Catalytic Hydroisomerisation of Fischer–Tropsch Waxes to Lubricating Oil and Investigation of the Correlation between Its Physical Properties and the Chemical Composition of the Corresponding Fuel Fractions
Abstract
:1. Introduction
2. Theoretical Background
2.1. Dewaxing
2.2. Hydroprocessing
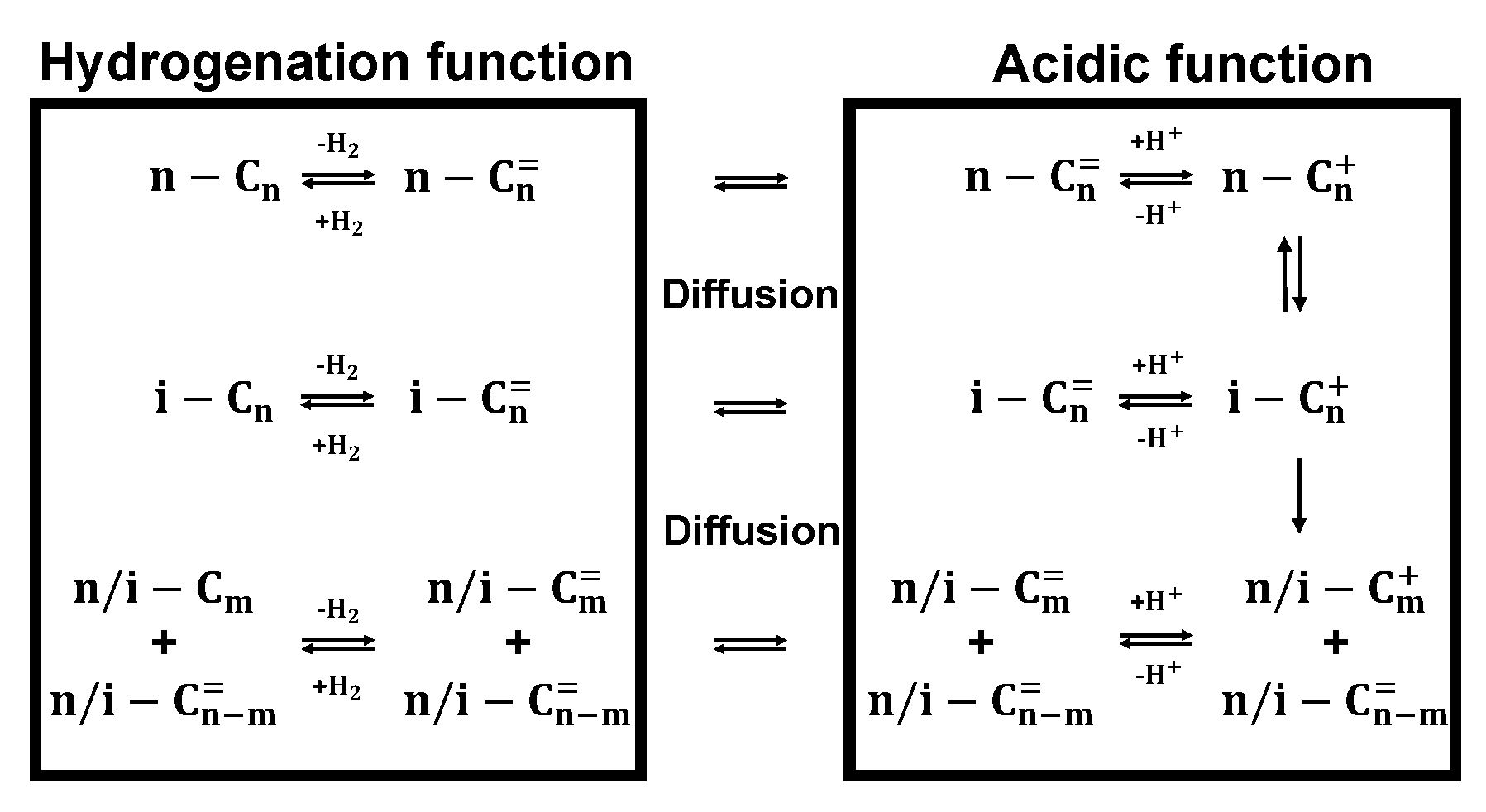
2.3. Catalytic Mechanism
2.4. Synthetic Lubricants from FT Waxes
2.5. Cloud Point
3. Materials and Methods
3.1. Reactor Setup and Materials Used
3.2. Sample Preparation
3.3. Analytics
4. Results
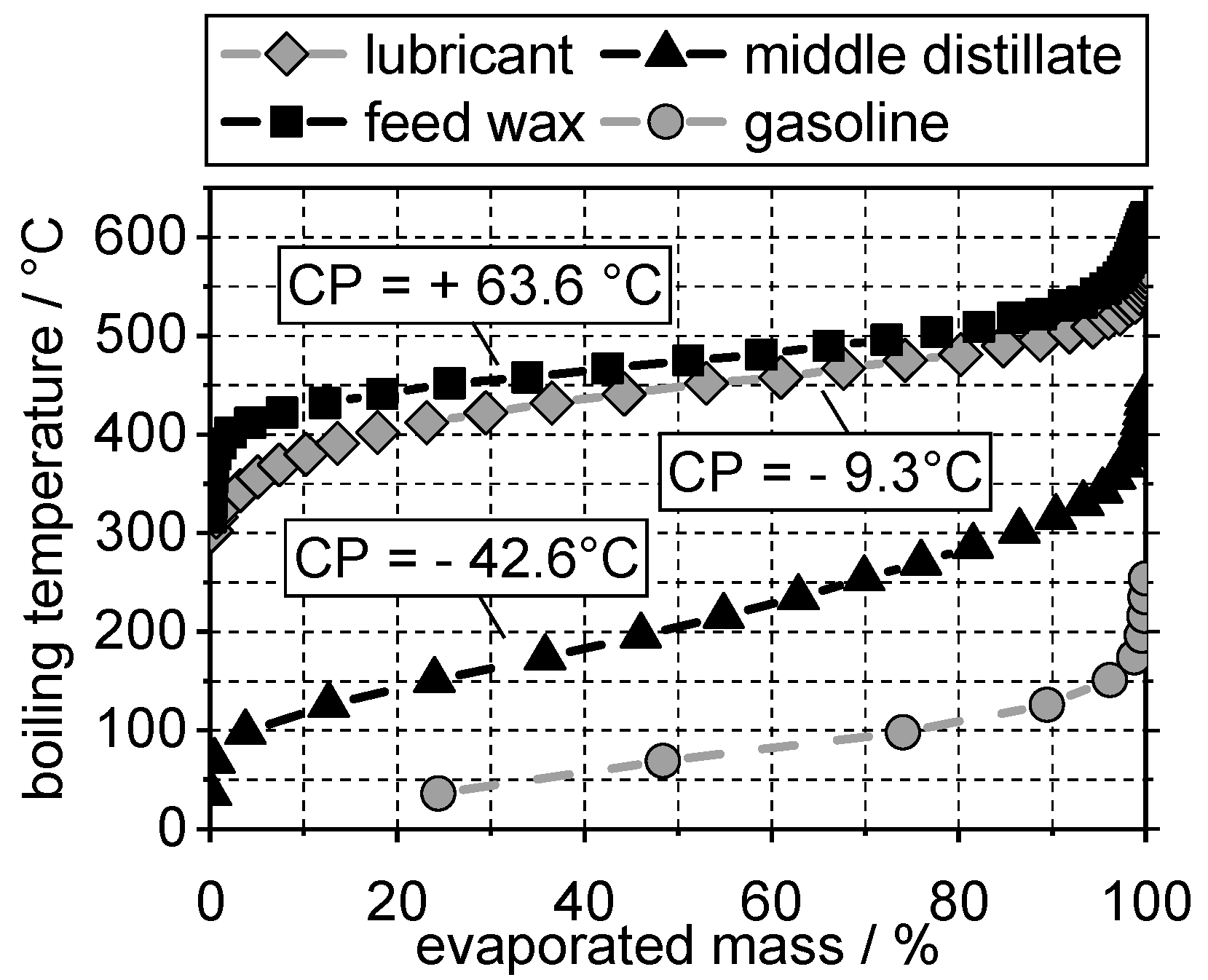
