Abstract
The steel industry is among the highest carbon-emitting industrial sectors. Since the steel production process is already exhaustively optimized, alternative routes are sought in order to increase carbon efficiency and reduce these emissions. During steel production, three main carbon-containing off-gases are generated: blast furnace gas, coke oven gas and basic oxygen furnace gas. In the present work, the addition of renewable hydrogen by electrolysis to those steelworks off-gases is studied for the production of methane and methanol. Different case scenarios are investigated using AspenPlusTM flowsheet simulations, which differ on the end-product, the feedstock flowrates and on the production of power. Each case study is evaluated in terms of hydrogen and electrolysis requirements, carbon conversion, hydrogen consumption, and product yields. The findings of this study showed that the electrolysis requirements surpass the energy content of the steelwork’s feedstock. However, for the methanol synthesis cases, substantial improvements can be achieved if recycling a significant amount of the residual hydrogen.
1. Introduction
The iron and steel industry is among the industrial sectors with the highest production volumes, having indispensable end-products for modern society [1]. The European steel industry, in particular, is a world leader in steel production accounting for approximately 16% of the world production (8.5% belongs to the European Union countries), coming second only to China. In market and economic terms, in 2019 it generated 140 bn € of gross added value and employed around 2.67 million people [2]. Steelworks, however, are one the most energy- and carbon-intensive industries in the world, accounting for 27% of the total industrial CO2 emissions and 4–5% of the total anthropogenic CO2 emissions [3]. Since world steel production is expected to rise in the following years, CO2 and carbon emissions will increase accordingly, if no proper countermeasures are adopted [1].
During the primary steel production route, carbonaceous off-gases are generated during the main production steps of: (1) conversion of coal to coke in the coke oven, (2) pig iron production in the blast furnace and (3) processing of pig iron to steel in the basic oxygen furnace [1]. Since the usage of fossil fuels (usually coal and natural gas) as reducing agents in the blast furnace is intensely optimized [4], alternative ways are investigated for the reduction in those emissions. Generally, a common way to avoid the flaring of steelmaking off-gases is their use as internal energy sources both for heating and power production. As a consequence, a reduction in natural gas and external produced electricity use is obtained, resulting in a decrease in emissions, primary resources consumption, and operating costs. Recent works focused on the optimization of the management of steelworks off-gases networks using a decision support system [5] including machine learning-based forecasting models [6,7,8] and advanced optimization strategies [9,10].
An alternative/complementary way of utilizing those gases without deviating from the already established steel production route is their conversion to added-value chemicals. The proposed utilization strategy involves the use of the carbonaceous feedstocks for the production of methane and methanol (MeOH) through the addition of renewable hydrogen by proton exchange membrane (PEM) electrolysis. Apart from the environmental perspective, target is to partially replace the fossil fuel demands of the steel plant and/or to generate revenue by utilizing a by-product stream. Methanol has already broad commercial uses, as chemical intermediate and fuel [11], whereas methane apart from its commercial value, can be used within the steel plant for power production and/or reused as reducing agent in the BF process [12]. The proposed strategy, however, has to surpass or match the benefits obtained through the conventional off-gases exploitation strategy (i.e., heating and power production) from an energetic, economic and environmental perspective.
The three mentioned steelworks off-gases (Blast Furnace Gas: BFG, Coke Oven Gas: COG, Basic Oxygen Furnace Gas: BOFG) are commonly stored in dedicated gasholders, that act as buffers. These off-gases contain more or less the same compounds but at different proportions: the most common are CO2, CO, H2, and N2. Small amounts of impurities are also contained; however, they do not pose environmental threats when combusted in their traditional use within the plant. However, when advanced catalytic processes are pursued using these gases as feedstock, then, further gas cleaning steps are required to avoid catalyst poisoning. The present work considers an already existing gas cleaning setup prior to the gas holder short storage as the starting point for the process formulation. In addition, further gas cleaning steps are proposed upstream the catalytic processes, considering a possible presence of residual impurities in the off-gases, before entering the catalytic syntheses units.
The scope of this work is to study the integration of renewable hydrogen into steelworks off-gases for the efficient production of methane and methanol and to exploit the largest amounts of steelworks off-gases as carbon sources. This is a novelty in respect to past works [13,14] that exploit only limited amounts of these off-gases and focus mainly on the exploitation of the COG as feedstock for the synthesis reactors (as it is or mixed with other off-gases, due to its high hydrogen content). This study has been conducted using flowsheet simulations in AspenPlusTM. The key points of this work can be summarized as follows:
- Based on the possible contained impurities, a gas cleaning strategy is proposed in order to avoid poisoning of the synthesis catalysts.
- The modelling methodology both for methane and methanol synthesis is presented, and sensitivity analyses are conducted to define specific operating parameters with a major influence on the overall process.
- Five case studies are analyzed, which correspond to different utilization amounts of the steelworks off-gases for the production of methane/methanol, whereas one of the investigated cases involves the combination of methane and methanol production. The defined case studies also include the discussion and analysis of PEM electrolysis for renewable hydrogen production.
- The overall benefits of these scenarios are compared to the traditional use of power production in energetic and efficiency terms. Process improvements are proposed to increase the overall efficiency of the integrated process.
This work is organized as follows: Section 2 describes the main features and characteristics of the considered off-gases; Section 3 illustrates the investigated process and the comprising sub-systems; and Section 4 presents and discusses the obtained results. Finally, Section 5 provides the conclusions of this work and hints for future work.
2. Steelworks Off-Gases
Table 1 depicts the total volumetric amounts and the mean composition of the steelworks off-gases, for a steel plant producing 6 MT steel per year [15].

Table 1.
Mean composition of steelworks off-gases.
As shown in Table 1 from an overall perspective, the component that prevails through the three gases is nitrogen. The inert nature of nitrogen lowers the partial pressures of the reactants and raises the volume of the feed gases. Thus, it increases the capital expenses and the costs associated to compression and could lead to accumulation within a recycling loop. The contained CO and CO2 can be used as feedstock for the production of chemicals (e.g., CH4 and/or CH3OH). The reactivity of CO is always higher than the one of CO2 for a considered chemical, resulting, thus, in higher activation energies for the CO2 conversion [15]. The insufficient amount of H2 contained in the off-gases, dictates the addition of additional hydrogen for the synthesis. In order to increase the carbon efficiency of the process, attention should be paid on the addition of renewable hydrogen instead of fossil based. In addition, it is assumed that after cleaning, the off-gases are water saturated. This water content should be removed prior to compression, in order to: (i) avoid condensation that could damage the compressors [15], and (ii) avoid the promotion of the Water Gas Shift (WGS) reaction that could lead to the consumption of CO for the formation of additional CO2 [17]. Finally, the off-gases also contain small amounts of oxygen, which need to be removed for safety reasons prior to the conduction of adsorption processes, such as pressure swing adsorption [18].
From a particular point of view, the BFG contains large quantities of nitrogen due to the use of hot air as oxidant within the furnace and low amount of H2 [6]; enrichment is required for its use as feedstock for methane and methanol production. The coke oven gas is generated in the coking plant during the heating of coal to produce coke. In contrast to the BFG, it contains large amounts of hydrogen and can be mixed with the other gases to reduce the required amounts of additional hydrogen by electrolysis. In addition, it can be easily valorized within the plant as fuel or feedstock for producing chemicals, due to the contained hydrogen and methane [19]. The BOFG is generated in the basic oxygen furnace, where oxygen is injected to oxidize part of the carbon in the pig iron produced from the blast furnace; it contains predominantly CO [15].
The three steelworks off-gases are generally used internally for heating and electricity production purposes. For instance, the COG is used for firing coke ovens, as heat input for rolling mills and to produce energy at the power plant [20]. The blast furnace gas serves also as a fuel for firing the coke ovens, the hot blast stoves heating the wind to be injected into the blast furnace and the power plant [21], whereas the basic oxygen furnace gas, apart from power applications, can also be used for upgrading the heating value of the BFG in a gas mixing station [16]. Gasholders are used for storing the surplus of those gases. However, in some cases the gasholders capacities are not sufficient to contain the generated quantities and as a consequence, the excess off-gases are flared. In other cases, the gases do not satisfy the internal requirements and natural gas is purchased. An optimized off-gas distribution management can improve the efficiency [5], as well as the consideration of an alternative use, such as for methane and methanol production. Regarding the available amounts for CH4 and MeOH production, it is assumed that 50% of the total generated amount is available, after the rest being utilized in internal applications within the plant [16,20].
3. Process Description
In this section, the outline of the five case studies is described. These case studies differ on the quantities of the utilized gases for the syntheses and on the produced chemical (methane/methanol). The case studies are:
- 100% utilization of the available by-product gases (BFG, COG, and BOFG) for the production of methane.
