3.1. Physicochemical Properties
The accumulation of clay fraction ranged from 4.22 to 14.8%. Soils are characterized by the alkaline reaction (pH in 1 M KCl 7.7–7.9 at the sites of influence of the emitter and 7.1 in the control). There was a significant variation found in the content of CaCO
3 (from 3.88% to 48.3%) and 1.85% in the control soil (
Table 1).
C, N, and P are the three macroelements that are necessary for plant growth and soil quality. The content of TOC ranged from 4.70 to 47.7 g kg
−1 (control 22.0 g kg
−1) (
Table 1). The concentration of TOC varied significantly depending on the soil sampling site. The highest concentration of TOC was at S6 (at the dried pond for ash waters). However, no significant differences in the content of TOC at S1, S2, and S5 were identified. According to Peinemann et al. [
36] and Wong et al. [
37], salinity adversely affects the content of organic carbon in salt-affected areas by increasing the dispersion of aggregates, which intensifies SOC mineralization, and by increasing bulk density, which restricts access to the substrate for mineralization. Saline soils contain carbonates that complicate the C dynamics and are also subjected to increased losses of SOM due to dispersion and leaching.
The content of total nitrogen varied across the sites. The highest TN was observed in the soil of S8 (4.36 g kg
−1), followed by S5 (3.29 g kg
−1). As compared to the control, it was 120% and 67% higher than the control (C—1.96 g kg
−1). The agricultural field (S7) showed a low TN value (1.77 g kg
−1) (
Table 1).
The value of TOC/NT ranged from 2.5 to 58.1 (control 1.1) (
Table 1). The differences of TOC/NT (
Table 1) demonstrate a varied rate of mineralization of organic C and N compounds. The narrow ratio of TOC/NT in the soils (S1, S2, S3, S4, S5, S7, S8, and C) indicates a very fast decomposition of organic matter by soil microorganisms, that nitrogen is more intensively mineralized, and that high amounts of N-NH
+4 are not used by the plants and gets accumulated in the ground. In the soil of S6, the value above 20 was recorded. Then, nitrogen immobilization occured. According to Lu and others [
38], this might be due to the higher cation contents in the S6 soil as confirmed by Dąbkowska-Naskręt and Bartkowiak [
39].
The value EC
e next to the soda plant ranged from 6.87 to 204 mS cm
−1 (control 4.11–5.97 mS cm
