Microwave Torrefaction of Oat Hull: Effect of Temperature and Residence Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Storage
2.2. Microwave Torrefaction
2.3. Physical Properties
2.4. Yields after Microwave Torrefaction
2.5. Grinding Energy Consumption
2.6. Cost Per Tonne of Torrefied Material
2.7. Organic Composition
3. Results and Discussion
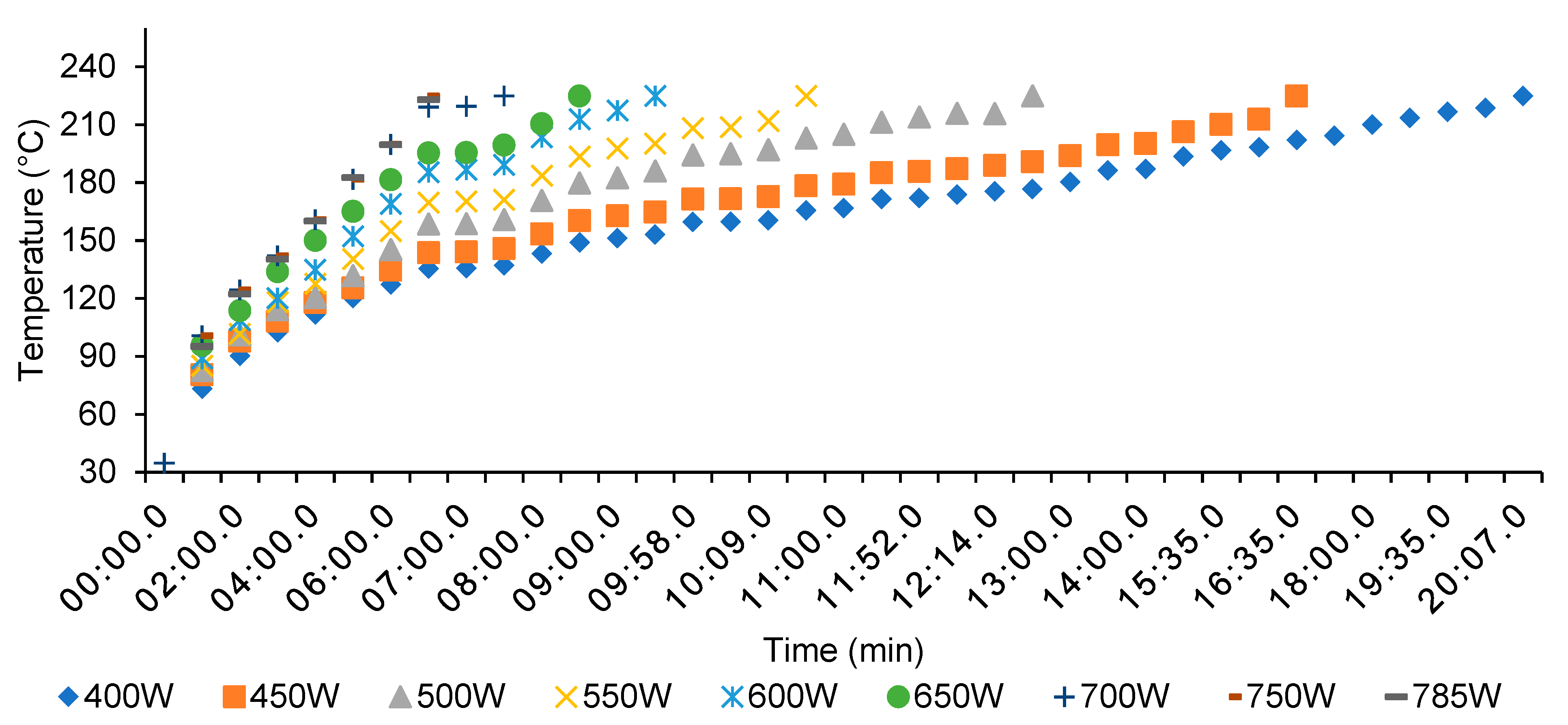
3.1. Microwave Torrefaction
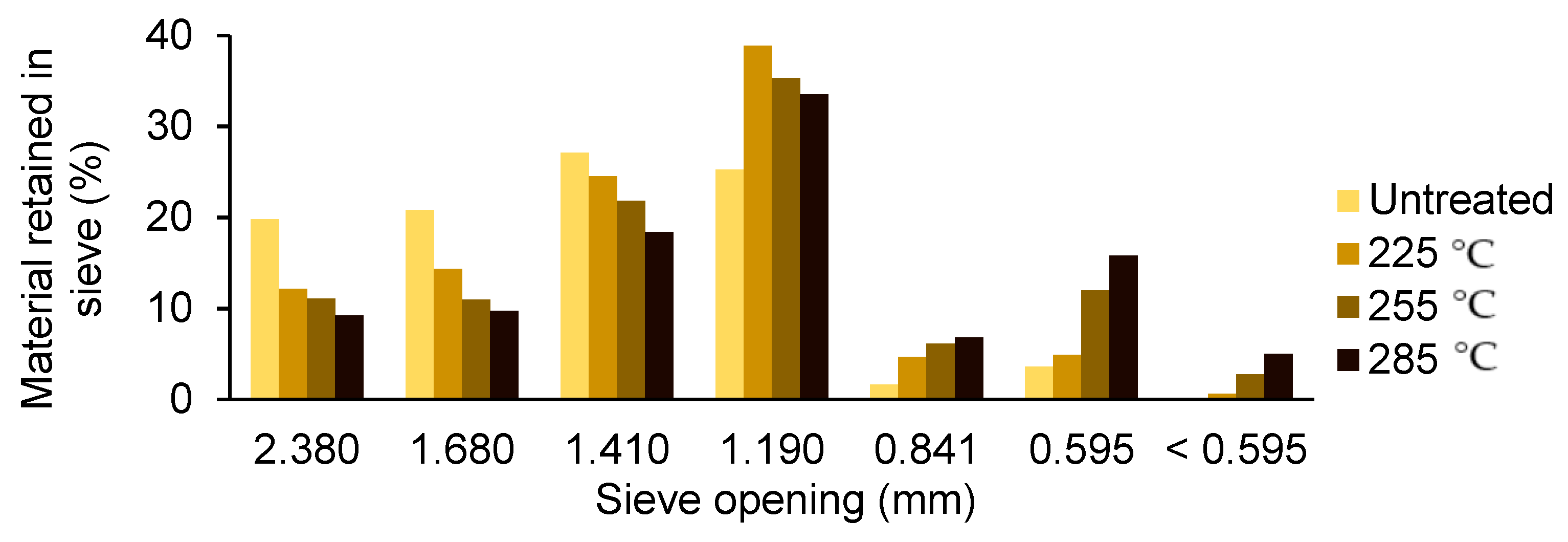
3.2. Physical Properties of the Samples