- Methanation of 80% of the available by-product gases and the remaining fraction is used in the power plant.
- Methanation of specific amounts of the by-product gases in order to replace the natural-gas demands of the plant and the remaining fraction is used in the power plant.
- Methanol synthesis of 80% of the available by-product gases and the remaining fraction is used in the power plant.
- Methanation of specific amounts of the by-product gases in order to replace the natural-gas demands of the plant and 50% of the quantity used in Case 4 is used for the production of significant quantities of MeOH (only the remaining by-product gases are used in the power plant).
The selected scenarios want to cover the short-, medium-, and long-term technology deployment horizon and to provide useful information in order to reduce the relevant costs when moving towards the large deployment of the proposed technological option. In particular, the Cases 1, 2, and 4 are adopted because they actually present the medium- to long-term capacities required to deploy the proposed technological option. On the other hand, Cases 3 and 5 represent a shorter-term demonstration of that technological option to move towards decarbonization of steelmaking.
After the description of the case studies in Section 3.1, the overall process scheme is presented in Section 3.2 that includes the aforementioned systems (gas cleaning, hydrogen production, methane, and methanol synthesis) as well as the power plant. A gas cleaning strategy is proposed in Section 3.3 based on the possible contained impurities, whereas Section 3.4 and Section 3.5 involve the description of the methanol and methane synthesis processes and the followed AspenPlusTM modelling methodology. Sensitivity analyses on crucial modelling approaches and operating parameters are conducted on both processes. Finally, Section 3.6 includes the description of PEM electrolysis for the production of renewable hydrogen.
3.1. Case Studies Description
The integration of methane and methanol synthesis is evaluated for the previously described scenarios considering a steelmaking plant of medium size with an annual steel production of about 6 MT. The different case scenarios are evaluated in terms of carbon conversion, product yields, hydrogen requirements and consumption, electrolysis demands, as well as overall efficiency of the process. For the cases where power is produced, it is assumed that a gas-fired boiler is in operation within the plant [16]. Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5 depict the different flowrates and utilization factors of the steelworks off-gases.
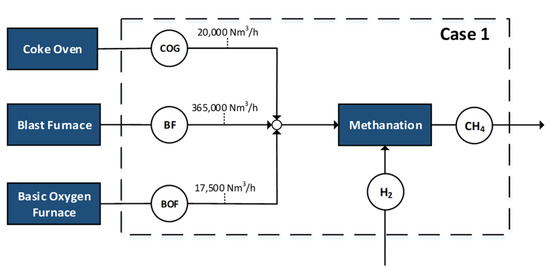
Figure 1.
Case 1—100% of off-gases as input for methanation.
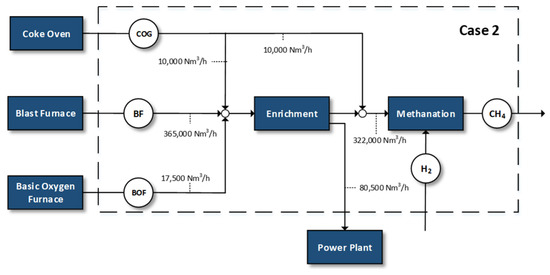
Figure 2.
Case 2—methanation of 80% of by-product gases.
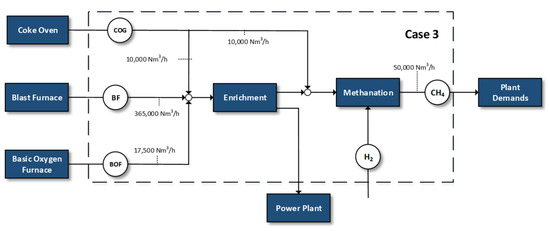
Figure 3.
Case 3—replacement of natural gas demands by methanation.
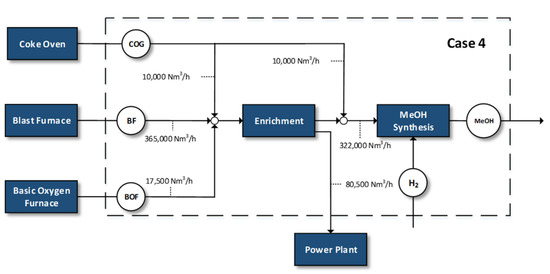
Figure 4.
Case 4—methanol synthesis of 80% of by-product gases.
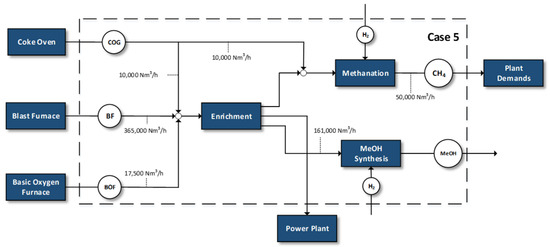
Figure 5.
Case 5—100% replacement of natural gas demands and methanol synthesis.
- 100% utilization of the produced by-product gases for the production of methane
The first case represents the utilization of the entire available amount of the three off-gases for the production of methane. Renewable hydrogen is added in basic stoichiometric ratio to produce methane. This represents a boundary scenario for the utilization of the steelwork gases, which is restrictive in terms of hydrogen flows and electrolysis power requirements.
- 2.
- Methanation of 80% of the available by-product gases and the remaining fraction used in the power plant
The steelworks off-gases have generally various uses within the steel plant and the main one is for the production of electrical power. However, in this scenario, 80% of the total amounts of the gases are used for the production of methane with the addition of renewable hydrogen and the other fraction is sent to the power plant. Before the methanation process, the enrichment step serves as mixing/upgrading process before entering the power plant. A part of the COG is dispatched directly to methane synthesis, due to its higher hydrogen content compared to the other gases. Although, the CH4 content of COG could have a negative impact in methanation activity, the amount of available COG is relatively small compared to the total utilized gases, reducing significantly the methane quantities (i.e., <1%) in the reactor inlet of the methanation cases (i.e., after the H2 addition).
- 3.
- Methanation of specific amounts of the by-product gases in order to replace the natural gas demands of the plant
This case investigates the possibility of valorizing the steelworks off-gases for the replacement of the internal steelworks needs of natural gas—assuming an overestimated case of approximately 50,000 Nm3/h internal natural gas demands for a 6 MT/year steel plant [12]. The remaining portion of the gases is combusted in the power plant.
- 4.
- Methanol synthesis of 80% of the by-product gases and the rest goes to the power plant
Similar with Case 2, 80% of the amounts of the gases are used for methanol production with the addition of renewable hydrogen, whereas the remaining portion is used in the power plant.
- 5.
- Methanation of specific amounts of the by-product gases in order to replace the natural gas demands and the production of significant quantities of MeOH
Case 5 represents the most integrated valorization scheme for steel gases for the simultaneous production of methane and methanol. After the enrichment step, half of the amount employed in Case 4 is used for methanol production and another part is used for the replacement of the industry’s natural gas demands. Finally, a part of the gases is sent to the power plant.
Figure 6 shows a comparison of the energy content of the mixed feedstock of Case 1 with respect to the energy content of the other cases, by highlighting the different off-gases contribution. In the first scenario, BFG comprises 73% of the total energy content of the feed stream to the syntheses processes, due to the larger used flowrate, whereas COG, although in lower quantity (20,000 Nm3/h compared to 365,000 Nm3/h of the BFG; 5% of the total amount) contains a significant portion of the overall energy content (19%). This indicates a higher energy content per m3, due to the contained CH4 and H2 and the lower CO2 and N2 contents in the COG feedstock. Regarding the energy contents of the feedstock used in the different scenarios, Cases 2 and 4 use the same feed quantities for the production of chemicals (81% of the energy compared to Case 1). Case 3 represents the feedstock energy content for the replacement of the natural gas demand of the plant, while Case 5 is a combined case for the replacement of natural gas as well as for methanol synthesis (33% and 70%, respectively).
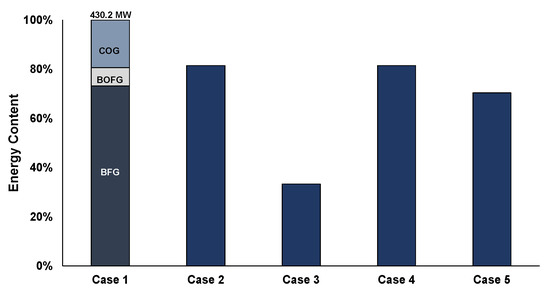
Figure 6.
Feedstock energy content for the different case scenarios.
3.2. Integration Options of CH4/MeOH Syntheses Concepts into Steelworks
Figure 7 shows the overall process flowsheet that includes the major sections of the proposed concept: gas conditioning, methane production, methanol synthesis, hydrogen production, and the power plant (for the cases where power is produced).