−1) (
Table 2). Earlier research of those soils [
40] showed that EC
e was increasing with depth. The highest EC
e value was found at a depth of 40–60 cm. The values varied significantly depending on the soil sampling site. The value EC
e S7 (agricultural field) was 66.35 mS cm
−1. The estimates show that by 2050, salinity will have affected 50% of all the global arable land [
41]. Most crops are sensitive to salinity [
1]. Crops grown in saline soils suffer due to the high osmotic stress and disturbed nutrients’ uptake, which decreases yields. According to Munns and Tester [
42], soils are saline when EC
e is 4 dS m
−1 or more (131), which is approximately 40 mM NaCl. The highest values (
p < 0.05) were found in the soil of S1 (up to 2000 places flooded with post-soda sludge), both in spring and autumn. The value EC
e in the soil was changing throughout the season. A significantly higher EC
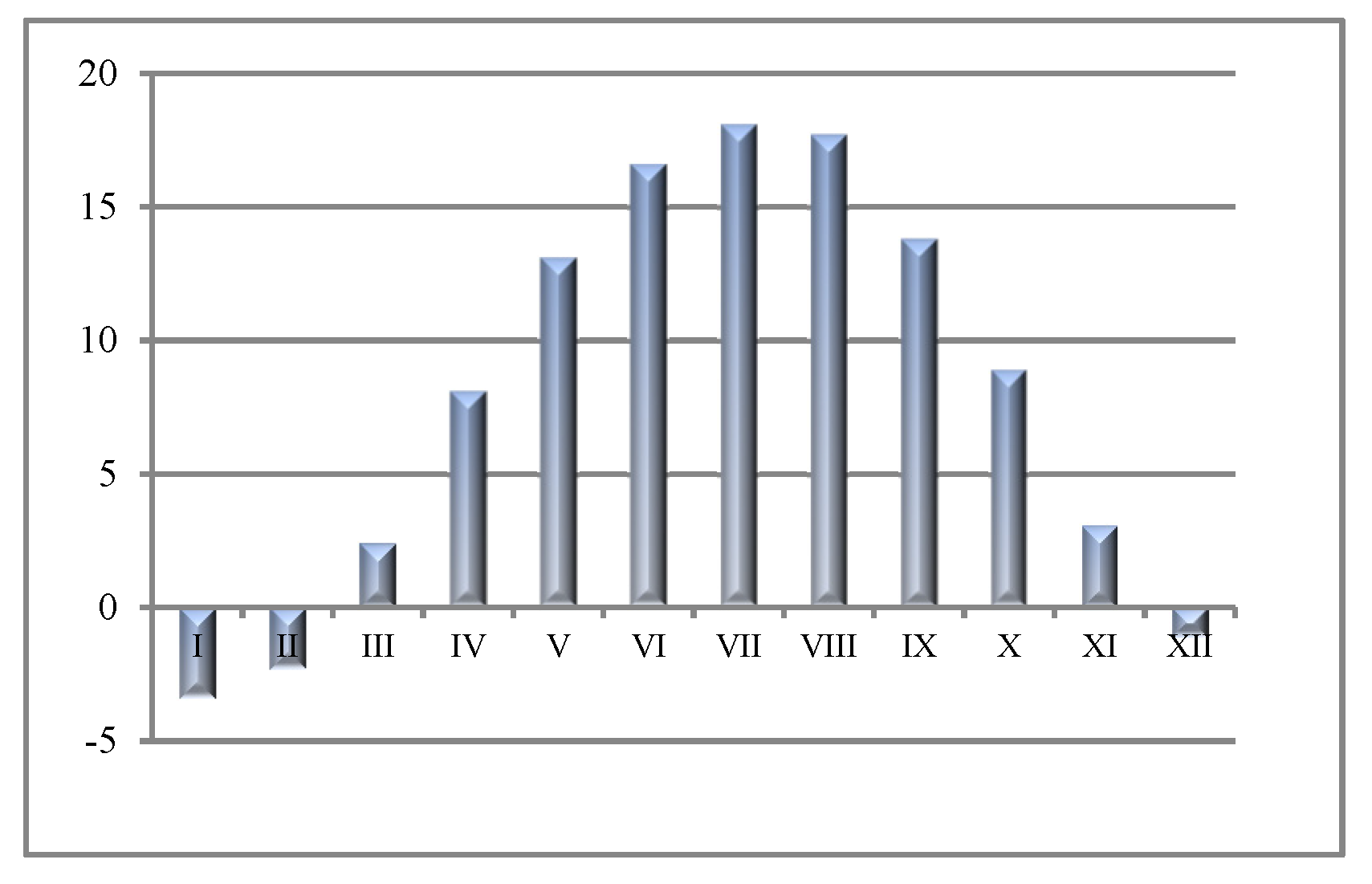
e value was recorded in spring compared to autumn. Changes in rainfall and evaporation are key factors in the content of salts in soil layers. During spring, there is less rain (April) than in autumn (October) in Inowrocław (
Table 1); thus, previously available moisture evaporated, leaving the salts on the soil surface. This increases the value of EC
e. Heavy rainfall can change the soil salinity (EC
e) and macro and micronutrient status of the soil due to leaching.
Total exchangeable bases (TEB) in the soil were significantly modified by the soil sampling site (in spring) (
Table 2). While higher TEB values were noted in the soil in autumn, there were no significant differences found in the TEB in the soils sampled from S1, S6, and S8.
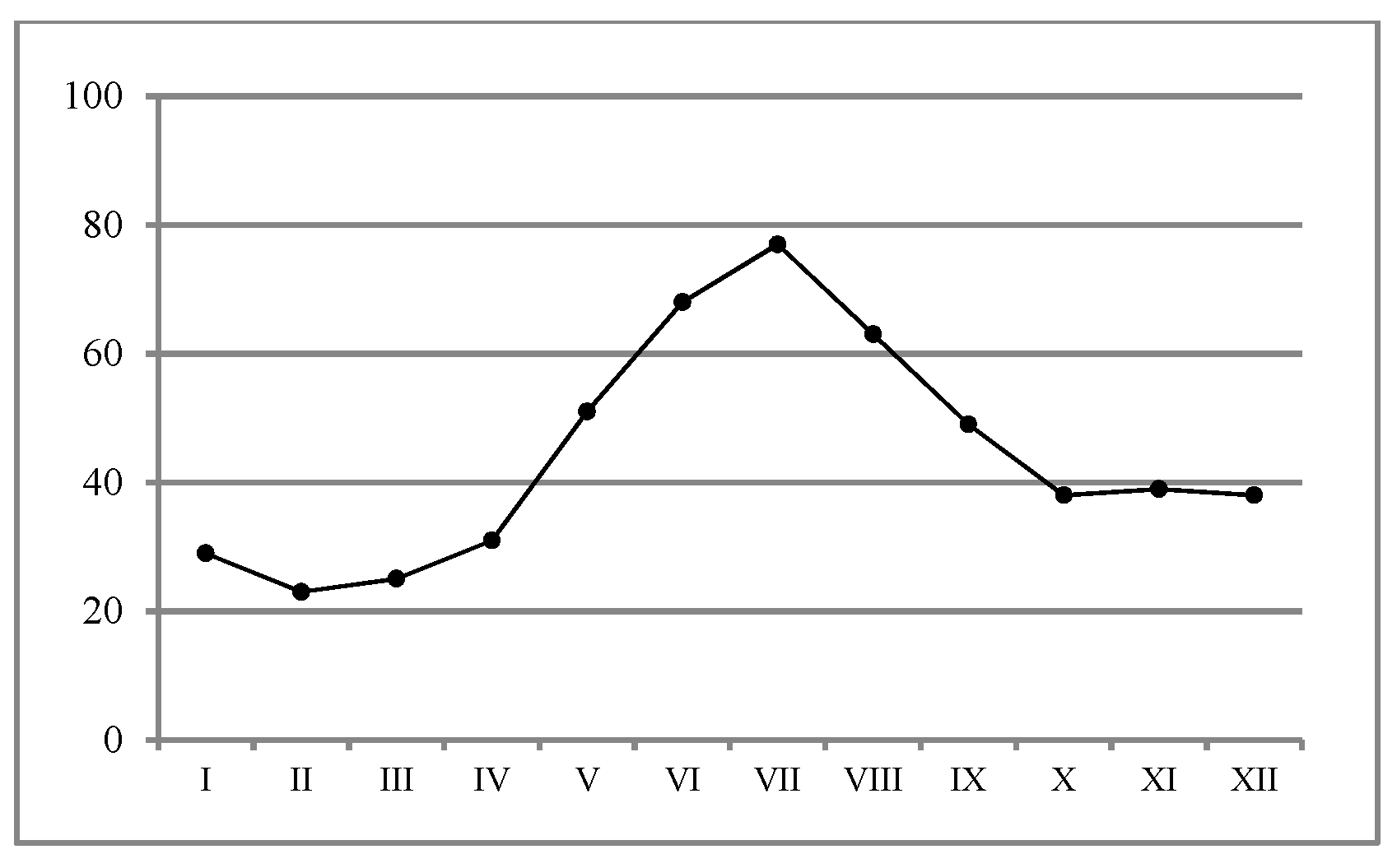
The content of AP in the soil ranged from 0.99 to 44.65 mg kg
−1 (in spring) (
Figure 4). In autumn, the content was significantly higher (
p < 0.05) and ranged from 3.92 to 49.5 mg kg
−1. The content of available phosphorus varied significantly depending on the soil sampling site. The highest accumulation was recorded for the control. In the soil within the influence of the soda plant, the AP decreased, and in the soil from the S1, S3, S6, and S8 sites (only in spring), the lowest AP content was recorded. The AP concentration was significantly negatively related to pH in KCl (
r = −0.652,
p = 0.0033) and EC
e (
r = −0.481,
