Influence of Graphene Nano Particles and Antioxidants with Waste Cooking Oil Biodiesel and Diesel Blends on Engine Performance and Emissions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Graphene Nano Material
2.2. Antioxidants
2.3. Test Fuel Preparation
2.4. Fuel Physical Property
3. Engine Specification and Test Procedure
4. Uncertainty Analysis of the Experimental Data
5. Results and Discussions
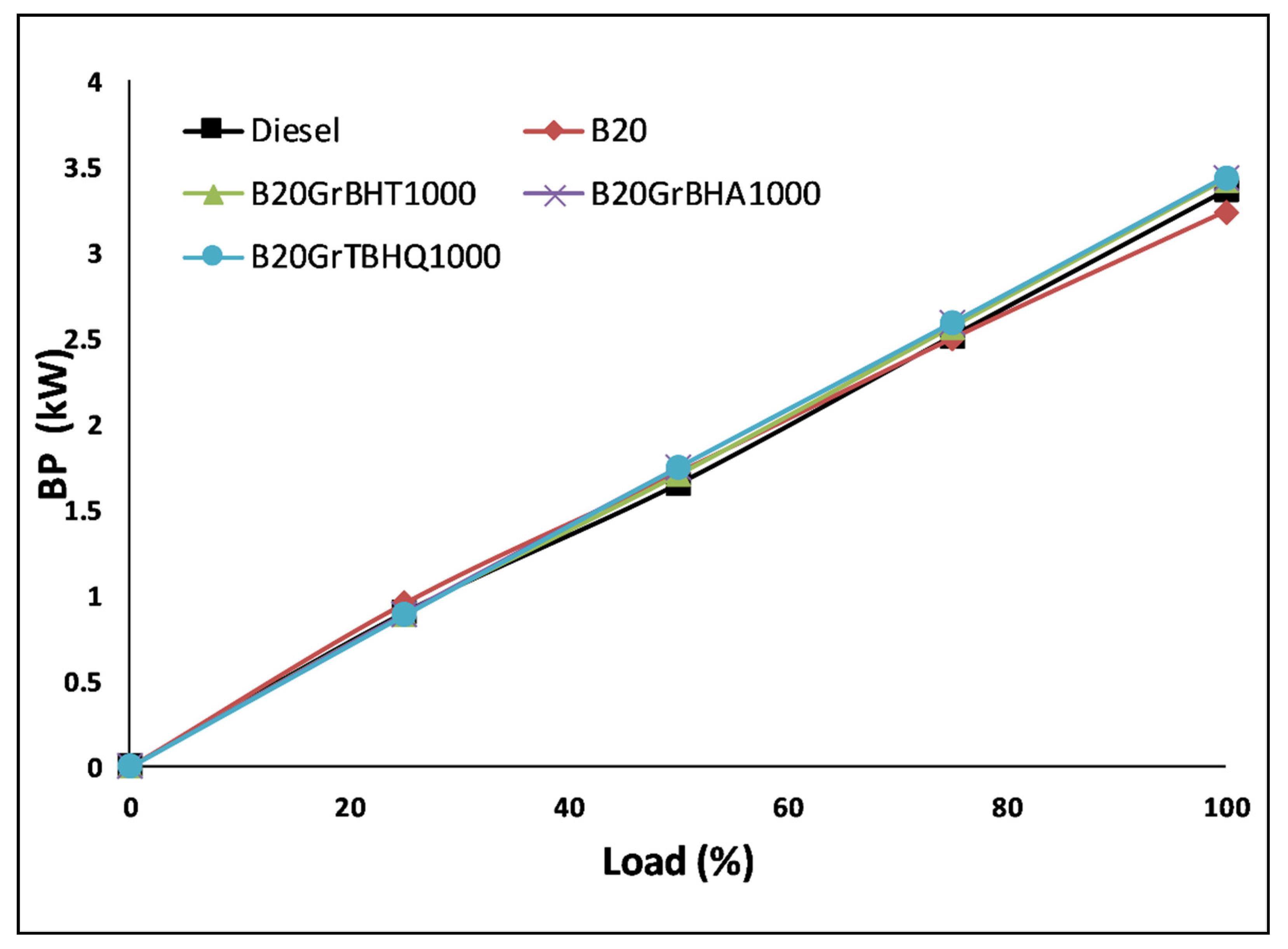
5.1. Brake Power
5.2. Brake Thermal Efficiency