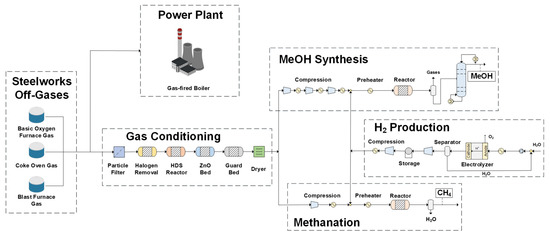
Figure 7.
Integration of synthesis units into steelworks—superstructure flowsheet.
The mixture of the steelworks off-gases, after an ad hoc conditioning for removal of unwanted impurities, is fed either to the methanol synthesis or to the methanation section. For the methanol synthesis, the feed gas undergoes compression in three stages in order to reduce the associated compression ratio costs; intermediate cooling between the stages is provided. Afterwards, hydrogen is added to reach the required stoichiometric number and the inlet mixture is preheated before inserted to the synthesis reactor. The produced mixture is separated using a flash separator into the liquid (mainly methanol and water) and gaseous products that consist of the unreacted hydrogen and the rest of the initial feedstock. The last step is the purification of the methanol product in a distillation column, which removes the contained product water.
The first step for methanation requires compression only in one step, since methanation takes place at low pressures (<10 bar). Renewable hydrogen is added to achieve the required stoichiometric ratio and the inlet feed is preheated and directed to the reactor. A flash separator is also used to separate the gaseous products from the produced liquid water. Table 2 depicts the assumptions that refer to the overall process flowsheet simulations. The off-gases composition reported in Table 1 is taken as the starting point of the subsequent flowsheet simulations.

Table 2.
General assumptions and specifications of the overall process.
3.3. Impurities and Gas Conditioning
The three steelwork off-gases undergo different cleaning steps in order to remove the contained, unwanted components before being stored in gas holders. Typical gas cleaning steps involve dust removal, cooling, scrubbing (for ammonia and BTX removal), and demistering [19,25]. After the initial steps, additional gas cleaning is required to protect the methane [26] and methanol [27] syntheses catalysts.
As shown in Table 1, several sulfur-containing compounds can be found in the steelworks off-gases, which cause corrosion and poisoning of Cu-based catalysts. Other common impurities include nitrogen-containing species such as ammonia or hydrogen cyanide. At the high temperatures of the steel production processes, nitrogen oxides NOx can be formed, which have to be removed from the exhaust gases, whereas at lower temperatures, NH3 can be adsorbed at catalyst sites, reducing the catalyst activity [28]. Halogens (HCl, HF, and HBr) are also contained in the off-gases and are known to cause corrosion and poison catalysts. In particular, experimental works have shown that HCl poisoning could cause loss of the active surface area of the catalyst and promote sintering of the copper crystallites [27]. Furthermore, additional reactions could occur between HCl and other contaminant-forming species such as NH4Cl and NaCl, which when condensed, could cause fouling and create deposits in cooler downstream pipes and equipment [29,30]. Finally, trace elements and heavy metals are also contained in the off-gases due to the diverse nature of the feedstocks. Besides corrosion problems, other trace elements pose a threat to human health and the environment. The distribution and partitioning of these contaminants play an important role on the undertaken cleaning strategy. For example, particle filters could be used for solid particles, but if those compounds appear in the gaseous phase, more advanced cleaning efforts should be employed, such as solid sorption. Whether a trace element appears in the gas or particulate phase and in which form, depends on following factors [31]:
- how the trace element resides in the incoming material,
- temperature and pressure,
- oxidizing or reducing conditions,
- presence of halogens, such as chlorine,
- presence of compounds that can act as sorbents, such as calcium.
Based on the contained impurities, Figure 8 depicts the proposed off-gases cleaning strategy. Each of the cleaning steps targets aims at a specific impurity group. However, possible interactions between an impurity and a precedent/succeeding step cannot be ruled out.
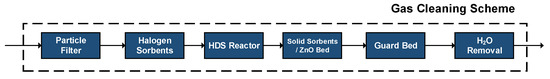
Figure 8.
Proposed gas cleaning scheme.
A first step is devoted to the removal of any contained solid particles through fine filters. Afterwards, the contained halogens (HCl, HF, etc.) are removed using inexpensive sorption materials such as NaHCO3 (Nahcolite) or Trona (Na2CO3-NaHCO3-2H2O) [28]. For instance, in the case of nahcolite, HCl is removed in the form of NaCl, whereas H2O and CO2 are also formed, according to the following reaction:
NaHCO3 + HCl → NaCl + H2O + CO2.
Regarding the sulfur-containing compounds, H2S is more easily removed at ppb levels with respect to other sulfur species. A common strategy consists in converting organic sulfur compounds to H2S and then employing adsorption technologies for the deep removal of H2S [32]. The avoidance of acid gas removal process, such as SelexolTM or RectisolTM, despite their efficiency in reducing H2S to ppm levels, lies within their affinity to physically absorb CO2, which should otherwise be used as feedstock for the production of methanol/methane [26].
At the hydrodesulfurization (HDS) reactor, organic sulfur compounds and COS are converted to H2S through the addition of hydrogen. The usual employed catalysts are based on cobalt and nickel. A possible reaction network for the conversion to hydrogen sulfide is the following [33]:
COS + H2 → H2S + CO
CS2 + 4H2 → 2H2S + CH4.
Afterwards, a sorption bed containing metal oxides, such as CaO and ZnO, can be used for the removal of H2S. For the case of ZnO, H2S is removed in the form of ZnS [34]:
H2S + ZnO → H2O + ZnS.
It is an exothermic process, conducted at T < 250 °C and as shown in the reaction stoichiometry, the reaction equilibrium is not affected by pressure, whereas the inlet content of water could affect the H2S removal efficiency. Studies have shown that H2S can be effectively removed at ppb levels employing the ZnO strategy [34,35]. However, due to the contained CO and CO2, additional reactions could occur, with a consequent deterioration of the H2S removal efficiency [34,36].
Finally, a guard bed is placed, containing nickel or other inexpensive material to protect the subsequent synthesis units. It restricts impurities that could have escaped from the former gas cleaning steps and acts as a final protection before the production of chemicals. In addition, the gases are dried to remove the contained water to avoid condensation during compression and/or the promotion of unwanted side-reactions.
3.4. Methanol Synthesis
3.4.1. Process Description and Modelling Approach
Methanol synthesis is based on the following three reactions:
CO + 2H2 → CH3OH ΔH0 = −90 kJ/molCO
CO2 + 3H2 → CH3OH + H2O ΔH0 = −49 kJ/molCO2
CO2 + H2 → CO + H2O ΔH0 = +41 kJ/molCO2.
The first two hydrogenation reactions can be combined to form the reverse water gas shift (RWGS) reaction, indicating thus a dependency in-between the reaction system [37]. The catalytic methanol synthesis is exothermic and thermodynamically favored by lower temperatures and higher pressures. Today most of the world methanol production is covered with natural gas derived synthesis gas that after H2/CO ratio adjustment is catalytically processed at 50–100 bar and temperatures between 200–300 °C (temperatures required for the activation of the employed catalyst) [38]. An alternative consideration could be a process occurring at much higher pressures (above 100 bar), which would result into an increase in the CO and CO2 conversion rates and thus lowering the needs for carbon recycling [39,40]. This would, however, result in increasing compression costs and power demands and therefore, it was not adopted in this study.
The most common MeOH catalyst employed in industrial scale is based on CuO/ZnO/Al2O3, which is also considered in this study. At higher synthesis temperatures, sintering could take place resulting in higher deactivation rate of the catalyst [41]. The produced water, mainly by CO2 hydrogenation, apart from affecting the equilibrium, could also adsorb on the catalyst sites and promote catalyst sintering [42]. In past works, in-situ water removal was proposed for the enhancement of the thermodynamic equilibrium concentration [43]. The methanol synthesis reaction is characterized by the stoichiometric number (S.N.) where [H2], [CO], and [CO2] refer to the molar flows of the feed components:. A value of S.N. = 2 refers to a stoichiometric correlation between the components, whereas the optimum case is slightly above the stoichiometric number [41].
Methanol synthesis applications result in conversion close to what the thermodynamic equilibrium dictates. Any additional hydrogen is not consumed throughout the process and remains unexploited [38]. Therefore, the process economics could benefit from separating the residual H2 and reuse it in the synthesis reactor.
In this work, MeOH synthesis reactor is simulated using two different approaches: a thermodynamic and a kinetic approach. The thermodynamic approach is represented by an AspenPlusTM RGibbs reactor model (based on Gibbs free energy minimization), which for a given pressure and temperature, calculates the equilibrium concentration of selected components. The kinetic approach utilizes the kinetic model developed by Vanden Bussche (with WHSV = 2 kgfeed kgcat−1 h−1 and Bed Voidage: 0.33) [44]. Simulation results have shown that for the studied conditions, the deviation of the two modelling approaches is within an acceptable range (<5%) and therefore, the thermodynamic approach is being employed in the investigations.
