Fuel Improvement Measures for Particulate Matter Emission Reduction during Corn Cob Combustion
Abstract
:1. Introduction
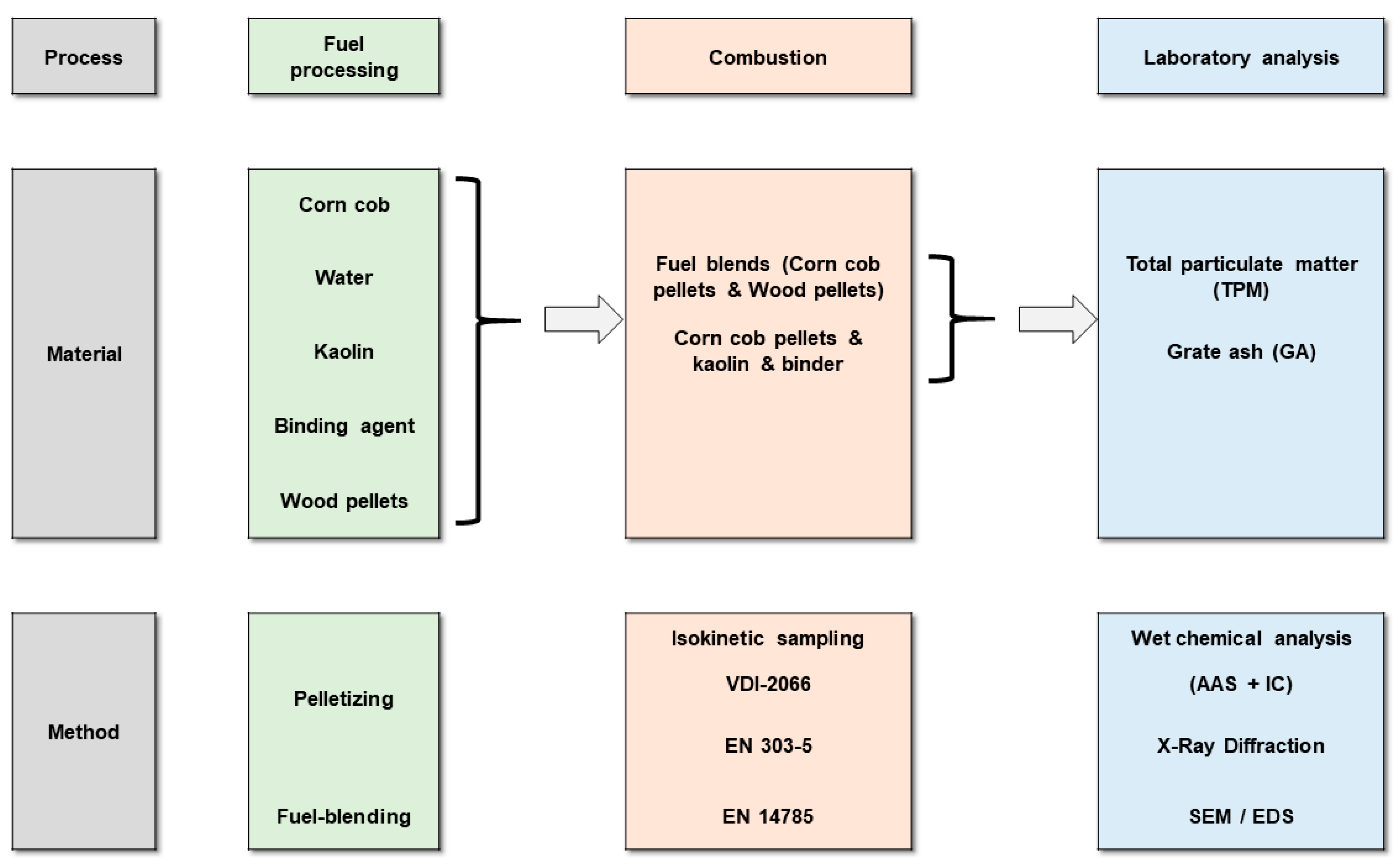
2. Materials and Methods
2.1. Feedstock and Additive
2.2. Experimental Setup
- Fuel blends made of corn cob pellets (no additive no binder) with wood pellets in specific ratios, represented by WP, 12.5CCP (e.g., 12.5 wt. % or 1/8 of corn cob pellets in the fuel blend), 25CCP, 50CCP, and CCP. This fuel