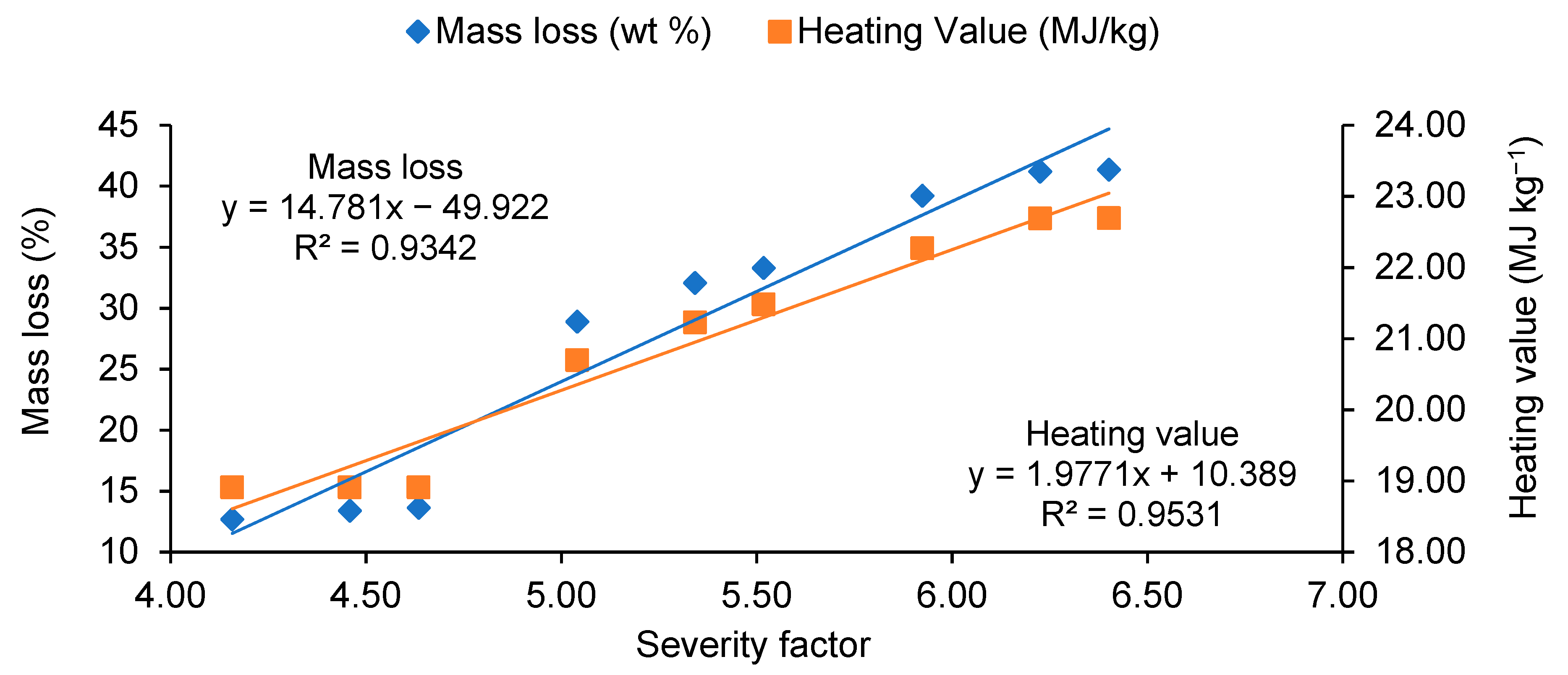
3.3. Torrefaction Performance
3.4. Energy Consumption during Grinding
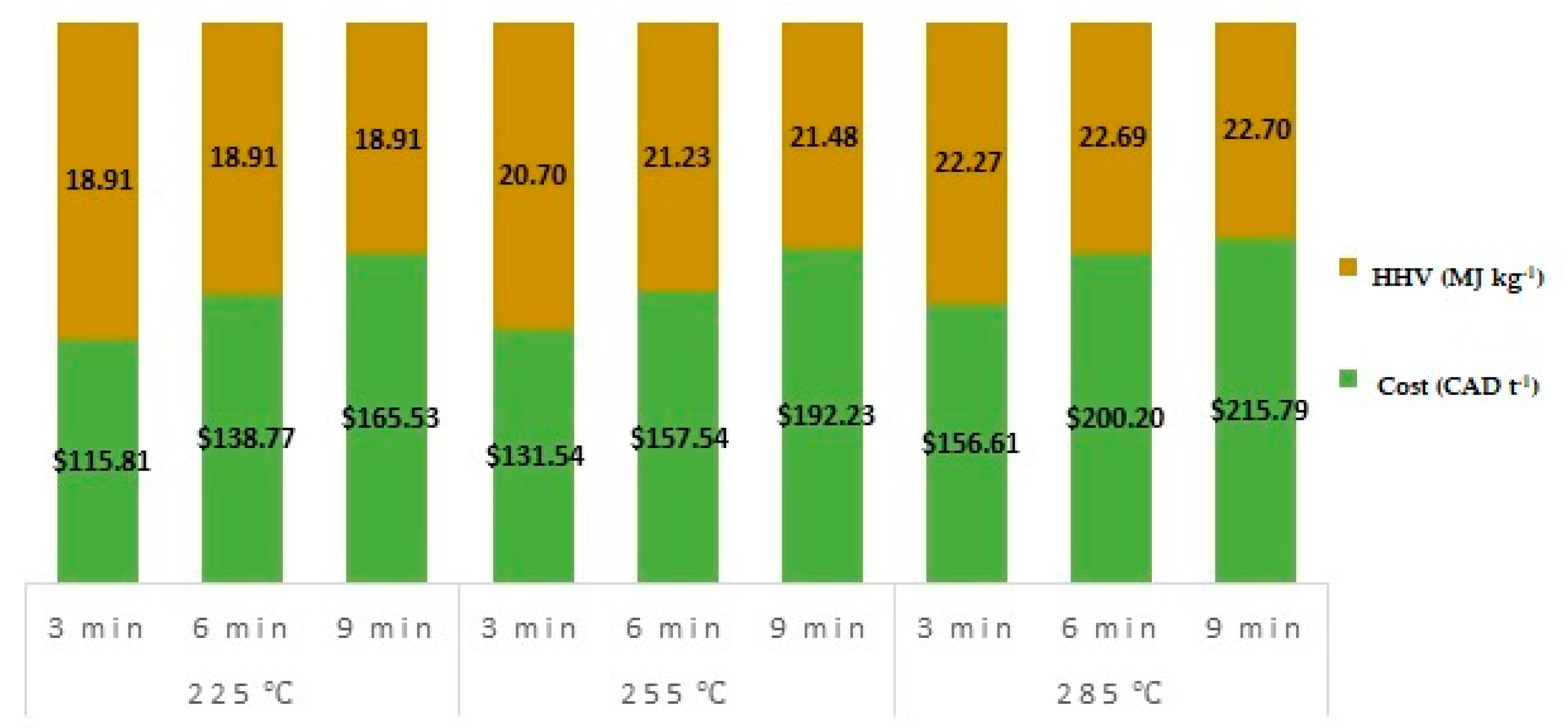
3.5. Cost Per Tonne of Torrefied Material
3.6. Organic Composition of Samples
3.7. By-Products of Torrefaction
4. Conclusions
- A higher microwave power level drastically decreased processing time, without modifying the final torrefied biomass energy and mass yields.
- Physical properties such as moisture content, particle size, moisture absorption, mass yield, ash content, and bulk density decreased with an increase in microwave torrefaction temperature.
- Increase in microwave torrefaction residence time from 3 to 9 min did not significantly modify the heating values, mass, or energy yields. However, the cost of torrefied material significantly increased by an average of 42% from 3 to 9 min.
- There was a drastic decrease in grinding energy consumption of 44 to 86% by water removal and degradation of biomass lignocellulosic structure through microwave torrefaction.
- At 285 °C, carbon content increased 130.16%, while oxygen and hydrogen contents decreased by 19.40% and 8.09%, whereas the hemicellulose decreased by 98.08% and lignin increased 398.34%.
- The changes in heating values were attributed to devolatilization of hemicellulose and incremented lignin content. These reactions resulted in color change, formation of carbon monoxide, carbon dioxide, water, acids, phenols, and carbonyls.