3.4.2. Modelling Assumptions
The modelling of methanol synthesis is based on chemical equilibrium by means of minimization of the Gibbs free energy. Certain components included in the feed mixture are assumed as inert components, having thus no influence in the reaction. Apart from nitrogen, ethane, and methane are also treated as inert gases that do not affect reaction equilibrium. The property method that is used in the flowsheet simulations is Soave–Redlich–Kwong equation of state [45], as past works have proven that it is suitable for methanol synthesis applications [46,47]. Table 3 shows the assumptions and specifications for the thermodynamic MeOH synthesis model.

Table 3.
General assumptions and specifications of the MeOH AspenPlusTM model.
3.4.3. Sensitivity Analysis
Since the methanol synthesis reaction is exothermic, it is thermodynamically favored by lower temperatures. However, the temperature range of the catalyst’s activation should also be taken into consideration in order to find the optimum operating conditions. Higher pressures are thermodynamically preferred for methanol production (Figure 9a–c), but the higher compression costs should also be taken into account. Figure 9c depicts the lower conversion rate of CO2 compared to CO, whereas at higher temperature and pressure values, CO conversion tends to decrease and CO2 to increase. This fact can be attributed to the WGS reaction, which is an endothermic reaction and is thermodynamically favored by higher temperatures.
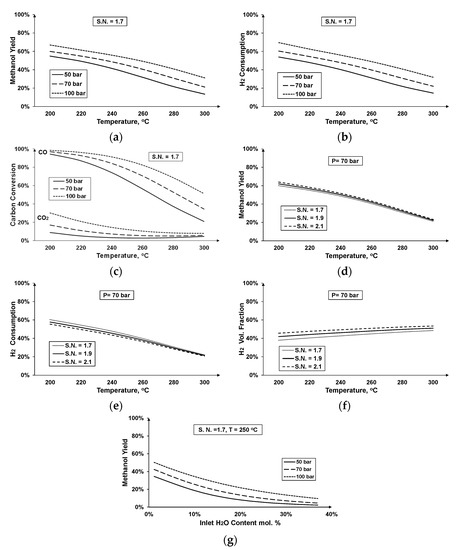
Figure 9.
Methanol synthesis at different operating conditions: (a) methanol yield at constant stoichiometric number, (b) hydrogen consumption at constant stoichiometric number, (c) carbon conversion at constant stoichiometric number, (d) methanol yield at constant pressure, (e) hydrogen consumption at constant pressure, (f) residual hydrogen at constant pressure, and (g) methanol yield—inlet water content at constant stoichiometric number and temperature.
Figure 9d–f shows the influence of increasing stoichiometric number, e.g., increasing input hydrogen. Higher stoichiometric numbers result in higher methanol yields for a given operating temperature (Figure 9d). However, relatively to the input, less hydrogen is consumed in the reactor (Figure 9e) and more remains unexploited in the outlet gaseous fraction (Figure 9f), which refers to the gaseous stream after the separation of methanol and water. Figure 9g illustrates the need for drying of the feed mixture before entering the synthesis reactor. It can be seen that an increase in the water content of the inlet feed leads to a strong decrease in the maximum attained methanol yield. This behavior can be attributed to the promotion of the WGS reaction and consequent CO conversion to additional CO2, at the expense of the methanol synthesis reactions.
The higher the input hydrogen flowrates, the higher the quantity that remains unexploited during the process. Even in sub-stoichiometric ratios, the remaining hydrogen is in considerable portions, which illustrates the need for efficient hydrogen management throughout the process. This could be achieved either through operating in sub-stoichiometric numbers or employing hydrogen recirculation technologies to lower the needs for additional hydrogen and increase the overall efficiency of the system.
3.5. Methane Production
3.5.1. Process Description and Modelling Approach
Syngas methanation is a highly exothermic process aiming at the production of Substitute Natural Gas (SNG) from CO and CO2 with the addition of H2 at the required stoichiometries. The simplicity and high efficiency of the process have been crucial parameters for the establishment of this technology for the production of methane from waste feedstocks such as biomass [48,49] or steelwork off-gases [19]. For the production of methane from syngas, the main occurring reactions are:
CO + 3H2 → CH4 + H2O ΔH0 = −206 kJ/molCO
CO2 + 4H2 → CH4 + 2H2O ΔH0 = −165 kJ/molCO2
CO2 + H2 → CO + H2O ΔH0 = +41 kJ/molCO2.
However, based on experimental results, it is assumed to consist of a more complex reaction network, taking provision also for the formation of solid carbon throughout the process [22].
A variety of catalysts are employed for the catalytic methanation process. In this work, a nickel-based catalyst is considered, since it is mostly employed in commercial applications due to the high activity and low associated costs [50]. Similar to methanol synthesis, methane production is also favored by low temperatures and higher pressures because it results in reduction in the total volume. There is currently a variety of established methanation concepts operating at different conditions and reactor configurations [50]. In this work, methanation is conducted in low temperatures 200–300 °C and pressures < 10 bar.
Again, two approaches based on kinetics and thermodynamics are compared using the reactor inlet composition of Case 1, as a comparison basis. The first approach is based on the Langmuir-Hinshelwood (LHHW) kinetics derived from Kopyscinski et al. (for WHSV = 2 × 10−5 kgfeed kgcat−1 h−1 and Bed Voidage = 0.33) [51]. Because the LHHW kinetics cannot be implemented directly in AspenPlusTM, a revised form was adopted [52]. The second approach is also based on the thermodynamic model using the Gibbs free energy minimization method, as already explained for the methanol synthesis system. Simulation results have shown that the two modelling approaches agree well for the studied conditions (deviation < 5%) and therefore, for the subsequent sensitivity analyses and case studies evaluation, the thermodynamic model was used.
3.5.2. Modelling Assumptions
Modelling of the methanation section is also based on the minimization of the Gibbs free energy. In this case, nitrogen and ethane are treated as inert gases and the used property method in the flowsheet simulations is Soave–Redlich–Kwong equation of state [22,23]. Table 4 illustrates the assumptions for the AspenPlusTM model of methane synthesis.

Table 4.
General assumptions and specifications of the AspenPlusTM methanation model.
3.5.3. Sensitivity Analysis
Figure 10 shows some crucial characteristics of the process at different operating parameters of methane production. As depicted in Figure 10, CO conversion is almost complete, irrespectively of the operating pressure and temperature range (T = 200–300 °C, P = 1–10 bar). On the other hand, CO2 conversion, strongly depends on the operating parameters; lower temperature and higher pressure favor the CO2 conversion thermodynamically. Higher conversion rates mean higher hydrogen consumption (see Figure 10b), resulting in >95% consumption in any pressure and temperature range. This results in low portion of the hydrogen remaining unexploited in the off-gases of the methanation process. In addition, Figure 10c shows that methane production is favored in any of these operating conditions obtaining a methane yield greater than 95%.
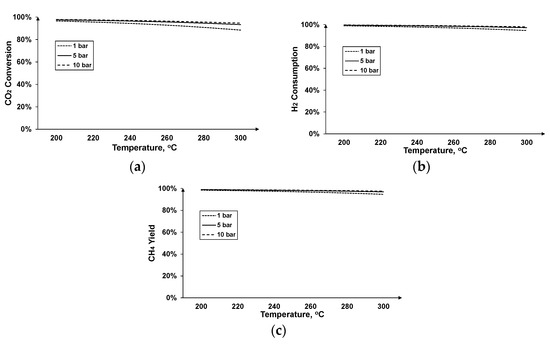
Figure 10.
Methane production at different operating temperature and pressure: (a) CO2 conversion, (b) hydrogen consumption, and (c) methane yield.
3.6. Hydrogen Production
Due to the composition of the steelworks off-gases, hydrogen addition is required in order to reach certain stoichiometric ratios and improve the efficiency of methane and methanol syntheses. In order to obtain both economic and environmental advantages, hydrogen needs to be produced in an environmentally friendly way, i.e., by exploiting renewable sources. In this work, the adopted process is water electrolysis fed by renewable energy.
During water electrolysis, the water molecules are split into hydrogen and oxygen by means of electricity. There are three main electrolysis processes, each one differing on the operating principles and conditions: alkaline exchange membrane (AEM), proton exchange membrane (PEM) and solid oxide electrolysis (SOE) [53]. In the present work, PEM electrolysis is considered as the option for renewable hydrogen production, since it is an already established technology, it is used in large-scale industrial applications and it is not sensitive to the fluctuations in power supply, such as in the case of renewable energy sources [24].
