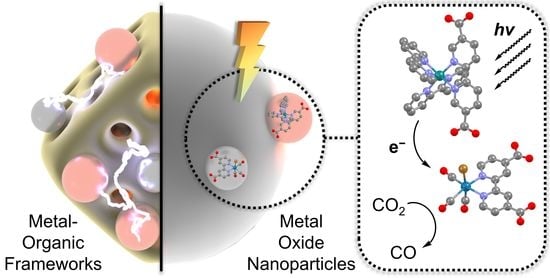
Molecular Dye-Sensitized Photocatalysis with Metal-Organic Framework and Metal Oxide Colloids for Fuel Production
Abstract
:1. Introduction
2. Materials and Methods
3. Results
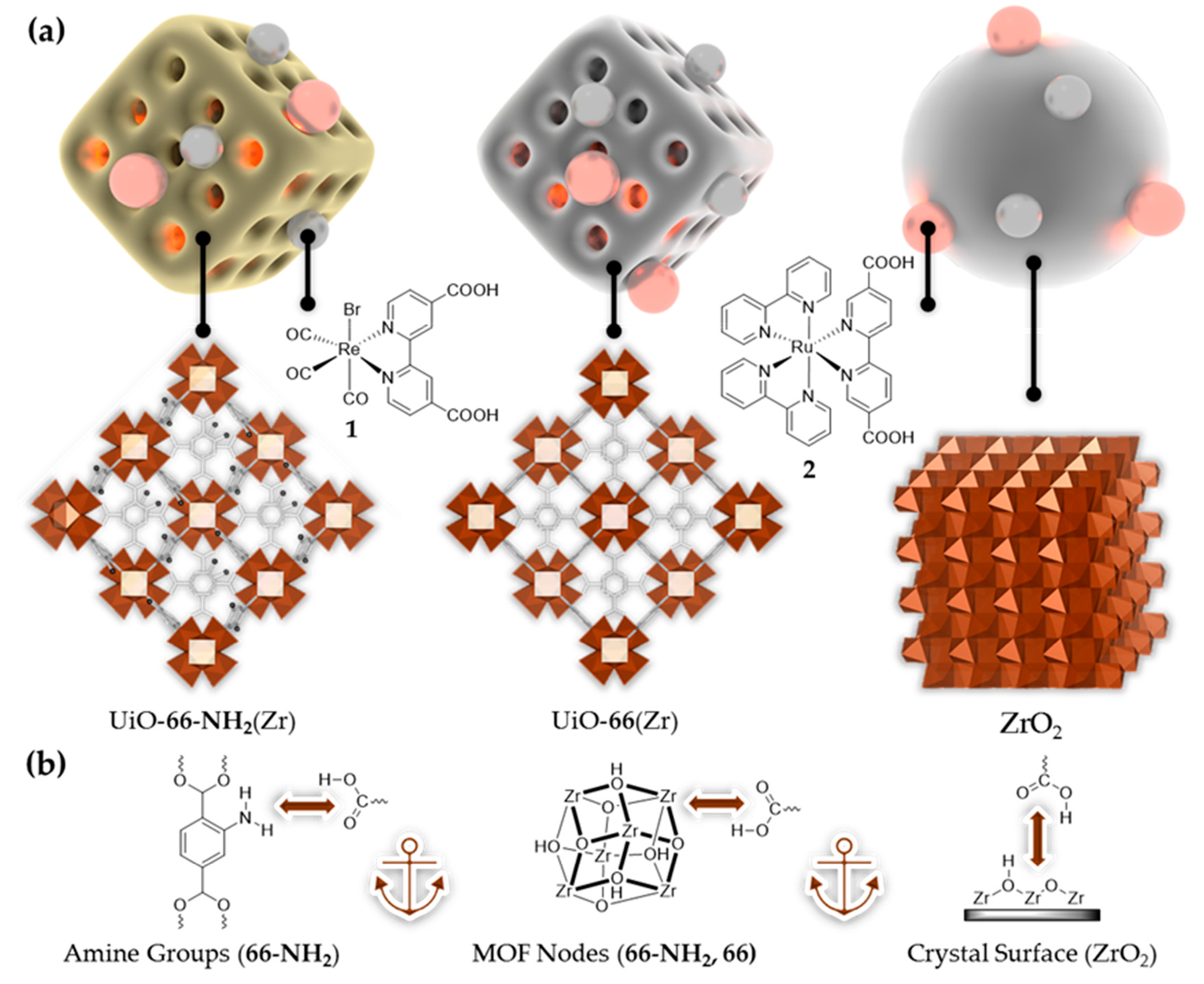
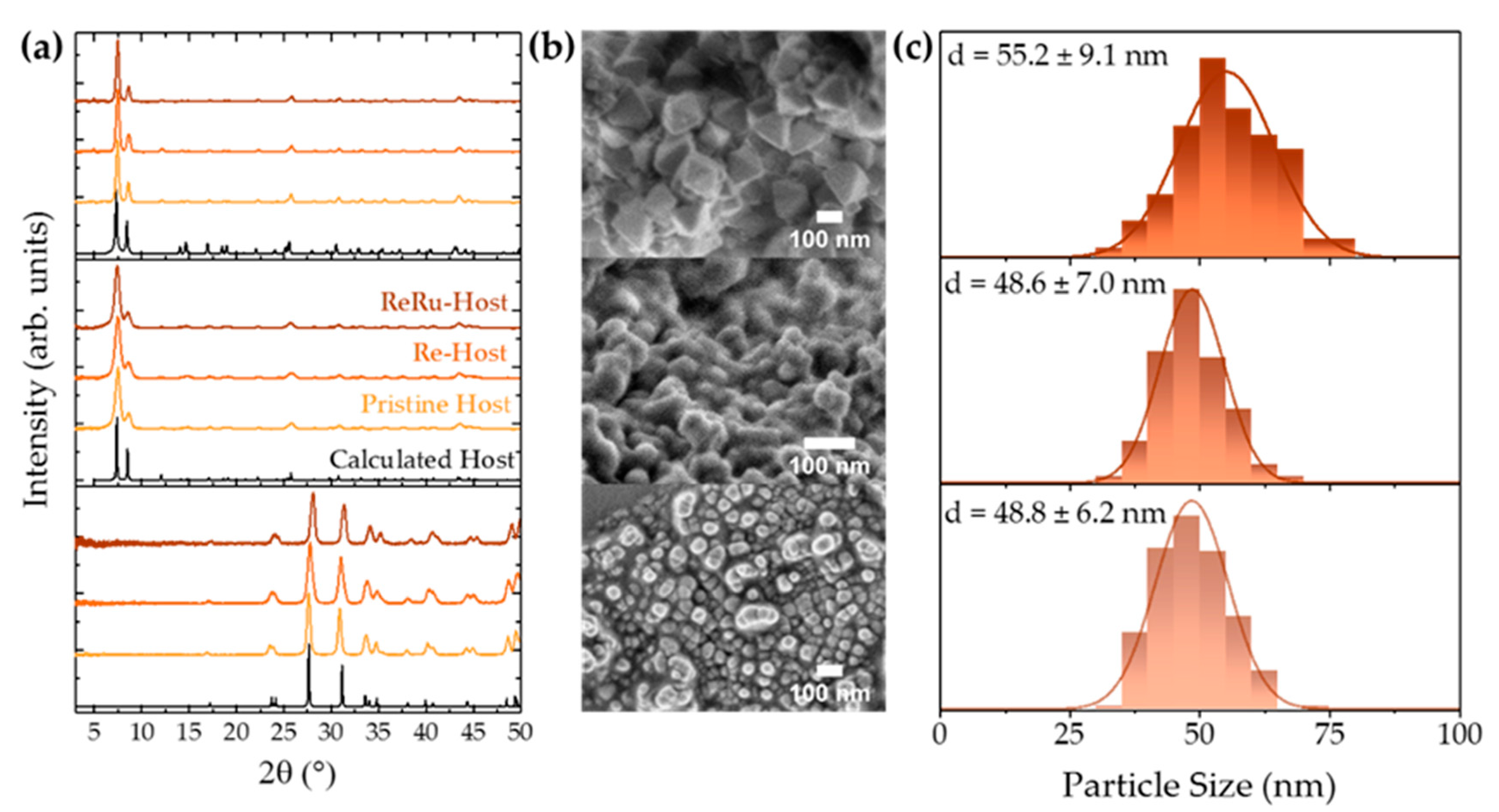
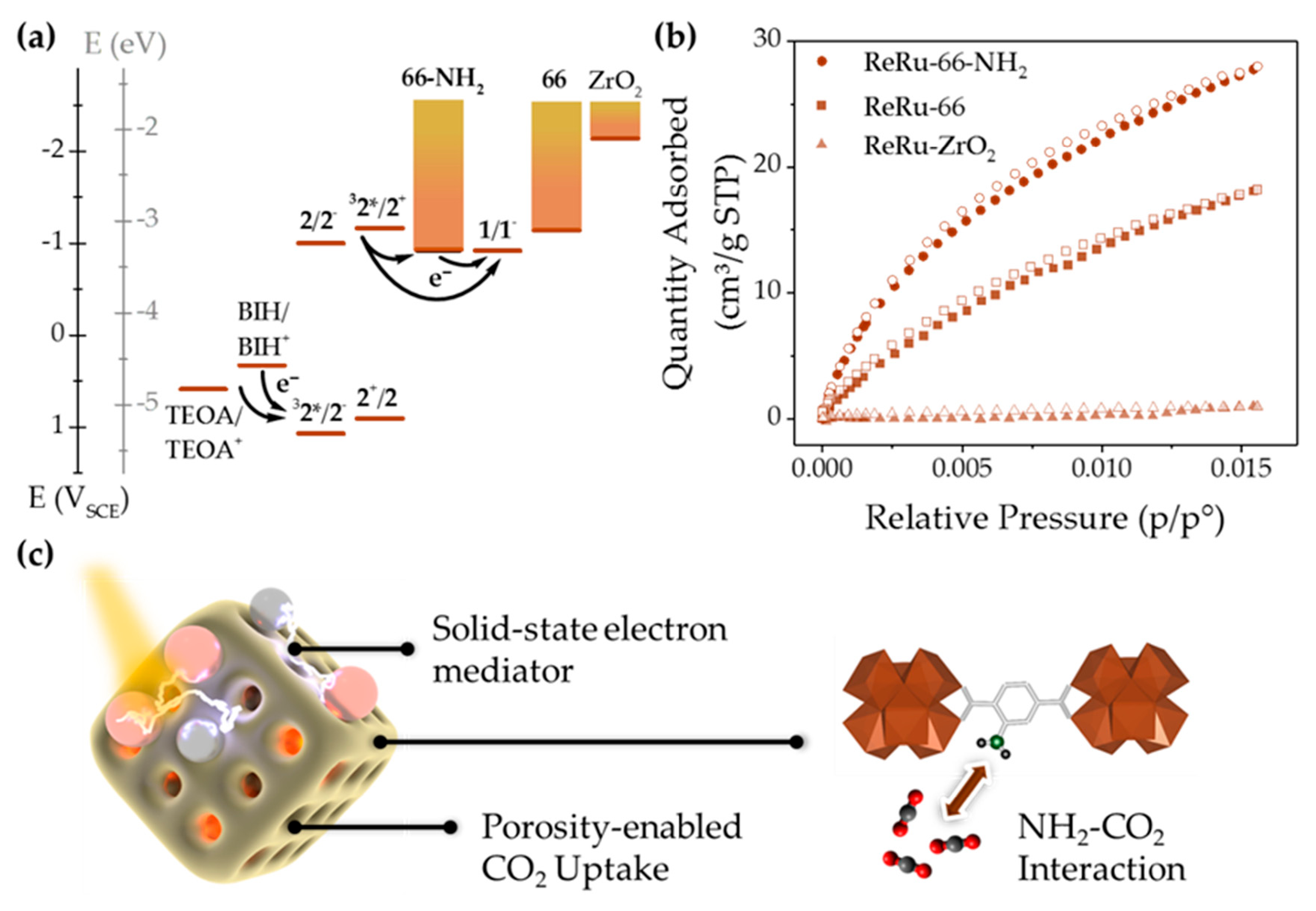
3.1. Catalyst, Dye, and Host Characterization
3.2. Host-Guest Photosystem Assembly
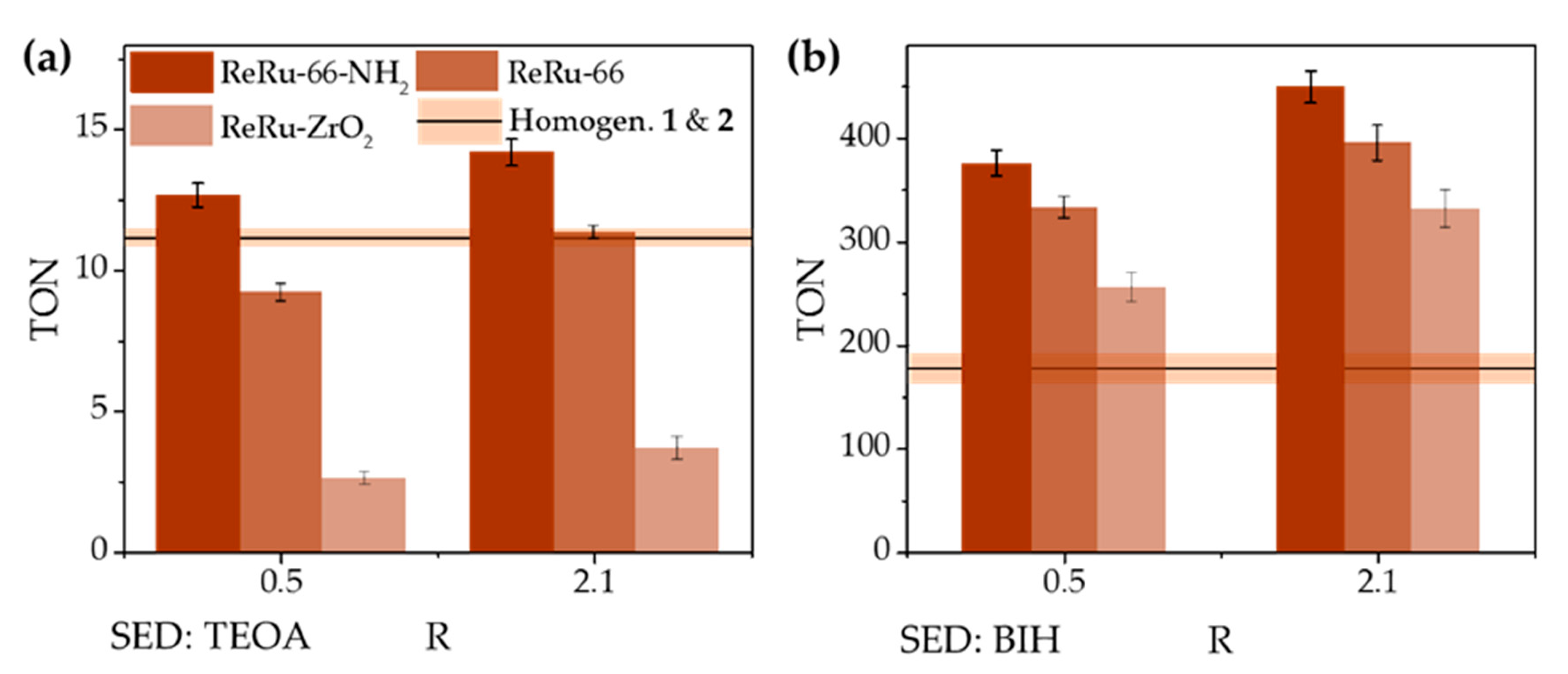
3.3. Photocatalytic Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA. World Energy Outlook 2019; IEA: Paris, France, 2019. [Google Scholar]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5, 486–519. [Google Scholar] [CrossRef] [Green Version]
- Dalle, K.E.; Warnan, J.; Leung, J.J.; Reuillard, B.; Karmel, I.S.; Reisner, E. Electro- and Solar-Driven Fuel Synthesis with First Row Transition Metal Complexes. Chem. Rev. 2019, 119, 2752–2875. [Google Scholar] [CrossRef]
- Sahara, G.; Ishitani, O. Efficient Photocatalysts for CO2 Reduction. Inorg. Chem. 2015, 54, 5096–5104. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, L. Artificial Photosynthesis: Opportunities and Challenges of Molecular Catalysts. Chem. Soc. Rev. 2019, 48, 2216–2264. [Google Scholar] [CrossRef] [Green Version]
- Willkomm, J.; Orchard, K.L.; Reynal, A.; Pastor, E.; Durrant, J.R.; Reisner, E. Dye-sensitised semiconductors modified with molecular catalysts for light-driven H2 production. Chem. Soc. Rev. 2016, 45, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Fabian, D.M.; Hu, S.; Singh, N.; Houle, F.A.; Hisatomi, T.; Domen, K.; Osterloh, F.E.; Ardo, S. Particle suspension reactors and materials for solar-driven water splitting. Energy Environ. Sci. 2015, 8, 2825–2850. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef]