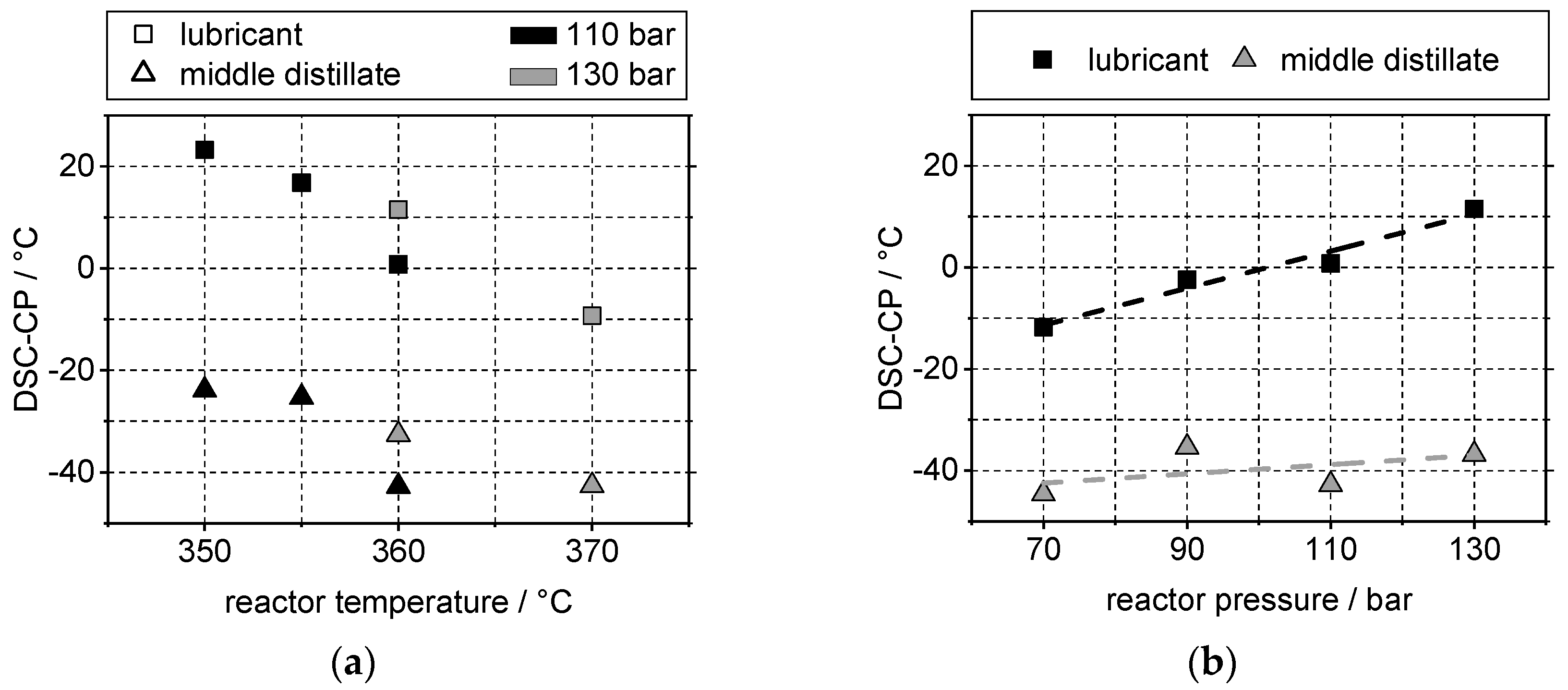
4.1. Boiling Range and Cloud Points
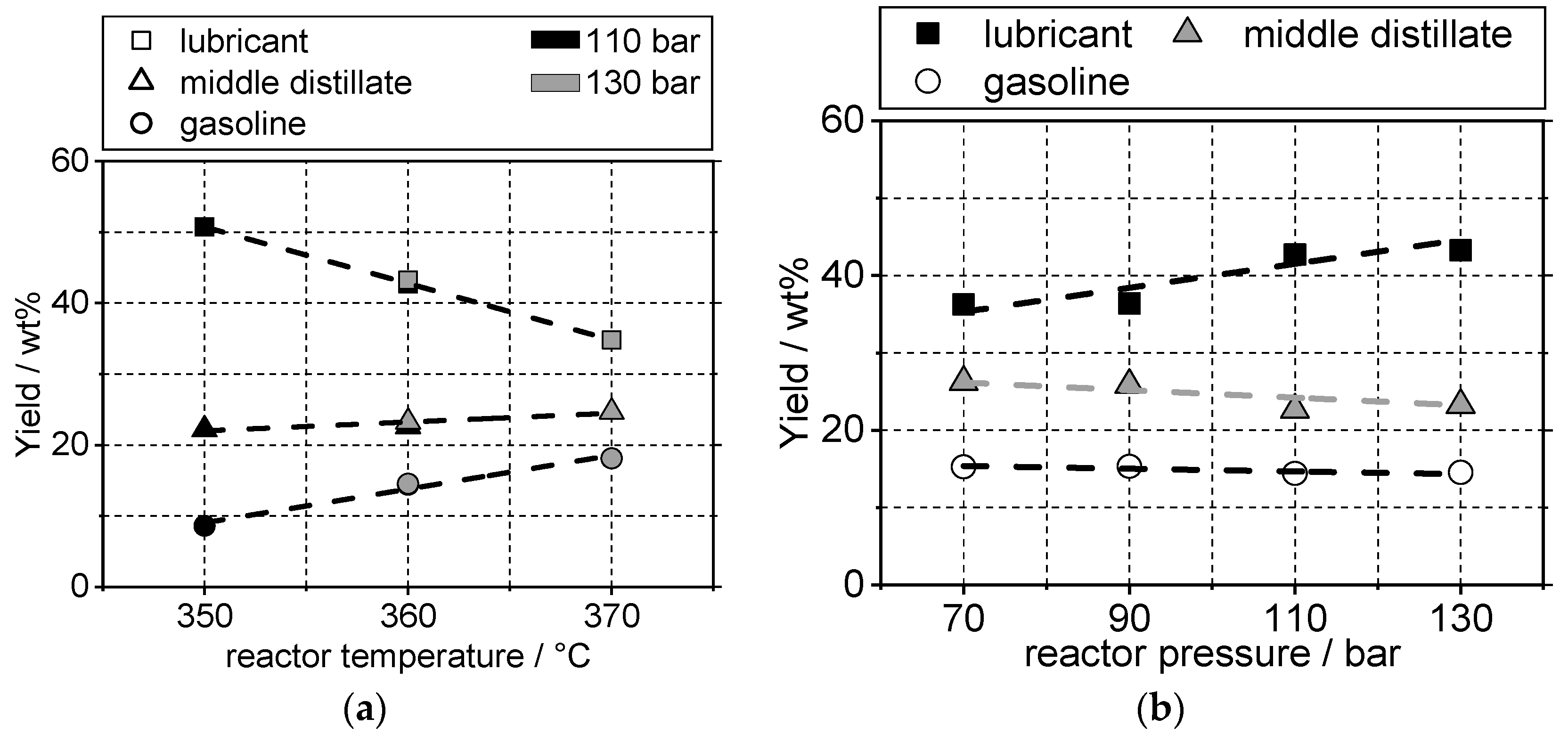
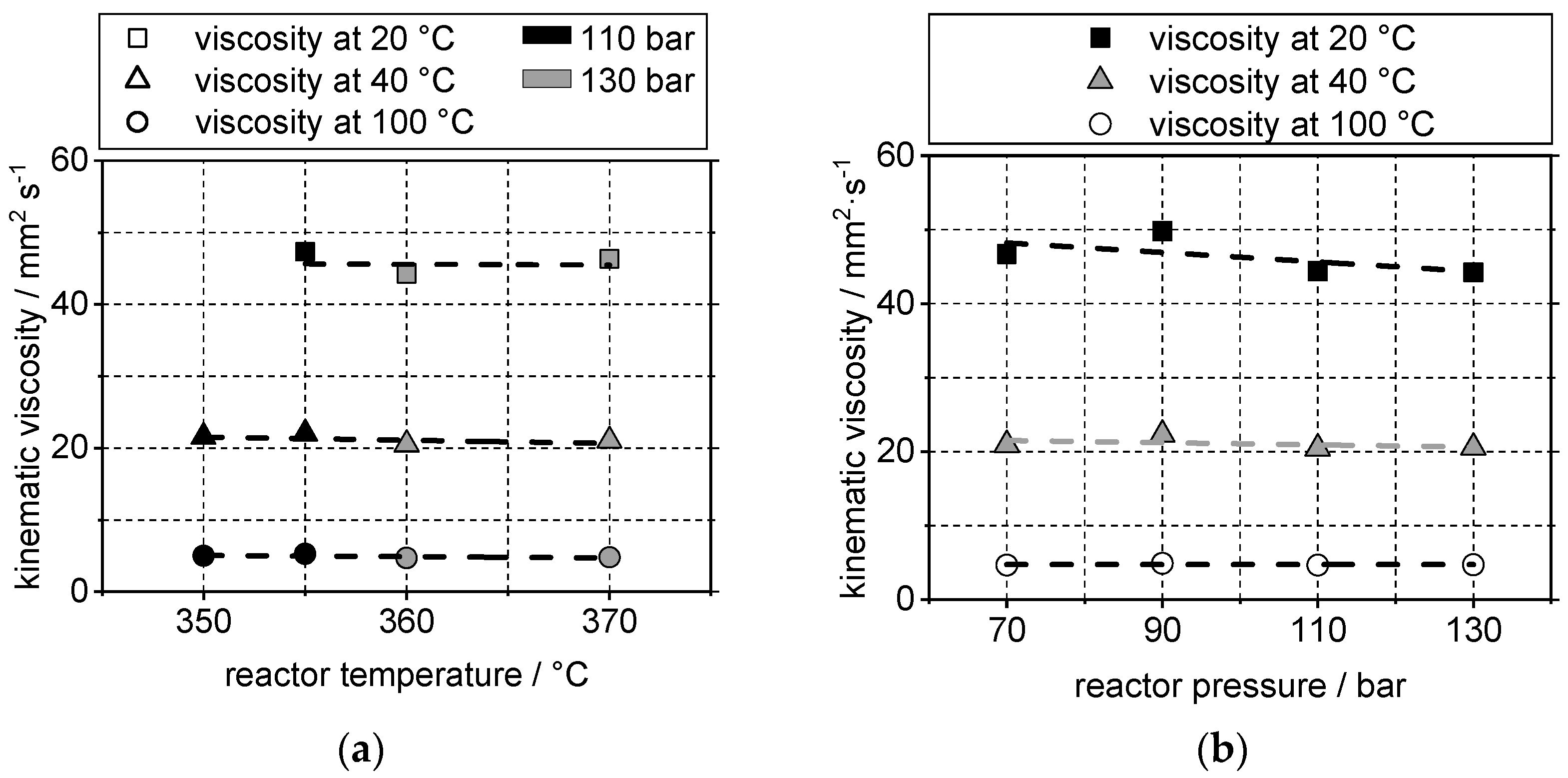
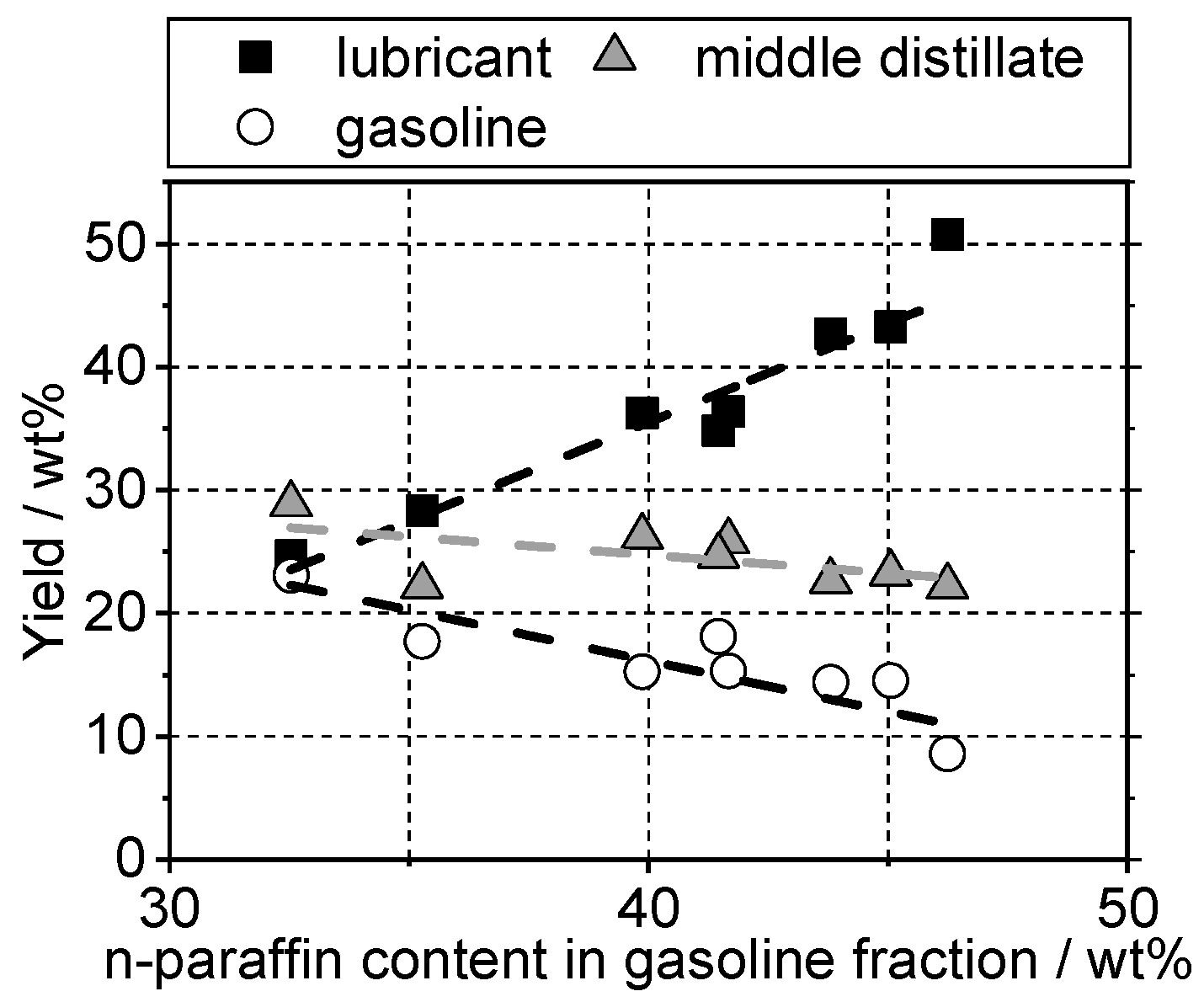
4.2. Yield and Viscosity
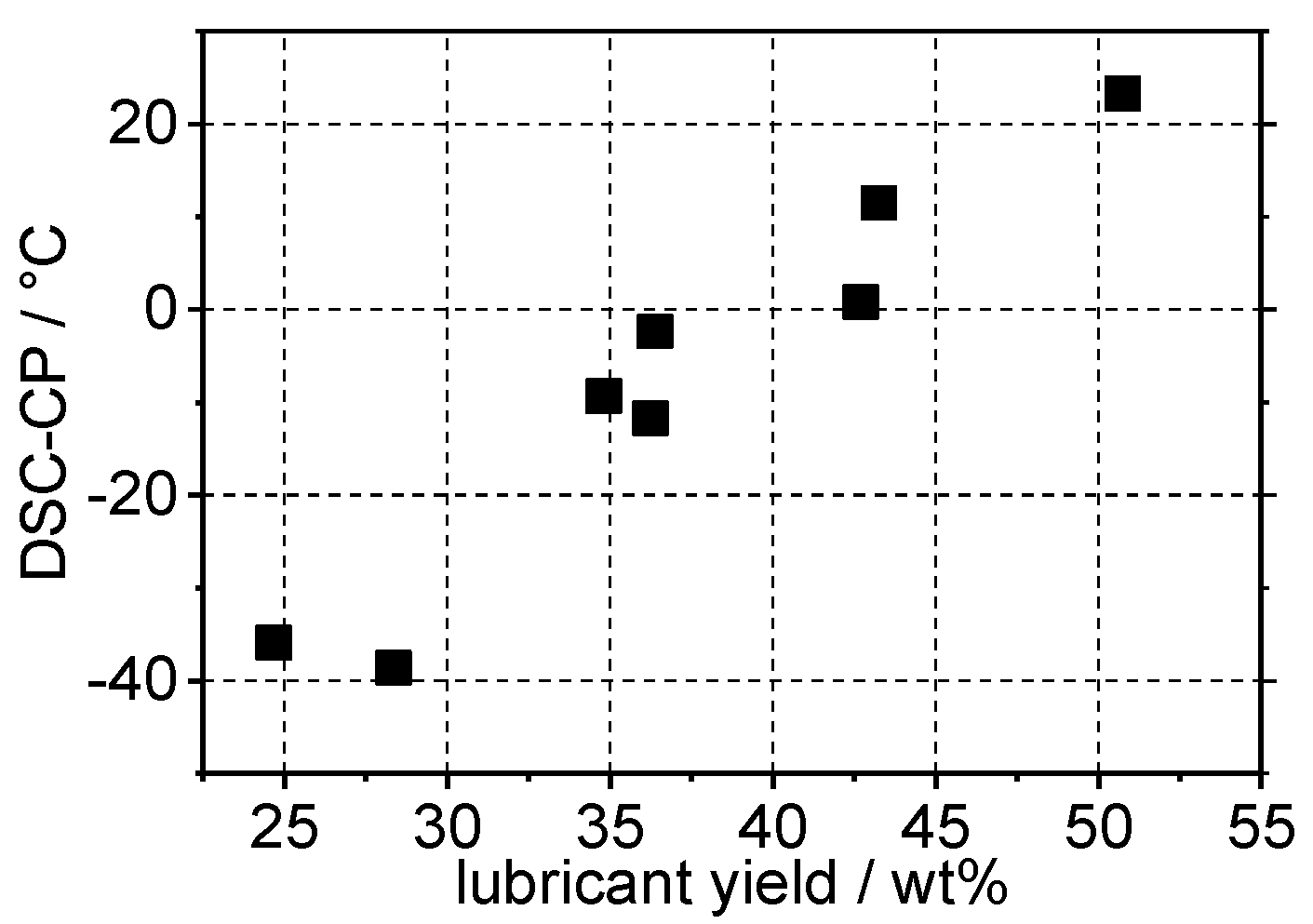
4.3. Determination of Cloud Point Using Composition of the Gasoline Fraction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
With: | ||
|---|---|---|
| TM | a | b |
| <−12.9 °C | 0.7841 | 0.8216 |
| >−12.9 °C <2.5 °C | 0.9900 | 3.0611 |
| >2.5°C | 0.9701 | 3.2361 |