For the calculation of the power requirements, an AspenPlusTM PEM electrolysis model has been developed. The model incorporates the following main reactions occurring in the two PEM sections:
2H2O → O2 + 4H+ + 2e− anode section
2H+ + 2e− → H2 cathode section.
In addition, further phenomena taking place inside a PEM electrolysis module are also considered such as hydrogen and oxygen permeations [54,55] and water diffusion [55,56], which are estimated in ad hoc configured calculator blocks. Highly pure hydrogen is assumed to be produced (<99.9 vol.%), and the overall electrical energy consumption for the stack is 54.8 kWh/kg H2. The produced hydrogen is stored into pressurized vessels and compressed to achieve the conditions required for the syntheses units.
A detailed description of the PEM electrolysis model, as well as of other renewable hydrogen production technologies that are considered possible solutions for the enrichment of steelworks off-gases, is included in another publication by the authors [57].
4. Results
In this section, the proposed case studies are evaluated using the aforementioned AspenPlusTM models. The results presented in Table 5 focus on the hydrogen requirements and consumption, electrolysis demands, product yields, and carbon conversion. Figure 11 shows these key results. Further specific indicative stream results are available in the Appendix A.

Table 5.
Case studies key results (methanation: T = 250 °C, P = 5 bar and stoichiometric H2, MeOH synthesis: T = 250 °C, P = 70 bar, and S.N. = 1.7).
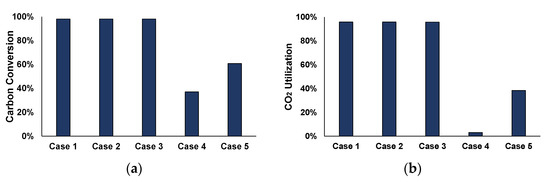
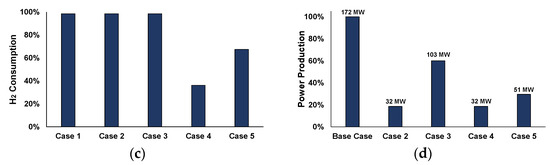
Figure 11.
Key Results of the case studies (methanation: T = 250 °C, P = 5 bar and stoichiometric H2, MeOH synthesis: T = 250 °C, P = 70 bar and S.N. = 1.7): (a) carbon conversion, (b) CO2 utilization, (c) hydrogen consumption, and (d) power production.
For the three first cases, which refer only to methanation, carbon is almost completely converted, compared to Case 4, which includes only methanol synthesis, and Case 5, which is a combination between methane and methanol syntheses. In Case 4, in particular, the low CO2 utilization rates indicate that CO (see Figure 11a) and not CO2 (see Figure 11b) is consumed for methanol synthesis. In addition, for the different case studies, the higher the carbon conversion rate, the higher the hydrogen consumption throughout the process (see Figure 11c). Figure 11d shows the produced electrical power of the different cases compared to the base-case, that refers to the traditional, full-scale utilization of the steelworks off-gases for power production. Case 1 is not included in the comparison, as the whole amount of off-gases is used for methane production. For Cases 2 and 4, the same power is produced since the same feedstock amount is used in the syntheses units (19% of the total power). Case 3 produces 60% of the power produced in the base-case and Case 5, which is the most integrated scheme, involves the production of 30% of the total power.
If different stoichiometric ratios are chosen (other than the stoichiometric for methane and 1.7 for methanol synthesis) the required hydrogen feed inputs are greatly affected, as illustrated in Figure 12. Case 1, which refers to the full-scale utilization of the steelworks by-product gases, requires more hydrogen compared to the other cases at any stoichiometric ratio. Although Cases 2 and 4 refer to utilization of the same feedstock flowrates, methane synthesis (Case 2) requires more hydrogen compared to methanol synthesis (Case 5), due to the higher carbon conversion. As a consequence of the lower carbon conversion, the rest of the hydrogen remains unexploited in the off-gases of the methanol synthesis process.
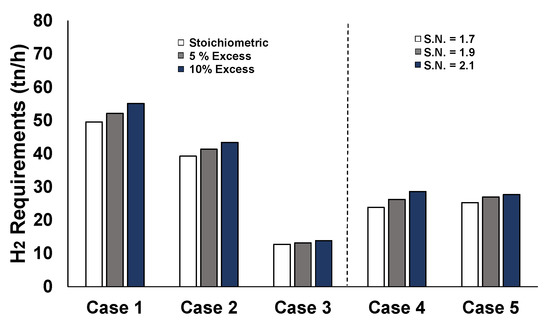
Figure 12.
Hydrogen requirements for the different stoichiometries per case.
This is also verified in Figure 13, which depicts the energy content of the residual off-gases after each synthesis processes and after the separation of the total amounts of produced methane and methanol, in each respective case. Especially for Cases 4 and 5, the remaining off-gases have a significant energetic value due to the large fraction of unreacted hydrogen. These residual off-gases could either be used for combustion to support heat-intensive processes, or hydrogen recycling should be included to avoid producing additional hydrogen by electrolysis. In conventional methanol synthesis loops, a flash drum separates the methanol and water products from the unreacted gaseous components, which are recycled back to the synthesis reactor [41]. Alternative processes to recover only the residual hydrogen include technologies such as pressure swing adsorption [58], membranes [59], and/or electrochemical hydrogen compression [60].
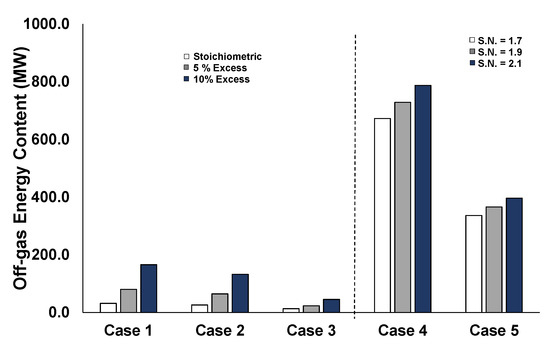
Figure 13.
Residual off-gases energy content for the different stoichiometries per case.
The PEM electrolysis requirements, as illustrated in Figure 14, are in the range of GWs, which are restrictive for employment in full-scale, by considering the capacities of currently available commercial electrolyzers.
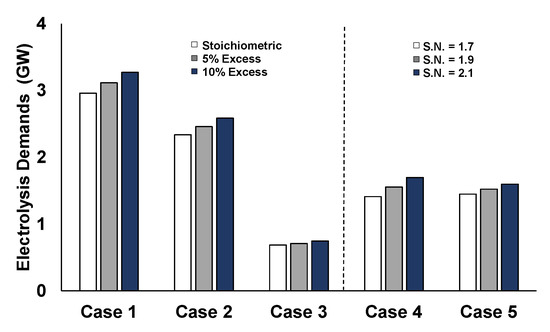
Figure 14.
PEM electrolysis requirements for the different cases.
In terms of comparison between the energy content of the total feedstock (used in each case) and the electrolysis requirements, Figure 15 shows the major energy streams of the Cases 1, 4, and 5. In Case 1, 383% of the energy content is contained in the methane product, 89% is contained in the methanol of Case 4, whereas 105% and 46% are contained in the methanol and methanol products of Case 5. The electrolysis requirements of each case are noticeable. In Case 1, 631% of the energy of the feedstock is required for electrolysis, in comparison to 304% and 322% in Cases 4 and 5. Regarding the MeOH synthesis cases, however, these figures could be further reduced, if certain amounts of the residual hydrogen are recycled.
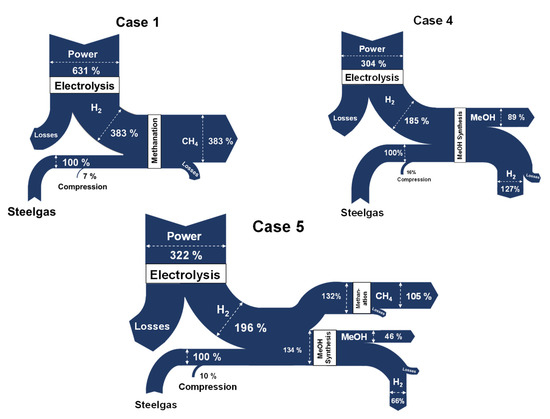
Figure 15.
Sankey diagrams—energy analysis for three key scenarios.
Figure 16 compares the PEM electrolysis energy requirements of the base case without H2 recycling, to recycling 25%, 50%, and 75% of the residual hydrogen. If recycling 75% of the residual hydrogen is pursued, almost 50% less power is required for electrolysis, indicating, thus opportunity for further optimization of the process.