- Through an increased microwave power level from 400 to 700 W, the total processing time and energy consumption decreased from 23.07 to 10.10 min and from 158.79 to CAD $109.38 ton−1.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraser, J.; McCartney, D. Chapter III—Fodder oats in North America. In Fodder Oats: A World Overview; Suttie, J.M., Reynolds, S.G., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubiera, F.; Pis, J.J. Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Tumuluru, J.; Sokhansanj, S.; Wright, C.T.; Hess, J.R.; Boardman, R.D. A review on biomass torrefaction process and product properties. Symposium on thermochemical conversion. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef] [Green Version]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Basu, P. (Ed.) Chapter 4—Torrefaction. In Biomass Gasification, Pyrolysis and Torrefaction, 2nd ed.; Academic Press: Boston, MA, USA, 2013; pp. 87–145. [Google Scholar]
- Basu, P. (Ed.) Chapter 4—Torrefaction. In Biomass Gasification, Pyrolysis and Torrefaction, 3rd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 93–154. [Google Scholar]
- Chen, W.-H.; Kuo, P.-C. Torrefaction and co-torrefaction characterization of hemicellulose, cellulose and lignin as well as torrefaction of some basic constituents in biomass. Energy 2011, 36, 803–811. [Google Scholar] [CrossRef]
- Nhuchhen, D.; Basu, P.; Acharya, B. A comprehensive review on biomass torrefaction. Int. J. Renew. Energy Biofuels 2014, 1–56. [Google Scholar] [CrossRef]
- Iroba, K.L.; Baik, O.-D.; Tabil, L.G. Torrefaction of biomass from municipal solid waste fractions II: Grindability characteristics, higher heating value, pelletability and moisture adsorption. Biomass Bioenergy 2017, 106, 8–20. [Google Scholar] [CrossRef]
- Abubakar, Z.; Salema, A.A.; Ani, F.N. A new technique to pyrolyse biomass in a microwave system: Effect of stirrer speed. Bioresour. Technol. 2013, 128, 578–585. [Google Scholar] [CrossRef]
- Abedi, A.; Dalai, A.K. Study on the quality of oat hull fuel pellets using bio-additives. Biomass Bioenergy 2017, 106, 166–175. [Google Scholar] [CrossRef]
- Satpathy, S.K.; Tabil, L.G.; Meda, M.; Narayana, S.; Rajendra, P. Torrefaction and grinding performance of wheat and barley straw after microwave heating. In Proceedings of the CSBE/SCGAB Annual Conference, Saskatoon, SK, Canada, 10–13 July 2013; p. 17. Available online: http://www.csbe-scgab.ca/docs/meetings/2013/CSBE13065.pdf (accessed on 6 December 2019).
- Huang, Y.F.; Chen, W.R.; Chiueh, P.T.; Kuan, W.H.; Lo, S.L. Microwave torrefaction of rice straw and pennisetum. Bioresour. Technol. 2012, 123, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Valdez, E. Microwave Torrefaction and Densification of oat Hulls for Heat and Power Production. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2021. [Google Scholar]
- ASAE S358.3. Moisture Measurement-Forages; American Society of Agricultural and Biological Engineers Standards: St. Joseph, MI, USA, 2012; Available online: https://webstore.ansi.org/standards/asabe/ansiasaes358may2012r2017 (accessed on 9 December 2019).
- ANSI/ASAE S319.3. Method of Determining and Expressing Fineness of Feed Materials by Sieving; American Society of Agricultural and Biological Engineers Standard: St. Joseph, MI, USA, 2003; Available online: https://webstore.ansi.org/standards/asabe/ansiasaes319feb2008r2017 (accessed on 9 December 2019).
- Adapa, P.; Tabil, L.; Schoenau, G. Grinding performance and physical properties of non-treated and steam exploded barley, canola, oat and wheat straw. Biomass Bioenergy 2011, 35, 549–561. [Google Scholar] [CrossRef]
- Lu, Q.; Tang, L.; Wang, S.; Chen, Y.; Chen, X.; Huang, B. Preparation and characterization of cellulose nanocrystals via ultrasonication-assisted FeCl3-catalyzed hydrolysis. Cellulose 2014, 21, 3497–3506. [Google Scholar] [CrossRef]
- Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Effect of thermal pretreatment on equilibrium moisture content of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 4849–4854. [Google Scholar] [CrossRef]
- Peng, J.; Bi, H.T.; Lim, C.J.; Sokhansanj, S. Study on density, hardness, and moisture uptake of torrefied wood pellets. Energy Fuels 2013, 27, 967–974. [Google Scholar] [CrossRef]
- Bridgeman, T.G.; Jones, J.M.; Shield, I.; Williams, P.T. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Bridgeman, T.G.; Jones, J.M.; Williams, A.; Waldron, D.J. An investigation of the grindability of two torrefied energy crops. Fuel 2010, 89, 3911–3918. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, S.; Moeller, P.J. Liquid smoke: Product of hardwood pyrolysis. Fuel Chem. Div. Prepr. 2002, 47, 366–367. [Google Scholar]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Moeller, P.W.; Ramakrishnan, S. Process for Making Liquid Smoke Brownin Agent Solution. WO 2001/028361, 11 April 2007. Available online: https://patentimages.storage.googleapis.com/bc/b7/e2/68375b2283834a/EP1221866B1.pdf (accessed on 10 August 2020).