group should asses the effect of fuel blending as a primary fuel-related measure.
- Corn cob pellets with and without kaolin (and starch-based binder), which are represented by fuels CCP, CCPB, CCPB0.5KAO, CCP1KAO, CCPB1.5KAO, and CCP2KAO. Fuels CCPB, CCPB0.5KAO, and CCPB1.5KAO are prepared with 2 wt. % starch-based binder and Kaolin B, whereas all other corn cob pellets are produced without the binder (additivized pellets) with Kaolin A. This fuel group should assess the effect of additivation and pelletizing as primary fuel-related measures.
2.3. Measurement and Analysis Methods
3. Results and Discussion
3.1. Performance Indicators
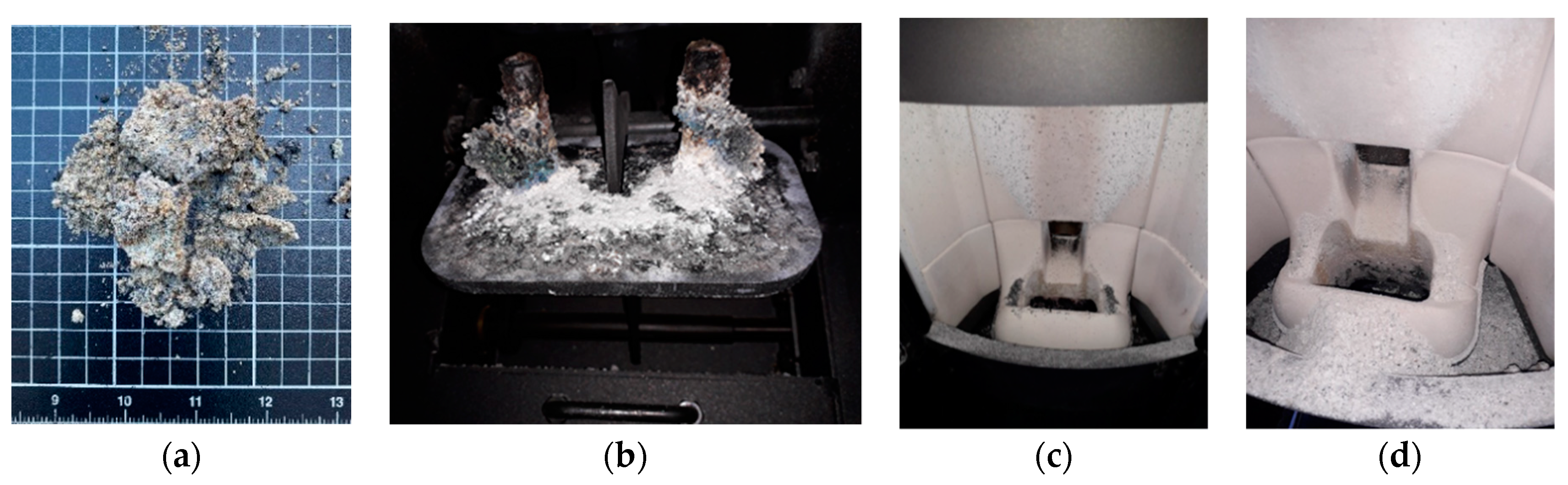
3.2. Ash Sintering
3.3. Emissions
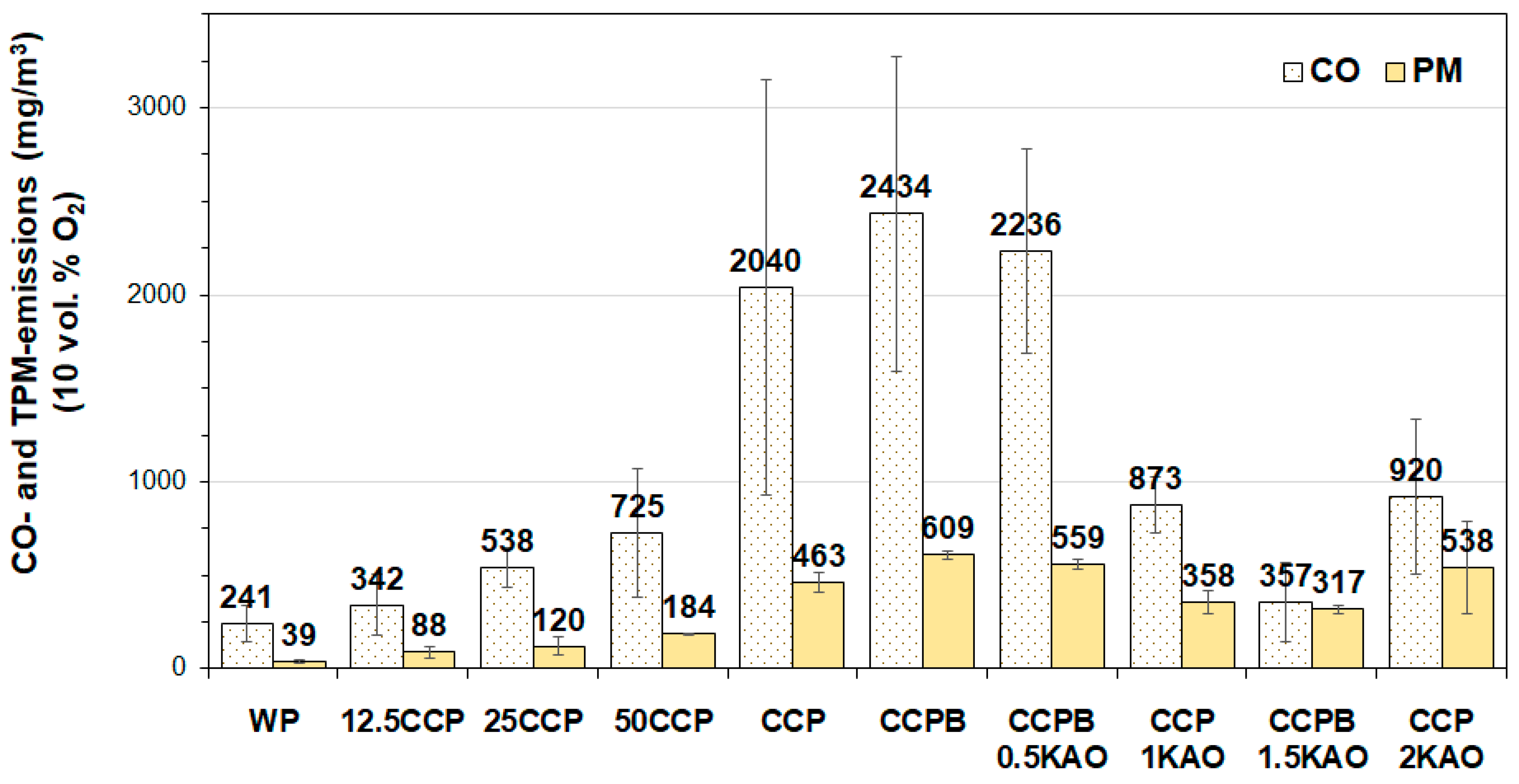
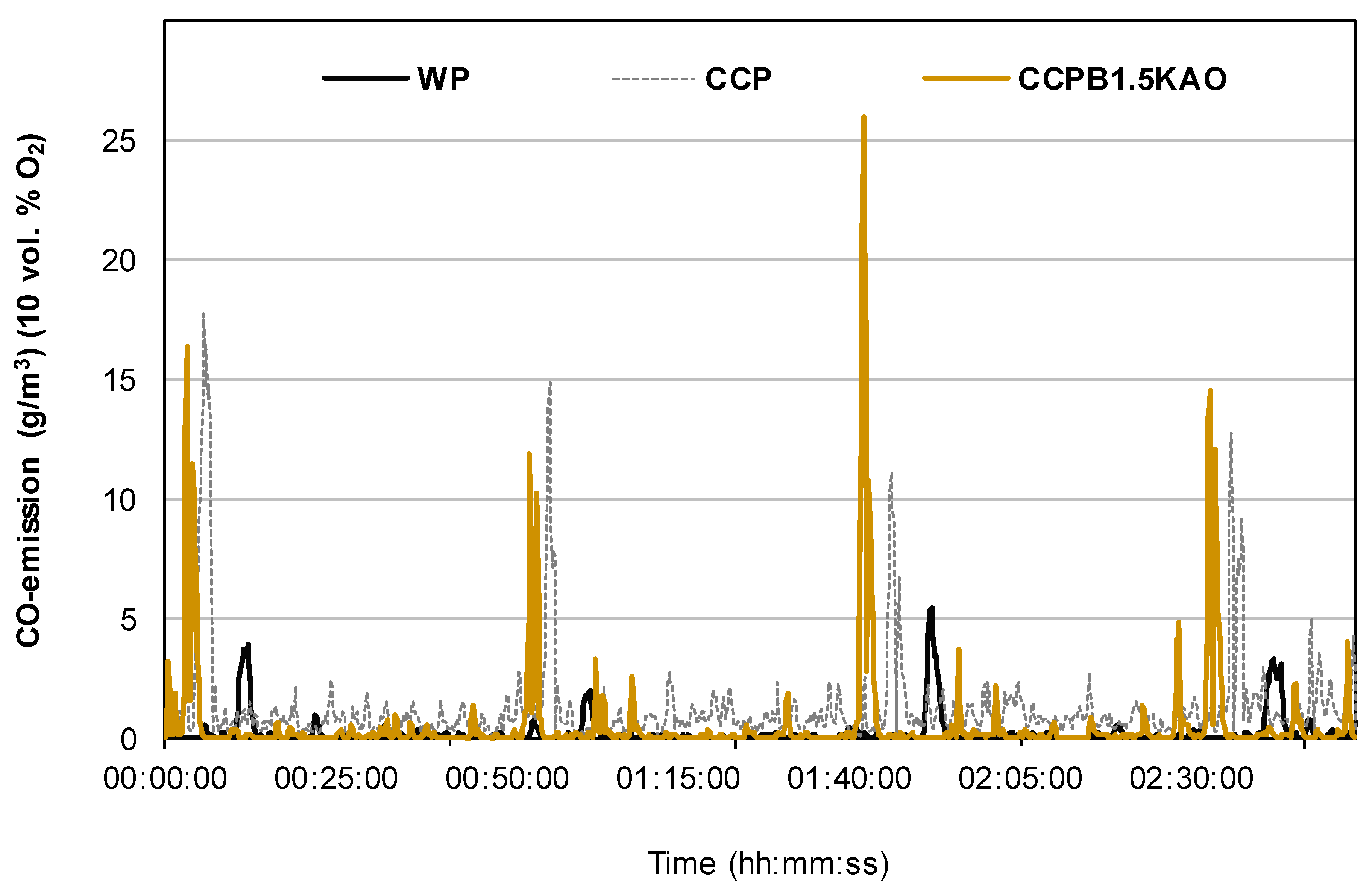
3.3.1. CO-Emissions
3.3.2. Total Particulate Matter Emissions
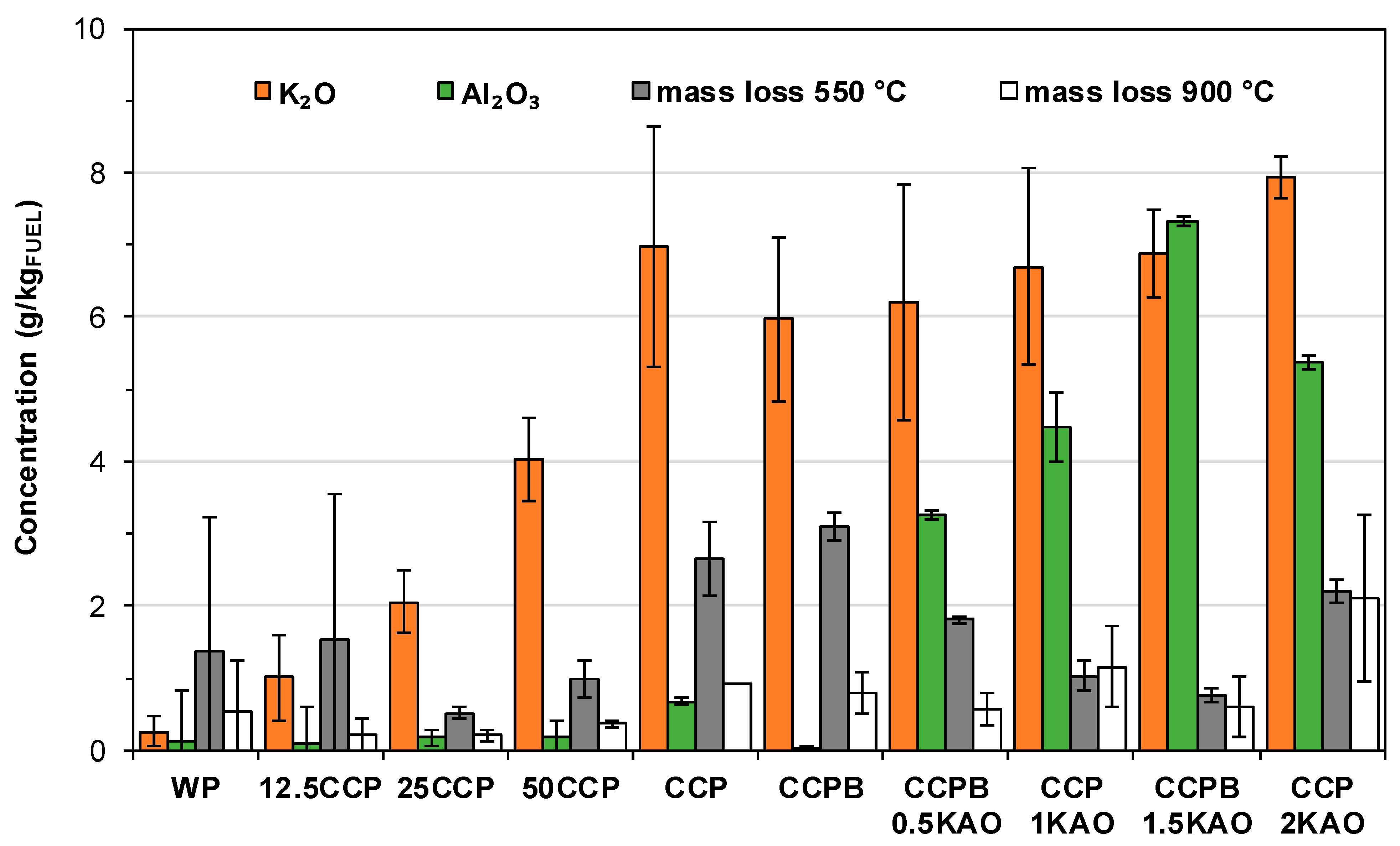
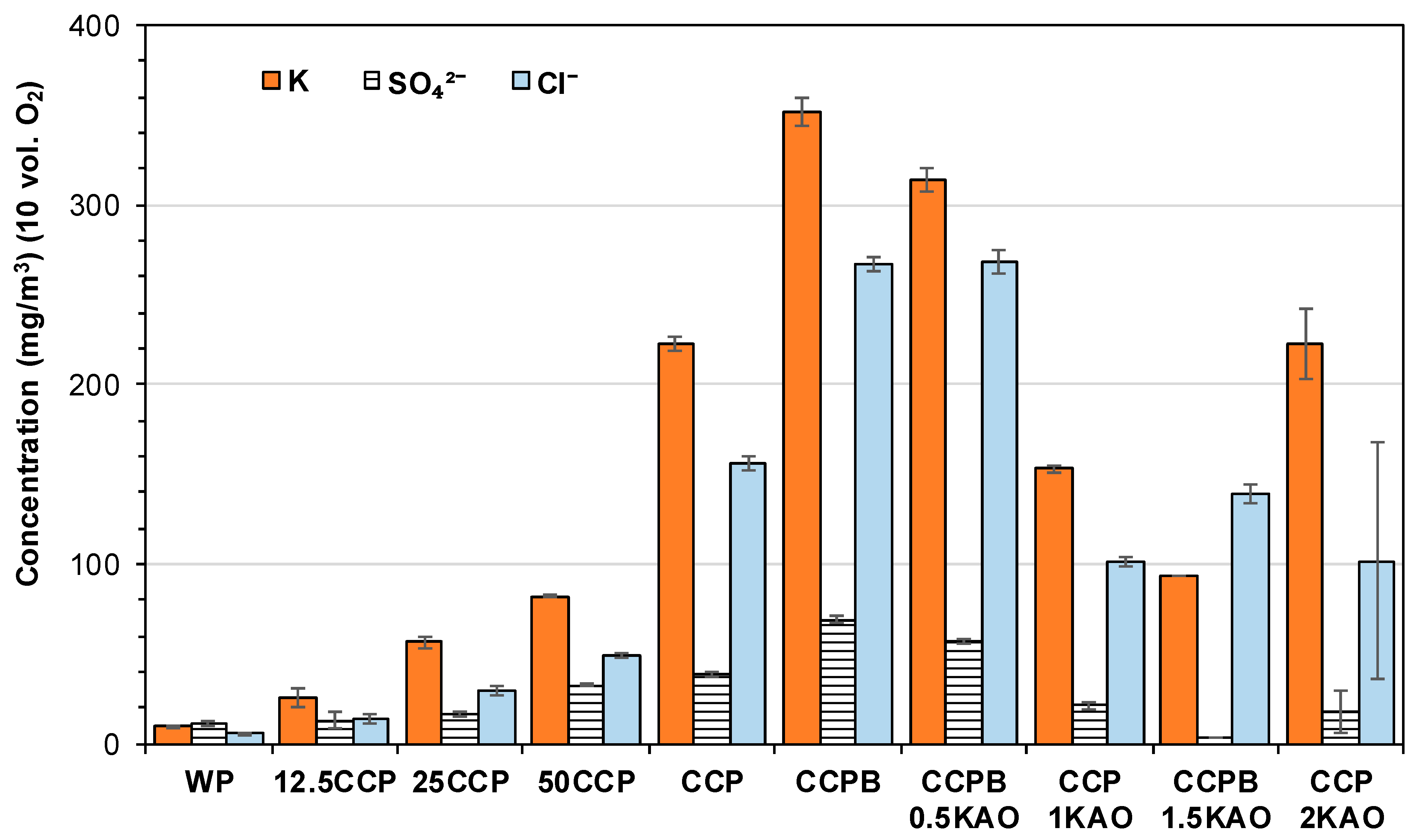