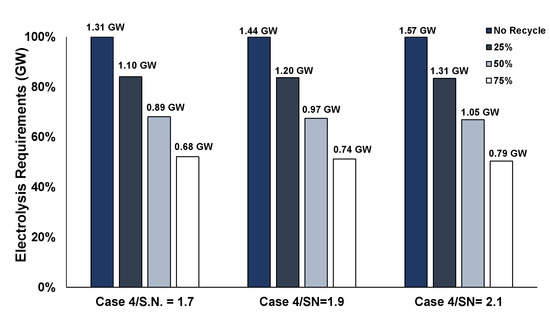
Figure 16.
Influence of hydrogen recycling in PEM electrolysis requirements of Case 4.
Regarding Case 5, the benefits of recycling 75% could result in almost 25% lower electrolysis requirements (see Figure 17). The lower savings percentage compared to Case 4 is due to the methanation section of Case 5, which consumes a major fraction of the input hydrogen, resulting in less available hydrogen for recycling, which is also verified in Figure 11.
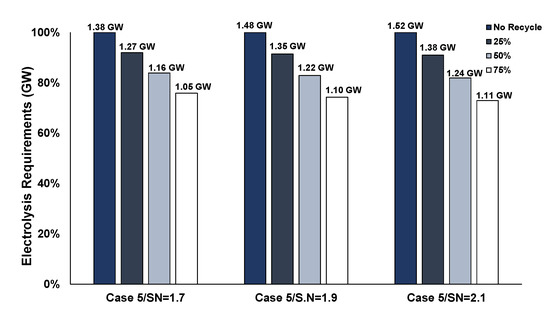
Figure 17.
Influence of hydrogen recycling in PEM electrolysis requirements of Case 5.
The benefits of recycling can also be seen in Figure 18, which refer to Cases 4 and 5 and the required electrolysis power to the energy content of the steelworks feedstock (as shown in Figure 15). The 304% of Case 4 could be reduced to 158% and from 322% to 244% for Case 5 if recycling 75% of the hydrogen is pursued.
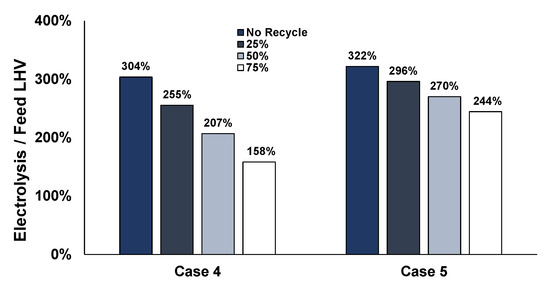
Figure 18.
Electrolysis requirements compared to feedstock energy content for the different recycling cases for Case 4 and Case 5.
5. Discussion
In this work, the integration of hydrogen-intensified methane and methanol synthesis is investigated for three available steelworks off-gases by means of AspenPlusTM flowsheet simulations. The composition of the off-gases is analyzed and a generic gas cleaning scheme is proposed for the removal of the contained impurities that can affect the catalyst operation, which is used in the CH4 and CH3OH syntheses. Thermodynamic and kinetic AspenPlusTM models are compared for the investigation of methanol and methane synthesis processes. The studied conditions for methane synthesis include 200–300 °C, stoichiometric number 1–1.1 and pressure 1–10 bar and for methanol synthesis: 200–300 °C, stoichiometric number 1.7–2.1, and pressure 50–100 bar. Furthermore, case studies corresponding to different usage of the steelworks off-gases for chemicals production are investigated in terms of hydrogen requirements and consumption, carbon conversion, product yields, and PEM electrolysis requirements. The cases of methane synthesis depict high CO and CO2 conversion rates that almost eliminate the CO2 emissions of the steel plant. In case of increasing carbon credits, this would represent a significant financial benefit and therefore, a carbon credit avoidance could be of high importance. On the other hand, methanol synthesis produces a product with higher market value, but only converts approximately 40% of the carbon emissions into a renewable fuel/chemical. The choice between methane and methanol production or a combination of the two, will be a result of an upcoming cost estimation study but also on the relevant prices of the products, as well as, on the carbon credits. The energy content of the hydrogen employed for this transformation far overcomes the energy off-set of the steel plants. However, recycling of the residual hydrogen in the methanol-involved cases could lead to substantial benefits in terms of electrolysis requirements. Calculations show that reductions in the range of 50% for Case 4 and 25% for Case 5 of electrolysis requirements could be achieved, when recycling 75% of the residual hydrogen. Future studies will involve the capital and operating cost estimation analysis of the case studies as well as the application of state-of-the art and alternative hydrogen recirculation technologies for recycling the residual hydrogen from the methanol synthesis cases. In addition, the application of an advanced dispatch controller will be investigated in order to optimize the management of steelworks off-gases among internal users, power plant and syntheses processes, and the related hydrogen requirements.
Author Contributions
Conceptualization, M.B., K.P., I.M. and V.C.; methodology, M.B., K.P., I.M., V.I. and V.C.; software, M.B., K.P. and S.V.; validation, M.B., K.P., I.M. and S.D.; formal analysis, K.P., T.A.B. and V.C.; investigation, M.B. and K.P.; resources, K.P., S.V., P.S. and V.C.; data curation, M.B., K.P., A.P. and A.Z.; writing—original draft preparation, M.B. and K.P.; writing—review and editing, M.B., K.P., I.M., V.C., S.D., V.I., A.P., A.Z., T.A.B., P.S. and S.V.; visualization, M.B. and K.P.; supervision, K.P., P.S. and S.V.; project administration, K.P. and V.C.; funding acquisition, K.P. and V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union through the Research Fund for Coal and Steel (RFCS), Grant Agreement No. 800659.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The work described in this paper was developed within the project entitled “Integrated and intelligent upgrade of carbon sources through hydrogen addition for the steel industry” (i3upgrade, GA No. 800659), which has received funding from the Research Fund for Coal and Steel of the European Union. The sole responsibility of the issues treated in this paper lies with the authors; the Commission is not responsible for any use that may be made of the information contained therein.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Indicative process stream results are presented in the following tables, namely, the steelworks off-gases feedstock, the added hydrogen, the reactor inlet, and outlet of the methanol and methane sections (see Figure 7). Note that the depicted cases refer to methane synthesis conducted at T = 250 °C, P = 5 bar, and stoichiometric H2, whereas for methanol synthesis, T = 250 °C, P = 70 bar, and S.N. = 1.7.

Table A1.
Stream results of Case 1.
Table A1.
Stream results of Case 1.
| Stream Name | Feed | H2 Feed | Reactor Inlet | Reactor Outlet |
|---|---|---|---|---|
| Stream number | 1 | 2 | 3 | 4 |
| m, kg/s | 133.68 | 13.75 | 144.11 | 144.11 |
| T, °C | 25 | 150 | 150 | 250 |
| P, bar | 1 | 5 | 5 | 5 |
| Mole fraction, % | ||||
| H2 | 6.51 | 99.9 | 63.16 | 1.32 |
| N2 | 43.34 | - | 17.81 | 27.86 |
| CO2 | 20.52 | - | 8.43 | 0.52 |
| CO | 23.86 | - | 9.81 | Trace |
| CH4 | 1.09 | - | 0.45 | 28.71 |
| O2 | 0.58 | - | 0.24 | Trace |
| H2O | 4.00 | 0.10 | 0.06 | 41.53 |
| C2H6 | 0.10 | - | 0.04 | 0.06 |
| CH3OH | - | - | - | - |

Table A2.
Stream results of Case 2.
Table A2.
Stream results of Case 2.
| Stream Name | Feed | H2 Feed | Reactor Inlet | Reactor Outlet |
|---|---|---|---|---|
| Stream number | 1 | 2 | 3 | 4 |
| m, kg/s | 106.48 | 10.91 | 114.74 | 114.74 |
| T, °C | 25 | 150 | 150 | 250 |
| P, bar | 1 | 5 | 5 | 5 |
| Mole fraction, % | ||||
| H2 | 6.85 | 99.9 | 63.11 | 1.32 |
| N2 | 43.1 | - | 17.80 | 27.83 |
| CO2 | 20.40 | - | 8.42 | 0.52 |
| CO | 23.74 | - | 9.81 | Trace |
| CH4 | 1.22 | - | 0.51 | 28.78 |
| O2 | 0.58 | - | 0.24 | Trace |
| H2O | 4.00 | 0.1 | 0.06 | 41.49 |
| C2H6 | 0.11 | - | 0.05 | 0.07 |
| CH3OH | - | - | - | - |

Table A3.
Stream results of Case 3.
Table A3.