Appendix B
| Component | Amount |
|---|---|
| Methane | 0.48 wt% |
| Ethane | 0.14 wt% |
| Propane | 5.26 wt% |
| Butanes | 7.10 wt% |
| n-Butane | 03.98 wt% |
| 2-M-Propane | 03.13 wt% |
| Pentanes | 3.67 wt% |
| n-Pentane | 01.64 wt% |
| 2-M-Butane | 02.02 wt% |
| Hexanes | 1.22 wt% |
| n-Hexane | 00.45 wt% |
| 2-M-Pentane | 00.51 wt% |
| 3-M-Pentane | 00.26 wt% |
| Heptanes | 0.29 wt% |
| n-Heptane | 00.08 wt% |
| 2-M-Hexane | 00.09 wt% |
| 3-M-Hexane | 00.12 wt% |
| Liquid product mix | 77.18 wt% |
| Lubricant (CP = −35.9 °C) | 24.68 wt% |
| Middle distillate (CP = −55.6 °C) | 28.88 wt% |
| Gasoline | 23.10 wt% |
| Distillation loss | 00.53 wt% |
| 95.34 wt% |
Appendix C
| Sample | Reactor Temperature (°C) | Reactor Pressure (bar) | Cloud Point (°C) | Reformulyzer Analysis (wt%) | Kinematic Viscosity (mm2/s) | Liquid Yields (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lubricant | Middle Distillate | Naphthenes | iso-Paraffins | n-Paraffins | 20 °C | 40 °C | 100 °C | Lubricant | Middle Distllate | Gasoline | |||
| 1 | 360 | 70 | −11.7 | −44.6 | 4.38 | 55.60 | 39.87 | 46.7 | 20.9 | 4.7 | 36.2 | 26.2 | 15.3 |