5.3. Specific Fuel Consumption
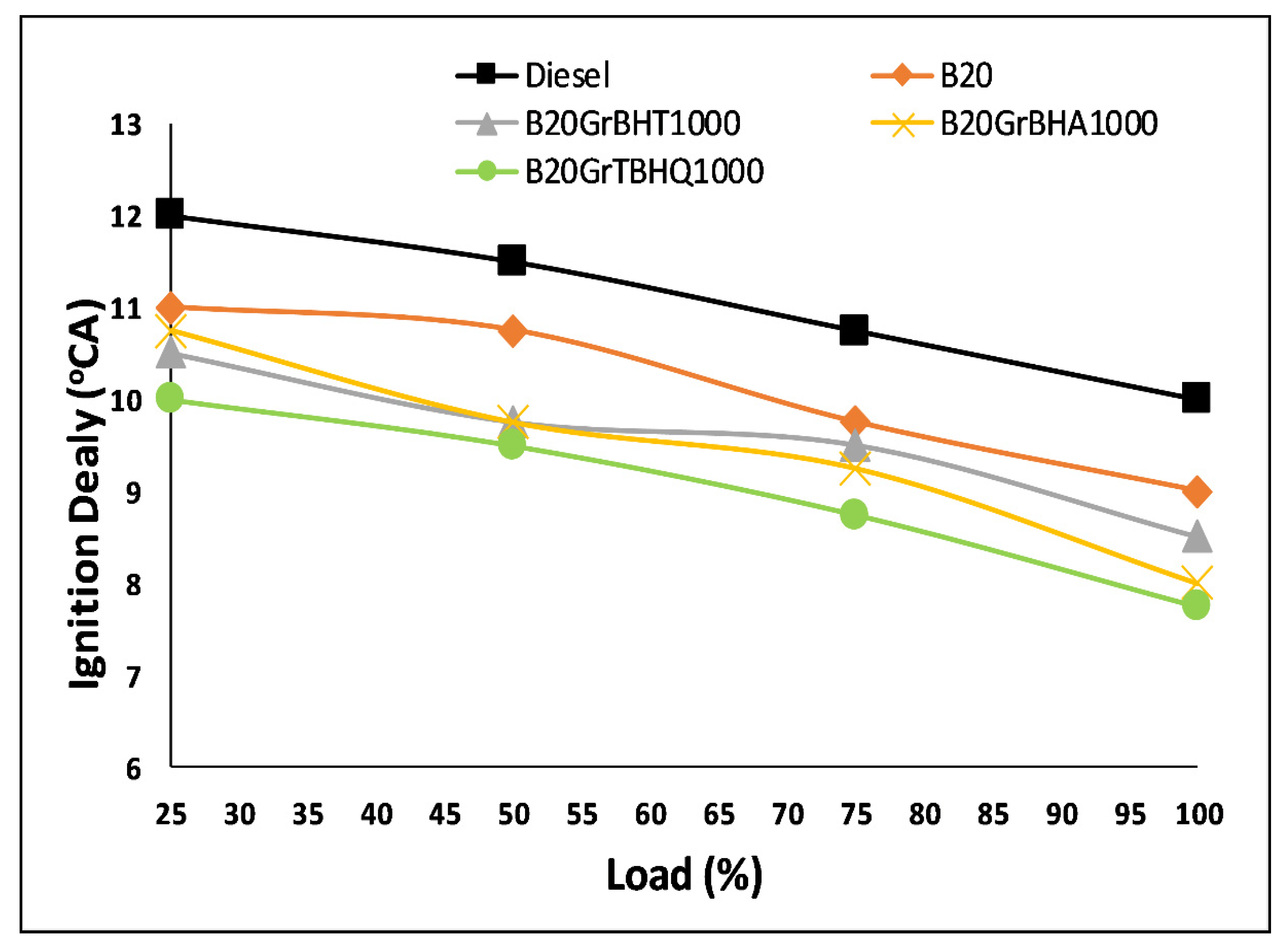
5.4. Ignition Delay
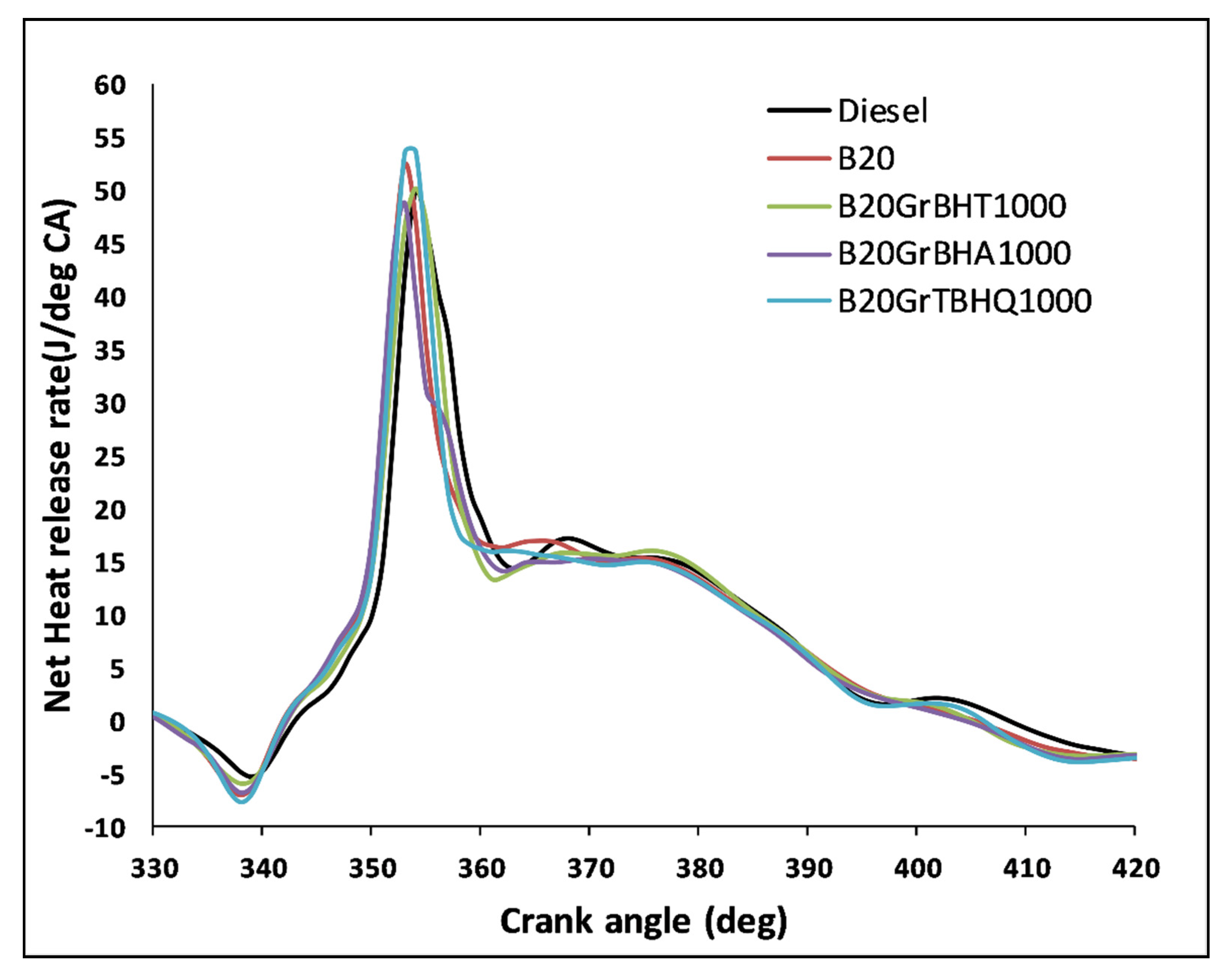
5.5. Heat Release Rate
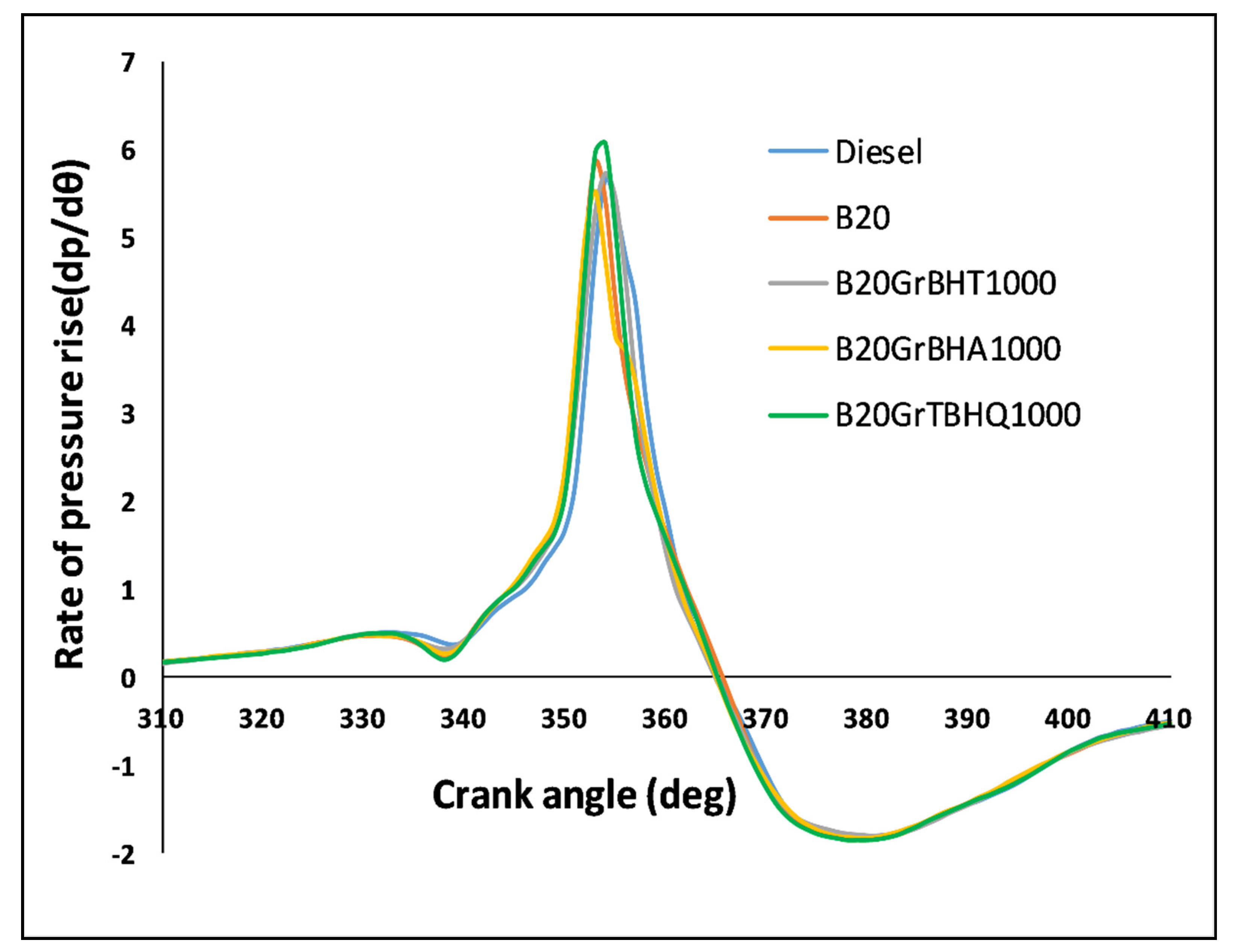
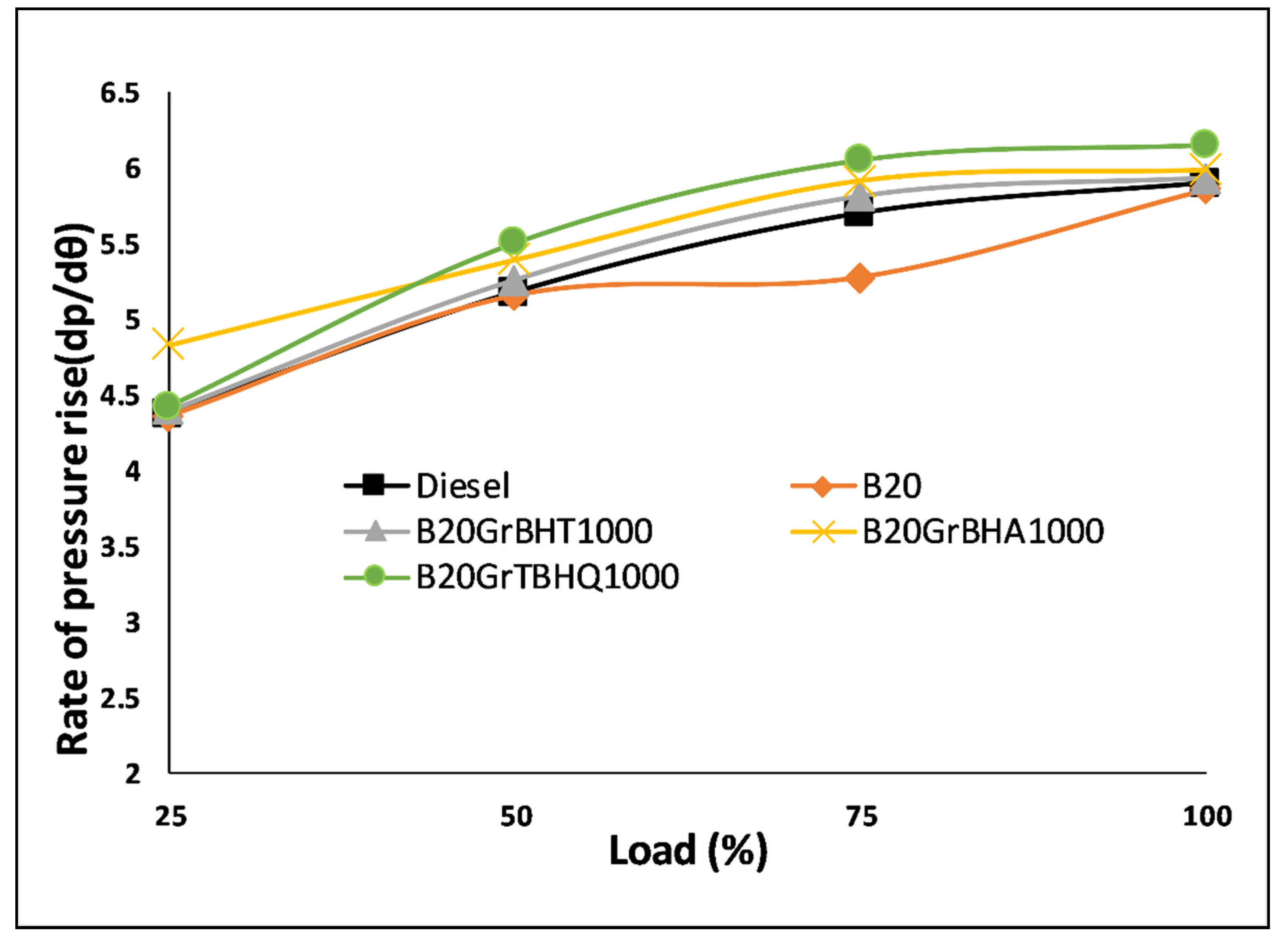
5.6. Pressure Rise Rate
5.7. CO Emission
5.8. HC Emission
5.9. NOX Emission
6. Conclusions
- A 20% blending of biodiesel derived from waste cooking oil met ASTM specification for diesel–biodiesel blend and can be used without any modification to the engine.
