Oxygen Transfer Effects in a Two-Phase System of an Aqueous Phase and Liquid Perfluorochemical Subjected to Continuous Wave-Assisted Agitation in Disposable Bioreactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Setup of Disposable Bioreactor Supporting Continuous Wave-Assisted Agitation
2.2. Operating Parameters of WAVE 25 System
- α and ω, which commonly characterize the vertical movements of the Cellbag fixed to the rocking tray of WAVE 25, i.e., the parameters directly associated with waves mechanically generated on liquids inside the Cellbag;
- QG, which characterizes the gaseous phase flowing over the liquid phases in the Cellbag during continuous wave-assisted agitation.
2.3. Composition of Liquid Phases and Gas Mixture
2.4. DoE
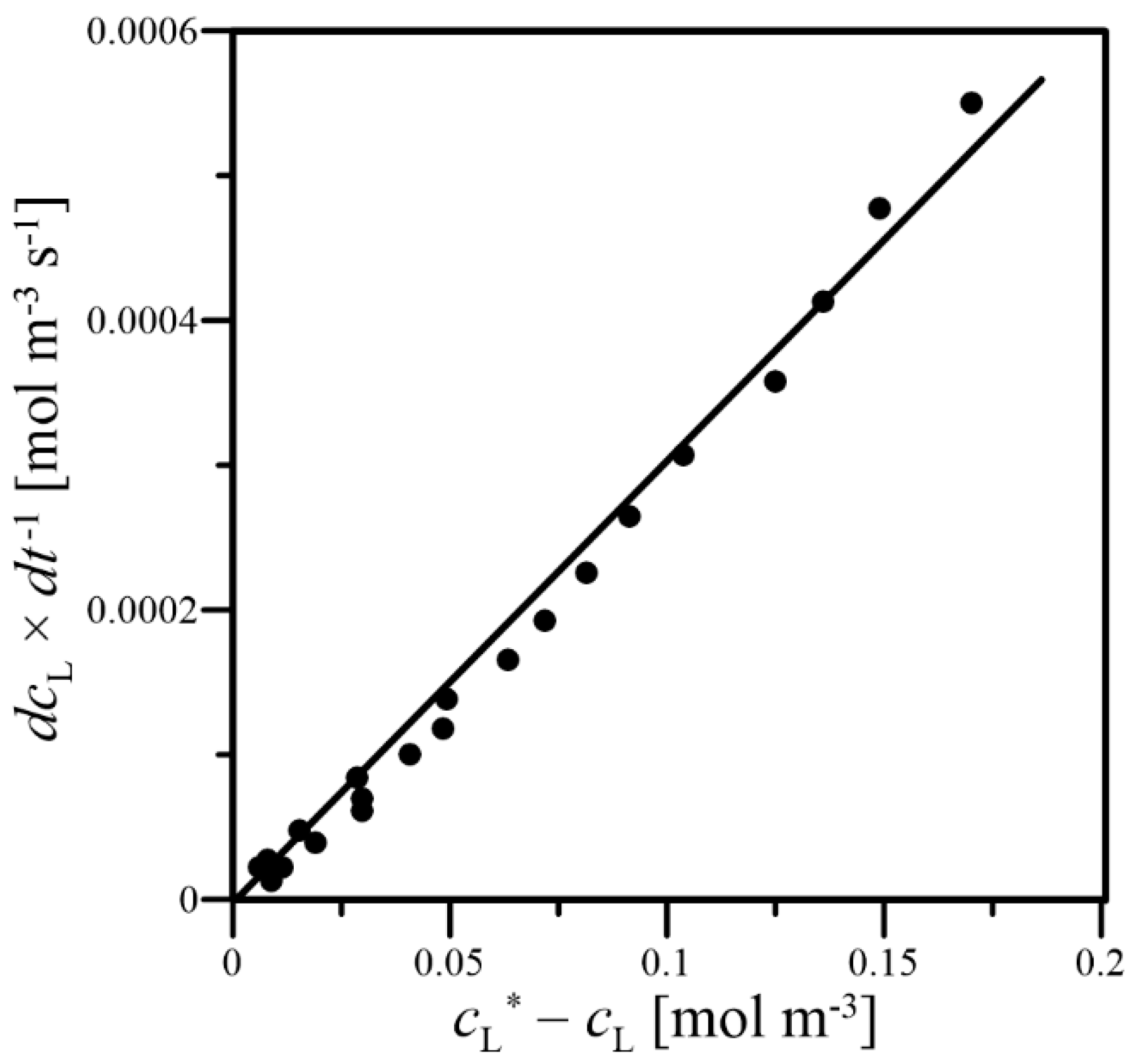
2.5. Determination of the kLa Coefficient
3. Results
4. Discussion
5. Conclusions
- Lower levels of the kLa coefficient noted for PBS-containing systems, i.e., PBS and PBS-PFD than for systems with dH2O, i.e., dH2O and dH2O-PFD, resulted from the lower value of oxygen diffusion coefficient for dH2O if compared to PBS (i.e., 3.06 × 10−9 m2 s−1 vs. 2.50 × 10−9 m2 s−1, respectively);
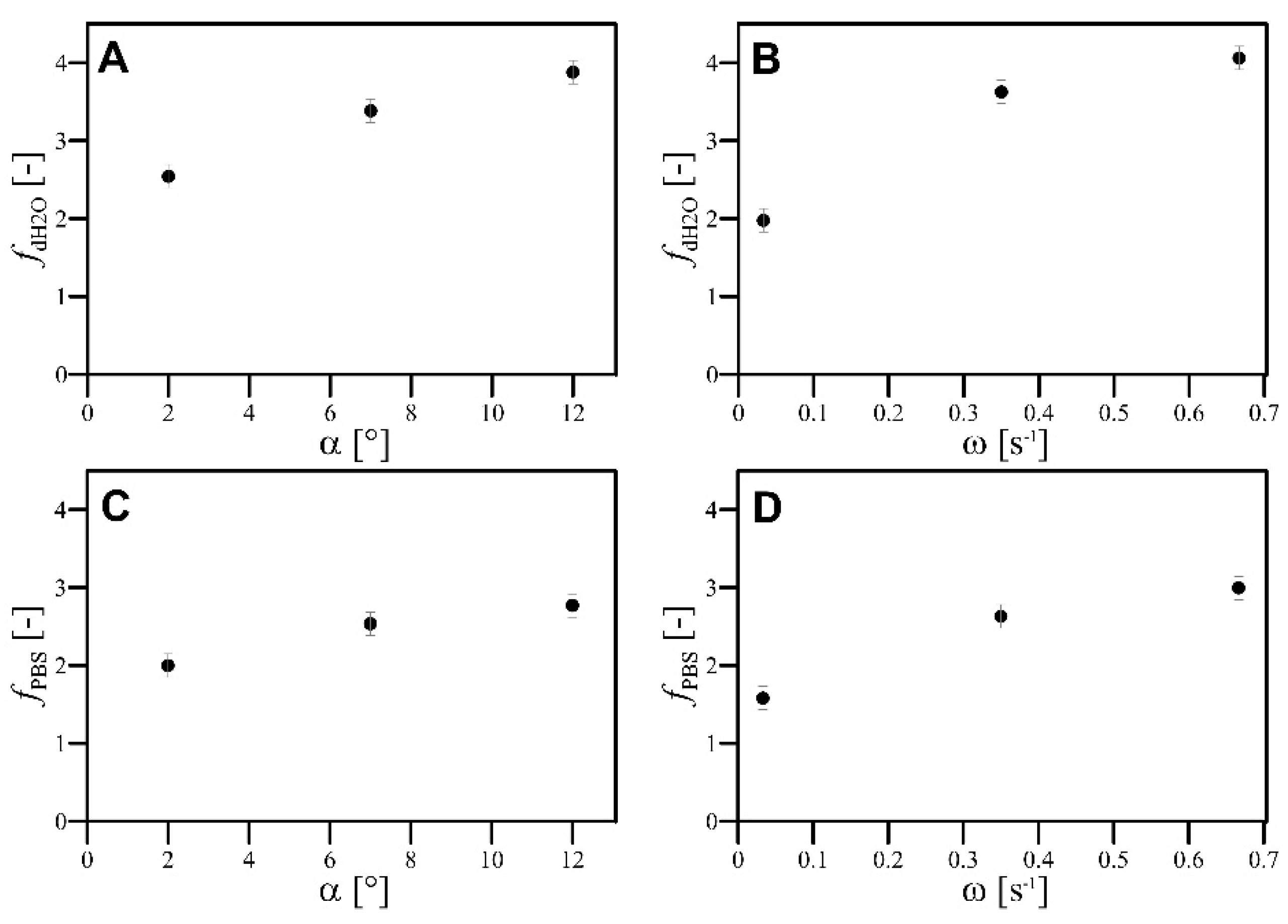
- The values of α and ω had monotonically increased influence on the value of the f factor;
- The values of both and factors increased monotonically according to the increase in α and ω, but the increase in the value of QG resulted in a monotonical decrease in values of both and ;
- Supplementation of dH2O with PFD caused the significant decrease in values of the kLa coefficient reached in the system of dH2O-PFD in comparison to the kLa values reached for pure dH2O, which resulted in values of and of less than 1.0 for the majority of the studied variants;
- The waving two-liquid system containing aqueous phase (as the upper liquid phase) and PFD (as the lower liquid phase) is characterized by different hydrodynamics than that of the waving individual aqueous phase, i.e., dH2O or PBS, without PFD;
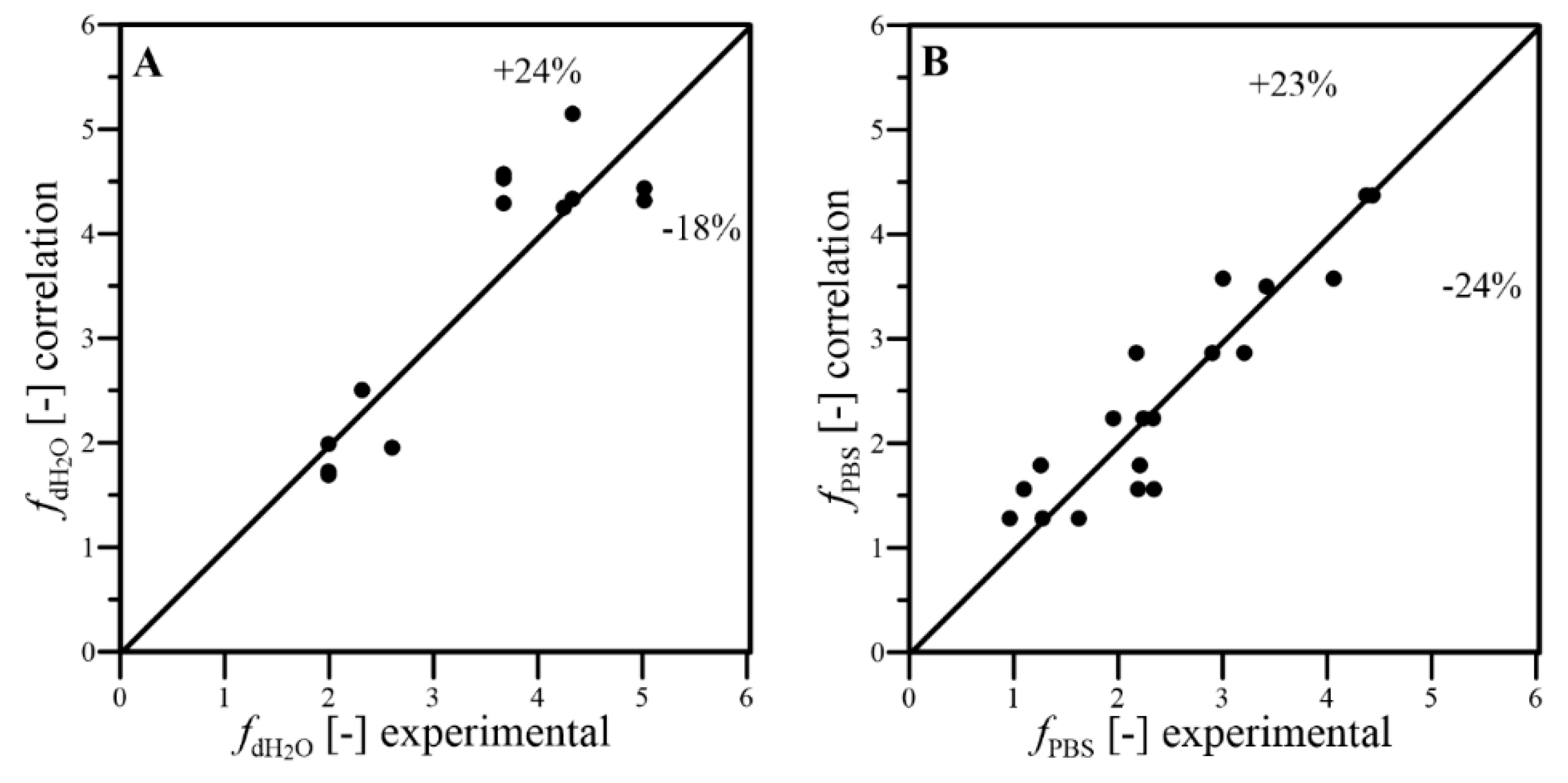
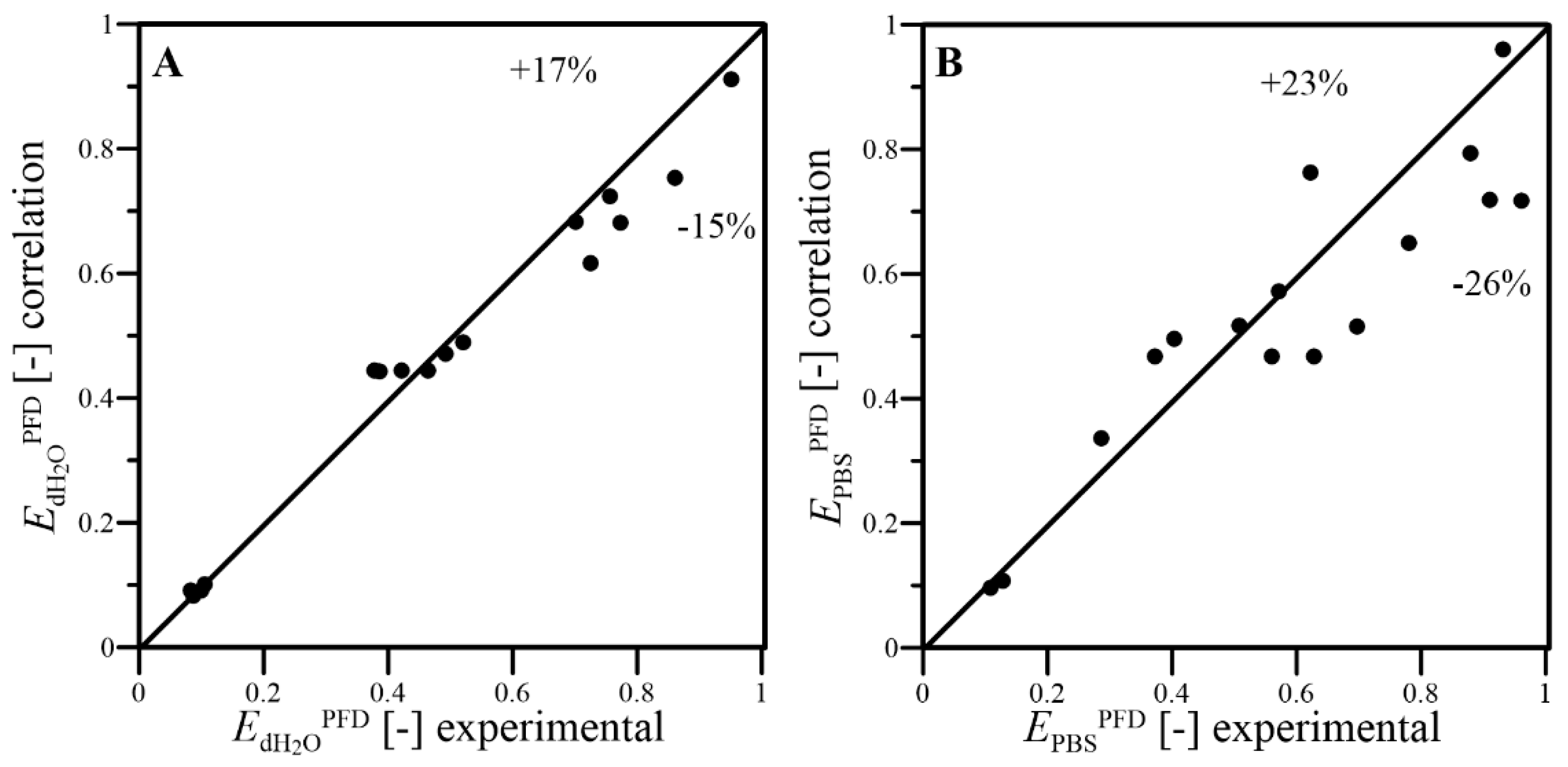