| 2 | 360 | 90 | −2.4 | −35.4 | 4.23 | 54.02 | 41.67 | 49.8 | 22.3 | 4.9 | 36.4 | 25.9 | 15.3 |
| 3 | 360 | 110 | 0.8 | −42.8 | 3.78 | 52.36 | 43.80 | 44.4 | 20.5 | 4.7 | 42.7 | 22.6 | 14.4 |
| 4 | 350 | 110 | 23.3 | −23.9 | 3.56 | 50.11 | 46.24 | - | 21.6 | 5.0 | 50.7 | 22.2 | 8.6 |
| 5 | 355 | 110 | 16.8 | −25.3 | 3.45 | 49.38 | 44.77 | 47.3 | 22.0 | 5.3 | 46.7 | 22.4 | 11.5 |
| 6 | 360 | 130 | 11.5 | −36.8 | 3.37 | 51.35 | 45.06 | 44.2 | 20.6 | 4.7 | 43.3 | 23.2 | 14.5 |
| 7 | 370 | 130 | −9.3 | −42.6 | 3.29 | 55.20 | 41.46 | 46.3 | 21.1 | 4.8 | 34.8 | 24.7 | 18.1 |
| 8 | 370 | 68.75 | −35.9 | −55.6 | 5.59 | 61.72 | 32.54 | 33.6 | 15.7 | 3.8 | 24.7 | 28.9 | 23.1 |
| 9 | 380 | 50 | −38.6 | −59.6 | 5.38 | 58.86 | 35.28 | 41.1 | 18.9 | 4.2 | 28.4 | 22.2 | 17.7 |
References
- German Federal Government, Effectively Reducing CO2 Emissions. 2020. Available online: https://bit.ly/3wivKDO (accessed on 6 July 2021).
- Eckert, V.; Heinrich, M. German Retail Gas Prices to Rise in 2021 due New CO2 Tax: Portal. 2020. Available online: https://reut.rs/3yqqAqH (accessed on 6 July 2021).