- The addition of graphene nanoparticle and BHT, BHA and TBHQ antioxidants yielded a diminutive reduction in calorific value of the fuel samples without altering kinematic viscosity and density compared to B20.
- There was an increase in brake thermal efficiency of 6.22%, 3.11% and 3.315%, respectively, for B20GrBHT1000, B20GrBHA1000 and B20GrTBHQ1000, respectively; this is higher than B20 at full load. The graphene nano particle played a tremendous role here for boosting the efficiency, even though the addition of antioxidants might not yield a good result in terms of performance of the engine is considered.
- BSFC of the reformed fuel showed lower specific fuel consumption compared to B20. BSFC of B20GrBHT1000, B20GrBHA1000 and B20GrTBHQ1000 were found to be 11.76%, 5.55% and 8.57% lower than that for B20. Even though the addition of graphene showed a slight dip in the calorific value of the fuel, it promotes the secondary atomization and accelerates combustion by increasing the power rendering slender drop in BSFC.
- There was a reduction in HC of 42.85%, 64.28% and 71.42% and oxides of nitrogen by 18.73%, 24.81% and 27.91% for B20GrBHT1000, B20GrBHA1000 and B20GrTBHQ1000, respectively, compare to B20.
- There was a slight increase in CO of about 11.11%, 22.22% and 16.66% for B20GrBHT1000, B20GrBHA1000 and B20GrTBHQ1000, respectively, compared to B20. The inclusion of antioxidants lowers the peroxyl and hydrogen peroxide radicals, which has a negative impact on the formation of OH radical as well as oxidation of CO.
