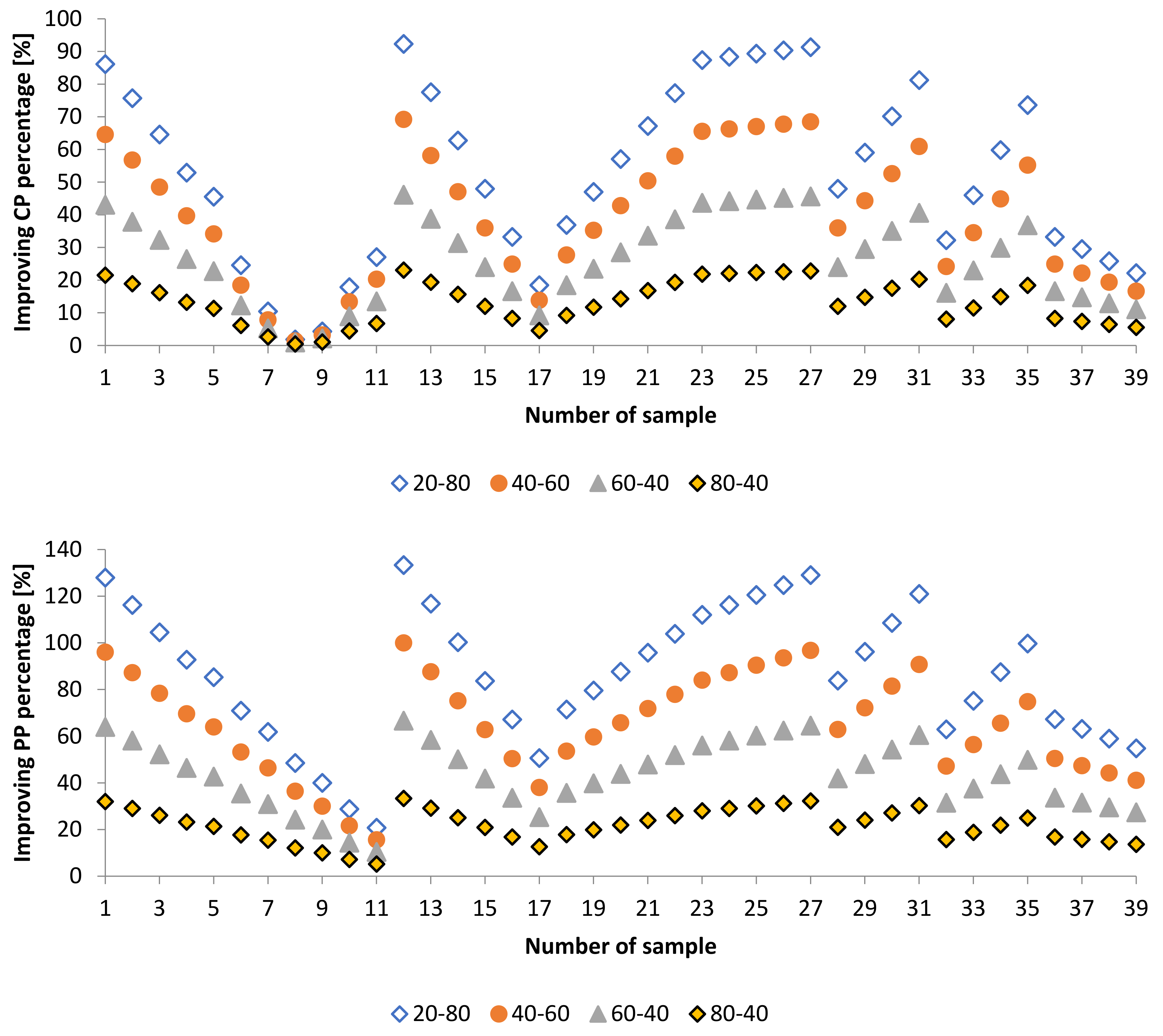
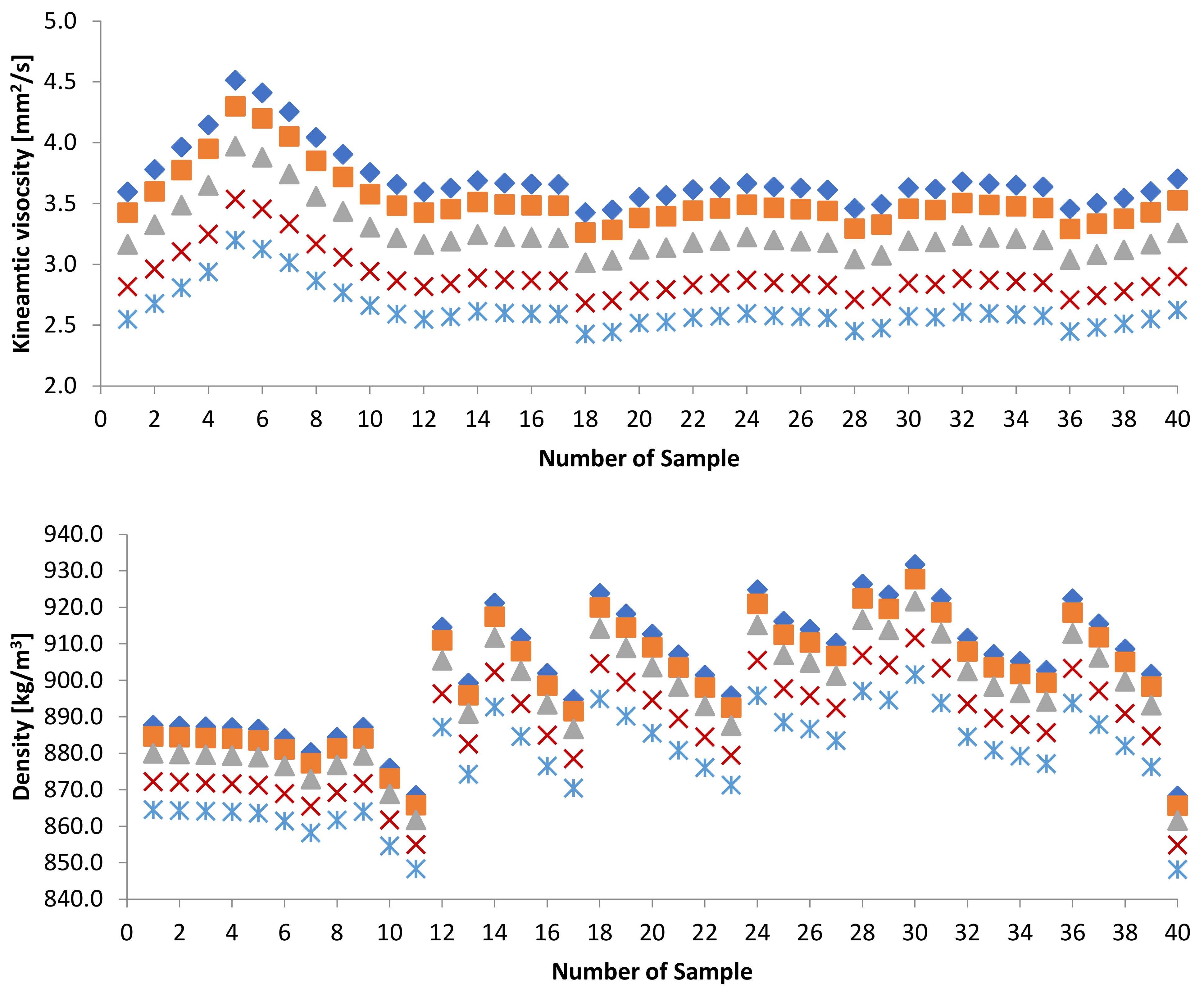
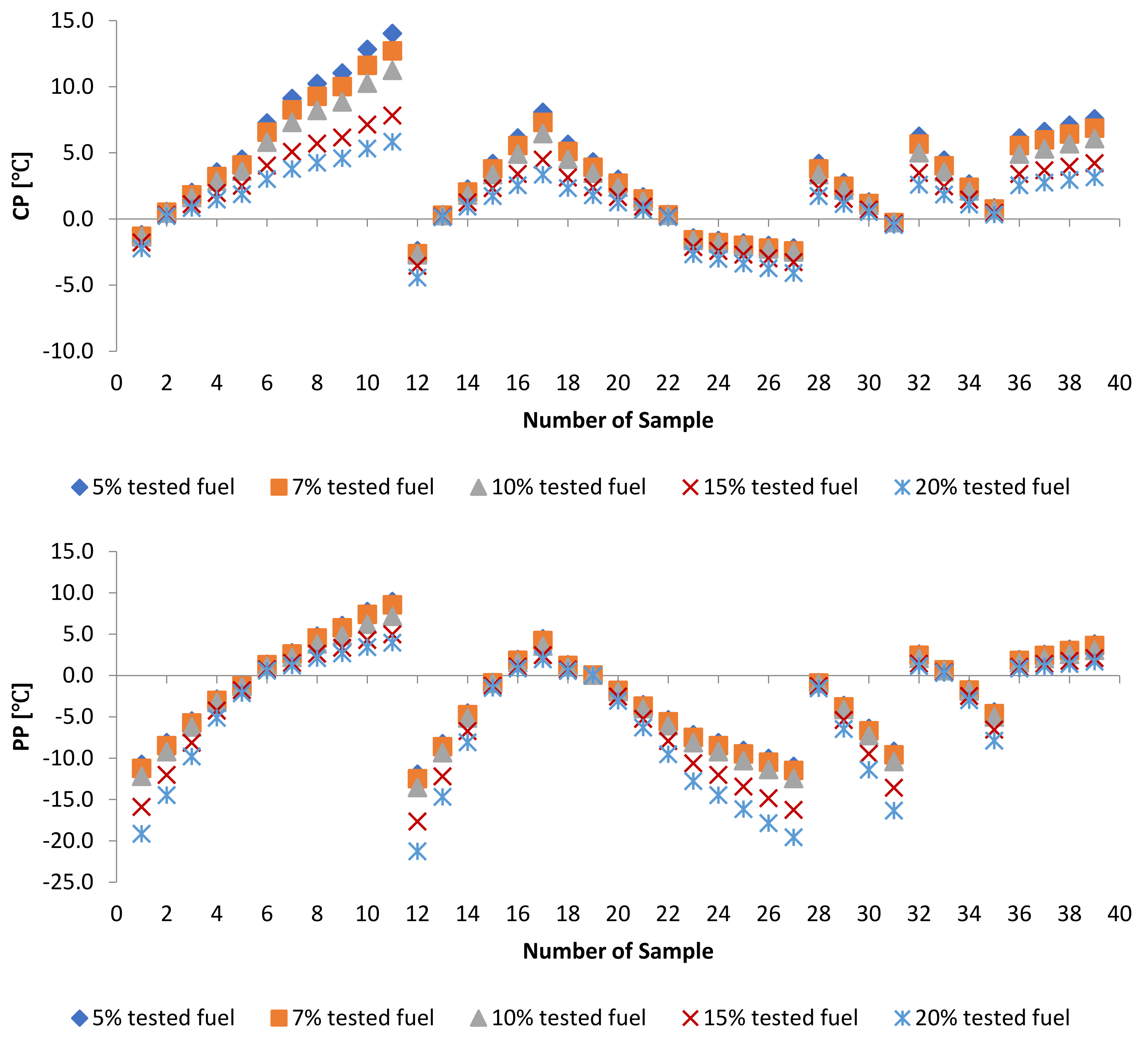
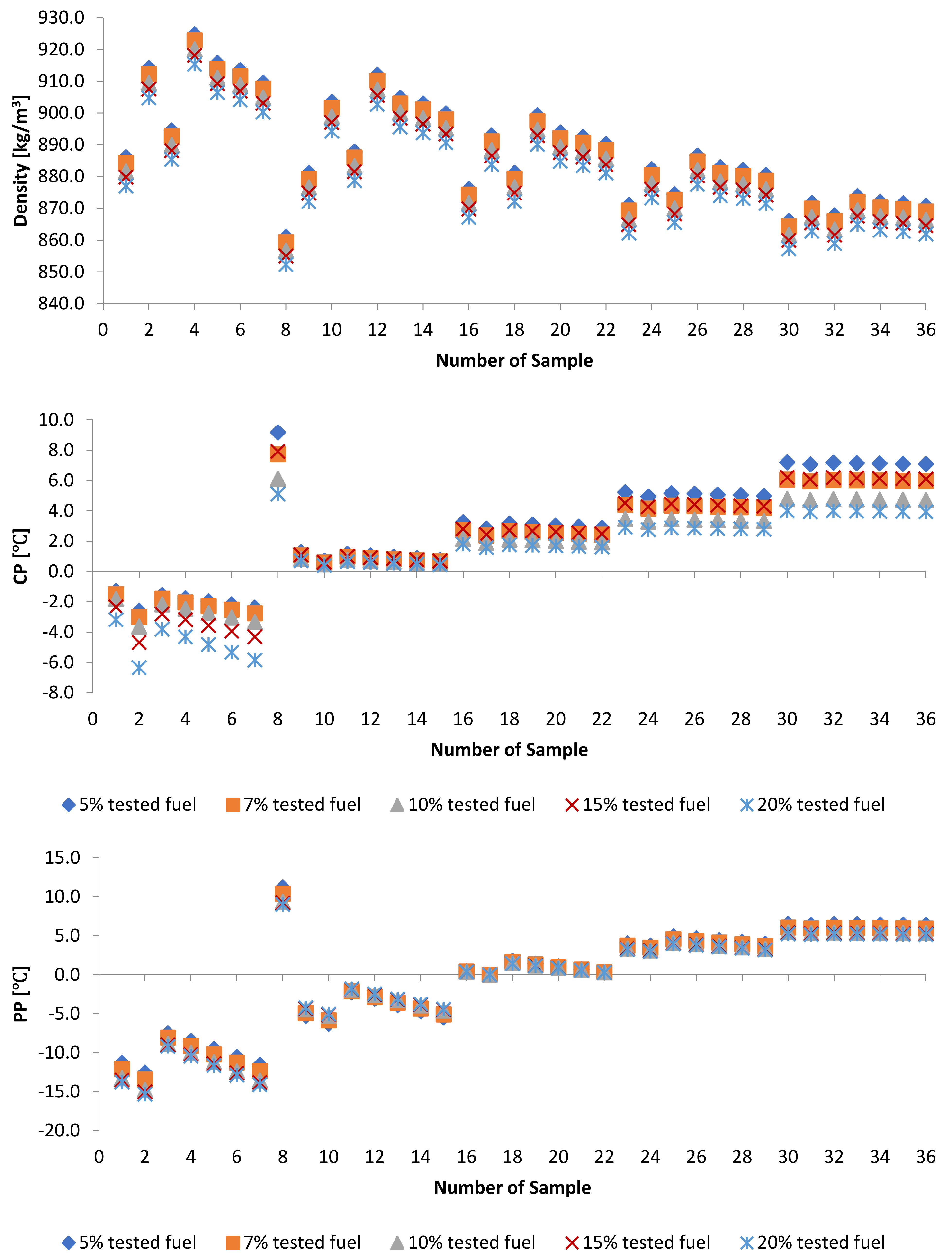
3.2. Effect of Blends of Palm Oil Biodiesel on Waste/Residential Frying Oil Biodiesel
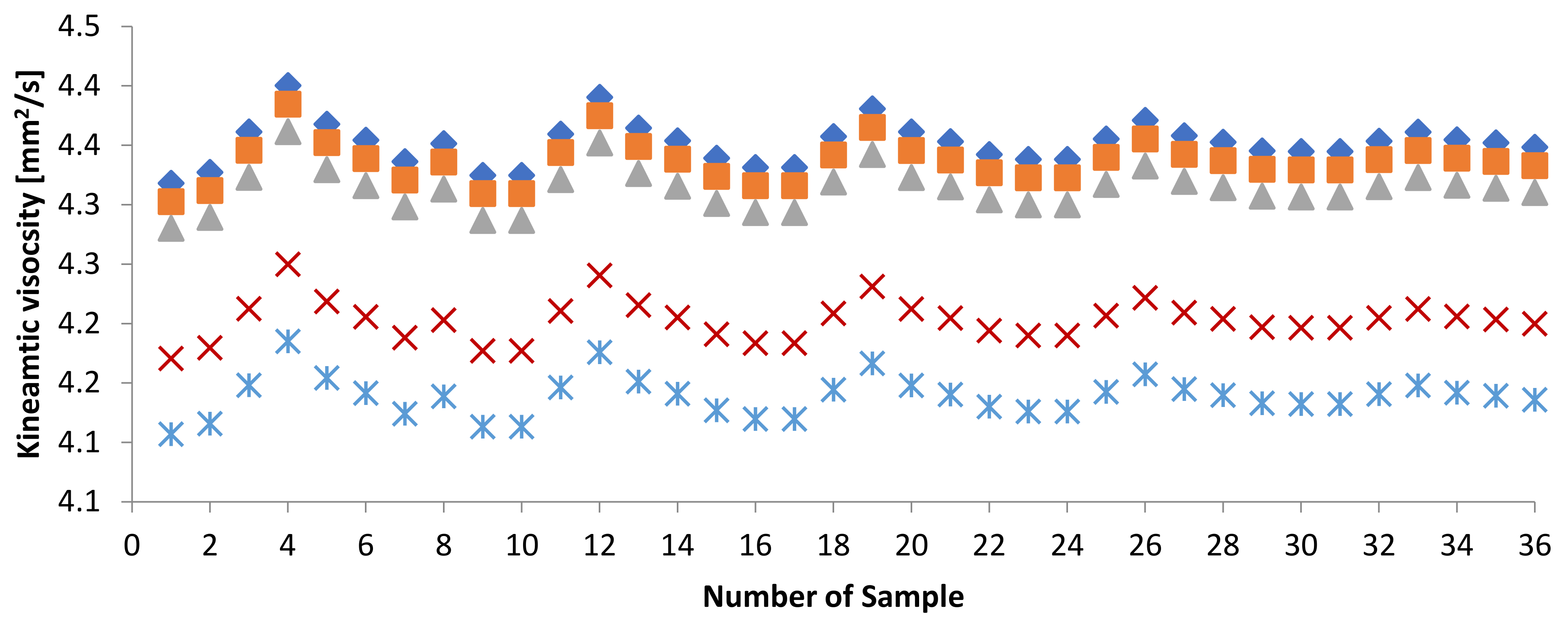
3.2.1. Kinematic Viscosity at 40 and Density at 15 of PME-WFME Blends
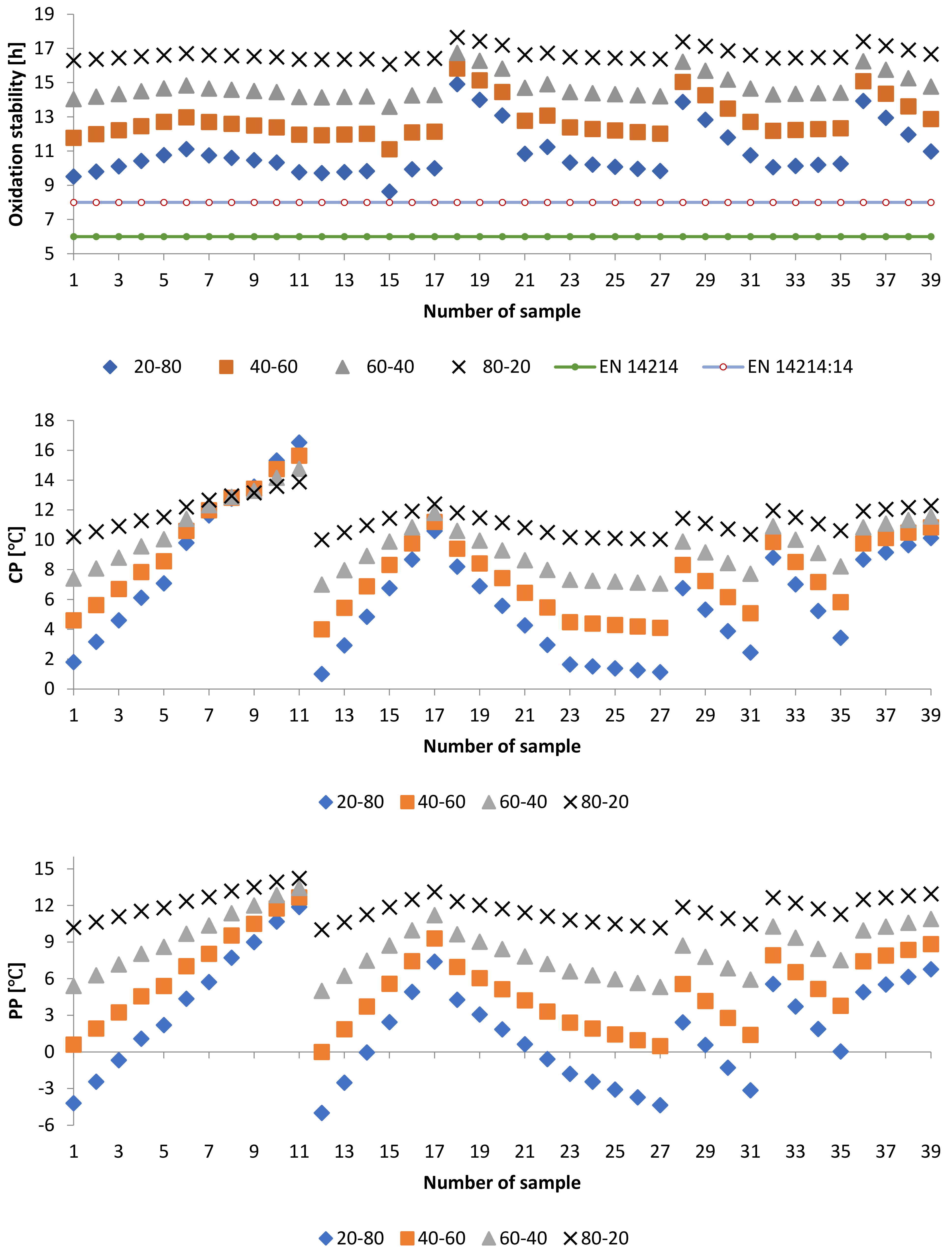
3.2.2. Oxidation Stability of PME-WFME Blends
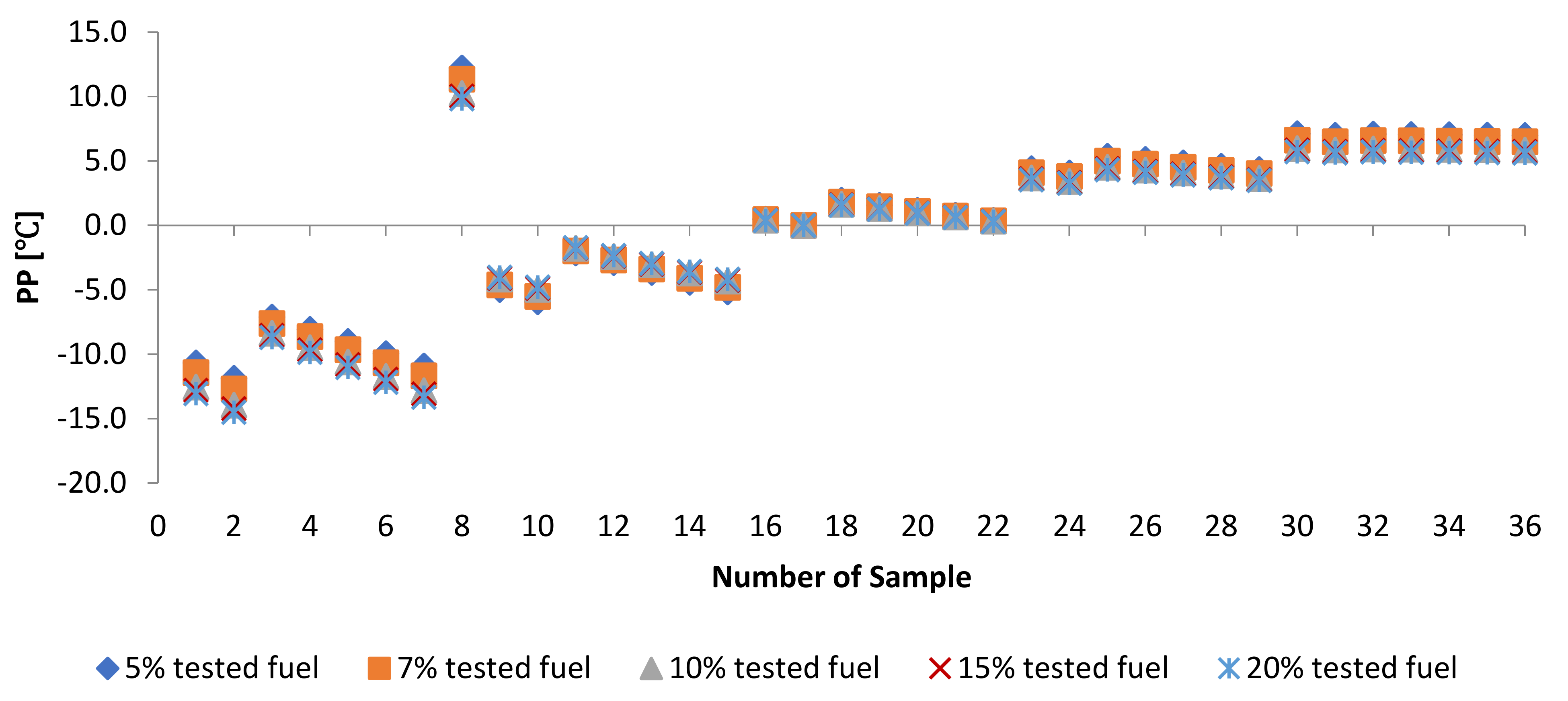
3.2.3. Cold Flow Properties of PME-WFME Blends
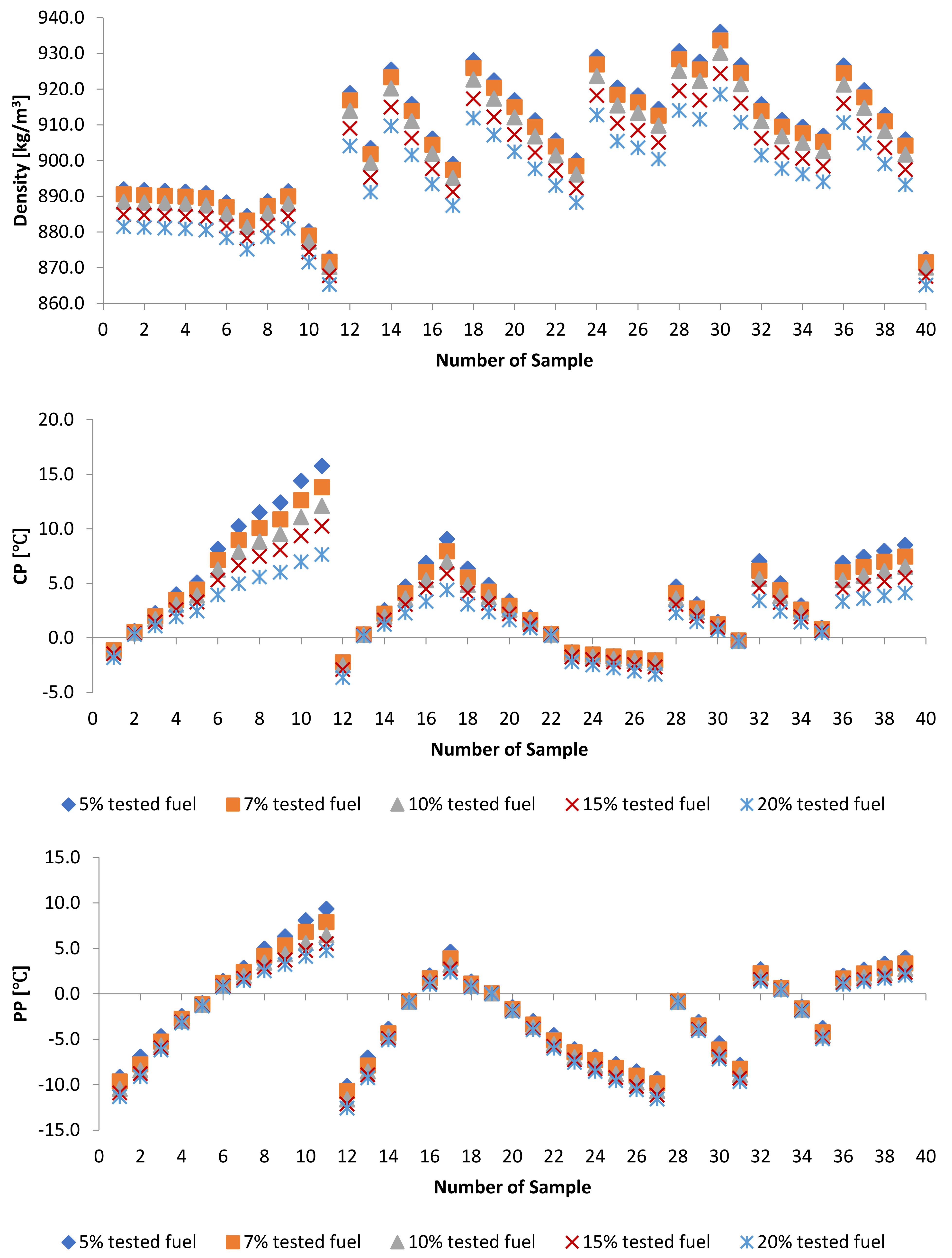
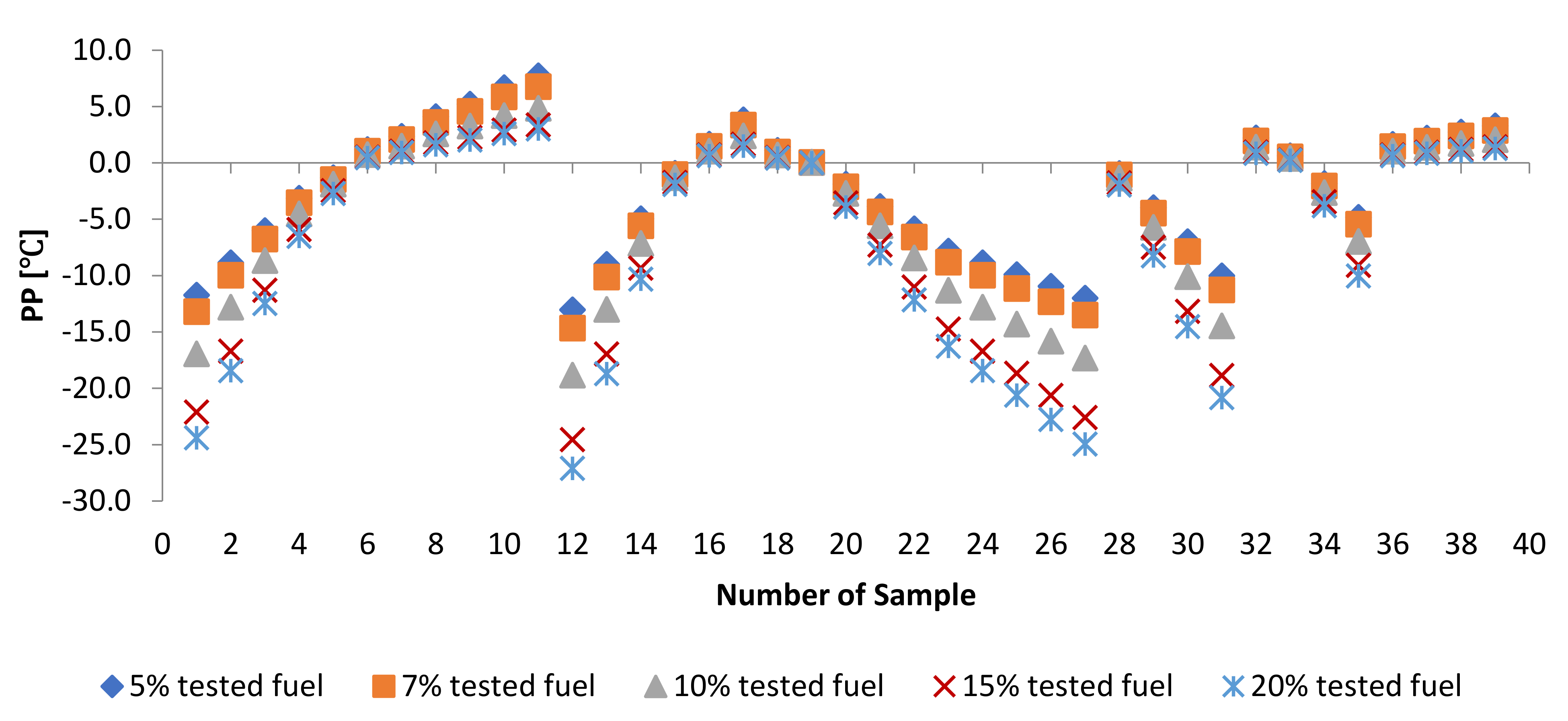
3.3. Effect of Adding Petroleum-Based Fuels to Pure Biodiesel Samples on the Properties of the Mixture
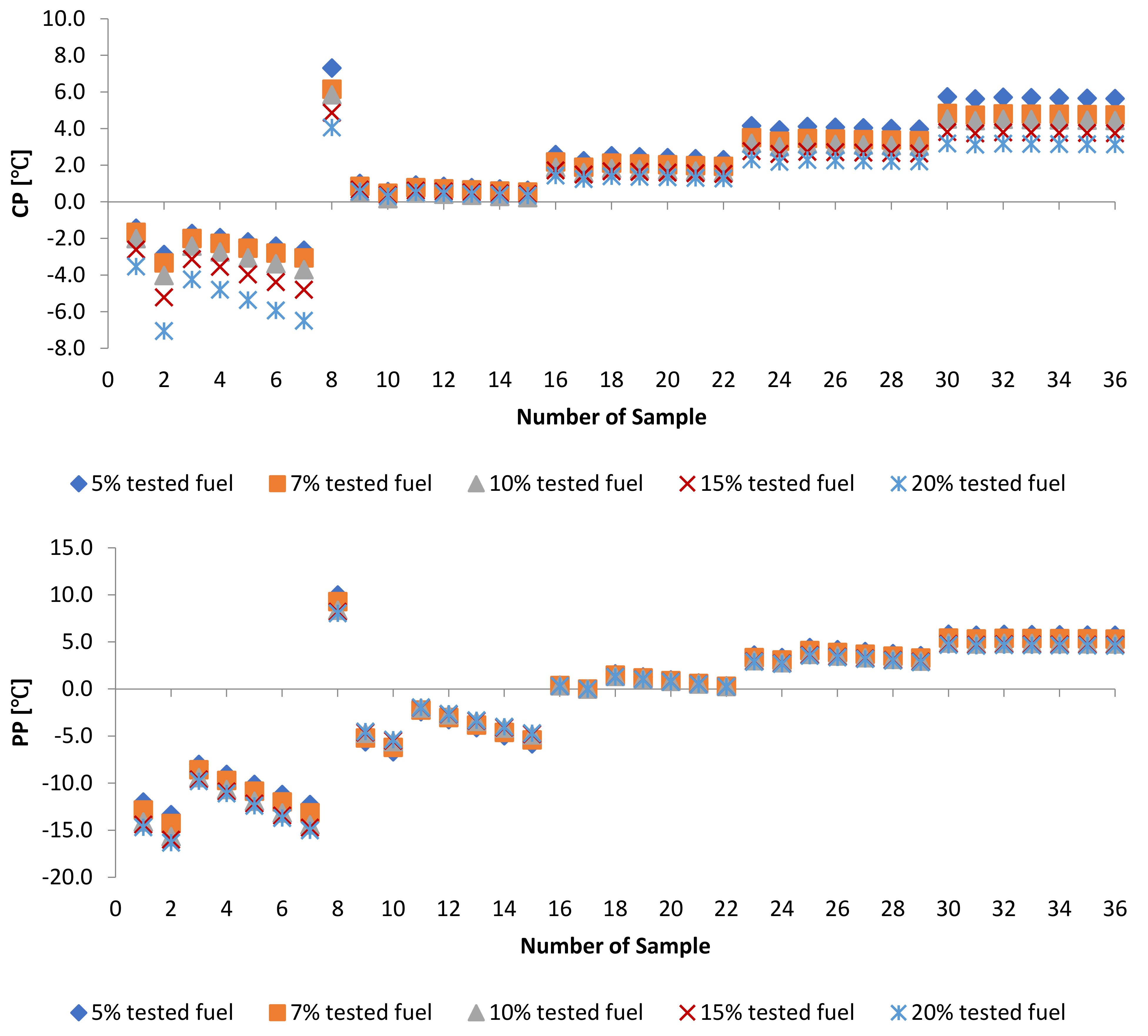
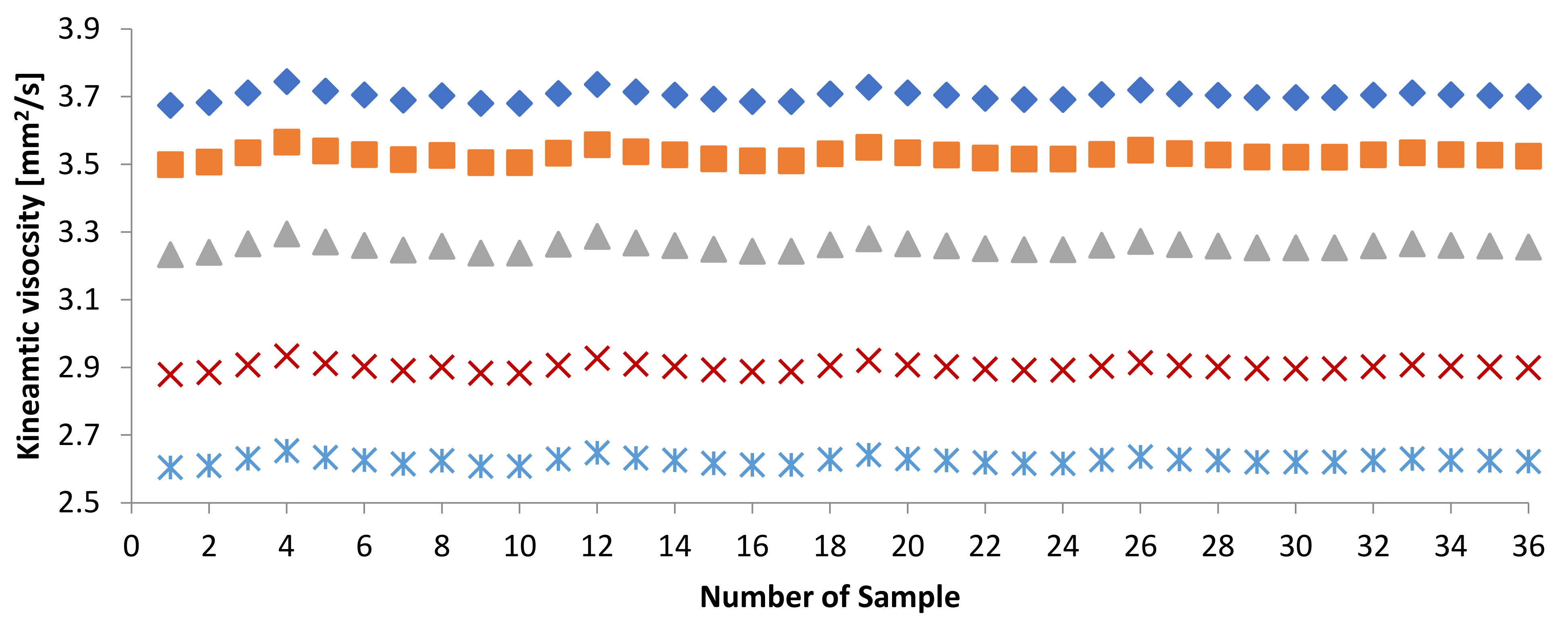
3.4. WFME–PME-Petroleum-Based Fuels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Symbols and Nomenclature
| ASTM | American Standard Test Method |
| BF | Benzene |
| CFP | Cold flow properties |
| CP | Cloud point |
| D | Density |
| DU | Degree of unsaturation |
| GC | Gas chromatography |
| KF | Kerosene |
| KV | Kinematic viscosity |
| LCSF | Long-chain saturated factor |
| MUFAMEs | Monounsaturated |
| NaOH | Sodium hydroxide |
| OX | Oxidation stability |
| PME | Palm methyl ester |
| PP | Pour point |
| PUFAMEs | Polyunsaturated |