Stream results of Case 3.
| Stream Name | Feed | H2 Feed | Reactor Inlet | Reactor Outlet |
|---|---|---|---|---|
| Stream number | 1 | 2 | 3 | 4 |
| m, kg/s | 35.89 | 3.54 | 38.50 | 38.50 |
| T, °C | 25 | 150 | 150 | 250 |
| P, bar | 1 | 5 | 5 | 5 |
| Mole fraction, % | ||||
| H2 | 10.03 | 99.90 | 62.71 | 1.31 |
| N2 | 40.90 | - | 17.71 | 27.60 |
| CO2 | 19.26 | - | 8.34 | 0.52 |
| CO | 22.57 | - | 9.78 | Trace |
| CH4 | 2.45 | - | 1.06 | 29.37 |
| O2 | 0.56 | - | 0.24 | Trace |
| H2O | 4.00 | 0.10 | 0.06 | 41.05 |
| C2H6 | 0.22 | - | 0.10 | 0.15 |
| CH3OH | - | - | - | - |

Table A4.
Stream results of Case 4.
Table A4.
Stream results of Case 4.
| Stream Name | Feed | H2 Feed | Reactor Inlet | Reactor Outlet |
|---|---|---|---|---|
| Stream number | 1 | 2 | 3 | 4 |
| m, kg/s | 106.48 | 6.62 | 114.77 | 114.77 |
| T, °C | 25 | 150 | 150 | 250 |
| P, bar | 1 | 70 | 70 | 70 |
| Mole fraction, % | ||||
| H2 | 6.85 | 99.90 | 51.67 | 40.25 |
| N2 | 43.1 | - | 23.34 | 28.47 |
| CO2 | 20.40 | - | 11.04 | 13.06 |
| CO | 23.74 | - | 12.85 | 5.30 |
| CH4 | 1.22 | - | 0.66 | 0.81 |
| O2 | 0.58 | - | 0.32 | trace |
| H2O | 4.00 | 0.10 | 0.05 | 1.24 |
| C2H6 | 0.11 | - | 0.06 | 0.07 |
| CH3OH | - | - | - | 10.80 |

Table A5.
Stream results of Case 5—MeOH synthesis.
Table A5.
Stream results of Case 5—MeOH synthesis.
| Stream Name | Feed | H2 Feed | Reactor Inlet | Reactor Outlet |
|---|---|---|---|---|
| Stream number | 1 | 2 | 3 | 4 |
| m, kg/s | 54.39 | 3.48 | 56.54 | 56.54 |
| T, °C | 25 | 150 | 150 | 250 |
| P, bar | 1 | 70 | 70 | 4 |
| Mole fraction, % | ||||
| H2 | 5.13 | 99.90 | 51.90 | 40.55 |
| N2 | 44.29 | - | 23.42 | 28.58 |
| CO2 | 21.01 | - | 11.11 | 13.17 |
| CO | 24.37 | - | 12.88 | 5.29 |
| CH4 | 0.56 | - | 0.30 | 0.36 |
| O2 | 0.59 | - | 0.31 | trace |
| H2O | 4.00 | 0.10 | 0.05 | 1.21 |
| C2H6 | 0.05 | - | 0.03 | 0.03 |
| CH3OH | - | - | - | 10.81 |

Table A6.
Stream results of Case 5—methane production.
Table A6.
Stream results of Case 5—methane production.
| Stream Name | Feed | H2 Feed | Reactor Inlet | Reactor Outlet |
|---|---|---|---|---|
| Stream number | 1 | 2 | 3 | 4 |
| m, kg/s | 35.89 | 3.54 | 38.50 | 38.50 |
| T, °C | 25 | 150 | 150 | 250 |
| P, bar | 1 | 5 | 5 | 5 |
| Mole fraction, % | ||||
| H2 | 10.03 | 99.90 | 62.71 | 1.31 |
| N2 | 40.90 | - | 17.71 | 27.60 |
| CO2 | 19.26 | - | 8.34 | 0.52 |
| CO | 22.57 | - | 9.78 | trace |
| CH4 | 2.45 | - | 1.06 | 29.37 |
| O2 | 0.56 | - | 0.24 | trace |
| H2O | 4.00 | 0.10 | 0.06 | 41.05 |
| C2H6 | 0.22 | - | 0.1 | 0.15 |
| CH3OH | - | - | - | - |
References
- Ramírez-Santos, Á.A.; Castel, C.; Favre, E. A review of gas separation technologies within emission reduction programs in the iron and steel sector: Current application and development perspectives. Sep. Purif. Technol. 2018, 194, 425–442. [Google Scholar] [CrossRef]
- The European Steel Association (EUROFER). European Steel in Figures 2020; The European Steel Association (EUROFER): Brussels, Belgium, 2020. [Google Scholar]
- Quader, M.A.; Ahmed, S.; Ghazilla, R.A.R.; Ahmed, S.; Dahari, M. A comprehensive review on energy efficient CO2 breakthrough technologies for sustainable green iron and steel manufacturing. Renew. Sustain. Energy Rev. 2015, 50, 594–614. [Google Scholar] [CrossRef]
- Frey, A.; Goeke, V.; Voss, C. Steel Gases as Ancient and Modern Challenging Resource; Historical Review, Description of the Present, and a Daring Vision. Chem. Ing. Tech. 2018, 90, 1384–1391. [Google Scholar] [CrossRef]
- Colla, V.; Matino, I.; Dettori, S.; Petrucciani, A.; Zaccara, A.; Weber, V.; Salame, S.; Zapata, N.; Bastida, S.; Wolff, A.; et al. Assessing the efficiency of the off-gas network management in integrated steelworks. Mater. Tech. 2019. [Google Scholar] [CrossRef]
- Matino, I.; Dettori, S.; Colla, V.; Weber, V.; Salame, S. Forecasting blast furnace gas production and demand through echo state neural network-based models: Pave the way to off-gas optimized management. Appl. Energy 2019. [Google Scholar] [CrossRef]
- Colla, V.; Matino, I.; Dettori, S.; Cateni, S.; Matino, R. Reservoir Computing Approaches Applied to Energy Management in Industry. In Proceedings of the International Conference on Engineering Applications of Neural Networks, Xersonisos, Greece, 24–26 May 2019; pp. 66–79. [Google Scholar]
- Dettori, S.; Matino, I.; Colla, V.; Speets, R. Deep Echo State Networks in Industrial Applications. In Proceedings of the IFIP International Conference on Artificial Intelligence Applications and Innovations, Neos Marmaras, Greece, 5–7 June 2020; pp. 53–63. [Google Scholar]
- Maddaloni, A.; Matino, R.; Matino, I.; Dettori, S.; Zaccara, A.; Colla, V. A quadratic programming model for the optimization of off-gas networks in integrated steelworks. Mater. Tech. 2019, 107. [Google Scholar] [CrossRef]
- Wolff, A.; Mintus, F.; Bialek, S.; Dettori, S.; Colla, V. Economical Mixed-Integer Model Predictive Controller for optimizing the sub-network of the BOF gas. In Proceedings of the METEC 4th ESTAD, Düsseldorf, Germany, 24–28 June 2019. [Google Scholar]
- Leonzio, G.; Zondervan, E.; Foscolo, P.U. Methanol production by CO 2 hydrogenation: Analysis and simulation of reactor performance. Int. J. Hydrogen Energy 2019. [Google Scholar] [CrossRef]
- Bender, M.; Roussiere, T.; Schelling, H.; Schuster, S.; Schwab, E. Coupled Production of Steel and Chemicals. Chem. Ing. Tech. 2018, 90, 1782–1805. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J. The optimal carbon and hydrogen balance for methanol production from coke oven gas and Linz-Donawitz gas: Process development and techno-economic analysis. Fuel 2020. [Google Scholar] [CrossRef]
- Man, Y.; Yang, S.; Qian, Y. Integrated process for synthetic natural gas production from coal and coke-oven gas with high energy efficiency and low emission. Energy Convers. Manag. 2016. [Google Scholar] [CrossRef]
- Uribe-Soto, W.; Portha, J.F.; Commenge, J.M.; Falk, L. A review of thermochemical processes and technologies to use steelworks off-gases. Renew. Sustain. Energy Rev. 2017, 74, 809–823. [Google Scholar] [CrossRef]
- Remus, R.; Roudier, S.; Aguado Monsonet, M.A.; Sancho, L.D. Best Available Techniques (BAT) Reference Document for Iron and Steel Production; European Commission: Sevilla, Spain, 2013; ISBN 9789279264757. [Google Scholar]
- Saito, M.; Murata, K. Development of high performance Cu/ZnO-based catalysts for methanol synthesis and the water-gas shift reaction. Catal. Surv. Asia 2004, 8, 285–294. [Google Scholar] [CrossRef]
- Wiesmann, T.; Hamel, C.; Kaluza, S. Techniques to Remove Traces of Oxygen by Catalytic Conversion from Gas Mixtures. Chem. Ing. Tech. 2018, 90, 1446–1452. [Google Scholar] [CrossRef]
- Razzaq, R.; Li, C.; Zhang, S. Coke oven gas: Availability, properties, purification, and utilization in China. Fuel 2013, 113, 287–299. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, E.-B. Optimization simulation, using steel plant off-gas for power generation: A life-cycle cost analysis approach. Energies 2018, 11, 2884. [Google Scholar] [CrossRef]
- He, H.; Guan, H.; Zhu, X.; Lee, H. Assessment on the energy flow and carbon emissions of integrated steelmaking plants. Energy Rep. 2017. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012. [Google Scholar] [CrossRef]