- There was a reduction of 18.73%, 24.81% and 27.91% lesser oxides of nitrogen emission for B20GrBHT1000, B20GrBHA1000 and B20GrTBHQ1000, respectively, compared to B20. Antioxidant inclusion showed a positive impact on reducing oxides of nitrogen emission.
- B20GrTBHQ1000 has yielded the best result in terms of power, efficiency and emission.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Our Bureau. Economy (Budget 2021), Business line. The Hindu. 22 July 2019. Available online: https://www.thehindubusinessline.com/archive/ (accessed on 20 May 2021).
- EPA, United States Environmental Protection Agency. Available online: https://www.epa.gov/environmental-economics/economics-biofuels (accessed on 1 December 2020).
- Ali, M.H.; Adam, A.; Yasin, M.H.M.; Kamarulzaman, M.K. Mitigation of NOx emission by monophenolic antioxidants blended in POME biodiesel blends. Greenh. Gases Sci. Technol. 2019, 10, 829–839. [Google Scholar] [CrossRef]
- Debbarma, S.; Misra, R.D.; Das, B. Performance of graphene added palm biodiesel in a diesel engine. Clean Technol. Environ. Policy 2020, 22, 523–534. [Google Scholar] [CrossRef]
- Office of Air Quality Planning and Standards Research Triangle Park, United States Environmental Protection Agency. Nitrogen Oxides (NOx), Why and How They Are Controlled; Clean Air Technology Center (MD-12): Durham, NC, USA, 2019. [Google Scholar]
- Aalam, C.S.; Saravanan, C.G. Effects of nano metal oxide blended Mahua biodiesel on CRDI diesel engine. Ain Shams Eng. J. 2017, 8, 689–696. [Google Scholar] [CrossRef] [Green Version]
- NOx. Available online: https://en.wikipedia.org/wiki/NOx (accessed on 1 December 2020).
- Xu, H.; Liu, F.; Wang, Z.; Ren, X.; Chen, J.; Li, Q.; Zhu, Z. A Detailed Numerical Study of NOx Kinetics in Counterflow Methane Diffusion Flames: Effects of Fuel-Side versus Oxidizer-Side Dilution. J. Combust. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Jolibois, N.; Aleksandrov, K.; Hauser, M.; Stapf, D.; Seifert, H.; Matthes, J.; Waibel, P.; Vogelbacher, M.; Keller, H.B.; Gehrmann, H.-J. Analysis of Oscillating Combustion for NOx−Reduction in Pulverized Fuel Boilers. Inventions 2021, 6, 9. [Google Scholar] [CrossRef]
- Miller, J.A.; Bowman, C.T. Mechanism and modeling of nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338. [Google Scholar] [CrossRef]
- Velmurugan, K.; Sathiyagnanam, A.P. Impact of antioxidants on NOx emissions from a mango seed biodiesel powered DI diesel engine. Alex. Eng. J. 2016, 55, 715–722. [Google Scholar] [CrossRef] [Green Version]
- Soudagar, M.E.M.; Nik-Ghazali, N.-N.; Kalam, M.A. The effects of graphene oxide nanoparticle additive stably dispersed in dairy scum oil biodiesel-diesel fuel blend on CI engine: Performance, emission and combustion characteristics. Fuel 2019, 257, 116015. [Google Scholar] [CrossRef]
- Paramashivaiah, B.; Banapurmath, N.; Rajashekhar, C.; Khandal, S. Studies on effect of graphene nanoparticles addition in different levels with simarouba biodiesel and diesel blends on performance, combustion and emission characteristics of CI engine. Arab. J. Sci. Eng. 2018, 43, 4793–4801. [Google Scholar] [CrossRef]
- Hoseini, S.; Najafi, G.; Ghobadian, B.; Ebadi, M.; Mamat, R.; Yusaf, T. Performance and emission characteristics of a CI engine using graphene oxide (GO) nano-particles additives in biodiesel-diesel blends. Renew. Energy 2020, 145, 458–465. [Google Scholar] [CrossRef]
- Prabu, A. Nanoparticles as additive in biodiesel on the working characteristics of a DI diesel engine. Ain Shams Eng. J. 2017, 9, 2343–2349. [Google Scholar] [CrossRef]