- The dimensional correlations proposed for prediction of kLa coefficient, in addition to f and EPFD factors, well fitted the experimental results with satisfactory levels of the relative errors; thus, they can be applied to predict the conditions of oxygen transfer effects reached under continuous wave-assisted agitation of systems containing aqueous phase and liquid PFC;
- Lower kLa values noted for PFD-supplemented systems may indicate hypoxia conditions affecting biomass maintained in the cell culture systems containing liquid PFC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Symbols
| a | interfacial area of mass transfer (m−1) |
| interfacial area of mass transfer for distilled water (m−1) | |
| interfacial area of mass transfer for phosphate-buffered saline (m−1) | |
| a’ | physical interfacial area estimated for non-mixed conditions (m−1) |
| molar concentration of oxygen dissolved in the liquid phase (mol m−3) | |
| equilibrium concentration of oxygen dissolved in the liquid phase (mol m−3) | |
| DL | coefficient of oxygen diffusivity in the liquid phase (m2 s−1) |
| EPFD | enhancement factor (-) |
| enhancement factor for distilled water and perfluorodecalin (-) | |
| enhancement factor for phosphate-buffered saline and perfluorodecalin (-) | |
| F | interfacial area between gas and liquid phases (m2) |
| f | interface development factor (-) |
| interface development factor for distilled water (-) | |
| interface development factor for phosphate-buffered saline (-) | |
| VL | volume of the liquid phase (m3) |
| kL | liquid-side mass transfer coefficient (s−1) |
| kLa | volumetric liquid-side mass transfer coefficient (s−1) |
| volumetric liquid-side mass transfer coefficient for distilled water (s−1) | |
| volumetric liquid-side mass transfer coefficient for distilled water and perfluorodecalin (s−1) | |
| volumetric liquid-side mass transfer coefficient for phosphate-buffered saline (s−1) | |
| volumetric liquid-side mass transfer coefficient for phosphate-buffered saline and perfluorodecalin (s−1) | |
| pN2 | nitrogen partial pressure in the gas phase (Pa) |
| pO2 | oxygen partial pressure in the gas phase (Pa) |
| t | time (s) |
| tC | temperature (°C) |
| QG | volumetric gas flow thought bag-like container (m3 s−1) |
Greek Symbols
| α | angle of oscillations (°) |
| τ | contact time (s) |
| ω | frequency of oscillations (s−1) |
Abbreviations
| BBD | Box–Behnken design |