| SFAMEs | Saturated |
| ULSDFS | Ultra-low sulfur diesel fuel summer |
| ULSDFW | Ultra-low sulfur diesel fuel winter |
| WFME | Waste frying methyl ester |
References
- Verma, P.; Sharma, M.P. Comparative analysis of the effect of methanol and ethanol on Karanja biodiesel production and its optimization. Fuel 2016, 180, 164–174. [Google Scholar] [CrossRef]
- Hassan, A.A.; Smith, J.D. Laboratory-scale research of non-catalyzed supercritical alcohol process for continuous biodiesel production. Catalysts 2021, 11, 435. [Google Scholar] [CrossRef]
- Hassan, A.A.; Alhameedi, H.A.; Smith, J.D. Two-step sub/supercritical water and ethanol processes for non-catalytic biodiesel production. Chem. Eng. Process. Process. Intensif. 2020, 150, 107881. [Google Scholar] [CrossRef]
- Hassan, A.A.; Alhameedi, H.A.; Smith, J.D. Using ethanol for continuous biodiesel production with trace catalyst and CO2 co-solvent. Fuel Process. Technol. 2020, 203, 106377. [Google Scholar] [CrossRef]
- Hoang, A.T. Prediction of the density and viscosity of biodiesel and the influence of biodiesel properties on a diesel engine fuel supply system. J. Mar. Eng. Technol. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Determination of the density and the viscosities of biodiesel–diesel fuel blends. Renew. Energy 2008, 33, 2623–2630. [Google Scholar] [CrossRef]