- Varatharajan, K.; Cheralathan, M.; Velraj, R. Mitigation of NOx emissions from a jatropha biodiesel fueled DI diesel engine using antioxidant additives. Fuel 2011, 90, 2721–2725. [Google Scholar] [CrossRef]
- Rashedul, H.K.; Masjuki, H.H.; Kalam, M.A.; Teoh, Y.H. Effect of antioxidant on the oxidation stability and combustion–performance–emission characteristics of a diesel engine fueled with diesel–biodiesel blend. Energy Convers. Manag. 2015, 106, 849–858. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Masjuki, H.; Kalam, A.; Mofijur, M.; Abedin, M. Effect of antioxidant on the performance and emission characteristics of a diesel engine fueled with palm biodiesel blends. Energy Convers. Manag. 2014, 79, 265–272. [Google Scholar] [CrossRef]
- Palash, S.; Kalam, A.; Masjuki, H.; Arbab, M.; Masum, B.; Sanjid, A. Impacts of NOx reducing antioxidant additive on performance and emissions of a multi-cylinder diesel engine fueled with Jatropha biodiesel blends. Energy Convers. Manag. 2014, 77, 577–585. [Google Scholar] [CrossRef]
- Paramashivaiah, B.M.; Rajashekhar, C.R. Studies on effect of various surfactants on stable dispersion of graphene nano particles in simarouba biodiesel. IOP Conf. Ser. Mater. Sci. Eng. 2016, 149, 012083. [Google Scholar] [CrossRef] [Green Version]
- Nagappan, B.; Devarajan, Y.; Kariappan, E. Influence of antioxidant additives on performance and emission characteristics of beef tallow biodiesel-fueled C.I engine. Environ. Sci. Pollut. Res. 2021, 28, 12041–12055. [Google Scholar] [CrossRef]
- Abed, K.; El Morsi, A.; Sayed, M.; El Shaib, A.; Gad, M.S. Effect of waste cooking-oil biodiesel on performance and exhaust emissions of a diesel engine. Egypt. J. Pet. 2018, 27, 985–989. [Google Scholar] [CrossRef]
- Guo, T.; Duan, X.; Liu, Y.; Liu, J. A comparative experimental study on emission characteristics of a turbocharged gasoline direct-injection (TGDI) engine fuelled with gasoline/ ethanol blends under transient cold-start and steady-state conditions. Fuel 2020, 277, 118153. [Google Scholar] [CrossRef]
- Rashed, M.M.; Kalam, M.A.; Masjuki, H.H.; Habibullah, M.; Imdadul, H.K.; Shahin, M.M.; Rahman, M.M. Improving oxidation stability and NOX reduction of biodiesel blends using aromatic and synthetic antioxidant in a light duty diesel engine. Ind. Crop. Prod. 2016, 89, 273–284. [Google Scholar] [CrossRef]
- Rashed, M.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Rahman, M.M.; Imdadul, H.K.; Rashedul, H.K. Study of the oxidation stability and exhaust emission analysis of Moringa olifera biodiesel in a multi-cylinder diesel engine with aromatic amine antioxidants. Renew. Energy 2016, 94, 294–303. [Google Scholar] [CrossRef]
- Abed, K.A.; Gad, M.S.; El Morsi, A.K.; Sayed, M.M.; Elyazeed, S.A. Effect of biodiesel fuels on diesel engine emissions. Egypt. J. Pet. 2019, 28, 183–188. [Google Scholar] [CrossRef]
- Lata, D.B.; Misra, A.; Medhekar, S. Investigations on the combustion parameters of a dual fuel diesel engine with hydrogen and LPG as secondary fuels. Int. J. Hydrog. Energy 2011, 36, 13808–13819. [Google Scholar] [CrossRef]
- Puneeth Kumar Reddy, V.; Senthil Kumar, D.; Thirumalini, S. Effect of antioxidants on the performance and emission characteristics of a diesel engine fueled by waste cooking sunflower methyl ester. IOP Conf. Ser. Mater. Sci. Eng. 2018, 310, 012116. [Google Scholar] [CrossRef]