- Stanley, P.M.; Haimerl, J.; Thomas, C.; Urstoeger, A.; Schuster, M.; Shustova, N.B.; Casini, A.; Rieger, B.; Warnan, J.; Fischer, R.A. Host-Guest Interactions in Metal-Organic Framework Isoreticular Series for Molecular Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2021. [Google Scholar] [CrossRef]
- Stanley, P.M.; Thomas, C.; Thyrhaug, E.; Urstoeger, A.; Schuster, M.; Hauer, J.; Rieger, B.; Warnan, J.; Fischer, R.A. Entrapped Molecular Photocatalyst and Photosensitizer in Metal-Organic Framework Nanoreactors for Enhanced Solar CO2 Reduction. ACS Catal. 2021, 11, 871–882. [Google Scholar] [CrossRef]
- Wang, X.; Wisser, F.M.; Canivet, J.; Fontecave, M.; Mellot-Draznieks, C. Immobilization of a Full Photosystem in the Large-Pore MIL-101 Metal-Organic Framework for CO2 reduction. ChemSusChem 2018, 11, 3315–3322. [Google Scholar] [CrossRef]
- Stanley, P.M.; Parkulab, M.; Rieger, B.; Warnan, J.; Fischer, R.A. Understanding entrapped molecular photosystem and metal-organic framework synergy for improved solar fuel production. Faraday Discuss. 2021. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Leith, G.A.; Martin, C.R.; Mayers, J.M.; Kittikhunnatham, P.; Larsen, R.W.; Shustova, N.B. Confinement-guided photophysics in MOFs, COFs, and cages. Chem. Soc. Rev. 2021. [Google Scholar] [CrossRef]
- Hendon, C.H.; Tiana, D.; Walsh, A. Conductive metal-organic frameworks and networks: Fact or fantasy? Phys. Chem. Chem. Phys. 2012, 14, 13120–13132. [Google Scholar] [CrossRef] [Green Version]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Winarta, J.; Shan, B.; Mcintyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A Decade of UiO-66 Research: A Historic Review of Dynamic Structure, Synthesis Mechanisms, and Characterization Techniques of an Archetypal Metal–Organic Framework. Cryst. Growth Des. 2019, 20, 1347–1362. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Tan, L.-L.; Liu, J.-M.; Qin, S.; Xie, Z.-Q.; Huang, J.-F.; Xu, Y.-W.; Xiao, L.-M.; Su, C.-Y. Calix[4]arene based dye-sensitized Pt@UiO-66-NH2 metal-organic framework for efficient visible-light photocatalytic hydrogen production. Appl. Catal. B-Environ. 2017, 206, 426–433. [Google Scholar] [CrossRef]