- Caspeta, L.; Buijs, N.A.A.; Nielsen, J. The role of biofuels in the future energy supply. Energy Environ. Sci. 2013, 6, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Ripfel-Nitsche, K.; Hofbauer, H.; Rauch, R.; Goritschnig, M. BTL—Biomass to liquid (Fischer Tropsch process at the bio-mass gasifier in Güssing). In Proceedings of the 15th European Biomass Conference & Exhibition, Berlin, Germany, 7–11 May 2007. [Google Scholar]
- González, M.I.; Eilers, H.; Schaub, G. Flexible Operation of Fixed-Bed Reactors for a Catalytic Fuel Synthesis-CO2 Hydrogenation as Example Reaction. Energy Technol. 2016, 4, 90–103. [Google Scholar] [CrossRef]
- Pöhlmann, F.; Jess, A. Influence of Syngas Composition on the Kinetics of Fischer-Tropsch Synthesis of using Cobalt as Catalyst. Energy Technol. 2015, 4, 55–64. [Google Scholar] [CrossRef]
- Puskas, I.; Hurlbut, R. Comments about the causes of deviations from the Anderson–Schulz–Flory distribution of the Fischer–Tropsch reaction products. Catal. Today 2003, 84, 99–109. [Google Scholar] [CrossRef]
- Snell, R. Deviations of Fischer-Tropsch products from an Anderson-Schulz-Flory distribution. Catal. Lett. 1988, 1, 327–330. [Google Scholar] [CrossRef] [Green Version]
- Jess, A.; Wasserscheid, P. Chemical Technology. In An Integral Textbook; Wiley-VCH: Weinheim, Germany, 2013; ISBN 978-3-527-30446-2. [Google Scholar]
- Van der Laan, G.; Beenackers, A.A.C.M. Kinetics and Selectivity of the Fischer–Tropsch Synthesis: A Literature Review. Catal. Rev. 1999, 41, 255–318. [Google Scholar] [CrossRef]
- Leckel, D. Diesel Production from Fischer−Tropsch: The Past, the Present, and New Concepts. Energy Fuels 2009, 23, 2342–2358. [Google Scholar] [CrossRef]
- Gill, S.; Tsolakis, A.; Dearn, K.D.; Fernández, J.R. Combustion characteristics and emissions of Fischer–Tropsch diesel fuels in IC engines. Prog. Energy Combust. Sci. 2011, 37, 503–523. [Google Scholar] [CrossRef]
- Miller, S.J.; Shah, N.; Huffman, G.P. Conversion of Waste Plastic to Lubricating Base Oil. Energy Fuels 2005, 19, 1580–1586. [Google Scholar] [CrossRef]
- De Klerk, A. Catalysis in the Refining of Fischer-Tropsch Syncrude; RSC Publishing: Cambridge, UK, 2010; ISBN 978-8-84973-080-8. [Google Scholar]
- Bouchy, C.; Hastoy, G.; Guillon, E.; Martens, J.A. Fischer-Tropsch Waxes UpgradingviaHydrocracking and Selective Hydroisomerization. Oil Gas Sci. Technol. Rev. l’IFP 2009, 64, 91–112. [Google Scholar] [CrossRef] [Green Version]
- Gamba, S.; Pellegrini, L.A.; Calemma, V.; Gambaro, C. Liquid fuels from Fischer–Tropsch wax hydrocracking: Isomer distribution. Catal. Today 2010, 156, 58–64. [Google Scholar] [CrossRef]
- Rossetti, I.; Gambaro, C.; Calemma, V. Hydrocracking of long chain linear paraffins. Chem. Eng. J. 2009, 154, 295–301. [Google Scholar] [CrossRef]
- Schulz, H.F.; Weitkamp, J.H. Zeolite Catalysts. Hydrocracking and Hydroisomerization of n-Dodecane. Ind. Eng. Chem. Prod. Res. Dev. 1972, 11, 46–53. [Google Scholar] [CrossRef]
- Weitkamp, J. Isomerization of long-chain n-alkanes on a Pt/CaY zeolite catalyst. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 550–558. [Google Scholar] [CrossRef]
- Royal Dutch Shell plc. Risella X. High-Quality Technical White Oils Based on Gas to Liquids (GTL) Technology. Available online: https://go.shell.com/2RnueBW (accessed on 4 May 2021).
- Gerasimov, D.N.; Kashin, E.V.; Pigoleva, I.V.; Maslov, I.A.; Fadeev, V.V.; Zaglyadova, S.V. Effect of Zeolite Properties on Dewaxing by Isomerization of Different Hydrocarbon Feedstocks. Energy Fuels 2019, 33, 3492–3503. [Google Scholar] [CrossRef]
- Kobayashi, M.; Saitoh, M.; Togawa, S.; Ishida, K. Branching Structure of Diesel and Lubricant Base Oils Prepared by Isomerization/Hydrocracking of Fischer−Tropsch Waxes and α-Olefins. Energy Fuels 2009, 23, 513–518. [Google Scholar] [CrossRef]
- Kobayashi, M.; Togawa, S.; Ishida, K. Properties and molecular structures of fuel fractions obtained from Hydrocracking / Isomerization of Fischer-Tropsch waxes. J. Jpn. Pet. Inst. 2005, 49, 194–201. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ishida, K.; Saito, M.; Yachi, H. Japan Energy Corporation. Lubricant Base Oil Method of Producing the Same. U.S. Patent 8.012,342 B2, 6 September 2011. Available online: https://bit.ly/3phwTtk (accessed on 3 June 2021).
- Miller, S.J.; Chevron Research and Technology Company. Wax Isomerization Using Catalyst of Specific Pore Geometry. U.S. Patent 5,246,566, 21 September 1993. Available online: https://bit.ly/3uMNVRf (accessed on 3 June 2021).