p = 0.0430). However, the two parameters only in 42.5% and 23.1% determined the variation in AP in the soil. The results of the investigations by Dąbkowska-Naskręt and Bartkowiak [
39] in the soil near the soda plant showed that with the cations affecting salinity measured with EC
e were calcium and, to a lesser extent, sodium. According to Fageria et al. [
43], phosphate availability in saline soils is highly controlled by both sorption processes and the low solubility of Ca-P minerals. However, Artamonova, et al. [
44] stated that in the case of salinization, the degree of Ca
2+ saturation of the soil is reduced due to its replacement with Na
+ and Mg
2+. The toxic effect of Na
+ increases in the presence of Cl
−, sharply reducing the absorption of N, P, and K. The regression equation shows that with an increase in pH in KCl by 1 unit, the content of P decreased, lined by 45.83 mg kg
−1. According to Bano and Fatima [
45], soil salinity and pH > 7.5 significantly reduces the plant P uptake as phosphate ions precipitate with Ca ions. However Pan et al. [
46], Mahmood et al. [
47], and Lemanowicz and Bartkowiak [
20] reported that most saline soils are adequately supplied with P as the content of sodium ions may result in more soluble Na
3PO
4 being formed. In the soil from S7 (arable field), the content of AP was 24.2 mg kg
−1 (spring) and 39.7 mg kg
−1 (autumn), which classifies the soil as part of the IV (low) class of concentrations in P [
48]. The soil showed higher AP contents in autumn as compared to spring. According to Lu et al. [
38], the differences in seasonal nutrient changes across different sampling areas could have been due to the differences in plant uptake, soil properties, and hydrological conditions.
3.2. Soil Enzyme Activities
The enzymes’ activities at all the sites across the months are provided in
Table 3. The activity of the enzymes is to the parameter specifying the quality soil (e.g., the content of organic matter and cycle of macronutrients including C, N, P, and S). Enzymes are involved in the breakdown of various types of pollutants (e.g., salinization and heavy metals). The impacts of the months and sites of the activities of soil enzymes varied. The months demonstrated the strongest effect on the activity of CAT (
η2 33.54%) and AlP (
η2 12.6%), and the weakest effect on the activity of AcP (
η2 8.38%).