| βTC-tet | mouse pancreatic islet β-cell line |
| B16 | mouse skin melanoma cell line |
| CBCU | central bioreactor control unit |
| C2C12 | mouse myoblast cell line |
| dH2O | sterilized distilled water |
| DO | dissolved oxygen |
| DoE | Design of Experiment |
| N2 | nitrogen |
| O2 | oxygen |
| PBS | phosphate-buffered saline |
| PFC | perfluorochemical |
| PFD | perfluorodecalin |
| RINm5F | rat pancreatic islet β-cell line |
| SV-T2 | mouse embryonic fibroblast cell line |
| WAVE 25 | ReadyToProcess WAVE™25 bioreactor |
| 3T3-L1 | primary mouse embryonic fibroblast cell line |
References
- Cabrales, P.; Intaglietta, M. Blood substitutes: Evolution from non-carrying to oxygen and gas carrying fluids. ASAIO J. 2013, 59, 337–354. [Google Scholar] [CrossRef]
- Sarkar, S.; Paswan, A.; Prakas, S. Liquid ventilation. Anesth. Essays Res. 2014, 8, 277–282. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Perfluorocarbons: Life sciences and biomedical uses. J. Polym. Sci. 2007, 45, 1185–1198. [Google Scholar] [CrossRef]
- Ntwampe, S.K.O.; Williams, C.C.; Sheldon, M.S. Water-immiscible dissolved oxygen carriers in combination with Pluronic F 68 in bioreactors. Afr. J. Biotechnol. 2010, 9, 1106–1114. [Google Scholar] [CrossRef]
- Pilarek, M. Liquid perfluorochemicals as flexible and efficient gas carriers applied in bioprocess engineering: An updated overview and future prospects. Chem. Process Eng. 2014, 35, 463–487. [Google Scholar] [CrossRef]
- Riess, J.G. Perfluorocarbon-based oxygen delivery. Artif. Cell Blood Sub. Biotechnol. 2006, 34, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Sobieszuk, P.; Pilarek, M. Absorption of CO2 into perfluorinated gas carrier in the Taylor gas-liquid flow in a microchannel system. Chem. Process Eng. 2012, 33, 595–602. [Google Scholar] [CrossRef][Green Version]
- Hanga, M.P.; Murasiewicz, M.; Pacek, A.W.; Nienow, A.W.; Coopman, K.; Hewitt, C.J. Expansion of bone marrow-derived human mesenchymal stem/stromal cells (hMSCs) using a two-phase liquid/liquid system. J. Chem. Technol. Biotechnol. 2017, 96, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, H.; Kasuya, M.C.; Hatanaka, K. Dodecafluoroheptanol: Oxygen reservoir for the culture of mouse melanoma B16 cells. J. Fluor. Chem. 2014, 163, 46–49. [Google Scholar] [CrossRef]