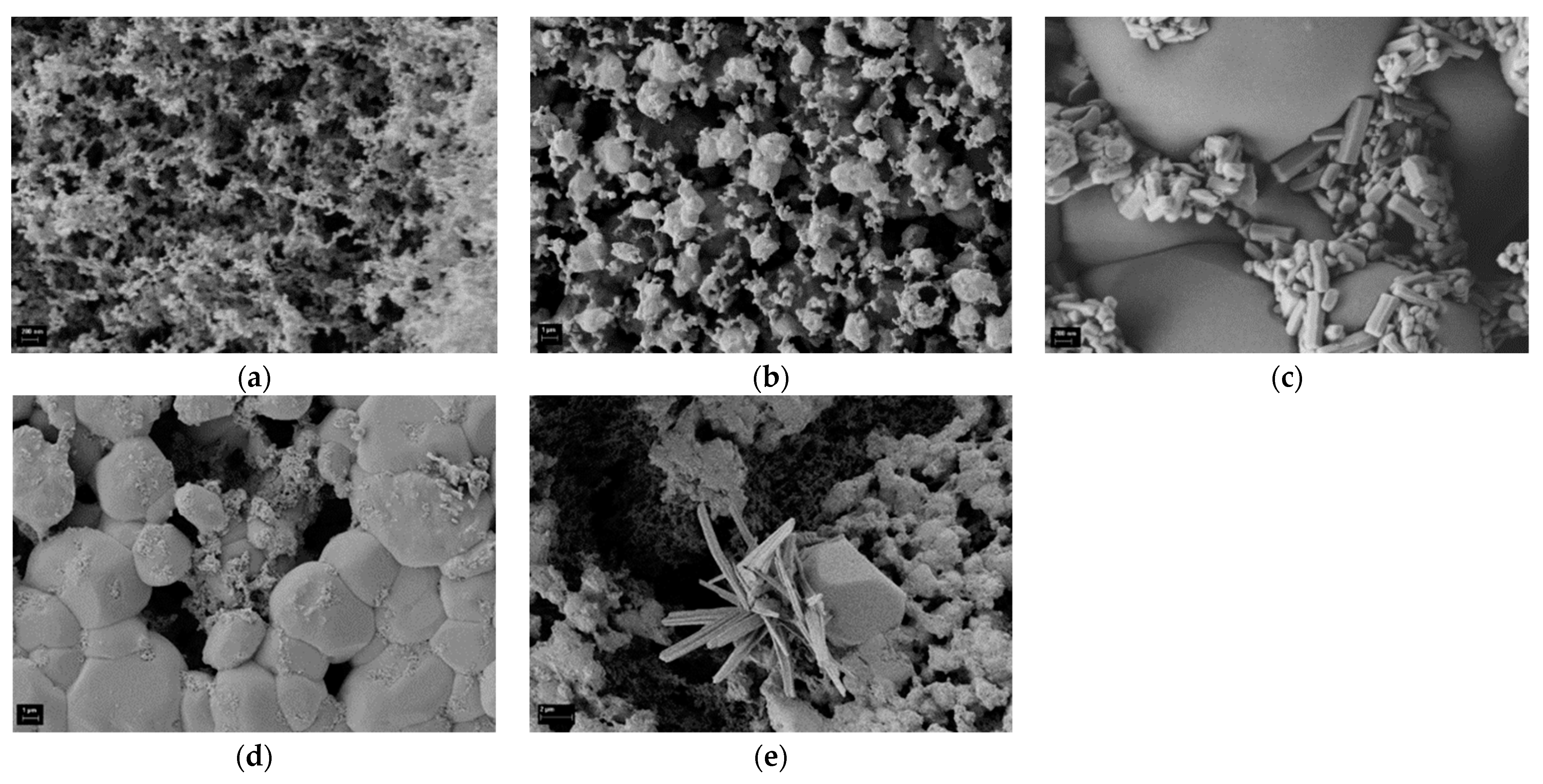
3.4. Composition of Solid Combustion Products
3.4.1. Grate Ash
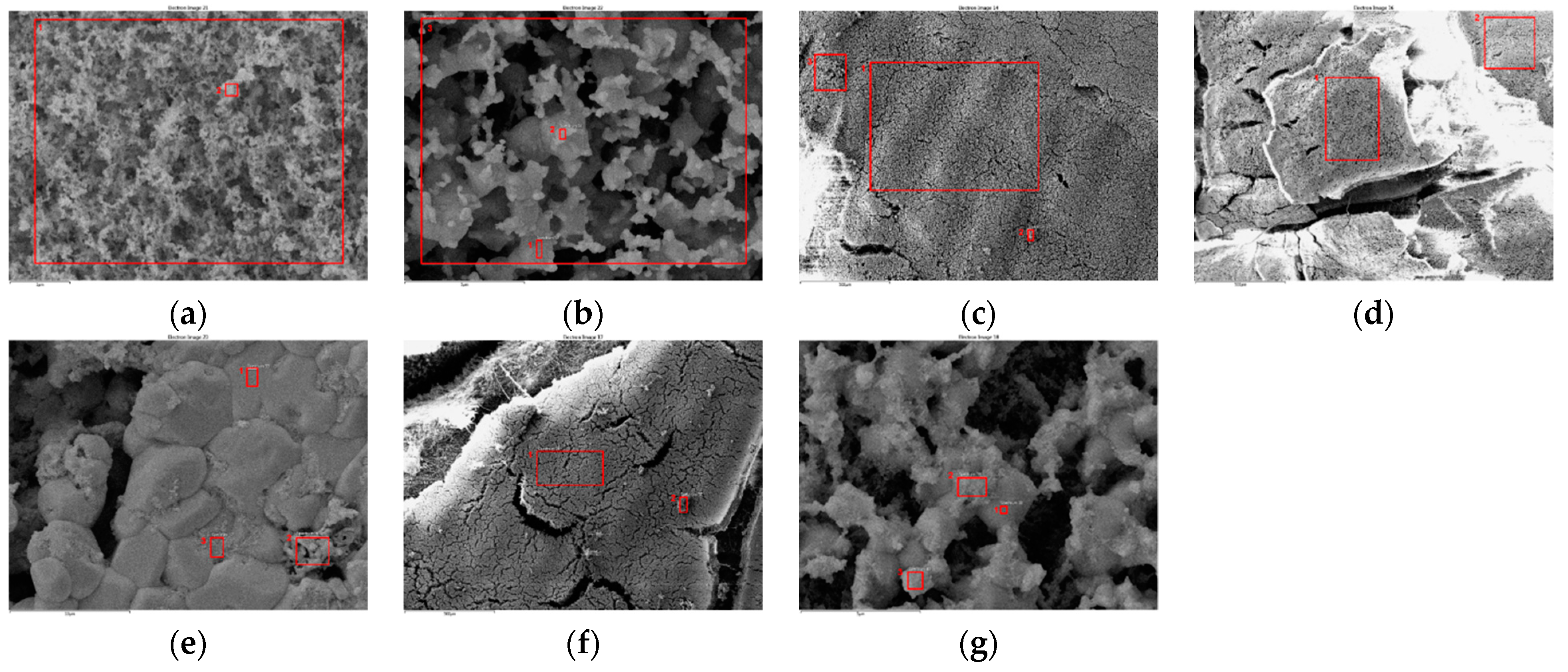
3.4.2. Particulate Matter
4. Conclusions
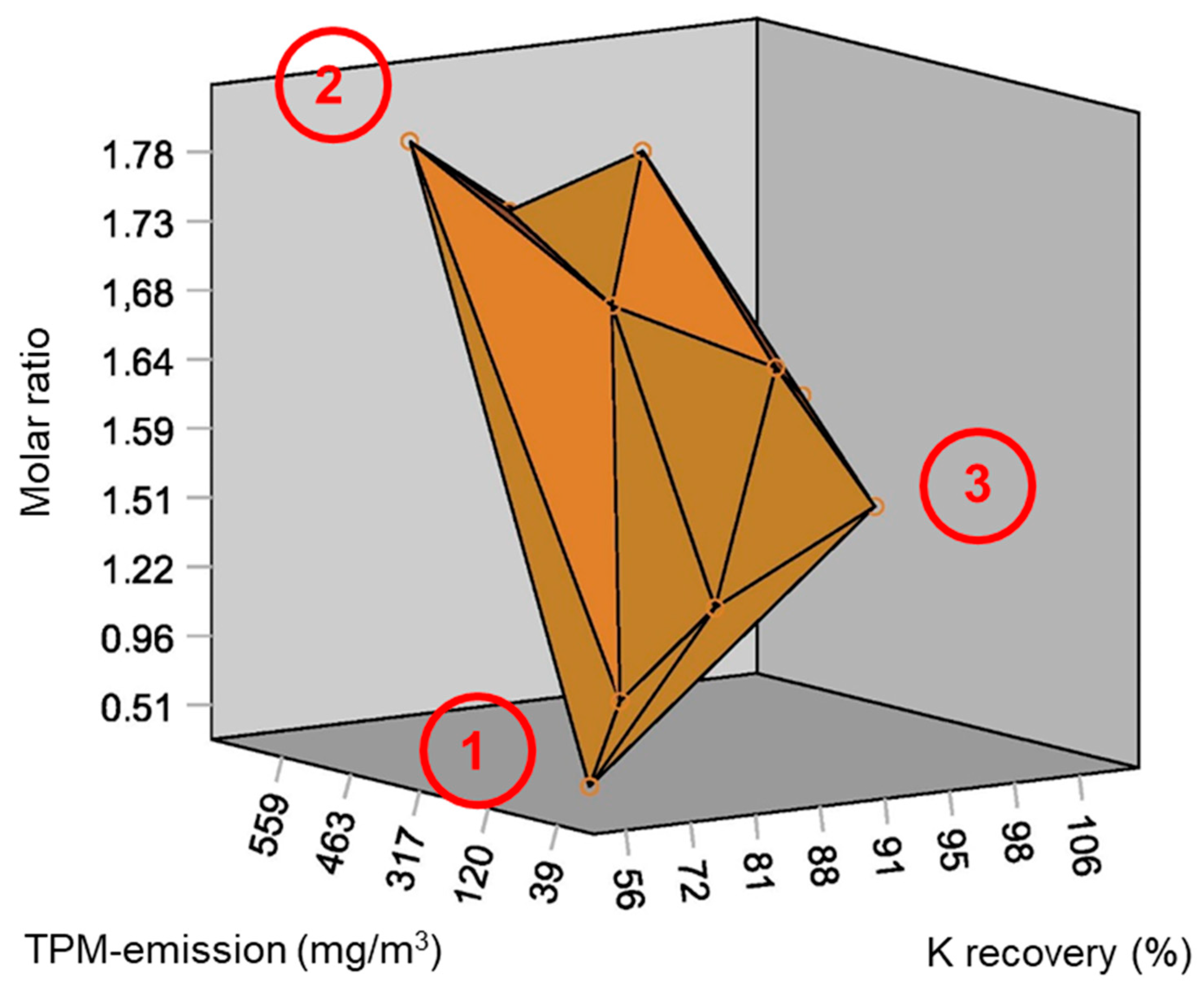
- Blending 50 wt. % corn cob pellets with 50 wt. % wood pellets resulted in 60 wt. % and 64 wt. % reduction in TPM-emissions and CO-emissions, respectively. Fuel blending influences TPM-emissions by reducing total K content of the fuel.