- He, J.; Wang, J.; Chen, Y.; Zhang, J.; Duan, D.; Wang, Y.; Yan, Z. A dye-sensitized Pt@UiO-66(Zr) metal-organic framework for visible-light photocatalytic hydrogen production. Chem. Commun. 2014, 50, 7063–7066. [Google Scholar] [CrossRef]
- Hendrickx, K.; Vanpoucke, D.E.P.; Leus, K.; Lejaeghere, K.; van Yperen-De Deyne, A.; van Speybroeck, V.; Van Der Voort, P.; Hemelsoet, K. Understanding Intrinsic Light Absorption Properties of UiO-66 Frameworks: A Combined Theoretical and Experimental Study. Inorg. Chem. 2015, 54, 10701–10710. [Google Scholar] [CrossRef]
- Chen, Q.; He, Q.; Lv, M.; Xu, Y.; Yang, H.; Liu, X.; Wei, F. Selective adsorption of cationic dyes by UiO-66-NH2. Appl. Surf. Sci. 2015, 327, 77–85. [Google Scholar] [CrossRef]
- Gross, M.A.; Reynal, A.; Durrant, J.R.; Reisner, E. Versatile photocatalytic systems for H2 generation in water based on an efficient DuBois-type nickel catalyst. J. Am. Chem. Soc. 2014, 136, 356–366. [Google Scholar] [CrossRef]
- Hawecker, J.; Lehn, J.-M.; Ziessel, R. Efficient Photochemical Reduction of CO2 to CO by Visible Light Irradiation of Systems Containing Re(bipy)(CO)3X or Ru(bipy)32+ – Co2+ Combinations as Homogeneous Catalysts. J. Chem. Soc. Chem. Commun. 1983, 9, 536–538. [Google Scholar] [CrossRef]
- Pfennig, B.W.; Chen, P.; Meyer, T.J. Photophysics and Photochemistry of Chromophore−Quencher Assemblies on Glass and Powdered Silica. Inorg. Chem. 1996, 35, 2898–2901. [Google Scholar] [CrossRef]
- Ha, E.-G.; Chang, J.-A.; Byun, S.-M.; Pac, C.; Jang, D.-M.; Park, J.; Kang, S.O. High-turnover visible-light photoreduction of CO2 by a Re(I) complex stabilized on dye-sensitized TiO2. Chem. Commun. 2014, 50, 4462–4464. [Google Scholar] [CrossRef]