- Pilarek, M.; Dąbkowska, K. Modelling of a hybrid culture system with a stationary layer of liquid perfluorochemical applied as oxygen carrier. Chem. Process Eng. 2016, 37, 149–158. [Google Scholar] [CrossRef]
- Bąk, A.; Pilarek, M.; Podgórska, W.; Markowska-Radomska, A.; Hubacz, R. Surface properties of perfluorodecalin-containing liquid/liquid systems: The influence of Pluronic F-68 dissolved in the aqueous phase. J. Fluor. Chem. 2018, 215, 36–43. [Google Scholar] [CrossRef]
- Fraker, C.A.; Mendez, A.J.; Inverardi, L.; Ricordia, C.; Stabler, C.L. Optimization of perfluoro nano-scale emulsions: The importance of particle size for enhanced oxygen transfer in biomedical applications. Colloids Surf. B 2012, 98, 26–35. [Google Scholar] [CrossRef]
- Murasiewicz, H.; Nienow, A.W.; Hanga, M.P.; Coopman, K.; Hewitt, C.J.; Pacek, A.W. Engineering considerations on the use of liquid/liquid two-phase systems as a cell culture platform. J. Chem. Technol. Biotechnol. 2017, 92, 1690–1698. [Google Scholar] [CrossRef]
- Lopes, A.G. Single-use in the biopharmaceutical industry: A review of current technology impact, challenges and limitations. Food Bioprod. Process. 2015, 93, 98–114. [Google Scholar] [CrossRef]
- Singh, V. Method for Culturing Cells Using Wave-Induced Agitation. U.S. Patent 6190913, 20 February 2001. [Google Scholar]
- Kazemzadeh, A.; Elias, C.; Tamer, M.; Lohi, A.; Ein-Mozaffari, F. Mass transfer in a single-use angled-shaft aerated stirred bioreactor applicable for animal cell culture. Chem. Eng. Sci. 2020, 219, 115606. [Google Scholar] [CrossRef]
- Müller, M.; Husemann, U.; Greller, G.; Meusel, W.; Kraume, M. Heat transfer characteristics of a stirred single-use bioreactor. Biochem. Eng. J. 2018, 140, 168–177. [Google Scholar] [CrossRef]
- Junne, S.; Neubauer, P. How scalable and suitable are single-use bioreactors? Curr. Opin. Biotechnol. 2018, 53, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowski, K.; Grabowska, I.; Pilarek, M. Efficient propagation of suspended HL-60 cells in a disposable bioreactor supporting wave-induced agitation at various Reynolds number. Bioprocess Biosyst. Eng. 2020, 43, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Löffelholz, C.; Kaiser, S.C.; Kraume, M.; Eibl, R.; Eibl, D. Dynamic single-use bioreactors used in modern liter- and m3-scale biotechnological processes: Engineering characteristics and scaling up. Adv. Biochem. Eng. Biotechnol. 2014, 138, 1–44. [Google Scholar] [CrossRef]