- Using 1.5 wt. % kaolin as additive, resulted in approximately 48 wt. % and 89 wt. % reduction in TPM- and CO-emissions respectively, as observed. Kaolin binds volatile alkali metals in high-temperature stabile K-Al-silicates in the grate ash, thereby minimizing TPM-emission and preventing ash sintering. However, entrainment of ash into the combustion chamber was considerable due to increased ash content.
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- International Renewable Energy Agency (IRENA). World Energy Transitions Outlook: 1.5 °C Pathway. Abu Dhabi, 2021. Available online: https://www.irena.org/publications/2021/Jun/World-Energy-Transitions-Outlook (accessed on 24 July 2021).
- Rebbling, A.; Sundberg, P.; Fagerström, J.; Carlborg, M.; Tullin, C.; Boström, D.; Öhman, M.; Boman, C.; Skoglund, N. Demonstrating Fuel Design To Reduce Particulate Emissions and Control Slagging in Industrial-Scale Grate Combustion of Woody Biomass. Energy Fuels 2020, 34, 2574–2583. [Google Scholar] [CrossRef]
- FAOSTAT. FAOSTAT Crop Statistics. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 16 November 2020).
- Kaltschmitt, M.; Hartmann, H.; Hofbauer, H. Energie aus Biomasse; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-47437-2. [Google Scholar]
- Wang, C.; Wu, Y.; Liu, Q.; Yang, H.; Wang, F. Analysis of the behaviour of pollutant gas emissions during wheat straw/coal cofiring by TG–FTIR. Fuel Process. Technol. 2011, 92, 1037–1041. [Google Scholar] [CrossRef]
- Brunner, T.; Kanzian, W.; Obernberger, I.; Theissl, A. Combustion properties of maize cobs-results from lab and pilot scale tests. In Proceedings of the 19th European Biomass Conference and Exhibition, Berlin, Germany, 6–9 June 2011. [Google Scholar]
- Sommersacher, P.; Brunner, T.; Obernberger, I. Fuel Indexes: A Novel Method for the Evaluation of Relevant Combustion Properties of New Biomass Fuels. Energy Fuels 2012, 26, 380–390. [Google Scholar] [CrossRef]
- Zeng, T.; Kuptz, D.; Schreiber, K.; Schön, C.; Schulmeyer, F.; Zelinski, V.; Pollex, A.; Borchert, H.; Loewen, A.; Hartmann, H.; et al. Impact of adhering soil and other extraneous impurities on the combustion and emission behavior of forest residue wood chips in an automatically stoked small-scale boiler. Biomass Conv. Bioref. 2019, 9, 99–116. [Google Scholar] [CrossRef]
- Xiong, S.; Burvall, J.; Örberg, H.; Kalen, G.; Thyrel, M.; Öhman, M.; Boström, D. Slagging Characteristics during Combustion of Corn Stovers with and without Kaolin and Calcite. Energy Fuels 2008, 22, 3465–3470. [Google Scholar] [CrossRef]
- Aho, M.; Paakkinen, K.; Taipale, R. Quality of deposits during grate combustion of corn stover and wood chip blends. Fuel 2013, 104, 476–487. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H.; Hui, S. Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Szymajda, A.; Łaska, G.; Joka, M. Assessment of Cow Dung Pellets as a Renewable Solid Fuel in Direct Combustion Technologies. Energies 2021, 14, 1192. [Google Scholar] [CrossRef]
- Comm/dg/unit. EU Climate Action and the European Green Deal—Climate Action—European Commission. Available online: https://ec.europa.eu/clima/policies/eu-climate-action_en (accessed on 14 July 2021).
- Aman, M. Measures to Address Air Pollution from Small Combustion Sources; International Institute for Applied Systems Analysis & Umweltbundesamt: Laxenburg, Austria, 2018. [Google Scholar]
- Rebbling, A. Dissertation: Application of Fuel Design to Mitigate Ash-Related Problems During Combustion of Biomass; Umea University: Umea, Sweden, 2019. [Google Scholar]
- Skoglund, N.; Werner, K.; Nylund, G.M.; Pavia, H.; Albers, E.; Broström, M. Combustion of seaweed—A fuel design strategy. Fuel Process. Technol. 2017, 165, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Skoglund, N.; Bäfver, L.; Fahlström, J.; Holmén, E.; Renström, C. Fuel design in co-combustion of demolition wood chips and municipal sewage sludge. Fuel Process. Technol. 2016, 141, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Djatkov, D.; Martinov, M.; Kaltschmitt, M. Influencing parameters on mechanical–physical properties of pellet fuel made from corn harvest residues. Biomass Bioenergy 2018, 119, 418–428. [Google Scholar] [CrossRef]
- Miranda, M.T.; Sepúlveda, F.J.; Arranz, J.I.; Montero, I.; Rojas, C.V. Analysis of pelletizing from corn cob waste. J. Environ. Manag. 2018, 228, 303–311. [Google Scholar] [CrossRef]
- Labbé, R.; Paczkowski, S.; Knappe, V.; Russ, M.; Wöhler, M.; Pelz, S. Effect of feedstock particle size distribution and feedstock moisture content on pellet production efficiency, pellet quality, transport and combustion emissions. Fuel 2020, 263, 116662. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, W.; Hu, D.; Ou, L.; Tong, Y.; Shen, G.; Shen, H.; Wang, X. Emissions of carbon monoxide and carbon dioxide from uncompressed and pelletized biomass fuel burning in typical household stoves in China. Atmos. Environ. 2012, 56, 136–142. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, Y.; Cheng, W.; Sang, H.; Xu, H.; Yang, H.; Chen, H. Effect of minerals and binders on particulate matter emission from biomass pellets combustion. Appl. Energy 2018, 215, 106–115. [Google Scholar] [CrossRef]
- Carvalho, L.; Wopienka, E.; Pointner, C.; Lundgren, J.; Verma, V.K.; Haslinger, W.; Schmidl, C. Performance of a pellet boiler fired with agricultural fuels. Appl. Energy 2013, 104, 286–296. [Google Scholar] [CrossRef]
- Zeng, T.; Pollex, A.; Weller, N.; Lenz, V.; Nelles, M. Blended biomass pellets as fuel for small scale combustion appliances: Effect of blending on slag formation in the bottom ash and pre-evaluation options. Fuel 2018, 212, 108–116. [Google Scholar] [CrossRef]
- Bäfver, L.; Rönnback, M.; Leckner, B.; Claesson, F.; Tullin, C. Particle emission from combustion of oat grain and its potential reduction by addition of limestone or kaolin. Fuel Process. Technol. 2009, 90, 353–359. [Google Scholar] [CrossRef]
- Boström, D.; Grimm, A.; Boman, C.; Björnbom, E.; Öhman, M. Influence of Kaolin and Calcite Additives on Ash Transformations in Small-Scale Combustion of Oat. Energy Fuels 2009, 23, 5184–5190. [Google Scholar] [CrossRef]
- Wang, L.; Hustad, J.E.; Skreiberg, Ø.; Skjevrak, G.; Grønli, M. A Critical Review on Additives to Reduce Ash Related Operation Problems in Biomass Combustion Applications. Energy Procedia 2012, 20, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Dragutinovic, N.; Höfer, I.; Kaltschmitt, M. Effect of additives on thermochemical conversion of solid biofuel blends from wheat straw, corn stover, and corn cob. Biomass Conv. Bioref. 2019, 9, 35–54. [Google Scholar] [CrossRef]