- Razzaq, L.; Farooq, M.; Mujtaba, M.A.; Sher, F.; Farhan, M.; Hassan, M.T.; Imran, M. Modeling viscosity and density of ethanol-diesel-biodiesel ternary blends for a sustainable environment. Sustainability 2020, 12, 5186. [Google Scholar] [CrossRef]
- Martínez, G.; Sánchez, N.; Encinar, J.M.; González, J.F. Fuel properties of biodiesel from vegetable oils and oil mixtures. Influence of methyl esters distribution. Biomass Bioenergy 2014, 63, 22–32. [Google Scholar] [CrossRef]
- Pandey, R.K.; Rehman, A.; Sarviya, R.M. Impact of alternative fuel properties on fuel spray behavior and atomization. Renew. Sustain. Energy Rev. 2012, 16, 1762–1778. [Google Scholar] [CrossRef]
- Demirbas, A. Relationships are derived from the physical properties of vegetable oil and biodiesel fuels. Fuel 2008, 87, 1743–1748. [Google Scholar] [CrossRef]
- Chen, P.C.; Wang, W.C.; Roberts, W.L.; Fang, T. Spray and atomization of diesel fuel and its alternatives from a single-hole injector using a common rail fuel injection system. Fuel 2013, 103, 850–861. [Google Scholar] [CrossRef]
- Pratas, M.J.; Freitas, S.; Oliveira, M.B.; Monteiro, S.C.; Lima, A.S.; Coutinho, J.A. Densities and viscosities of fatty acid methyl and ethyl esters. J. Chem. Eng. Data 2010, 55, 3983–3990. [Google Scholar] [CrossRef]