- Bertaux, J.-M.A.; Germaine, G.R.B.; Janssen, M.M.P.; Hoek, A. Shell Internationale Research Maatschappij. Process for producing lubricating base oils. U.S. Patent EP 0776959 A2, 4 June 1997. Available online: https://bit.ly/2S7Az4H (accessed on 3 June 2021).
- Sirota, E.B.; Johnson, J.W.; Simpson, R.R.; Exxonmobil Research and Engineering Company. Production of Extra Heavy Lube Oils from Fischer-Tropsch Wax. U.S. Patent 7465389B2, 16 December 2008. Available online: https://bit.ly/2SXXNdt (accessed on 3 June 2021).
- Speight, J.G. Hydrocarbons from Petroleum; Elsevier BV: Amsterdam, The Netherlands, 2011; pp. 85–126. [Google Scholar]
- Hsu, C.S.; Robinson, P.R. Petroleum Science and Technology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 93rd ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-1-4398-8049-4. [Google Scholar]
- Pichler, H.; Schulz, H.; Reitemeyer, H.O.; Weitkamp, J. Über das Hydrokracken gesättigter Kohlenwasserstoffe. Erdöl und Kohle Erdgas, Petrochemie Vereinigt mit Brennstoff-Chemie 1972, 25, 494–505. [Google Scholar]
- ASTM D2502-14. Standard Test Method for Estimation of Mean Relative Molecular Mass of Petroleum Oils from Viscosity Measurements; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- Coutinho, J.; Mirante, F.; Ribeiro, J.; Sansot, J.; Daridon, J. Cloud and pour points in fuel blends. Fuel 2002, 81, 963–967. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M. Impact of cold flow properties of biodiesel on engine performance. Renew. Sustain. Energy Rev. 2014, 31, 650–656. [Google Scholar] [CrossRef]
- Deldari, H. Suitable catalysts for hydroisomerization of long-chain normal paraffins. Appl. Catal. A Gen. 2005, 293, 1–10. [Google Scholar] [CrossRef]
- Höhne, G. Problems with the calibration of differential-temperature-scanning-calorimeters. Thermochim. Acta 1983, 69, 175–197. [Google Scholar] [CrossRef]
- Heino, E.-L. Determination of cloud point for petroleum middle distillates by differential scanning calorimetry. Thermochim. Acta 1987, 114, 125–130. [Google Scholar] [CrossRef]
- Rossi, A. Wax and Low Temperature Engine Oil Pumpability. SAE Tech. Paper Ser. 1985, 94, 768–776. [Google Scholar] [CrossRef]
- ASTM D4419-90. Standard Test Method for Measurement of Transition Temperatures of Petroleum Waxes by Differential Scanning Calorimetry (DSC); ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar] [CrossRef]
- ASTM F3418-20. Standard Test Method for Measurement of Transition Temperatures of Slack Waxes Used in Equine Sports Surfaces by Differential Scanning Calorimetry (DSC); ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar] [CrossRef]
- ASTM D2500-17a. Standard Test Method for Cloud Point of Petroleum Products and Liquid Fuels; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar] [CrossRef]
- Claudy, P.; Létoffé, J.-M.; Neff, B.; Damin, B. Diesel fuels: Determination of onset crystallization temperature, pour point and filter plugging point by differential scanning calorimetry. Correlation with standard test methods. Fuel 1986, 65, 861–864. [Google Scholar] [CrossRef]
- Pellegrini, L.; Locatelli, S.; Rasella, S.; Bonomi, S.; Calemma, V. Modeling of Fischer–Tropsch products hydrocracking. Chem. Eng. Sci. 2004, 59, 4781–4787. [Google Scholar] [CrossRef]





| Group | Manufacturing Process |
|---|---|
| I | solvent processing |
| II | hydroprocessing |
| III | GTL; wax isomerisation, severe hydroprocessing |
| IV | polyalphaolefins (POA) |
| V | all other base stocks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuner, P.; Graf, D.; Mild, H.; Rauch, R. Catalytic Hydroisomerisation of Fischer–Tropsch Waxes to Lubricating Oil and Investigation of the Correlation between Its Physical Properties and the Chemical Composition of the Corresponding Fuel Fractions. Energies 2021, 14, 4202. https://doi.org/10.3390/en14144202
Neuner P, Graf D, Mild H, Rauch R. Catalytic Hydroisomerisation of Fischer–Tropsch Waxes to Lubricating Oil and Investigation of the Correlation between Its Physical Properties and the Chemical Composition of the Corresponding Fuel Fractions. Energies. 2021; 14(14):4202. https://doi.org/10.3390/en14144202
Chicago/Turabian StyleNeuner, Philipp, David Graf, Heiko Mild, and Reinhard Rauch. 2021. "Catalytic Hydroisomerisation of Fischer–Tropsch Waxes to Lubricating Oil and Investigation of the Correlation between Its Physical Properties and the Chemical Composition of the Corresponding Fuel Fractions" Energies 14, no. 14: 4202. https://doi.org/10.3390/en14144202
APA StyleNeuner, P., Graf, D., Mild, H., & Rauch, R. (2021). Catalytic Hydroisomerisation of Fischer–Tropsch Waxes to Lubricating Oil and Investigation of the Correlation between Its Physical Properties and the Chemical Composition of the Corresponding Fuel Fractions. Energies, 14(14), 4202. https://doi.org/10.3390/en14144202