- Höfer, I.; Kaltschmitt, M. Assessment of additives avoiding the release of problematic species into the gas phase during biomass combustion—Development of a fast screening method based on TGA. Biomass Conv. Bioref. 2019, 9, 21–33. [Google Scholar] [CrossRef]
- Huelsmann, T.; Mack, R.; Kaltschmitt, M.; Hartmann, H. Influence of kaolinite on the PM emissions from small-scale combustion. Biomass Conv. Bioref. 2019, 9, 55–70. [Google Scholar] [CrossRef]
- Khalil, R.A.; Todorovic, D.; Skreiberg, O.; Becidan, M.; Backman, R.; Goile, F.; Skreiberg, A.; Sørum, L. The effect of kaolin on the combustion of demolition wood under well-controlled conditions. Waste Manag. Res. 2012, 30, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Konsomboon, S.; Pipatmanomai, S.; Madhiyanon, T.; Tia, S. Effect of kaolin addition on ash characteristics of palm empty fruit bunch (EFB) upon combustion. Appl. Energy 2011, 88, 298–305. [Google Scholar] [CrossRef]
- Gollmer, C.; Höfer, I.; Kaltschmitt, M. Additives as a fuel-oriented measure to mitigate inorganic particulate matter (PM) emissions during small-scale combustion of solid biofuels. Biomass Conv. Bioref. 2019, 9, 3–20. [Google Scholar] [CrossRef]
- Fournel, S.; Palacios, J.; Godbout, S.; Heitz, M. Effect of Additives and Fuel Blending on Emissions and Ash-Related Problems from Small-Scale Combustion of Reed Canary Grass. Agriculture 2015, 5, 561–576. [Google Scholar] [CrossRef] [Green Version]
- Mediavilla, I.; Fernández, M.J.; Esteban, L.S. Optimization of pelletisation and combustion in a boiler of 17.5 kWth for vine shoots and industrial cork residue. Fuel Process. Technol. 2009, 90, 621–628. [Google Scholar] [CrossRef]
- Miranda, M.T.; Arranz, J.I.; Román, S.; Rojas, S.; Montero, I.; López, M.; Cruz, J.A. Characterization of grape pomace and pyrenean oak pellets. Fuel Process. Technol. 2011, 92, 278–283. [Google Scholar] [CrossRef]
- Kallio, M.; Oravainen, H. Agrofuels—Challenging Fuel for the Future. Mix. Proj. 2011, 2011, 253–260. [Google Scholar]
- Zeng, T.; Mlonka-Mędrala, A.; Lenz, V.; Nelles, M. Evaluation of bottom ash slagging risk during combustion of herbaceous and woody biomass fuels in a small-scale boiler by principal component analysis. Biomass Conv. Bioref. 2019, 1–19. [Google Scholar] [CrossRef]
- Nunes, L.; Matias, J.; Catalao, J.; Nunes, L.; Catalão, J. Mixed biomass pellets for thermal energy production—A review of combustion models//Mixed biomass pellets for thermal energy production: A review of combustion models. Appl. Energy 2014, 127, 135–140. [Google Scholar] [CrossRef]
- Höfer, I.; Kaltschmitt, M. Effect of additives on particulate matter formation of solid biofuel blends from wood and straw. Biomass Conv. Bioref. 2017, 7, 101–116. [Google Scholar] [CrossRef]
- Schmitt, V. Dissertation am Institut für Umwelttechnik und Energiewirtschaft an der Technischen Universität Hamburg-Harburg; Kovac: Hamburg, Germany, 2013. [Google Scholar]
- Carroll, J.P.; Finnan, J.M. The use of additives and fuel blending to reduce emissions from the combustion of agricultural fuels in small scale boilers. Biosyst. Eng. 2015, 129, 127–133. [Google Scholar] [CrossRef]
- Lundholm, K.; Nordin, A.; Öhman, M.; Boström, D. Reduced Bed Agglomeration by Co-combustion Biomass with Peat Fuels in a Fluidized Bed. Energy Fuels 2005, 19, 2273–2278. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Yang, W.; Zhao, Q.; Dai, Y. Evaluation of combustion properties and pollutant emission characteristics of blends of sewage sludge and biomass. Sci. Total Environ. 2020, 720, 137365. [Google Scholar] [CrossRef] [PubMed]
- Hülsmann, T. Feinstaubemissionen bei der Verbrennung von Holz- und Holz/Stroh-MIschpellets -Einfluss Einder Brennstoffadditivierung; Technische Universität Hamburg: Hamburg, Germany, 2018. [Google Scholar]
- Zeng, T.; Weller, N.; Pollex, A.; Lenz, V. Blended biomass pellets as fuel for small scale combustion appliances: Influence on gaseous and total particulate matter emissions and applicability of fuel indices. Fuel 2016, 184, 689–700. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, W.; Yan, Y.; Ren, X.; Ismail, T.M.; Sun, R. Effects of preheating primary air and fuel size on the combustion characteristics of blended pinewood and corn straw in a fixed bed. Energy 2020, 210, 118481. [Google Scholar] [CrossRef]
- Örberg, H.; Jansson, S.; Kalén, G.; Thyrel, M.; Xiong, S. Combustion and Slagging Behavior of Biomass Pellets Using a Burner Cup Developed for Ash-Rich Fuels. Energy Fuels 2014, 28, 1103–1110. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Xiao, Z.; Liang, J.; Li, H.; Cao, L.; Wang, H.; Chen, X.; Zeng, G. A comparative study of biomass pellet and biomass-sludge mixed pellet: Energy input and pellet properties. Energy Convers. Manag. 2016, 126, 509–515. [Google Scholar] [CrossRef]
- Wzorek, M.; Junga, R.; Yilmaz, E.; Niemiec, P. Combustion behavior and mechanical properties of pellets derived from blends of animal manure and lignocellulosic biomass. J. Environ. Manag. 2021, 290, 112487. [Google Scholar] [CrossRef] [PubMed]
- Boman, C.; Boström, D.; Öhman, M. (Eds.) Effect of Fuel Additive Sorbents (Kaolin and Calcite) on Aerosol Particle Emissions and Characteristics During Combustion of Pelletized Wood Biomass. In Proceedings of the European Biomass Conference, Valensia, Spain, 2–6 June 2008. [Google Scholar]
- Schmitt, V.; Kaltschmitt, M. Effect of straw proportion and Ca- and Al-containing additives on ash composition and sintering of wood–straw pellets. Fuel 2013, 109, 551–558. [Google Scholar] [CrossRef]
- Brunner, T.; Obernberger, I.; Boman, C.; Rebbing, A.; Mack, R.; Hartmann, H. Guidelines for Advanced Fuel and Boiler Design; BIOS BIOENERGIESYSTEME GmbH: Graz, Austria, 2019. [Google Scholar]
- Meng, X.; Sun, R.; Ismail, T.M.; Zhou, W.; Ren, X.; Zhang, R. Parametric studies on corn straw combustion characteristics in a fixed bed: Ash and moisture content. Energy 2018, 158, 192–203. [Google Scholar] [CrossRef]
- Dragutinovic, N.; Höfer, I.; Kaltschmitt, M. Joint examination of fuel-related measures for the improvement of corn cob combustion properties. J. Renew. Sustain. Energy 2021, 13. [Google Scholar] [CrossRef]
- Ye, Y.; Jiang, W.; Wang, H.; An, X. A New Method for Calculating Excess Air Ratio. In Frontier Computing; Hung, J.C., Yen, N.Y., Hui, L., Eds.; Springer: Singapore, 2019; pp. 767–776. ISBN 978-981-13-3647-8. [Google Scholar]
- Paniagua, S.; Prado-Guerra, A.; Neto, A.I.; Nunes, T.; Tarelho, L.; Alves, C.; Calvo, L.F. Influence of Varieties and Organic Fertilizer in the Elaboration of a New Poplar-Straw Pellet and Its Emissions in a Domestic Boiler. Energies 2020, 13, 6332. [Google Scholar] [CrossRef]