- Wierzchowski, K.; Kuźmińska, A.; Pilarek, M. Intensification of chondrocytes proliferation by microcarriers and wave-induced mixing: Reynolds number influence on CP5 cells growth. Chem. Eng. Process. 2021, 166, 108472. [Google Scholar] [CrossRef]
- Pilarek, M.; Sobieszuk, P.; Wierzchowski, K.; Dąbkowska, K. Impact of operating parameters on values of a volumetric mass transfer coefficient in a single-use bioreactor with wave-induced agitation. Chem. Eng. Res. Des. 2018, 136, 1–10. [Google Scholar] [CrossRef]
- Higbie, R. The rate of absorption of a pure gas into a still liquid during short periods of exposure. Trans. Am. Inst. Chem. Eng. 1935, 31, 365–389. [Google Scholar]
- DeCoursey, W.J. Enhancement factors for gas absorption with reversible reaction. Chem. Eng. Sci. 1982, 37, 1483–1489. [Google Scholar] [CrossRef]
- Ju, L.K. Enhancing oxygen transfer in bioreactors by perfluorocarbon emulsions. Biotechnol. Prog. 1991, 7, 323–329. [Google Scholar] [CrossRef]
- Kumar, A.; Narta, U.K.; Azmi, W. The emergence of oxygen vectors in overcoming the challenges of oxygen transfer rate in aerobic bioprocesses. Curr. Eng. J. 2017, 4, 164–171. [Google Scholar] [CrossRef]
- Hillig, F.; Annemüller, S.; Chmielewska, M.; Pilarek, M.; Junne, S.; Neubauer, P. Bioprocess development in single-use systems for heterotrophic marine microalgae. Chem. Ing. Tech. 2013, 85, 153–161. [Google Scholar] [CrossRef]
- Goh, F.; Gross, J.D.; Simpson, N.E.; Sambanis, A. Limited beneficial effects of perfluorocarbon emulsions on encapsulated cells in culture: Experimental and modeling studies. J. Biotechnol. 2010, 150, 232–239. [Google Scholar] [CrossRef]
- Maillard, E.; Juszczak, M.T.; Langlois, A.; Kleiss, C.; Sencier, M.C.; Bietiger, W.; Sanchez-Dominguez, M.; Krafft, M.P.; Johnson, P.R.V.; Pinget, M.; et al. Perfluorocarbon emulsions prevent hypoxia of pancreatic β-cells. Cell Transplant. 2012, 21, 657–669. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. Behavior of cells at fluid interfaces. Proc. Natl. Acad. Sci. USA 1983, 80, 219–222. [Google Scholar] [CrossRef]