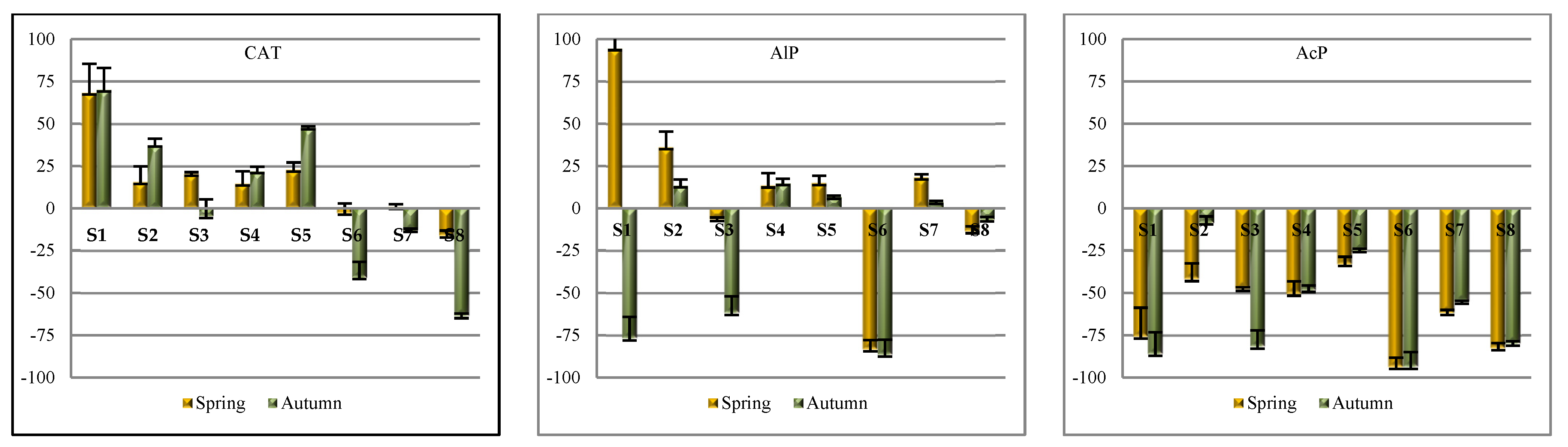
The activity of the other enzymes was higher in spring than autumn, which is also seen from the values of coefficient
TI < 0 (except for the activity of AlP assayed at soil site 8) (
Figure 5).
The seasonal changes in soil enzyme activity are a derivative of the hydrothermal conditions; the distribution of temperature and precipitation as the factors with the highest effect on the biological activity of soil [
49]. Shao et al. [
50] stated that the activity of some enzymes (urease, invertase, and alkaline phosphatase) had lower activity in winter and higher in summer. The analysis of coefficient
η2 has demonstrated that the soil sampling around the soda plant accounted for the variation in the activity of enzymes (alkaline phosphatase accounted for 51%, catalase for 60%, and acid phosphatase for 75.57%) more considerably as compared to the research months (
Table 3). There were no significant differences found in the activity of catalase in the soil at sites S2, S3, S4, and S5 (
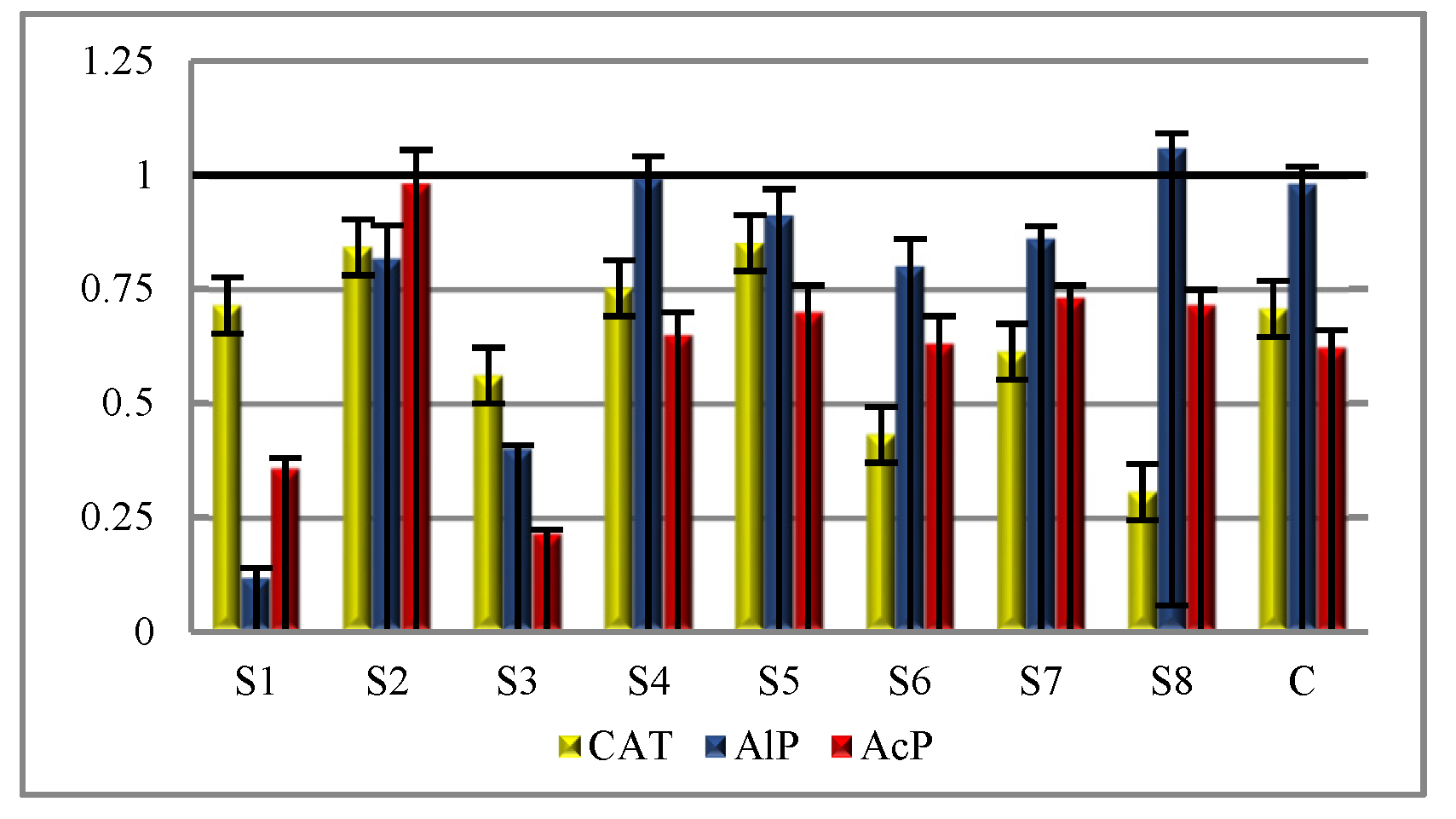
Table 3). The significantly highest activity of that enzyme was reported at site S1 and the lowest at site S8 (spring and autumn). The study of the
RCh index of the catalase activities showed that in the soil from sites S1, S2, S3, S4, and S5, the activity increased in comparison to the control. In soil sites S6, S7, and S8, the decrease was greater, from 2.88% to 63.94% (
Figure 6). The available literature shows that of all the soil enzymes, the ones most sensitive to salinization are oxidoreductases [
51,
52,
53], especially catalase, which is also confirmed by the present study.