- Won, D.-I.; Lee, J.-S.; Ji, J.-M.; Jung, W.-J.; Son, H.-J.; Pac, C.; Kang, S.O. Highly Robust Hybrid Photocatalyst for Carbon Dioxide Reduction: Tuning and Optimization of Catalytic Activities of Dye/TiO2/Re(I) Organic-Inorganic Ternary Systems. J. Am. Chem. Soc. 2015, 137, 13679–13690. [Google Scholar] [CrossRef]
- Windle, C.D.; Pastor, E.; Reynal, A.; Whitwood, A.C.; Vaynzof, Y.; Durrant, J.R.; Perutz, R.N.; Reisner, E. Improving the photocatalytic reduction of CO2 to CO through immobilisation of a molecular Re catalyst on TiO2. Chem. Eur. J. 2015, 21, 3746–3754. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Dubois, K.D.; Louis, M.E.; Vorushilov, A.S.; Li, G. Photocatalytic CO2 Reduction and Surface Immobilization of a Tricarbonyl Re(I) Compound Modified with Amide Groups. ACS Catal. 2013, 3, 655–662. [Google Scholar] [CrossRef]
- Kouva, S.; Honkala, K.; Lefferts, L.; Kanervo, J. Review: Monoclinic zirconia, its surface sites and their interaction with carbon monoxide. Catal. Sci. Technol. 2015, 5, 3473–3490. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.-C.; Li, T.-T.; Cao, S.; Chen, Y.; Fu, W.-F. Incorporation of a [Ru(dcbpy)(bpy)2]2+ Photosensitizer and a Pt(dcbpy)Cl2 Catalyst into Metal–Organic Frameworks for Photocatalytic Hydrogen Evolution from Aqueous Solution. J. Mater. Chem. A 2015, 3, 10386–10394. [Google Scholar] [CrossRef]
- Sun, D.; Fu, Y.; Liu, W.; Ye, L.; Wang, D.; Yang, L.; Fu, X.; Li, Z. Studies on photocatalytic CO2 reduction over NH2-UiO-66(Zr) and its derivatives: Towards a better understanding of photocatalysis on metal-organic frameworks. Chem. Eur. J. 2013, 19, 14279–14285. [Google Scholar] [CrossRef]
- Hou, L.; Wang, L.; Zhang, N.; Xie, Z.; Dong, D. Polymer brushes on metal–organic frameworks by UV-induced photopolymerization. Polym. Chem. 2016, 7, 5828–5834. [Google Scholar] [CrossRef]