- Sykłowska-Baranek, K.; Rymaszewski, W.; Gaweł, M.; Rokicki, P.; Pilarek, M.; Grech-Baran, M.; Hennig, J.; Pietrosiuk, A. Comparison of elicitor-based effects on metabolic responses of Taxus × media hairy roots in perfluorodecalin-supported two-phase culture system. Plant Cell Rep. 2019, 38, 85–99. [Google Scholar] [CrossRef]
- Minami, K.; Mori, T.; Nakanishi, W.; Shigi, N.; Nakanishi, J.; Hill, J.P.; Komiyama, M.; Ariga, K. Suppression of myogenic differentiation of mammalian cells caused by fluidity of a liquid-liquid interface. ACS Appl. Mater. Interfaces 2017, 9, 30553–30560. [Google Scholar] [CrossRef] [PubMed]
- Meusel, W.; Loffelholz, C.; Husemann, U.; Dreher, T.; Greller, G.; Kauling, J.; Eibl, D.; Kleebank, S.; Bauer, I.; Glockler, R.; et al. Recommendations for Process Engineering Characterisation of Single-Use Bioreactors and Mixing Systems by Using Experimental Methods, 2nd ed.; Dechema Biotechnologie: Frankfurt am Main, Germany, 2020. [Google Scholar]
- Stroe-Biezen, S.A.M.; Janssen, A.P.M.; Janssen, L.J.J. Solubility of oxygen in glucose solutions. Anal. Chim. Acta 1993, 280, 217–222. [Google Scholar] [CrossRef]




| Factor | Range of Parameter Accessible in WAVE 25 | Value of Parameter Employed in BBD | Unit | ||
|---|---|---|---|---|---|
| −1 (Minimal) | 0 (Centre) | +1 (Maximal) | |||
| α | 2–12 | 2 | 7 | 12 | [°] |
| ω | 0.033–0.667 | 0.033 | 0.350 | 0.667 | [s−1] |
| QG | 0.17 × 10−5–1.67 × 10−5 | 0.17 × 10−5 | 0.92 × 10−5 | 1.67 × 10−5 | [m3 s−1] |
| Run | Variable Levels | kLa Characterized Gas-Liquid(s) Systems | |||||
|---|---|---|---|---|---|---|---|
| α | ω | QG | [s−1] | [s−1] | [s−1] | [s−1] | |
| 1 | −1 | 0 | −1 | 0.00055 | 0.00027 | 0.00056 | 0.00023 |
| 2 | −1 | 0 | 0 | 0.00255 | 0.00036 | 0.00186 | 0.00030 |
| 3 | −1 | 0 | 1 | 0.00319 | 0.00030 | 0.00101 | 0.00029 |
| 4 | −1 | 1 | −1 | 0.00058 | 0.00044 | 0.00057 | 0.00035 |
| 5 | −1 | 1 | 0 | 0.00308 | 0.00073 | 0.00139 | 0.00079 |
| 6 | −1 | 1 | 1 | 0.00458 | 0.00081 | 0.00156 | 0.00079 |
| 7 | 0 | −1 | −1 | 0.00048 | 0.00017 | 0.00041 | 0.00010 |
| 8 | 0 | −1 | 0 | 0.00131 | 0.00013 | 0.00057 | 0.00010 |
| 9 | 0 | −1 | 1 | 0.00150 | 0.00013 | 0.00102 | 0.00016 |
| 10 | 0 | 0 | −1 | 0.00061 | 0.00051 | 0.00055 | 0.00050 |
| 11 | 0 | 0 | 0 | 0.00282 | 0.00107 | 0.00173 | 0.00108 |