The ANOVA analysis indicated that alkaline phosphatase was affected by the method of use of the soil next to the soda plant (
p < 0.05) (
Table 4). The highest AlP activity in spring was reported in at soil site S1 (3.124 mM pNP kg
−1 h
−1) and in autumn (1.803 mM pNP kg
−1 h
−1) at soil site S4, whereas the activity of acid phosphatase was highest in the control in spring (4.645 mM pNP kg
−1 h
−1) and autumn (2.894 mM pNP kg
−1 h
−1). In the soil under the impact of the soda industry, the activity of AcP decreased as compared to the control, which is seen from the negative values of coefficient
RCh (
Figure 6). There were no significant differences identified (
p < 0.05) in the activity of AcP across S2, S3, S4, and S5 (spring) (
Table 4). The alkaline phosphatase activities did not exhibit a response to the salinity. Due to the concentration of ions Na
+ and Cl
− in the soil solution, salting-out of enzymatic proteins occurred. This involved the reduction in enzyme solubility through dehydration, thus altering the enzyme ‘catalytic site’ and resulting in a decrease in the activity of enzymes [
54]. In addition, this affects enzyme activities by denaturing proteins and decreasing their solubility. The study by Garcia-Gil, et al. [
55] showed that soil salinity disperses the fraction clays, resulting in the enzymes becoming less protected and denaturated.
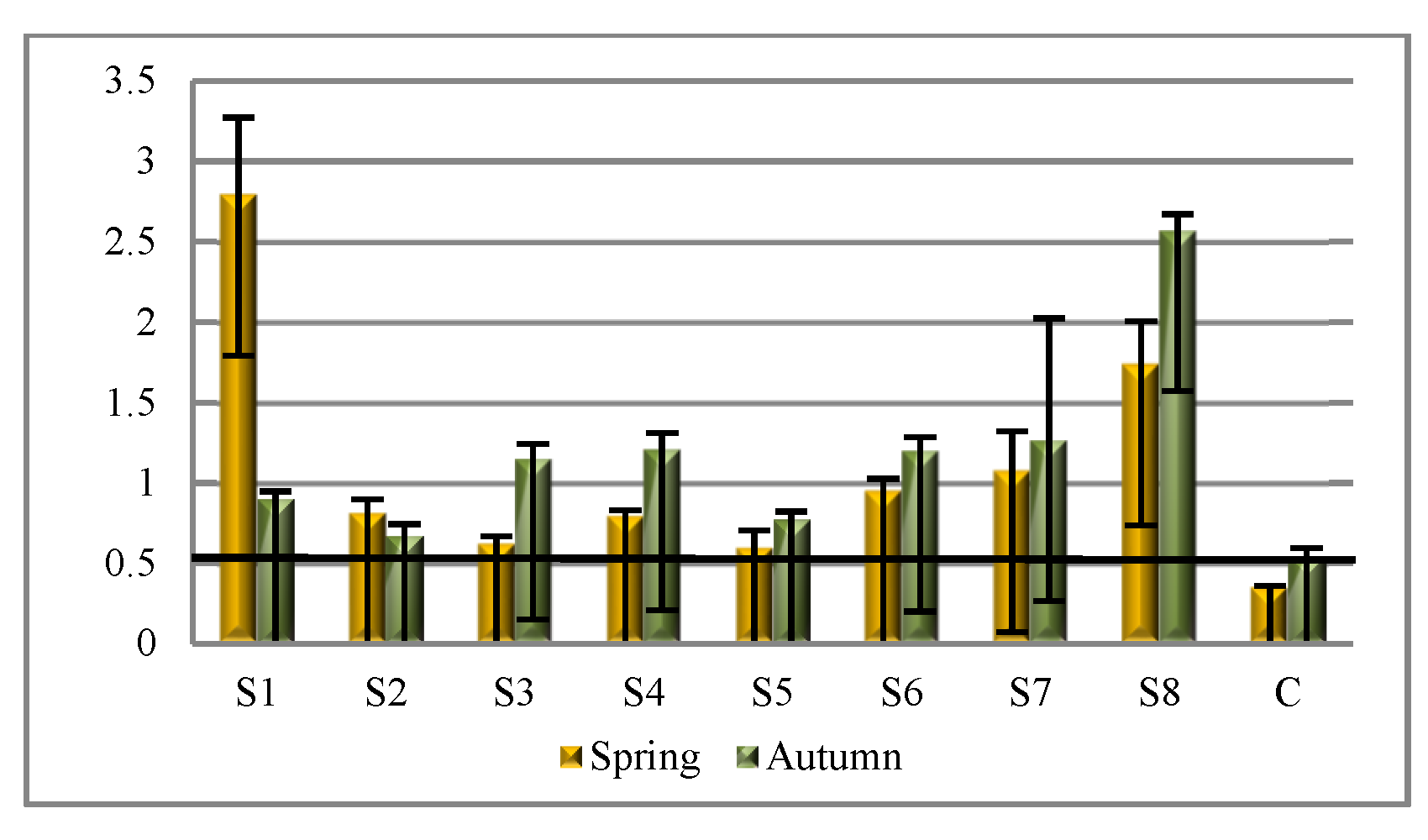
Based on the results of the activity of both phosphatases, the enzymatic pH index was calculated (
Figure 7). The values at all the soil sampling sites within the impact of the soda plant exceeded the value of 0.5, above which we can consider the soil to be alkaline [
31], which is confirmed by the values of pH in KCl. The highest values were recorded at S6, S7, and S8.