- Smieja, J.M.; Kubiak, C.P. Re(bipy-tBu)(CO)3Cl-improved Catalytic Activity for Reduction of Carbon Dioxide: IR-spectroelectrochemical and mechanistic studies. Inorg. Chem. 2010, 49, 9283–9289. [Google Scholar] [CrossRef]
- Sun, H.; Hoffman, M.Z. Reductive Quenching of the Excited States of Ruthenium(II) Complexes Containing 2,2′-Bipyridine, 2,2′-Bipyrazine, and 2,2′-Bipyrimidine Ligands. J. Phys. Chem. 1994, 98, 11719–11726. [Google Scholar] [CrossRef]
- Pellegrin, Y.; Odobel, F. Sacrificial Electron Donor Reagents for Solar Fuel Production. Cr. Chim. 2017, 20, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Gholamkhass, B.; Mametsuka, H.; Koike, K.; Tanabe, T.; Furue, M.; Ishitani, O. Architecture of supramolecular metal complexes for photocatalytic CO2 reduction: Ruthenium-rhenium bi- and tetranuclear complexes. Inorg. Chem. 2005, 44, 2326–2336. [Google Scholar] [CrossRef]
- Tamaki, Y.; Ishitani, O. Supramolecular Photocatalysts for the Reduction of CO2. ACS Catal. 2017, 7, 3394–3409. [Google Scholar] [CrossRef]
- Semrau, A.L.; Stanley, P.M.; Urstoeger, A.; Schuster, M.; Cokoja, M.; Fischer, R.A. Substantial Turnover Frequency Enhancement of MOF Catalysts by Crystallite Downsizing Combined with Surface Anchoring. ACS Catal. 2020, 10, 3203–3211. [Google Scholar] [CrossRef]
- Semrau, A.L.; Pujari, S.P.; Stanley, P.M.; Wannapaiboon, S.; Albada, B.; Zuilhof, H.; Fischer, R.A. Selective Positioning of Nanosized Metal–Organic Framework Particles at Patterned Substrate Surfaces. Chem. Mater. 2020, 32, 9954–9963. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Stat. Sol. 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Jun, J.W.; Hasan, Z.; Matrosova, M.M.; Jhung, S.H. Effects of linker substitution on catalytic properties of porous zirconium terephthalate UiO-66 in acetalization of benzaldehyde with methanol. Appl. Catal. A Gen. 2014, 471, 91–97. [Google Scholar] [CrossRef]