| 12 | 0 | 0 | 1 | 0.00460 | 0.00126 | 0.00311 | 0.00092 |
| 13 | 0 | 1 | −1 | 0.00057 | 0.00054 | 0.00057 | 0.00053 |
| 14 | 0 | 1 | 0 | 0.00285 | 0.00200 | 0.00178 | 0.00162 |
| 15 | 0 | 1 | 1 | 0.00456 | 0.00331 | 0.00325 | 0.00254 |
| 16 | 1 | −1 | −1 | 0.00032 | 0.00048 | 0.00028 | 0.00015 |
| 17 | 1 | −1 | 0 | 0.00165 | 0.00017 | 0.00139 | 0.00018 |
| 18 | 1 | −1 | 1 | 0.00184 | 0.00015 | 0.00176 | 0.00019 |
| 19 | 1 | 0 | 0 | 0.00279 | 0.00145 | 0.00203 | 0.00141 |
| 20 | 1 | 0 | 1 | 0.00432 | 0.00167 | 0.00160 | 0.00173 |
| 21 | 1 | 1 | −1 | 0.00061 | 0.00057 | 0.00058 | 0.00051 |
| 22 | 1 | 1 | 0 | 0.00292 | 0.00251 | 0.00260 | 0.00228 |
| 23 | 1 | 1 | 1 | 0.00382 | 0.00295 | 0.00355 | 0.00341 |
| 24 | 0 | 0 | 0 | 0.00300 | 0.00094 | 0.00191 | 0.00107 |
| 25 | 0 | 0 | 0 | 0.00298 | 0.00138 | 0.00271 | 0.00101 |
| 26 | 0 | 0 | 0 | 0.00363 | 0.00153 | 0.00270 | 0.00098 |
| Run | Variable Levels | Factors Characterized Gas-Liquid(s) Systems | |||||
|---|---|---|---|---|---|---|---|
| α | ω | QG | [-] | [-] | [-] | [-] | |
| 1 | −1 | 0 | −1 | 1.953 | 2.205 | 0.492 | 0.403 |
| 2 | −1 | 0 | 0 | 3.876 | 3.129 | 0.140 | 0.163 |
| 3 | −1 | 0 | 1 | 3.605 | 1.259 | 0.093 | 0.287 |
| 4 | −1 | 1 | −1 | 2.072 | 2.237 | 0.756 | 0.623 |
| 5 | −1 | 1 | 0 | 4.687 | 2.338 | 0.237 | 0.572 |
| 6 | −1 | 1 | 1 | 5.165 | 1.953 | 0.177 | 0.508 |
| 7 | 0 | −1 | −1 | 1.725 | 1.623 | 0.345 | 0.243 |
| 8 | 0 | −1 | 0 | 1.986 | 0.963 | 0.100 | 0.180 |
| 9 | 0 | −1 | 1 | 1.693 | 1.269 | 0.087 | 0.153 |
| 10 | 0 | 0 | −1 | 2.181 | 2.172 | 0.841 | 0.914 |
| 11 | 0 | 0 | 0 | 4.294 | 2.904 | 0.378 | 0.628 |
| 12 | 0 | 0 | 1 | 5.194 | 3.878 | 0.274 | 0.295 |
| 13 | 0 | 1 | −1 | 2.022 | 2.259 | 0.951 | 0.932 |
| 14 | 0 | 1 | 0 | 4.332 | 3.002 | 0.701 | 0.910 |
| 15 | 0 | 1 | 1 | 5.147 | 4.061 | 0.725 | 0.780 |
| 16 | 1 | −1 | −1 | 1.140 | 1.097 | 1.496 | 0.550 |
| 17 | 1 | −1 | 0 | 2.506 | 2.343 | 0.105 | 0.128 |
| 18 | 1 | −1 | 1 | 2.078 | 2.192 | 0.083 | 0.108 |
| 19 | 1 | 0 | 0 | 4.252 | 3.419 | 0.520 | 0.696 |
| 20 | 1 | 0 | 1 | 4.874 | 2.001 | 0.386 | 1.080 |
| 21 | 1 | 1 | −1 | 2.161 | 2.281 | 0.945 | 0.875 |
| 22 | 1 | 1 | 0 | 4.438 | 4.373 | 0.861 | 0.879 |
| 23 | 1 | 1 | 1 | 4.313 | 4.432 | 0.773 | 0.961 |
| 24 | 0 | 0 | 0 | 4.569 | 3.208 | 0.314 | 0.560 |