- Devi, A.; Das, V.K.; Deka, D. A green approach for enhancing oxidation stability including long storage periods of biodiesel via Thuja oreantalis L. as an antioxidant additive. Fuel 2019, 253, 1264–1273. [Google Scholar] [CrossRef]
- Schober, S.; Mittelbach, M. The impact of antioxidants on biodiesel oxidation stability. Eur. J. Lipid Sci. Technol. 2014, 106, 382–389. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.S.; Corscadden, K.; Caldwell, C. Improvement on oxidation and storage stability of biodiesel derived from an emerging feedstock camelina. Fuel Process. Technol. 2017, 157, 90–98. [Google Scholar] [CrossRef]
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: Composition, specifications and prediction models. Renew. Sustain. Energy Rev. 2016, 63, 62–92. [Google Scholar] [CrossRef]
- Lanjekar, R.D.; Deshmukh, D. A review of the effect of the composition of biodiesel on NOx emission, oxidative stability and cold flow properties. Renew. Sustain. Energy Rev. 2016, 54, 1401–1411. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M.P. Cold flow behavior of biodiesel-A review. Int. J. Renew. Energy Res. 2013, 3, 827–836. [Google Scholar]
- Pérez, Á.; Casas, A.; Fernández, C.M.; Ramos, M.J.; Rodríguez, L. Winterization of peanut biodiesel to improve the cold flow properties. Bioresour. Technol. 2010, 101, 7375–7381. [Google Scholar] [CrossRef]