- Horti, N.C.; Kamatagi, M.D.; Nataraj, S.K.; Wari, M.N.; Inamdar, S.R. Structural and optical properties of zirconium oxide (ZrO2) nanoparticles: Effect of calcination temperature. Nano Ex. 2020, 1, 10022. [Google Scholar] [CrossRef]
- Vlček, A. Ultrafast Excited-State Processes in Re(I) Carbonyl-Diimine Complexes: From Excitation to Photochemistry. In Photophysics of Organometallics; Lees, A.J., Castellano, F.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 115–158. ISBN 9783642047282. [Google Scholar]
- Tamaki, Y.; Koike, K.; Morimoto, T.; Ishitani, O. Substantial Improvement in the Efficiency and Durability of a Photocatalyst for Carbon Dioxide Reduction Using a Benzoimidazole Derivative as an Electron Donor. J. Catal. 2013, 304, 22–28. [Google Scholar] [CrossRef]
- Fujita, E.; Szalda, D.J.; Creutz, C.; Sutin, N. Carbon dioxide activation: Thermodynamics of carbon dioxide binding and the involvement of two cobalt centers in the reduction of carbon dioxide by a cobalt(I) macrocycle. J. Am. Chem. Soc. 1988, 110, 4870–4871. [Google Scholar] [CrossRef]
- Meister, S.; Reithmeier, R.O.; Tschurl, M.; Heiz, U.; Rieger, B. Unraveling Side Reactions in the Photocatalytic Reduction of CO2: Evidence for Light-Induced Deactivation Processes in Homogeneous Photocatalysis. ChemCatChem 2015, 7, 690–697. [Google Scholar] [CrossRef]
- Orchanian, N.M.; Hong, L.E.; Skrainka, J.A.; Esterhuizen, J.A.; Popov, D.A.; Marinescu, S.C. Surface-Immobilized Conjugated Polymers Incorporating Rhenium Bipyridine Motifs for Electrocatalytic and Photocatalytic CO2 Reduction. ACS Appl. Energy Mater. 2018, 2, 110–123. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; He, X.; Liu, B. Preparation of a g-C3N4/UiO-66-NH2/CdS Photocatalyst with Enhanced Visible Light Photocatalytic Activity for Tetracycline Degradation. Nanomaterials 2020, 10, 1824. [Google Scholar] [CrossRef]