The resistance of saline soil is not covered in the literature. The resistance of soil and its ability to renew the balance is for important quality elements and basic indicators between the impact caused by salinization and the soil capacity for regeneration [
56]. The
RS values showed that the activities of enzymes varied in their sensitivity to the soil next to the soda plant. According to Orwin and Wardle [
32], Borowik and others [
57], and Bartkowiak et al. [
53], the soil resistance factor is an effective measure of enzymatic responses to environmental stress. Higher values of index
RS show that the disturbances had an inconsiderable effect (maximum resistance). The highest average
RS value was noted for catalase (in spring) (
RS = 0.702) and the lowest for acid phosphatase (in spring) (
RS = 0.261) (
Table 4). Low
RS values are indicative of long-term toxic effects of salinity. The lowest values of
RS for alkaline and acid phosphatase were recorded in the soil at S6. Regardless of the soil sampling site, enzyme resistance was generally lower in spring than autumn, excluding catalase, the resistance of which in spring was 29.6% higher than in autumn.
The resilience (
RL) index is provided in
Table 5. The study demonstrated the recovery of acid phosphatase activity (the mean
RL = 0.288) and alkaline phosphatase (
RL = 0.142), which was proven by the mean positive
RL values. In some samples, especially for catalase, the soil resilience (
RL) index was negative. According to Baćmaga et al. [
58], it reflects the progressing harmful anthropogenic impact on soil biology from spring to autumn.
Statistical analysis correlation proved that the enzymes and AP in the soil were correlated with some parameters at
p < 0.05 (
Table 6). In our research, catalase proved to be more affectionate to soil salinization. With the analysis of the relation, there was a positive dependence found between the activity of catalase and EC
e (
r = 0.623,
p = 0.0057), and a negative dependence with TEB (
r = −0.523,
p = 0.0258), while the inhibition of the activity of catalase depended on the increasing content of salt in the soil due to the introduction of sodium chloride as reported by Telesiński [
52]. The activity of acid phosphatase was also negatively correlated with TEB (
r = −0.497,
p = 0.0355). According to Pan et al. [
45], the enzyme activity related to the salinity of the soil varied depending on the type of enzyme and degree of salinization. Catalase takes part in the defense of the plants against external factors triggering oxidation stress in plants such as growing in saline soils. In saline soils, the process of protein salting-out occurs, therefore enzymes lose their biological activity. The study by Aechra et al. [
59] revealed that the application of high soil salinity decreased the concentration of TP and AP, and the activity of DEH and AlP. In contrast, the study by Shirale et al. [
60] demonstrated that the soils containing high amounts of Na
+ hindered the microbial number under the restricted use of organic supplements, which resulted in a decrease in the activity of AcP of Mollic Gleysols.
Acid phosphatase activity was positively related with AP (
r = 0.597,
p = 0.0089), which indicates that the soil was more effectively organic P-mineralizing. The explanation of that phenomenon is complex because there is a probability of a long-term occurrence of soil enzymes in bonds with colloids. The activities of enzymes are correlated with soil organic matter concentration as they plays the key role as initiators for enzyme synthesis. However, in our study, the correlation between TOC and AcP was negative (
r = −0.509,
p = 0.0355). There was a negative correlation found between the content of CaCO
3, the AlP (
r = −0.595,
p = 0.0092), and AcP (
r = 0.527,
p = 0.0244). However, according to Acosta-Martínez and Tabatabai [
61], the alkaline and acid phosphatases react to lime differently because these enzymes are inductive and the intensity of their excretion by plant roots and microorganisms is determined by their requirement for orthophosphate, which is affected by soil pH. The research showed that CAT, AlP, and AcP were all positively significantly related with each other (
Table 6), indicating that the enzyme activity can other the activity of another enzyme in the soil to a large extent. Another measure to evaluate the dependence of the activity of CAT, AlP, and AcP on physical and chemical soil properties is the regression equation and the
R2. With the value of
R2, it was found that the activity of catalase was only 38.8% dependent on EC
e and 27.3% on TEB. Similarly, the activity of AcP was only 24.7% dependent on TEB and 25.9% on TOC, while about 75% can be accounted for by other soil parameters. In terms of the content of AP, only 23.1% was dependent onEC
e, 42.5% by pH KCl, and 37.2% by CaCO
3. The regression equation shows that with an increase in pH by 1 unit, the content of AP decreased by 45.83 mg kg
−1.