- Saskpower. Power Supply Rates. Power Company. 2019. Available online: https://www.saskpower.com/Accounts-and-Services/Billing/Power-Rates/Power-Supply-Rates (accessed on 9 December 2019).
- ANKOM Technology. Method 5: Acid Detergent Fiber in Feeds—Filter Bag Technique (for A200 and A200I). Available online: https://www.ankom.com/sites/default/files/document-files/Method_5_ADF_A200.pdf (accessed on 1 April 2020).
- ANKOM Technology. Method 6: Neutral Detergent Fiber in Feeds—Filter Bag Technique (for A200 and A200I). Available online: https://www.ankom.com/sites/default/files/document-files/Method_6_NDF_A200.pdf (accessed on 1 April 2020).
- ANKOM Technology. Method 8: Determining Acid Detergent Lignin in Beakers. Available online: https://www.ankom.com/sites/default/files/document-files/Method_8_Lignin_in_beakers_0.pdf (accessed on 1 April 2020).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; Technical Report NREL/TP-510-42622; Office of Energy Efficiency and Renewable Energy, National Renewable Energy Laboratory: Golden, CO, USA, 2008; Available online: https://www.researchgate.net/publication/309108211_Determination_of_Ash_in_Biomass/citations (accessed on 1 April 2020).
- Lin, Y.-L. Effects of microwave-induced torrefaction on waste straw upgrading. Int. J. Chem. Eng. Appl. 2015, 6, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.J.; Huang, Y.F.; Chiueh, P.T.; Kuan, W.H.; Lo, S.L. Microwave-induced torrefaction of rice husk and sugarcane residues. Energy 2012, 37, 177–184. [Google Scholar] [CrossRef]
- Bergman, P.; Kiel, J. Torrefaction for biomass upgrading. In Proceedings of the 14th European Biomass Conference & Exhibition, Paris, France, 17–21 October 2005; p. 8. [Google Scholar]
- Rodrigues, T.; Rousset, P. Effects of Torrefaction on Energy Properties of Eucalyptus Grandis Wood. Cerne 2009, 15, 446–452. Available online: https://www.researchgate.net/publication/41392935_Effects_of_Torrefaction_on_Energy_Properties_of_Eucalyptus_grandis_Wood (accessed on 15 December 2019).
- Verhoeff, F.; Adell I Arnuelos, A.; Boersma, A.R.; Pels, J.R.; Lenselink, J.; Kiel, J.H.A.; Schukken, H. TorTech: Torrefaction Technology for the Production of Solid Bioenergy Carriers from Biomass and Waste|ECN Report, ECN-E--11-039. 2011. Available online: https://library.wur.nl/WebQuery/titel/2012815 (accessed on 11 April 2021).
- Almeida, G.; Brito, J.O.; Perré, P. Alterations in energy properties of eucalyptus wood and bark subjected to torrefaction: The potential of mass loss as a synthetic indicator. Bioresour. Technol. 2010, 101, 9778–9784. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Kim, Y.-H.; Lee, S.-M.; Lee, H.-W. Optimizing the torrefaction of mixed softwood by response surface methodology for biomass upgrading to high energy density. Bioresour. Technol. 2012, 116, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Schniewind, A.P. Concise Encyclopedia of Wood & Wood-Based Materials, 1st ed.; MIT Press: Elmsford, NY, USA, 1989. [Google Scholar]
- Repellin, V.; Govin, A.; Rolland, M.; Guyonnet, R. Energy requirement for fine grinding of torrefied wood. Biomass Bioenergy 2010, 34, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Phanphanich, M.; Mani, S. Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef]
- Govin, A.; Repellin, V.; Rolland, M.; Duplan, J.-L. Effect of Torrefaction on Grinding Energy Requirement for Thin Wood Particle Production. In Proceedings of the XII° Congrès de la Société Française de Génie des Procédés Pour relever les défis Industriels du XXI° Siècle A la Croisée des Sciences et des Cultures, Marseille, France; 2009; p. 7. Available online: https://www.researchgate.net/publication/41817373_Effect_of_torrefaction_on_grinding_energy_requirement_for_thin_wood_particle_production (accessed on 28 March 2020).