- Juszczak, M.; Lossy, K. Pollutant emission from a heat station supplied with agriculture biomass and wood pellet mixture. Chem. Process. Eng. 2012, 33, 231–242. [Google Scholar] [CrossRef]
- Roy, M.M.; Corscadden, K.W. An experimental study of combustion and emissions of biomass briquettes in a domestic wood stove. Appl. Energy 2012, 99, 206–212. [Google Scholar] [CrossRef]
- Bäfver, L.S.; Leckner, B.; Tullin, C.; Berntsen, M. Particle emissions from pellets stoves and modern and old-type wood stoves. Biomass Bioenergy 2011, 35, 3648–3655. [Google Scholar] [CrossRef]
- Yao, X.; Xu, K. Comparative study of characterization and utilization of corncob ashes from gasification process and combustion process. Constr. Build. Mater. 2016, 119, 215–222. [Google Scholar] [CrossRef]
- Mack, R.; Kuptz, D.; Schön, C.; Hartmann, H. Schwierige Pelletbrennstoffe für Kleinfeuerungsanlagen: Verbrennungstechnische Optimierung durch Additivierung und Mischung; Technologie- und Förderzentrum im Kompetenzzentrum für Nachwachsende Rohstoffe (TFZ): Straubing, Germany, 2020. [Google Scholar]
- Schmidl, C.; Luisser, M.; Padouvas, E.; Lasselsberger, L.; Rzaca, M.; Ramirez-Santa Cruz, C.; Handler, M.; Peng, G.; Bauer, H.; Puxbaum, H. Particulate and gaseous emissions from manually and automatically fired small scale combustion systems. Atmos. Environ. 2011, 45, 7443–7454. [Google Scholar] [CrossRef]
- Verma, V.K.; Bram, S.; Delattin, F.; Laha, P.; Vandendael, I.; Hubin, A.; de Ruyck, J. Agro-pellets for domestic heating boilers: Standard laboratory and real life performance. Applied Energy 2012, 90, 17–23. [Google Scholar] [CrossRef]
- Krugly, E.; Martuzevicius, D.; Puida, E.; Buinevicius, K.; Stasiulaitiene, I.; Radziuniene, I.; Minikauskas, A.; Kliucininkas, L. Characterization of Gaseous- and Particle-Phase Emissions from the Combustion of Biomass-Residue-Derived Fuels in a Small Residential Boiler. Energy Fuels 2014, 28, 5057–5066. [Google Scholar] [CrossRef]
- He, Y.; Tang, S.; Yin, S.; Li, S. Research progress on green synthesis of various high-purity zeolites from natural material-kaolin. J. Clean. Prod. 2021, 306, 127248. [Google Scholar] [CrossRef]
- Ye, N.; Li, Y.; Yang, Z.; Zheng, J.; Zuo, S. Rare earth modified kaolin-based Cr2O3 catalysts for catalytic combustion of chlorobenzene. Appl. Catal. A Gen. 2019, 579, 44–51. [Google Scholar] [CrossRef]
- Cheng, W.; Zhu, Y.; Shao, J.; Zhang, W.; Wu, G.; Jiang, H.; Hu, J.; Huang, Z.; Yang, H.; Chen, H. Mitigation of ultrafine particulate matter emission from agricultural biomass pellet combustion by the additive of phosphoric acid modified kaolin. Renew. Energy 2021, 172, 177–187. [Google Scholar] [CrossRef]
- Jeguirim, M.; Kraiem, N.; Lajili, M.; Guizani, C.; Zorpas, A.; Leva, Y.; Michelin, L.; Josien, L.; Limousy, L. The relationship between mineral contents, particle matter and bottom ash distribution during pellet combustion: Molar balance and chemometric analysis. Environ. Sci. Pollut. Res. Int. 2017, 24, 9927–9939. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, T. Aerosols from Biomass Combustion: Technical report on behalf of the IEA Bioenergy Task 32; IEA Bioenergy: Zurich, Switzerland, 2017. [Google Scholar]
- Gollmer, C.; Höfer, I.; Kaltschmitt, M. Laboratory-scale additive content assessment for aluminum-silicate-based wood chip additivation. Renew. Energy 2021, 164, 1471–1484. [Google Scholar] [CrossRef]
- Johansson, L.S.; Tullin, C.; Leckner, B.; Sjövall, P. Particle emissions from biomass combustion in small combustors. Biomass Bioenergy 2003, 25, 435–446. [Google Scholar] [CrossRef]
- Tissari, J.; Sippula, O.; Kouki, J.; Vuorio, K.; Jokiniemi, J. Fine Particle and Gas Emissions from the Combustion of Agricultural Fuels Fired in a 20 kW Burner. Energy Fuels 2008, 22, 2033–2042. [Google Scholar] [CrossRef]
- Gollmer, C.; Höfer, I.; Harms, D.; Kaltschmitt, M. Potential additives for small-scale wood chip combustion—Laboratory-scale estimation of the possible inorganic particulate matter reduction potential. Fuel 2019, 254, 115695. [Google Scholar] [CrossRef]
- Míguez, J.L.; Porteiro, J.; Behrendt, F.; Blanco, D.; Patiño, D.; Dieguez-Alonso, A. Review of the use of additives to mitigate operational problems associated with the combustion of biomass with high content in ash-forming species. Renew. Sustain. Energy Rev. 2021, 141, 110502. [Google Scholar] [CrossRef]
- Llorente, M.F.; Arocas, P.D.; Nebot, L.G.; García, J.C. The effect of the addition of chemical materials on the sintering of biomass ash. Fuel 2008, 87, 2651–2658. [Google Scholar] [CrossRef]
- PubChem. Potassium Carbonate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Potassium-carbonate (accessed on 1 June 2021).
- PubChem. Calcium Carbonate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Calcium-carbonate (accessed on 1 June 2021).
- Misra, M.K.; Ragland, K.W.; Baker, A.J. Wood ash composition as a function of furnace temperature. Biomass Bioenergy 1993, 4, 103–116. [Google Scholar] [CrossRef]
- Demirbas, A. Potential applications of renewable energy sources, biomass combustion problems in boiler power systems and combustion related environmental issues. Prog. Energy Combust. Sci. 2005, 31, 171–192. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Knudsen, J.N.; Jensen, P.A.; Dam-Johansen, K. Transformation and Release to the Gas Phase of Cl, K, and S during Combustion of Annual Biomass. Energy Fuels 2004, 18, 1385–1399. [Google Scholar] [CrossRef]
- Lighty, J.S.; Veranth, J.M.; Sarofim, A.F. Combustion aerosols: Factors governing their size and composition and implications to human health. J. Air Waste Manag. Assoc. 2000, 50, 1565–1618; discussion 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Höfer, I.; Gollmer, C.; Kaltschmitt, M. Inorganic PM and K emissions during ashing of solid biofuels and Kaolinite—Data measurement in laboratory scale. Fuel 2021, 296, 120704. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Cheng, W.; Zhang, W.; Hu, J.; Zeng, K.; Yang, H.; Shao, J.; Chen, H. Reduction of fine particulate matter emissions from cornstalk combustion by calcium phosphates additives. Fuel 2021, 283, 119303. [Google Scholar] [CrossRef]
- Johansen, J.M.; Jakobsen, J.G.; Frandsen, F.J.; Glarborg, P. Release of K, Cl, and S during Pyrolysis and Combustion of High-Chlorine Biomass. Energy Fuels 2011, 25, 4961–4971. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Ye, J.; Deng, L.; Che, D. Condensation Behaviors of Potassium during Biomass Combustion. Energy Fuels 2017, 31, 2951–2958. [Google Scholar] [CrossRef]