- Buyukkaya, E. Effects of biodiesel on a DI diesel engine performance, emission and combustion characteristics. Fuel 2010, 89, 3099–3105. [Google Scholar] [CrossRef]






| Purity | 99% |
|---|---|
| Thickness (Z) | 0.8–1.6 nm |
| Dimension (X and Y) | <1 µm |
| Number of Layers | 1–5 |
| Surface Area | >200 m2/g |
| Bulk Density | 0.006 g/cm3 |
| Properties | Unit | ASTM D7467 Standard | Diesel | Biodiesel from Waste Cooking Oil (B20) | Waste Cooking Oil Biodiesel (B100) | BBD + GR-BHT1000 ppm | BBD + GR-BHA1000 ppm | BBD + GR-TBHQ1000 ppm |
|---|---|---|---|---|---|---|---|---|
| Flash point | °C | Min 52 | 65 | 71 | 160 | 79 | 79 | 79 |
| Kinematic Viscosity, 40 °C | CST | 1.9–4.1 (ASTM D93) | 2 to 3.8 | 4.06 | 4.2 | 4.05 | 4.05 | 4.05 |
| Calorific Value | kJ/kg | N/s | 42,600 | 41,320 | 36,848 | 40,750 | 40,090 | 40,090 |
| Density | kg/m3 | N/s | 820 | 880 | 920 | 880 | 880 | 880 |
| Product | 1 Cylinder, 4 Strokes, Multifuel, VCR Research Engine |
|---|---|
| Engine | Kirloskar made |
| Type of cooling | water cooled |
| Stroke, Bore, Cubic capacity | 110 mm, 87.5 mm, 661 cc |
| Rated Power | 3.5 kW at 1500 rpm |
| Dynamometer | Type eddy current, water cooled with loading unit |
| Load sensor | Make VPG Sensotronics, Load cell, type strain gauge, range 0–50 Kg |
| Overall dimensions | W 2000 × D 2500 × H 1500 mm |
| B20 | 20% Biodiesel from Waste Cooking Oil + 80% Diesel |
|---|---|
| B20GrBHT1000 | 20% Biodiesel from waste cooking oil + 80% Diesel + 30 PPM of Graphene + 1000 ppm of BHT antioxidant |
| B20GrBHA1000 | 20% Biodiesel from waste cooking oil + 80% Diesel + 30 PPM of Graphene + 1000 ppm of BHA antioxidant |
| B20GrTBHQ1000 | 20% Biodiesel from waste cooking oil + 80% Diesel + 30 PPM of Graphene + 1000 ppm of TBHQ antioxidant |
| Sl. No. | Parameter | Variables | Accuracy (±) | Uncertainty (%) (±) |
|---|---|---|---|---|
| 1 | Accuracy of the engine parameter | Engine load (N) | 0.05 | - |
| 2 | Engine speed (rpm) | 2 | - | |
| 3 | Fuel flow rate, cc/min | 0.1 | - | |
| 4 | Accuracy of the obtained emission value | Hydrocarbon emission | - | 0.8 |
| 5 | Carbon monoxide emission | - | 1.3 | |
| 6 | Oxides of nitrogen | - | 1.7 | |
| 7 | Determined parameters | Brake Thermal Efficiency (%) | - | 1 |
| 8 | Heat release rate (J/oCA) | - | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnakumar, S.; Khan, T.M.Y.; Rajashekhar, C.R.; M. Soudagar, M.E.; Afzal, A.; Elfasakhany, A. Influence of Graphene Nano Particles and Antioxidants with Waste Cooking Oil Biodiesel and Diesel Blends on Engine Performance and Emissions. Energies 2021, 14, 4306. https://doi.org/10.3390/en14144306
Krishnakumar S, Khan TMY, Rajashekhar CR, M. Soudagar ME, Afzal A, Elfasakhany A. Influence of Graphene Nano Particles and Antioxidants with Waste Cooking Oil Biodiesel and Diesel Blends on Engine Performance and Emissions. Energies. 2021; 14(14):4306. https://doi.org/10.3390/en14144306
Chicago/Turabian StyleKrishnakumar, Sandeep, T. M. Yunus Khan, C. R. Rajashekhar, Manzoore Elahi M. Soudagar, Asif Afzal, and Ashraf Elfasakhany. 2021. "Influence of Graphene Nano Particles and Antioxidants with Waste Cooking Oil Biodiesel and Diesel Blends on Engine Performance and Emissions" Energies 14, no. 14: 4306. https://doi.org/10.3390/en14144306
APA StyleKrishnakumar, S., Khan, T. M. Y., Rajashekhar, C. R., M. Soudagar, M. E., Afzal, A., & Elfasakhany, A. (2021). Influence of Graphene Nano Particles and Antioxidants with Waste Cooking Oil Biodiesel and Diesel Blends on Engine Performance and Emissions. Energies, 14(14), 4306. https://doi.org/10.3390/en14144306