- Stojković, M.; Pašti, I.A. Strain Engineering for Tuning the Photocatalytic Activity of Metal-Organic Frameworks-Theoretical Study of the UiO-66 Case. Catalysts 2021, 11, 264. [Google Scholar] [CrossRef]
- Gritsenko, V.A.; Novikov, Y.N.; Perevalov, T.V.; Kruchinin, V.N.; Aliev, V.S.; Gerasimova, A.K.; Erenburg, S.B.; Trubina, S.V.; Kvashnina, K.O.; Prosvirin, I.P.; et al. Nanoscale Potential Fluctuations in Zirconium Oxide and the Flash Memory Based on Electron and Hole Localization. Adv. Electron. Mater. 2018, 4, 1700592. [Google Scholar] [CrossRef]
- Neumann, T.; Liu, J.; Wächter, T.; Friederich, P.; Symalla, F.; Welle, A.; Mugnaini, V.; Meded, V.; Zharnikov, M.; Wöll, C.; et al. Superexchange Charge Transport in Loaded Metal Organic Frameworks. ACS Nano 2016, 10, 7085–7093. [Google Scholar] [CrossRef]
- Hod, I.; Farha, O.K.; Hupp, J.T. Modulating the rate of charge transport in a metal-organic framework thin film using host:guest chemistry. Chem. Commun. 2016, 52, 1705–1708. [Google Scholar] [CrossRef]
- Santiago-Portillo, A.; Baldoví, H.G.; Carbonell, E.; Navalón, S.; Álvaro, M.; García, H.; Ferrer, B. Ruthenium(II) Tris(2,2′-bipyridyl) Complex Incorporated in UiO-67 as Photoredox Catalyst. J. Phys. Chem. C 2018, 122, 29190–29199. [Google Scholar] [CrossRef] [Green Version]
- Asbury, J.B.; Wang, Y.-Q.; Hao, E.; Ghosh, H.N.; Lian, T. Evidences of hot excited state electron injection from sensitizer molecules to TiO2 nanocrystalline thin films. Res. Chem. Intermed. 2001, 27, 393–406. [Google Scholar] [CrossRef]