- Bouaid, A.; Hahati, K.; Martinez, M.; Aracil, J. Biodiesel production from biobutanol. Improvement of cold flow properties. Chem. Eng. J. 2014, 238, 234–241. [Google Scholar] [CrossRef]
- Kassem, Y.; Çamur, H. Effects of storage under different conditions on the fuel properties of biodiesel admixtures derived from waste frying and canola oils. Biomass Convers. Biorefin. 2018, 8, 825–845. [Google Scholar] [CrossRef]
- Kassem, Y.; Çamur, H.; Alassi, E. Biodiesel production from four residential waste frying oils: Proposing blends for improving the physicochemical properties of methyl biodiesel. Energies 2020, 13, 4111. [Google Scholar] [CrossRef]
- Evcil, A.; Al-Shanableh, F.; Savas, M.A. Variation of solid fraction with cold flow properties of biodiesel produced from waste frying oil. Fuel 2018, 215, 522–527. [Google Scholar] [CrossRef]
- El-Araby, R.; Amin, A.; El Morsi, A.K.; El-Ibiari, N.N.; El-Diwani, G.I. Study on the characteristics of palm oil–biodiesel–diesel fuel blend. Egypt. J. Pet. 2018, 27, 187–194. [Google Scholar] [CrossRef]
- Dunn, R.O. Fuel properties of biodiesel/ultra-low sulfur diesel (ULSD) blends. J. Am. Oil Chem. Soc. 2011, 88, 1977–1987. [Google Scholar] [CrossRef]
- ASTM D 445–09. In Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity); ASTM: West Conshohocken, PA, USA, 2009.