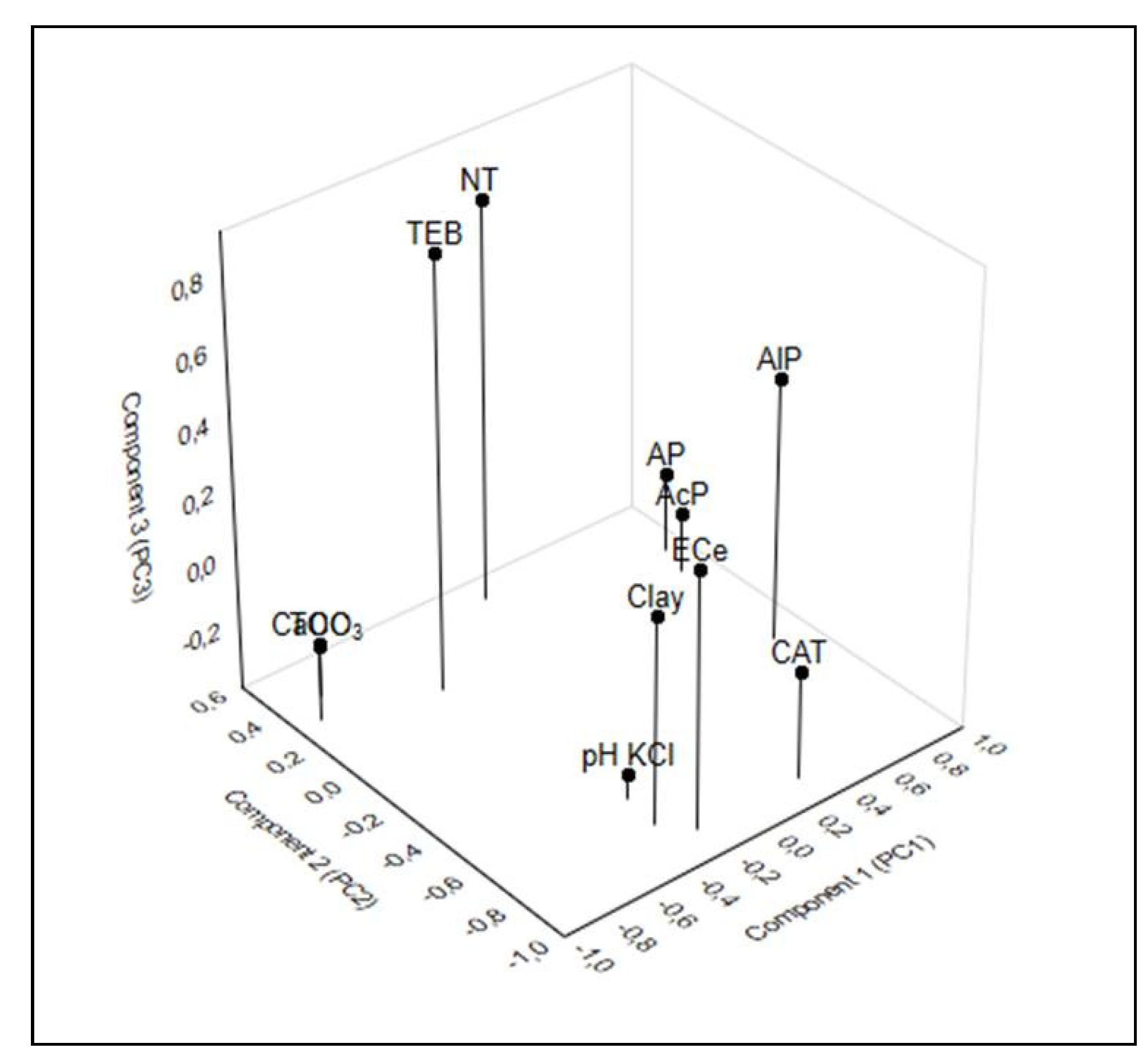
To define the nature and strength of dependencies of CAT, AlP, and AcP to the selected soil properties (grain size composition, pH in KCl, TOC, NT, AP, and TEB) and environmental variables, the principal component analysis (PCA) was applied. The drawing (
Figure 8) shows that three principal hypothetical reasons of variation (PC1, PC2 and PC3) allowed to account for a total of 92.72% of the variation.