- Musho, T.D.; Yasin, A.S. Ab-initio Study of the Electron Mobility in a Functionalized UiO-66 Metal Organic Framework. J. Electron. Mater. 2018, 47, 3692–3700. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Ding, J.; Huang, X.; Wei, X.; Wang, W. Experimental and Computational Investigation of CO2 Capture on Mix-ligand Metal-organic Framework UiO-66. Energy Procedia 2017, 105, 4395–4401. [Google Scholar] [CrossRef]
- Flaig, R.W.; Osborn Popp, T.M.; Fracaroli, A.M.; Kapustin, E.A.; Kalmutzki, M.J.; Altamimi, R.M.; Fathieh, F.; Reimer, J.A.; Yaghi, O.M. The Chemistry of CO2 Capture in an Amine-Functionalized Metal-Organic Framework under Dry and Humid Conditions. J. Am. Chem. Soc. 2017, 139, 12125–12128. [Google Scholar] [CrossRef] [Green Version]
- Jakobsen, J.B.; Rønne, M.H.; Daasbjerg, K.; Skrydstrup, T. Are Amines the Holy Grail for Facilitating CO2 Reduction? Angew. Chem. Int. Ed. 2021, 60, 9174–9179. [Google Scholar] [CrossRef]
- Waki, M.; Ikai, M.; Goto, Y.; Maegawa, Y.; Inagaki, S. Re(bpy)(CO)3Cl Immobilized on Bipyridine Organosilica Nanotubes for Photocatalytic CO2 Reduction. Eur. J. Inorg. Chem. 2021, 4, 1259. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanley, P.M.; Warnan, J. Molecular Dye-Sensitized Photocatalysis with Metal-Organic Framework and Metal Oxide Colloids for Fuel Production. Energies 2021, 14, 4260. https://doi.org/10.3390/en14144260
Stanley PM, Warnan J. Molecular Dye-Sensitized Photocatalysis with Metal-Organic Framework and Metal Oxide Colloids for Fuel Production. Energies. 2021; 14(14):4260. https://doi.org/10.3390/en14144260
Chicago/Turabian StyleStanley, Philip M., and Julien Warnan. 2021. "Molecular Dye-Sensitized Photocatalysis with Metal-Organic Framework and Metal Oxide Colloids for Fuel Production" Energies 14, no. 14: 4260. https://doi.org/10.3390/en14144260
APA StyleStanley, P. M., & Warnan, J. (2021). Molecular Dye-Sensitized Photocatalysis with Metal-Organic Framework and Metal Oxide Colloids for Fuel Production. Energies, 14(14), 4260. https://doi.org/10.3390/en14144260