- ASTM D854–14. In Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer; ASTM: West Conshohocken, PA, USA, 2014.
- Kassem, Y.; Çamur, H. A laboratory study of the effects of wide range temperature on the properties of biodiesel produced from various waste vegetable oils. Waste Biomass Valorization 2017, 8, 1995–2007. [Google Scholar] [CrossRef][Green Version]
- EN 14112. In Fat and Oil Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Oxidation Stability (Accelerated Oxidation Test); CEN: Brussels, Belgium, 2003.
- ASTM D2500-17a. In Standard Test Method for Cloud Point of Petroleum Products; ASTM: West Conshohocken, PA, USA, 2017.
- ASTM D97-17b. In Standard Test Method for Pour Point of Petroleum Products; ASTM: West Conshohocken, PA, USA, 2017.
- Doğan, T.H. The testing of the effects of cooking conditions on the quality of biodiesel produced from waste cooking oils. Renew. Energy 2016, 94, 466–473. [Google Scholar] [CrossRef]
- Fu, J.; Turn, S.Q.; Takushi, B.M.; Kawamata, C.L. Storage and oxidation stabilities of biodiesel derived from waste cooking oil. Fuel 2016, 167, 89–97. [Google Scholar] [CrossRef]
- Mejia, J.D.; Salgado, N.; Orrego, C.E. Effect of blends of diesel and palm-castor biodiesels on viscosity, cloud point and flash point. Ind. Crop. Prod. 2013, 43, 791–797. [Google Scholar] [CrossRef]
- Atabani, A.E.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Yussof, H.W.; Chong, W.T.; Lee, K.T. A comparative evaluation of physical and chemical properties of biodiesel synthesized from edible and non-edible oils and study on the effect of biodiesel blending. Energy 2013, 58, 296–304. [Google Scholar] [CrossRef]
- Campus, P. Comparative analysis of fuel characteristics of bio-diesel produced from selected oil-bearing seeds in Nigeria. Eur. J. Sci. Res. 2011, 58, 238–246. [Google Scholar]
- Balat, M. Potential alternatives to edible oils for biodiesel production–A review of current work. Energy Convers. Manag. 2011, 52, 1479–1492. [Google Scholar] [CrossRef]
- Karmakar, A.; Karmakar, S.; Mukherjee, S. Properties of various plants and animals feedstocks for biodiesel production. Bioresour. Technol. 2010, 101, 7201–7210. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Zuleta, E.C.; Rios, L.A.; Benjumea, P.N. Oxidative stability and cold flow behavior of palm, sacha-inchi, jatropha and castor oil biodiesel blends. Fuel Process. Technol. 2012, 102, 96–101. [Google Scholar] [CrossRef]
- Dey, S.; Reang, N.M.; Das, P.K.; Deb, M. A comprehensive study on prospects of economy, environment and efficiency of palm oil biodiesel as a renewable fuel. J. Clean. Prod. 2020, 286, 124981. [Google Scholar] [CrossRef]
- Mosarof, M.H.; Kalam, M.A.; Masjuki, H.H.; Ashraful, A.M.; Rashed, M.M.; Imdadul, H.K.; Monirul, I.M. Implementation of palm biodiesel based on economic aspects, performance, emission and wear characteristics. Energy Convers. Manag. 2015, 105, 617–629. [Google Scholar] [CrossRef]
- Wafti, A.N.S.; Lau, H.L.N.; Choo, Y.M. Production technology of biodiesel from palm fatty acid distillate using mild acid catalyst. J. Oil Palm Res. 2015, 27, 352–359. [Google Scholar]
- May, C.Y.; Liang, Y.C.; Foon, C.S.; Ngan, M.A.; Hook, C.C.; Basiron, Y. Key fuel properties of palm oil alkyl esters. Fuel 2005, 84, 1717–1720. [Google Scholar] [CrossRef]
- Banani, R.; Youssef, S.; Bezzarga, M.; Abderrabba, M. Waste frying oil with high levels of free fatty acids as one of the prominent sources of biodiesel production. J. Mater. Environ. Sci. 2015, 6, 1178–1185. [Google Scholar]
- Benjumea, P.; Agudelo, J.R.; Agudelo, A.F. Effect of the degree of unsaturation of biodiesel fuels on engine performance, combustion characteristics, and emissions. Energy Fuels 2011, 25, 77–85. [Google Scholar]
- Sokoto, M.A.; Hassan, L.G.; Dangoggo, S.M.; Ahmad, H.G.; Uba, A. Influence of fatty acid methyl esters on fuel properties of biodiesel produced from the seeds oil of Curcubita pepo. Niger. J. Basic Appl. Sci. 2011, 19, 81–86. [Google Scholar] [CrossRef]
- Kafuku, G.; Mbarawa, M. Effects of biodiesel blending with fossil fuel on flow properties of biodiesel produced from non-edible oils. Int. J. Green Energy 2010, 7, 434–444. [Google Scholar] [CrossRef]
- Kalam, M.A.; Masjuki, H.H. Biodiesel from palmoil—an analysis of its properties and potential. Biomass Bioenergy 2002, 23, 471–479. [Google Scholar] [CrossRef]
- Monirul, I.M.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Rashedul, H.K.; Rashed, M.M.; Mosarof, M.H. A comprehensive review on biodiesel cold flow properties and oxidation stability along with their improvement processes. RSC Adv. 2015, 5, 86631–86655. [Google Scholar] [CrossRef]
- Pölczmann, G.; Tóth, O.; Beck, Á.; Hancsók, J. Investigation of storage stability of diesel fuels containing biodiesel produced from waste cooking oil. J. Clean. Prod. 2016, 111, 85–92. [Google Scholar] [CrossRef]
- De Almeida, V.F.; García-Moreno, P.J.; Guadix, A.; Guadix, E.M. Biodiesel production from mixtures of waste fish oil, palm oil and waste frying oil: Optimization of fuel properties. Fuel Process. Technol. 2015, 133, 152–160. [Google Scholar] [CrossRef]
- Uzun, B.B.; Kılıç, M.; Özbay, N.; Pütün, A.E.; Pütün, E. Biodiesel production from waste frying oils: Optimization of reaction parameters and determination of fuel properties. Energy 2012, 44, 347–351. [Google Scholar] [CrossRef]
- Bharti, R.; Singh, B. Green tea (Camellia assamica) extract as an antioxidant additive to enhance the oxidation stability of biodiesel synthesized from waste cooking oil. Fuel 2020, 262, 116658. [Google Scholar] [CrossRef]
- Benjumea, P.; Agudelo, J.; Agudelo, A. Basic properties of palm oil biodiesel–diesel blends. Fuel 2008, 87, 2069–2075. [Google Scholar] [CrossRef]
- Mairizal, A.Q.; Awad, S.; Priadi, C.R.; Hartono, D.M.; Moersidik, S.S.; Tazerout, M.; Andres, Y. Experimental study on the effects of feedstock on the properties of biodiesel using multiple linear regressions. Renew. Energy 2020, 145, 375–381. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; Rodríguez-Rodríguez, J.E.; del Rayo Jaramillo-Jacob, A. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Giakoumis, E.G. A statistical investigation of biodiesel physical and chemical properties, and their correlation with the degree of unsaturation. Renew. Energy 2013, 50, 858–878. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Kumar, N. Oxidative stability of biodiesel: Causes, effects and prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Knothe, G. Some aspects of biodiesel oxidative stability. Fuel Process. Technol. 2007, 88, 669–677. [Google Scholar] [CrossRef]