| 25 | 0 | 0 | 0 | 4.526 | 4.569 | 0.463 | 0.373 |
| 26 | 0 | 0 | 0 | 5.520 | 4.550 | 0.421 | 0.362 |
| Type of Two Liquids System | Total Volume | Vessel | Biomass | Reference |
|---|---|---|---|---|
| Dispersion of PFC in aqueous phase | 100 cm3 | 500 cm3 TubeSpin disposable bioreactor | Crypthecodinium cohnii | [27] |
| 5 cm3 | T−75 (150 cm3) flasks on rocking platform | βTC-tet cells | [28] | |
| 2 cm3 | 6-wells disposable plates, static culture | RINm5F β-cells | [29] | |
| 0.3 cm3 | 1.0 cm3 cylindrical well, static culture | 3T3-L1 and SV-T2 cells | [30] | |
| Contacting continuous layers of PFC and aqueous phase | 100 cm3 | 300 cm3 Erlenmeyer flasks on orbital shaker | Nicotiana tabacum BY-2 | [10] |
| 35 cm3 | 250 cm3 Erlenmeyer flasks on orbital shaker | hairy roots of Taxus × media | [31] | |
| 3 cm3 | 6-/24-wells disposable plates, static culture | human mesenchymal stem cells | [8] | |
| 2 cm3 | 24-wells disposable plates, static culture | C2C12 cells | [32] | |
| 1.5 cm3 | 1.5 cm3 centrifuging tubes, static cultures | B16 cells | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzchowski, K.; Sobieszuk, P.; Pilarek, M. Oxygen Transfer Effects in a Two-Phase System of an Aqueous Phase and Liquid Perfluorochemical Subjected to Continuous Wave-Assisted Agitation in Disposable Bioreactor. Energies 2021, 14, 4381. https://doi.org/10.3390/en14144381
Wierzchowski K, Sobieszuk P, Pilarek M. Oxygen Transfer Effects in a Two-Phase System of an Aqueous Phase and Liquid Perfluorochemical Subjected to Continuous Wave-Assisted Agitation in Disposable Bioreactor. Energies. 2021; 14(14):4381. https://doi.org/10.3390/en14144381
Chicago/Turabian StyleWierzchowski, Kamil, Paweł Sobieszuk, and Maciej Pilarek. 2021. "Oxygen Transfer Effects in a Two-Phase System of an Aqueous Phase and Liquid Perfluorochemical Subjected to Continuous Wave-Assisted Agitation in Disposable Bioreactor" Energies 14, no. 14: 4381. https://doi.org/10.3390/en14144381
APA StyleWierzchowski, K., Sobieszuk, P., & Pilarek, M. (2021). Oxygen Transfer Effects in a Two-Phase System of an Aqueous Phase and Liquid Perfluorochemical Subjected to Continuous Wave-Assisted Agitation in Disposable Bioreactor. Energies, 14(14), 4381. https://doi.org/10.3390/en14144381