- Koytsoumpa, E.I.; Karellas, S. Equilibrium and kinetic aspects for catalytic methanation focusing on CO2 derived Substitute Natural Gas (SNG). Renew. Sustain. Energy Rev. 2018. [Google Scholar] [CrossRef]
- Bertuccioli, L.; Chan, A.; Hart, D.; Lehner, F.; Madden, B.; Standen, E. Development of water electrolysis in the EU. Fuel Cells Hydrog. Jt. Undert. 2014. [Google Scholar] [CrossRef]
- Lajtonyi, A. Blast furnace gas cleaning systems. Millenium Steel 2006, 2006, 57–65. [Google Scholar]
- Koytsoumpa, E.I.; Atsonios, K.; Panopoulos, K.D.; Karellas, S.; Kakaras, E.; Karl, J. Modelling and assessment of acid gas removal processes in coal-derived SNG production. Appl. Therm. Eng. 2015, 74, 128–135. [Google Scholar] [CrossRef]
- Spencer, M.S. The role of zinc oxide in Cu/ZnO catalysts for methanol synthesis and the water-gas shift reaction. Top. Catal. 1999, 8, 259–266. [Google Scholar] [CrossRef]
- Rhyner, U. Gas cleaning. In Synthetic Natural Gas from Coal and Dry Biomass, and Power-to-Gas Applications; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; ISBN 9781119191339. [Google Scholar]
- Woolcock, P.J.; Brown, R.C. A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 2013, 52, 54–84. [Google Scholar] [CrossRef]
- Ud Din, Z.; Zainal, Z.A. Biomass integrated gasification-SOFC systems: Technology overview. Renew. Sustain. Energy Rev. 2016, 53, 1356–1376. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Kilpinen, P. Control of Pollutants in Flue Gases and Fuel Gases; Helsinki University of Technology: Espoo/Turku, Finland, 2001; ISBN 951-22-5527-8. [Google Scholar]
- Dou, B.; Wang, C.; Chen, H.; Song, Y.; Xie, B.; Xu, Y.; Tan, C. Research progress of hot gas filtration, desulphurization and HCl removal in coal-derived fuel gas: A review. Chem. Eng. Res. Des. 2012, 90, 1901–1917. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, M.; Gao, J.; Shen, W.; Sha, X. High-temperature removal of NH3, organic sulfur, HCl, and tar component from coal-derived gas. Ind. Eng. Chem. Res. 2002, 41, 4195–4200. [Google Scholar] [CrossRef]
- Li, L.; King, D.L. H2S removal with ZnO during fuel processing for PEM fuel cell applications. Catal. Today 2006, 116, 537–541. [Google Scholar] [CrossRef]
- Novochinskii, I.I.; Song, C.; Ma, X.; Liu, X.; Shore, L.; Lampert, J.; Farrauto, R.J. Low-temperature H2S removal from steam-containing gas mixtures with ZnO for fuel cell application. 1. ZnO particles and extrudates. Energy Fuels 2004, 18, 576–583. [Google Scholar] [CrossRef]
- Sasaoka, E.; Taniguchi, K.; Hirano, S.; Uddin, M.A.; Kasaoka, S.; Sakata, Y. Catalytic Activity of ZnS Formed from Desulfurization Sorbent ZnO for Conversion of COS to H2S. Ind. Eng. Chem. Res. 1995. [Google Scholar] [CrossRef]
- Graaf, G.H.; Winkelman, J.G.M.; Stamhuis, E.J.; Beenackers, A.A.C.M. Kinetics of the three phase methanol synthesis. Chem. Eng. Sci. 1988. [Google Scholar] [CrossRef]
- Schittkowski, J.; Ruland, H.; Laudenschleger, D.; Girod, K.; Kähler, K.; Kaluza, S.; Muhler, M.; Schlögl, R. Methanol Synthesis from Steel Mill Exhaust Gases: Challenges for the Industrial Cu/ZnO/Al2O3 Catalyst. Chem. Ing. Tech. 2018, 90, 1419–1429. [Google Scholar] [CrossRef]
- Gaikwad, R.; Bansode, A.; Urakawa, A. High-pressure advantages in stoichiometric hydrogenation of carbon dioxide to methanol. J. Catal. 2016. [Google Scholar] [CrossRef]
- Bansode, A.; Urakawa, A. Towards full one-pass conversion of carbon dioxide to methanol and methanol-derived products. J. Catal. 2014. [Google Scholar] [CrossRef]
- Bozzano, G.; Manenti, F. Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 2016, 56, 71–105. [Google Scholar] [CrossRef]
- Liu, G.; Willcox, D.; Garland, M.; Kung, H.H. The role of CO2 in methanol synthesis on CuZn oxide: An isotope labeling study. J. Catal. 1985. [Google Scholar] [CrossRef]
- Zachopoulos, A.; Heracleous, E. Overcoming the equilibrium barriers of CO2 hydrogenation to methanol via water sorption: A thermodynamic analysis. J. CO2 Util. 2017. [Google Scholar] [CrossRef]
- Vanden Bussche, K.M.; Froment, G.F. A steady-state kinetic model for methanol synthesis and the water gas shift reaction on a commercial Cu/ZnO/Al2O3 catalyst. J. Catal. 1996. [Google Scholar] [CrossRef]
- Soave, G. Equilibrium constants from a modified Redlich-Kwong equation of state. Chem. Eng. Sci. 1972. [Google Scholar] [CrossRef]
- Graaf, G.H.; Sijtsema, P.J.J.M.; Stamhuis, E.J.; Joosten, G.E.H. Chemical equilibria in methanol synthesis. Chem. Eng. Sci. 1986. [Google Scholar] [CrossRef]
- Graaf, G.H.; Winkelman, J.G.M. Chemical Equilibria in Methanol Synthesis Including the Water-Gas Shift Reaction: A Critical Reassessment. Ind. Eng. Chem. Res. 2016. [Google Scholar] [CrossRef]
- Schildhauer, T.J. Methanation for Synthetic Natural Gas Production—Chemical Reaction Engineering Aspects. In Synthetic Natural Gas from Coal and Dry Biomass, and Power-to-Gas Applications; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; ISBN 9781119191339. [Google Scholar]
- Koytsoumpa, E.I.; Karellas, S.; Kakaras, E. Modelling of Substitute Natural Gas production via combined gasification and power to fuel. Renew. Energy 2019. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation -From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Vogel, F.; Biollaz, S.M.A.; Wokaun, A. Applying spatially resolved concentration and temperature measurements in a catalytic plate reactor for the kinetic study of CO methanation. J. Catal. 2010. [Google Scholar] [CrossRef]
- Er-rbib, H.; Bouallou, C. Modeling and simulation of CO methanation process for renewable electricity storage. Energy 2014. [Google Scholar] [CrossRef]
- Bessarabov, D.; Wang, H.; Li, H.; Zhao, N. PEM Electrolysis for Hydrogen Production: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Sol. Energy 2005. [Google Scholar] [CrossRef]
- Rivera-Tinoco, R.; Farran, M.; Bouallou, C.; Auprêtre, F.; Valentin, S.; Millet, P.; Ngameni, J.R. Investigation of power-to-methanol processes coupling electrolytic hydrogen production and catalytic CO2 reduction. Int. J. Hydrogen Energy 2016. [Google Scholar] [CrossRef]
- Medina, P.; Santarelli, M. Analysis of water transport in a high pressure PEM electrolyzer. Int. J. Hydrogen Energy 2010. [Google Scholar] [CrossRef]
- Zaccara, A.; Petrucciani, A.; Matino, I.; Branca, T.A.; Dettori, S.; Iannino, V.; Colla, V.; Bampaou, M.; Panopoulos, K. Renewable hydrogen production processes for the off-gas valorization in integrated steelworks through hydrogen intensified methane and methanol syntheses. Metals 2020, 10, 1535. [Google Scholar] [CrossRef]
- Yang, S.I.; Choi, D.Y.; Jang, S.C.; Kim, S.H.; Choi, D.K. Hydrogen separation by multi-bed pressure swing adsorption of synthesis gas. Adsorption 2008. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009. [Google Scholar] [CrossRef]
- Bampaou, M.; Panopoulos, K.D.; Papadopoulos, A.I.; Seferlis, P.; Voutetakis, S. An electrochemical hydrogen compression model. Chem. Eng. Trans. 2018, 70, 1213–1218. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).