- Devi, A.; Das, V.K.; Deka, D. Ginger extract as a nature based robust additive and its influence on the oxidation stability of biodiesel synthesized from non-edible oil. Fuel 2017, 187, 306–314. [Google Scholar] [CrossRef]
- Wang, J.; Cao, L.; Han, S. Effect of polymeric cold flow improvers on flow properties of biodiesel from waste cooking oil. Fuel 2014, 117, 876–881. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P.; Dwivedi, G. Evaluation and enhancement of cold flow properties of palm oil and its biodiesel. Energy Rep. 2016, 2, 8–13. [Google Scholar] [CrossRef]
- Lv, P.; Cheng, Y.; Yang, L.; Yuan, Z.; Li, H.; Luo, W. Improving the low temperature flow properties of palm oil biodiesel: Addition of cold flow improver. Fuel Process. Technol. 2013, 110, 61–64. [Google Scholar] [CrossRef]
- Nainwal, S.; Sharma, N.; Sharma, A.S.; Jain, S.; Jain, S. Cold flow properties improvement of Jatropha curcas biodiesel and waste cooking oil biodiesel using winterization and blending. Energy 2015, 89, 702–707. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M.P. Investigation and improvement in cold flow properties of Pongamia biodiesel. Waste Biomass Valoriz. 2015, 6, 73–79. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R.; Bhatia, S. Palm oil: Addressing issues and towards sustainable development. Renew. Sustain. Energy Rev. 2009, 13, 420–427. [Google Scholar] [CrossRef]
- Silalertruksa, T.; Gheewala, S.H. Environmental sustainability assessment of palm biodiesel production in Thailand. Energy 2012, 43, 306–314. [Google Scholar] [CrossRef]
- Sarin, R.; Sharma, M.; Sinharay, S.; Malhotra, R.K. Jatropha–palm biodiesel blends: An optimum mix for Asia. Fuel 2007, 86, 1365–1371. [Google Scholar] [CrossRef]



| Variable | WFME1 | WFME2 | WFME3 | WFME4 | WFME5 | WFME6 | WFME7 |
|---|---|---|---|---|---|---|---|
| MUFAMEs | 64.01 | 62.83 | 61.65 | 60.47 | 58.11 | 55.75 | 52.22 |
| PUFAMEs | 6.99 | 6.65 | 6.31 | 5.97 | 5.29 | 4.61 | 3.59 |
| SFAMEs | 7.74 | 9.50 | 11.27 | 13.03 | 16.55 | 20.08 | 25.38 |
| DU | 120.67 | 118.46 | 116.25 | 114.04 | 109.62 | 105.20 | 98.58 |
| LCSF | 1.81 | 2.01 | 2.20 | 2.40 | 2.79 | 3.19 | 3.78 |
| Variable | WFME8 | WFME9 | WFME10 | WFME11 | WFME12 | WFME13 | WFME14 |
| MUFAMEs | 48.68 | 46.32 | 42.78 | 40.42 | 65.43 | 60.43 | 55.43 |
| PUFAMEs | 2.56 | 1.88 | 0.86 | 0.18 | 6.99 | 5.63 | 4.27 |
| SFAMEs | 30.65 | 34.20 | 39.47 | 42.99 | 7.74 | 14.79 | 21.84 |
| DU | 91.93 | 87.52 | 80.88 | 76.46 | 120.09 | 110.96 | 101.84 |
| LCSF | 4.37 | 4.77 | 5.35 | 5.75 | 1.81 | 2.60 | 3.38 |
| Variable | WFME15 | WFME16 | WFME17 | WFME18 | WFME19 | WFME20 | WFME21 |
| MUFAMEs | 50.42 | 45.42 | 40.42 | 52.07 | 51.00 | 49.93 | 48.85 |
| PUFAMEs | 2.90 | 1.54 | 0.18 | 5.20 | 4.96 | 4.72 | 4.47 |
| SFAMEs | 28.89 | 35.94 | 42.99 | 22.13 | 22.70 | 23.28 | 23.85 |
| DU | 92.71 | 83.59 | 74.46 | 103.03 | 103.43 | 103.83 | 104.23 |
| LCSF | 4.17 | 4.96 | 5.75 | 4.13 | 4.20 | 4.28 | 4.35 |
| Variable | WFME22 | WFME23 | WFME24 | WFME25 | WFME26 | WFME27 | WFME28 |
| MUFAMEs | 47.78 | 46.71 | 50.45 | 54.20 | 57.94 | 61.69 | 54.74 |
| PUFAMEs | 4.23 | 3.99 | 4.59 | 5.19 | 5.79 | 6.39 | 5.56 |
| SFAMEs | 24.43 | 25.00 | 21.55 | 18.10 | 14.64 | 11.19 | 19.25 |
| DU | 104.63 | 105.03 | 108.04 | 111.05 | 114.07 | 117.08 | 106.44 |
| LCSF | 4.43 | 4.50 | 3.96 | 3.42 | 2.89 | 2.35 | 3.66 |
| Variable | WFME29 | WFME30 | WFME31 | WFME32 | WFME33 | WFME34 | WFME35 |
| MUFAMEs | 57.41 | 60.09 | 62.76 | 41.68 | 42.94 | 44.19 | 45.45 |
| PUFAMEs | 5.92 | 6.27 | 6.63 | 0.94 | 1.70 | 2.47 | 3.23 |
| SFAMEs | 16.37 | 13.50 | 10.62 | 39.39 | 35.79 | 32.20 | 28.60 |
| DU | 109.85 | 113.27 | 116.68 | 80.57 | 86.69 | 92.80 | 98.92 |
| LCSF | 3.20 | 2.74 | 2.27 | 5.50 | 5.25 | 5.00 | 4.75 |
| Variable | WFME36 | WFME37 | WFME38 | WFME39 | PME | ||
| MUFAMEs | 49.74 | 47.41 | 45.08 | 42.75 | 43.50 | ||
| PUFAMEs | 4.20 | 3.19 | 2.19 | 1.18 | 0.20 | ||
| SFAMEs | 26.30 | 30.47 | 34.65 | 38.82 | 44.90 | ||
| DU | 97.32 | 91.60 | 85.89 | 80.17 | 65.90 | ||
| LCSF | 4.45 | 4.78 | 5.10 | 5.42 | 6.36 |
| Property | Unit | Test Method | ULSDFW | ULSDFS | KF | BF |
|---|---|---|---|---|---|---|
| Density at 15 °C | kg/m3 | ASTMD 4052 | 832.5 | 825.8 | 798.6 | 740.94 |
| Viscosity at 40 °C | mm2/s | ASTM D 455 | 3.0 | 2.7 | 1.173 | 0.590 |
| Viscosity at -20 °C | mm2/s | ASTM D 455 | 3.899 | |||
| Flash Point | °C | ASTM D93 | 68 | 64 | ||
| °C | IP 170 | 47 | ||||
| Cetane Number | - | ASTM D613 | 55.0 | 54.0 | ||
| Oxidation Stability (at 110 °C) | h | EN 14112 | ||||
| Oxidation Stability | mg/L | ASTM D2274 | 3 | |||
| Total acid number | mg KOH/g | ASTM D3242 | 0.004 | |||
| Water content | mg/kg | ASTM D6304 | 53 | 41 | ||
| CFPP | °C | EN 116 | ||||
| IP 309 | -20 | -8 | ||||
| Cloud point | °C | ASTM D5771 | -15 | -6 | -87 | -75 |
| Pour point | °C | ASTM D5950 | -23 | -13 | -75 | |
| Sulphur content | mg/kg | EN ISO 20846 | ||||
| mg//kg | ASTM D5453 | 4.8 | 4.9 | |||
| WT PCT | IP 336 | 0.07 | ||||
| Ash content | WT PCT | ASTM D 482 | 0.0 | 0.0 | ||
| Octane number, Mon | ASTM D2700 | 85.1 | ||||
| Octane number, Ron | ASTM D 2699 | 95 | ||||
| Evaporated at 70 °C | VOL PCT | ASTM D86 | 41.4 | |||
| Evaporated at 100 °C | VOL PCT | ASTM D86 | 58.6 | |||
| Evaporated at 150 °C | VOL PCT | ASTM D86 | 81.9 | |||
| Distillation residue | VOL PCT | ASTM D86 | 1.4 | 1 |
| Sample | KV [mm2/s] | Sample | KV [mm2/s] | Sample | KV [mm2/s] |
|---|---|---|---|---|---|
| WFME1 | 4.55 | WFME15 | 4.64 | WFME29 | 4.42 |
| WFME2 | 4.78 | WFME16 | 4.63 | WFME30 | 4.59 |
| WFME3 | 5.01 | WFME17 | 4.62 | WFME31 | 4.57 |
| WFME4 | 5.24 | WFME18 | 4.33 | WFME32 | 4.65 |
| WFME5 | 5.71 | WFME19 | 4.36 | WFME33 | 4.63 |
| WFME6 | 5.57 | WFME20 | 4.49 | WFME34 | 4.61 |
| WFME7 | 5.38 | WFME21 | 4.51 | WFME35 | 4.60 |
| WFME8 | 5.11 | WFME22 | 4.57 | WFME36 | 4.37 |
| WFME9 | 4.94 | WFME23 | 4.59 | WFME37 | 4.43 |
| WFME10 | 4.75 | WFME24 | 4.63 | WFME38 | 4.48 |
| WFME11 | 4.62 | WFME25 | 4.60 | WFME39 | 4.55 |