The PC1 conveys 41.37% of the information on the soil properties fitting into input variables. Most of the variance in the PC1 was negatively correlated with the content of TOC (−0.791) and CaCO
3 (−0.887), and positively with the content of AP (0.901), AlP (0.833), and AcP (0.854) (
Table 7). The PC2 accounts for 31.98% of the variable data. It showed a negative correlation with the activity of CAT (−0.865), EC
e (−0.860), and clay (−0.758). The PC3 describes the role of NT (0.793) and TEB (0.859), and it accounts for 19.37% of the total variation (
Figure 8). Research by Liu et al. [
62] showed that these factors can be assumed as strong (>0.750). The use of the PCA is important in the interpretation of how the soil is used because it can identify soil-next-to-soda-industry variables that can be excluded to remove repetitive and difficult-to-measure information. PCA is found through the method for selecting more effective indexes in soil sustainability [
63].
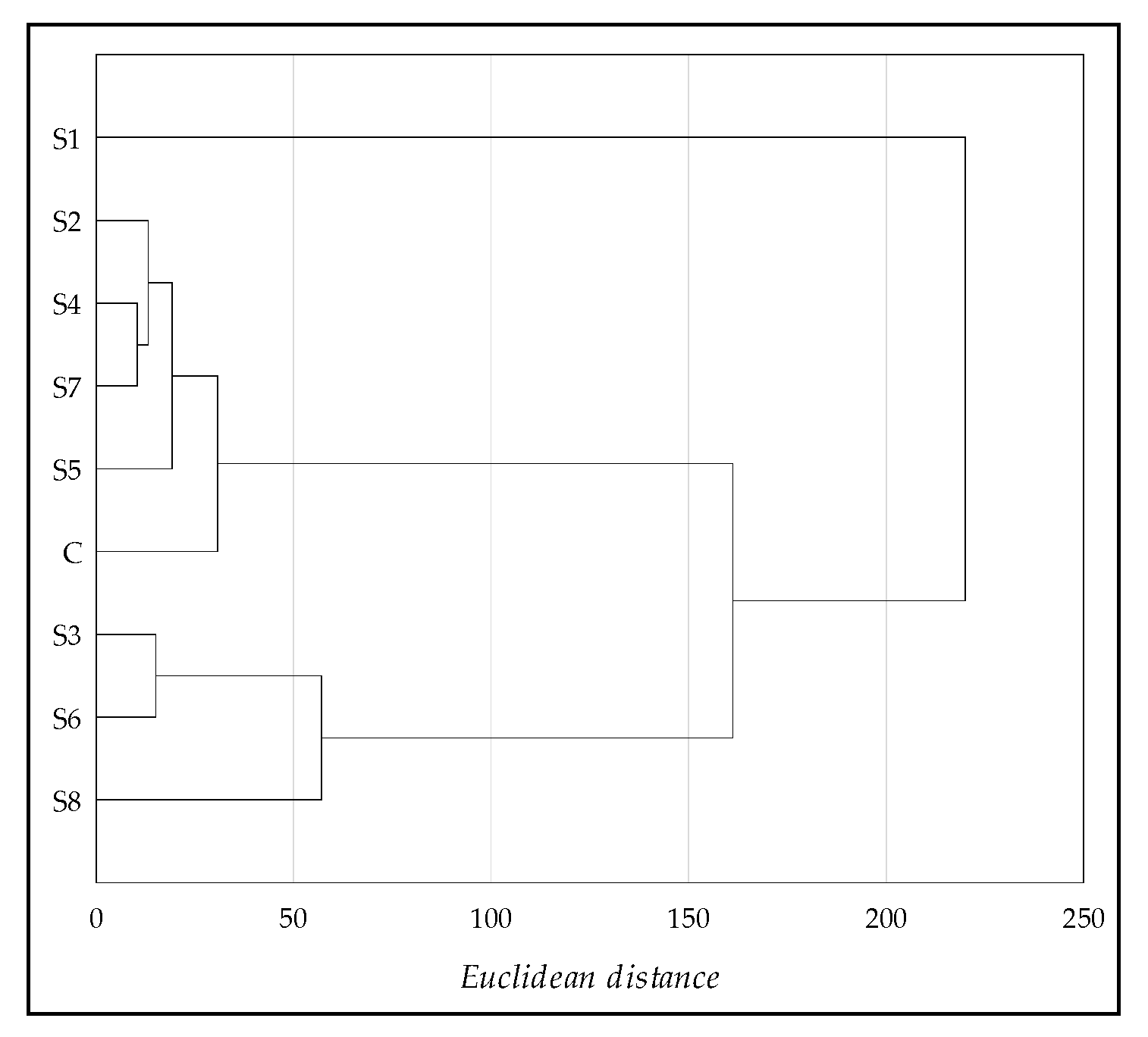
To determine the similarities across the eight sites next to the soda plant and the control, the grouping method by Ward [
35] was used. Physicochemical and enzymatic soil properties were applied. The results of the cluster analysis (CA) are shown with the dendrogram in
Figure 9. The grouping procedure differentiated two clusters with soils with similar properties and one outlier (S1 is the lowest phosphorus content and the highest EC
e). Cluster 1 groups soils S2, S4, S5, S7, and the control (the highest phosphorus content and the lowest EC
e), while cluster 2 groups soils S3, S6, and S8, which differed from the control soil in terms of the properties studied (the highest TOC, TEB, and CaCO
3 content).