- Lanigan, B.A. Microwave Processing of Lignocellulosic Biomass for Production of Fuels. Ph.D. Thesis, University of York, York, UK, 2010. [Google Scholar]
- Van Heiningen, A. Converting a Kraft pulp mill into an integrated forest biorefinery. Pulp Paper Can. 2006, 107, 38–43. [Google Scholar]
- Montazeri, N.; Oliveira, A.C.; Himelbloom, B.H.; Leigh, M.B.; Crapo, C.A. Chemical characterization of commercial liquid smoke products. Food Sci. Nutr. 2013, 1, 102–115. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.; Lei, H.; Wang, L.; Bu, Q.; Wei, Y.; Liang, J.; Liu, Y.; Julson, J.; Chen, S.; Wu, J.; et al. Microwave torrefaction of douglas fir sawdust pellets. Energy Fuels 2012, 26, 5936–5943. [Google Scholar] [CrossRef]
- Bartocci, P.; Barbanera, M.; Skreiberg, Ø.; Wang, L.; Bidini, G.; Fantozzi, F. Biocarbon pellet production: Optimization of pelletizing process. Chem. Eng. Trans. 2018, 65, 6. [Google Scholar]
- Budaraga, K.; Arnim, A.; Marlida, Y.; Bulanin, U. Liquid smoke production quality from raw materials variation and different pyrolysis temperature. Int. J. Adv. Sci. Eng. Inf. Technol. 2016, 6, 306–315. [Google Scholar] [CrossRef]
- Güllü, D.; Demirbaş, A. Biomass to methanol via pyrolysis process. Energy Convers. Manag. 2001, 42, 1349–1356. [Google Scholar] [CrossRef]
- Wood, L. Global Hot Dogs and Sausages Markets, 2020–2025: Market Is Expected to Grow at a CAGR of 4.08%. Research and Markets. 21 December 2020. Available online: https://www.globenewswire.com/news-release/2020/12/21/2148457/28124/en/Global-Hot-Dogs-and-Sausages-Markets-2020-2025-Market-is-Expected-to-Grow-at-a-CAGR-of-4-08.html (accessed on 7 April 2021).



| Power (Watts) | Total Processing Time (min) | Estimated Cost Per Tonne (CAD t−1) | Total kWh | kilo Volt Ampere (kVA) | Torrefaction Mass Yield (% t−1) |
|---|---|---|---|---|---|
| 400 | 23.07 | $158.79 | 0.1483 | 1.3879 | 88 |
| 450 | 19.35 | $148.65 | 0.1386 | 1.5377 | 86 |
| 500 | 15.35 | $129.05 | 0.1262 | 1.5820 | 87 |
| 550 | 13.50 | $121.60 | 0.1210 | 1.6336 | 85 |
| 600 | 12.14 | $117.34 | 0.1136 | 1.8522 | 88 |
| 625 | 11.55 | $116.31 | 0.1135 | 1.8616 | 85 |
| 650 | 11.44 | $115.81 | 0.1134 | 1.8754 | 87 |
| 675 | 10.47 | $110.53 | 0.1087 | 1.9680 | 86 |
| 700 | 10.10 | $109.38 | 0.1043 | 2.1175 | 87 |
| 750 | 9.59 | $110.95 | 0.1095 | 2.0894 | 87 |
| 785 | 9.48 | $112.05 | 0.1111 | 2.1943 | 85 |
| Temperature | Moisture Content (% wb) | dgw (mm) | Sgw (mm) | Ash Content (% db) | Bulk Density (kg m−3) |
|---|---|---|---|---|---|
| Untorrefied | 10.40 | 1.664 | 0.717 | 5.30 | 166.34 |
| 225 °C | 0.93 | 1.509 | 0.650 | 5.51 | 164.60 |