| WFME12 | 4.55 | WFME26 | 4.58 | PME | 4.68 |
| WFME13 | 4.58 | WFME27 | 4.56 | ||
| WFME14 | 4.66 | WFME28 | 4.37 |
| Sample | D [kg/m3] | Sample | D [kg/m3] | Sample | D [kg/m3] |
|---|---|---|---|---|---|
| WFME1 | 895.4 | WFME15 | 920.6 | WFME29 | 933 |
| WFME2 | 895.2 | WFME16 | 910.4 | WFME30 | 941.8 |
| WFME3 | 895 | WFME17 | 902,9 | WFME31 | 932 |
| WFME4 | 894.8 | WFME18 | 933.5 | WFME32 | 920.5 |
| WFME5 | 894.3 | WFME19 | 927.5 | WFME33 | 915.8 |
| WFME6 | 891.6 | WFME20 | 921.7 | WFME34 | 913.9 |
| WFME7 | 887.6 | WFME21 | 915.8 | WFME35 | 911.2 |
| WFME8 | 891.9 | WFME22 | 909.9 | WFME36 | 931.9 |
| WFME9 | 894.8 | WFME23 | 903.9 | WFME37 | 924.7 |
| WFME10 | 883.1 | WFME24 | 934.5 | WFME38 | 917.4 |
| WFME11 | 875.2 | WFME25 | 925.4 | WFME39 | 910.1 |
| WFME12 | 923.7 | WFME26 | 923.1 | PME | 875.0 |
| WFME13 | 907.6 | WFME27 | 919.1 | ||
| WFME14 | 930.7 | WFME28 | 936.1 |
| Sample | OX [h] | Sample | OX [h] | Sample | OX [h] |
|---|---|---|---|---|---|
| WFME1 | 7.25 | WFME15 | 6.14 | WFME29 | 11.40 |
| WFME2 | 7.61 | WFME16 | 7.78 | WFME30 | 10.10 |
| WFME3 | 7.99 | WFME17 | 7.85 | WFME31 | 8.80 |
| WFME4 | 8.39 | WFME18 | 14.00 | WFME32 | 7.93 |
| WFME5 | 8.81 | WFME19 | 12.85 | WFME33 | 8.02 |
| WFME6 | 9.25 | WFME20 | 11.71 | WFME34 | 8.10 |
| WFME7 | 8.79 | WFME21 | 8.91 | WFME35 | 8.19 |
| WFME8 | 8.61 | WFME22 | 9.42 | WFME36 | 12.77 |
| WFME9 | 8.44 | WFME23 | 8.27 | WFME37 | 11.54 |
| WFME10 | 8.27 | WFME24 | 8.12 | WFME38 | 10.31 |
| WFME11 | 7.56 | WFME25 | 7.96 | WFME39 | 9.08 |
| WFME12 | 7.50 | WFME26 | 7.81 | PME | 18.35 |
| WFME13 | 7.57 | WFME27 | 7.65 | ||
| WFME14 | 7.64 | WFME28 | 12.70 |
| Sample | CP [] | Sample | ] | Sample | ] |
|---|---|---|---|---|---|
| WFME1 | −1.00 | WFME15 | 7.60 | WFME29 | 3.40 |
| WFME2 | 0.70 | WFME16 | 10.00 | WFME30 | 1.60 |
| WFME3 | 2.50 | WFME17 | 7.00 | WFME31 | −0.20 |
| WFME4 | 4.40 | WFME18 | 5.36 | WFME32 | 7.76 |
| WFME5 | 5.60 | WFME19 | 3.72 | WFME33 | 5.52 |
| WFME6 | 9.00 | WFME20 | 2.08 | WFME34 | 3.28 |
| WFME7 | 11.30 | WFME21 | 0.44 | WFME35 | 1.04 |
| WFME8 | 12.70 | WFME22 | −1.20 | WFME36 | 7.60 |
| WFME9 | 13.70 | WFME23 | −1.36 | WFME37 | 8.20 |
| WFME10 | 15.90 | WFME24 | −1.52 | WFME38 | 8.80 |
| WFME11 | 17.40 | WFME25 | −1.68 | WFME39 | 9.40 |
| WFME12 | −2.00 | WFME26 | −1.84 | PME | 15.00 |
| WFME13 | 0.40 | WFME27 | 5.20 | ||
| WFME14 | 2.80 | WFME28 | 7.60 |
| Sample | ] | Sample | PP [] | Sample | ] |
|---|---|---|---|---|---|
| WFME1 | −9.00 | WFME15 | −0.70 | WFME29 | −3.04 |
| WFME2 | −6.80 | WFME16 | 2.40 | WFME30 | −5.36 |
| WFME3 | −4.60 | WFME17 | 5.50 | WFME31 | −7.68 |
| WFME4 | −2.40 | WFME18 | 1.60 | WFME32 | 3.20 |
| WFME5 | −1.00 | WFME19 | 0.08 | WFME33 | 0.90 |
| WFME6 | 1.70 | WFME20 | −1.44 | WFME34 | −1.40 |
| WFME7 | 3.40 | WFME21 | −2.96 | WFME35 | −3.70 |
| WFME8 | 5.90 | WFME22 | −4.48 | WFME36 | 2.38 |
| WFME9 | 7.50 | WFME23 | −6.00 | WFME37 | 3.16 |
| WFME10 | 9.60 | WFME24 | −6.80 | WFME38 | 3.94 |
| WFME11 | 11.10 | WFME25 | −7.60 | WFME39 | 4.72 |
| WFME12 | −10.00 | WFME26 | −8.40 | PME | 15.00 |
| WFME13 | −6.90 | WFME27 | −9.20 | ||
| WFME14 | −3.80 | WFME28 | −0.72 |
| Sample No. | Sample Name | Sample No. | Sample Name | Sample No. | Sample Name |
|---|---|---|---|---|---|
| 1 | PMWFME1 | 14 | PMWFME14 | 27 | PMWFME27 |
| 2 | PMWFME2 | 15 | PMWFME15 | 28 | PMWFME28 |
| 3 | PMWFME3 | 16 | PMWFME16 | 29 | PMWFME29 |
| 4 | PMWFME4 | 17 | PMWFME17 | 30 | PMWFME30 |
| 5 | PMWFME5 | 18 | PMWFME18 | 31 | PMWFME31 |
| 6 | PMWFME6 | 19 | PMWFME19 | 32 | PMWFME32 |
| 7 | PMWFME7 | 20 | PMWFME20 | 33 | PMWFME33 |
| 8 | PMWFME8 | 21 | PMWFME21 | 34 | PMWFME34 |
| 9 | PMWFME9 | 22 | PMWFME22 | 35 | PMWFME35 |
| 10 | PMWFME10 | 23 | PMWFME23 | 36 | PMWFME36 |
| 11 | PMWFME11 | 24 | PMWFME24 | 37 | PMWFME37 |
| 12 | PMWFME12 | 25 | PMWFME25 | 38 | PMWFME38 |
| 13 | PMWFME13 | 26 | PMWFME26 | 39 | PMWFME39 |
| Sample No. | Sample Name | Sample No. | Sample Name | Sample No. | Sample Name | Sample No. | Sample Name | Sample No. | Sample Name |
|---|---|---|---|---|---|---|---|---|---|
| 1 | WFME1 | 9 | WFME9 | 17 | WFME17 | 25 | WFME25 | 33 | WFME33 |
| 2 | WFME2 | 10 | WFME10 | 18 | WFME18 | 26 | WFME26 | 34 | WFME34 |
| 3 | WFME3 | 11 | WFME11 | 19 | WFME19 | 27 | WFME27 | 35 | WFME35 |
| 4 | WFME4 | 12 | WFME12 | 20 | WFME20 | 28 | WFME28 | 36 | WFME36 |
| 5 | WFME5 | 13 | WFME13 | 21 | WFME21 | 29 | WFME29 | 37 | WFME37 |
| 6 | WFME6 | 14 | WFME14 | 22 | WFME22 | 30 | WFME30 | 38 | WFME38 |
| 7 | WFME7 | 15 | WFME15 | 23 | WFME23 | 31 | WFME31 | 39 | WFME39 |
| 8 | WFME8 | 16 | WFME16 | 24 | WFME24 | 32 | WFME32 | 40 | PME |
| Sample No. | Sample Name | Sample No. | Sample Name | Sample No. | Sample Name |
|---|---|---|---|---|---|
| 1 | WFME1 | 13 | P20WFME25 | 25 | P60WFME23 |
| 2 | WFME12 | 14 | P20WFME26 | 26 | P60WFME24 |
| 3 | WFME23 | 15 | P20WFME27 | 27 | P60WFME25 |
| 4 | WFME24 | 16 | P40WFME1 | 28 | P60WFME26 |
| 5 | WFME25 | 17 | P40WFME12 | 29 | P60WFME27 |
| 6 | WFME26 | 18 | P40WFME23 | 30 | P80WFME1 |
| 7 | WFME27 | 19 | P40WFME24 | 31 | P80WFME12 |
| 8 | PME | 20 | P40WFME25 | 32 | P80WFME23 |
| 9 | P20WFME1 | 21 | P40WFME26 | 33 | P80WFME24 |
| 10 | P20WFME12 | 22 | P40WFME27 | 34 | P80WFME25 |
| 11 | P20WFME23 | 23 | P60WFME1 | 35 | P80WFME26 |
| 12 | P20WFME24 | 24 | P60WFME12 | 36 | P80WFME27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çamur, H.; Alassi, E. Physicochemical Properties Enhancement of Biodiesel Synthesis from Various Feedstocks of Waste/Residential Vegetable Oils and Palm Oil. Energies 2021, 14, 4928. https://doi.org/10.3390/en14164928
Çamur H, Alassi E. Physicochemical Properties Enhancement of Biodiesel Synthesis from Various Feedstocks of Waste/Residential Vegetable Oils and Palm Oil. Energies. 2021; 14(16):4928. https://doi.org/10.3390/en14164928
Chicago/Turabian StyleÇamur, Hüseyin, and Ebaa Alassi. 2021. "Physicochemical Properties Enhancement of Biodiesel Synthesis from Various Feedstocks of Waste/Residential Vegetable Oils and Palm Oil" Energies 14, no. 16: 4928. https://doi.org/10.3390/en14164928
APA StyleÇamur, H., & Alassi, E. (2021). Physicochemical Properties Enhancement of Biodiesel Synthesis from Various Feedstocks of Waste/Residential Vegetable Oils and Palm Oil. Energies, 14(16), 4928. https://doi.org/10.3390/en14164928