- Sippula, O.; Hokkinen, J.; Puustinen, H.; Yli-Pirilä, P.; Jokiniemi, J. Particle Emissions from Small Wood-fired District Heating Units. Energy Fuels 2009, 23, 2974–2982. [Google Scholar] [CrossRef]
- Boman, C.; Nordin, A.; Boström, D.; Öhman, M. Characterization of Inorganic Particulate Matter from Residential Combustion of Pelletized Biomass Fuels. Energy Fuels 2004, 18, 338–348. [Google Scholar] [CrossRef]
- Strand, M.; Lillieblad, L.; Sanati, M.; Pagels, J.; Szpila, A.; Boghard, M.; Rissler, J.; Swietlicki, E. Aerosol Formation and Effect in Biomass Combustion and Gasification; Report nr 2/2005; Vaxjo University: Vaxjo, Sweden, 2005. [Google Scholar]
- Du, S.; Yang, H.; Qian, K.; Wang, X.; Chen, H. Fusion and transformation properties of the inorganic components in biomass ash. Fuel 2014, 117, 1281–1287. [Google Scholar] [CrossRef]

| Parameter | Unit | Kaolin A | Kaolin B | Corn Cob Pellets | ISO 17225-6 Class A | Wood Pellets | ISO 17225-2 Class A1 |
|---|---|---|---|---|---|---|---|
| C | wt. %dm * | - | - | 48.88 | - | 50.15 | - |
| H | 1.56 | - | 7.77 | - | 6.49 | - | |
| N | - | - | 0.11 | ≤1.50 | 0.21 | ≤0.30 | |
| S | - | - | 0.11 | ≤1.50 | <0.22 | ≤0.04 | |
| O | 55.81 | - | 40.23 | - | 42.97 | - | |
| K/K2O | g/kgdm | - | 0.02 | 7.66 | - | 0.58 | - |
| Na/Na2O | - | 0.19 | <0.06 | - | <0.20 | - | |
| P/P2O5 | - | 0.18 | 0.19 | - | <0.13 | - | |
| Mg/MgO | - | 0.01 | 0.19 | - | 0.16 | - | |
| Ca/CaO | - | <0.01 | 0.06 | - | 0.76 | - | |
| Al/Al2O3 | 20.94 | 37.50 | <0.01 | - | 0.03 | - | |
| Cl | - | - | 3.67 | - | <0.50 | - | |
| Si/SiO2 | 21.70 | 44.60 | 0.19 | - | <0.20 | - | |
| Zn | mg/kgdm | - | - | 14.00 | - | 9.00 | - |
| Cu | - | - | 4.70 | - | 5.50 | - | |
| Cd | - | - | <0.20 | - | <0.40 | - | |
| Pb | - | - | <0.50 | - | <1.00 | - | |
| Hg | - | - | - | - | <1.00 | - | |
| Ash content | wt. %dm * | ≤15.00 | 14.30 | 1.83 | ≤6.00 | 0.54 | ≤0.70 |
| Moisture content | wt. %ar ** | - | 10.00 | 9.97 | ≤12.00 | 6.97 | ≤10.00 |
| Lower heating value (LHV) | MJ/kgar | - | - | 15.48 | ≥14.50 | 17.51 | ≥16.50 |
| Mechanical durability | wt. % | - | - | 95.87 | ≥97.50 | 97.10 | ≥97.50 |
| Bulk density | kg/m3 | - | - | 659.00 | ≥600.00 | 608.00 | ≥600.00 |
| Fuel | Abbreviation |
|---|---|
| Wood pellets | WP |
| 12.5 wt. % corn cob pellets and 87.5 wt. % wood pellets | 12.5CCP |
| 25 wt. % corn cob pellets and 75 wt. % wood pellets | 25CCP |
| 50 wt. % corn cob pellets and 50 wt. % wood pellets | 50CCP |
| Corn cob pellets | CCP |
| Corn cob pellets with 2 wt. % binder | CCPB |
| Corn cob pellets with 0.5 wt. % kaolin B and 2 wt. % binder | CCPB0.5KAO |
| Corn cob pellets with 1 wt. % kaolin A | CCP1KAO |
| Corn cob pellets with 1.5 wt. % kaolin B and 2 wt. % binder | CCPB1.5KAO |
| Corn cob pellets with 2 wt. % kaolin A | CCP2KAO |
| Fuel | Fuel Consumption | Efficiency (η) | Excess Air (λ) | O2 | NOx | CO | TPM |
|---|---|---|---|---|---|---|---|
| kg/h | % | - | vol. % | mg/m3 | |||
| WP | 1.3 | 92.5 | 2.6 | 13.0 | 152 | 241 | 39 |
| 12.5CCP | 1.4 | 93.0 | 2.5 | 12.7 | 157 | 342 | 88 |
| 25CCP | 1.3 | 92.9 | 2.5 | 12.7 | 165 | 538 | 120 |
| 50CCP | 1.2 | 93.0 | 2.3 | 12.0 | 160 | 725 | 184 |
| CCP | 1.8 | 92.2 | 2.3 | 11.7 | 170 | 2040 | 463 |
| CCPB | 2.0 | 90.5 | 3.1 | 13.9 | 163 | 2434 | 609 |
| CCPB0.5KAO | 1.8 | 90.3 | 2.8 | 13.5 | 149 | 2236 | 559 |
| CCP1KAO | 1.7 | 92.7 | 2.2 | 11.2 | 176 | 873 | 358 |
| CCPB1.5KAO | 1.9 | 93.0 | 2.2 | 11.6 | 161 | 357 | 317 |
| CCP2 KAO | 1.8 | 91.2 | 2.8 | 13.1 | 171 | 920 | 538 |
| EN 303-5 class 3 | - | >89.0 | - | 10.0 | - | <3000 | <150 |
| EN 303-5 class 4 | - | >83.0 | - | 10.0 | - | <1000 | <60 |
| EN 303-5 class 5 | - | >75.0 | - | 10.0 | - | <500 | <40 |
| EN 14785 | - | >75.0 | - | 10.0 | - | <500 | - |
| EcoDesign | - | ≥77.0 | - | 10.0 | <200 | <500 | <40 |
| Crystalline Phase | WP | 12.5CCP | 25CCP | 50CCP | CCP | CCPB | CCPB0.5KAO | CCP1KAO | CCPB1.5KAO | CCP2KAO | Literature |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | x | x | x | [43,76,77,78,79,80,81] | |||||||
| CaCO3 | x | x | x | [30,47,78,82,83,84,85,86] | |||||||
| KCl | x | x | x | [30,79,81,85,87,88,89] | |||||||
| MgO | x | x | [30,43,47,81,83] | ||||||||
| K2CO3 | x | x | |||||||||
| CaMn14SiO24 | x | x | x | [90] | |||||||
| KAlSiO4 | x | x | x | [63,78,79,85] |
| (norm, wt. %) | WP TPM | 50 CCP TPM | CCP TPM | CCP1KAO TPM | CCP2KAO TPM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 * | 2 ** | 1 ** | 2 ** | 3 * | 1 * | 2 ** | 3 * | 1 * | 2 * | 3 ** | 4 ** | 5 ** | 1 * | 2 ** | 3 ** | 4 ** | 5 ** | |
| K | 19.1 | 22.2 | 57.4 | 36.1 | 43.1 | 35.9 | 36.0 | 35.0 | 38.2 | 39.4 | 54.5 | 35.9 | 59.5 | 26.7 | 24.3 | 45.0 | 46.3 | 40.9 |
| Na | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.2 | - | - | - |
| Si | 1.1 | - | - | - | - | 0.2 | - | 0.6 | - | 0.3 | - | 12.2 | - | 0.3 | 0.3 | - | - | - |
| Al | - | - | - | - | - | 0.1 | - | 0.1 | - | - | - | 8.3 | - | - | - | - | - | - |
| P | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.0 | 0.8 | - | - | - |
| S | 4.2 | - | - | 13.0 | 6.6 | 3.8 | 3.7 | 3.4 | 1.8 | 1.4 | - | - | - | 1.7 | 1.4 | - | 2.6 | 4.5 |
| C | 53.6 | 53.5 | - | 15.7 | 14.7 | 18.5 | 16.9 | 19.9 | 20.8 | 18.2 | - | - | - | 39.7 | 44.0 | 16.7 | 11.2 | 15.5 |
| O | 14.5 | 15.9 | - | 26.8 | 12.3 | 15.5 | 18.0 | 15.5 | 9.0 | 8.5 | - | 36.8 | - | 11.4 | 10.7 | 5.9 | 7.5 | 15.2 |
| Cl | 7.7 | 8.4 | 42.6 | 8.4 | 23.3 | 26.0 | 25.5 | 25.4 | 30.2 | 32.2 | 45.6 | 6.8 | 40.5 | 19.3 | 18.3 | 32.4 | 32.4 | 23.9 |
| K/Cl *** | 2.3 | 2.4 | 1.2 | 3.9 | 1.7 | 1.3 | 1.3 | 1.3 | 1.2 | 1.1 | 1.1 | 4.8 | 1.3 | 1.3 | 1.2 | 1.3 | 1.3 | 1.6 |
| K/(Cl + 2S) | 1.0 | 2.4 | 1.2 | 0.9 | 1.0 | 0.9 | 1.0 | 1.0 | 1.0 | 1.0 | 1.1 | 4.8 | 1.3 | 1.1 | 1.0 | 1.3 | 1.1 | 1.1 |
| K/(Cl + 2S + Al) | 1.0 | 2.4 | 1.2 | 0.9 | 1.0 | 0.9 | 1.0 | 1.0 | 1.0 | 1.0 | 1.1 | 1.8 | 1.3 | 1.1 | 1.0 | 1.3 | 1.1 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragutinović, N.; Höfer, I.; Kaltschmitt, M. Fuel Improvement Measures for Particulate Matter Emission Reduction during Corn Cob Combustion. Energies 2021, 14, 4548. https://doi.org/10.3390/en14154548
Dragutinović N, Höfer I, Kaltschmitt M. Fuel Improvement Measures for Particulate Matter Emission Reduction during Corn Cob Combustion. Energies. 2021; 14(15):4548. https://doi.org/10.3390/en14154548
Chicago/Turabian StyleDragutinović, Nataša, Isabel Höfer, and Martin Kaltschmitt. 2021. "Fuel Improvement Measures for Particulate Matter Emission Reduction during Corn Cob Combustion" Energies 14, no. 15: 4548. https://doi.org/10.3390/en14154548
APA StyleDragutinović, N., Höfer, I., & Kaltschmitt, M. (2021). Fuel Improvement Measures for Particulate Matter Emission Reduction during Corn Cob Combustion. Energies, 14(15), 4548. https://doi.org/10.3390/en14154548