| 255 °C | 0.81 | 1.378 | 0.593 | 6.59 | 152.87 |
| 285 °C | 0.78 | 1.287 | 0.554 | 7.59 | 145.67 |
| Temperature (°C) | Residence Time (min) | Higher Heating Value (MJ kg−1) | Mass Yield (%) | Liquid Yield (%) | Gas Yield (%) | Energy Yield (%) | Severity Factor |
|---|---|---|---|---|---|---|---|
| Raw material | - | 16.80 | 100.00 | - | - | 100 | - |
| 225 | 3 | 18.91 | 87.30 | 9.97 | 2.73 | 98.28 | 4.16 |
| 6 | 18.91 | 86.60 | 10.97 | 2.43 | 97.50 | 4.46 | |
| 9 | 18.91 | 86.37 | 10.67 | 2.96 | 97.24 | 4.63 | |
| 255 | 3 | 20.70 | 71.10 | 19.70 | 9.20 | 87.61 | 5.04 |
| 6 | 21.23 | 67.93 | 22.37 | 9.70 | 85.85 | 5.34 | |
| 9 | 21.48 | 66.70 | 22.23 | 11.07 | 85.30 | 5.52 | |
| 285 | 3 | 22.27 | 60.77 | 27.57 | 11.66 | 80.56 | 5.92 |
| 6 | 22.69 | 58.80 | 29.00 | 12.20 | 79.42 | 6.23 | |
| 9 | 22.70 | 58.63 | 28.73 | 12.64 | 79.21 | 6.40 |
| Sample | Hemicellulose (%, db) | Cellulose (%, db) | Lignin (%, db) | HHV (MJ kg−1, wt) | HHV (MJ kg−1, db) |
|---|---|---|---|---|---|
| Raw | 37.54 | 34.22 | 11.46 | 16.80 | 18.76 |
| 225 °C | 27.34 | 34.40 | 15.40 | 18.91 | 19.09 |
| 255 °C | 0.81 | 38.92 | 35.18 | 20.70 | 20.87 |
| 285 °C | 0.72 | 33.52 | 45.65 | 22.27 | 22.45 |
| Sample | C (%, db) | H (%, db) | N (%, db) | S (%, db) | O (%, db) |
|---|---|---|---|---|---|
| Raw | 43.16 | 4.60 | 0.39 | 0.49 | 51.36 |
| 225 °C | 47.05 | 4.68 | 0.43 | 0.30 | 47.55 |
| 255 °C | 51.98 | 4.55 | 0.46 | 0.12 | 42.89 |
| 285 °C | 55.71 | 4.15 | 0.57 | 0.25 | 39.32 |
| Sample | pH | Total Acid (%) | Tar (%) |
|---|---|---|---|
| Commercial | 2.35 | 4.00 | ND * |
| Liquid at 225 °C | 2.73 | 2.30 | ND * |
| Liquid at 255 °C | 2.50 | 15.00 | 2.99 |
| Liquid at 285 °C | 2.48 | 16.50 | 4.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdez, E.; Tabil, L.G.; Mupondwa, E.; Cree, D.; Moazed, H. Microwave Torrefaction of Oat Hull: Effect of Temperature and Residence Time. Energies 2021, 14, 4298. https://doi.org/10.3390/en14144298
Valdez E, Tabil LG, Mupondwa E, Cree D, Moazed H. Microwave Torrefaction of Oat Hull: Effect of Temperature and Residence Time. Energies. 2021; 14(14):4298. https://doi.org/10.3390/en14144298
Chicago/Turabian StyleValdez, Esteban, Lope G. Tabil, Edmund Mupondwa, Duncan Cree, and Hadi Moazed. 2021. "Microwave Torrefaction of Oat Hull: Effect of Temperature and Residence Time" Energies 14, no. 14: 4298. https://doi.org/10.3390/en14144298
APA StyleValdez, E., Tabil, L. G., Mupondwa, E., Cree, D., & Moazed, H. (2021). Microwave Torrefaction of Oat Hull: Effect of Temperature and Residence Time. Energies, 14(14), 4298. https://doi.org/10.3390/en14144298









