Heat of Decomposition and Fire Retardant Behavior of Polyimide-Graphene Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene/Polyamic Acid
2.2. Fabrication of Graphene-Polyimide Films
2.3. Thermal Analysis
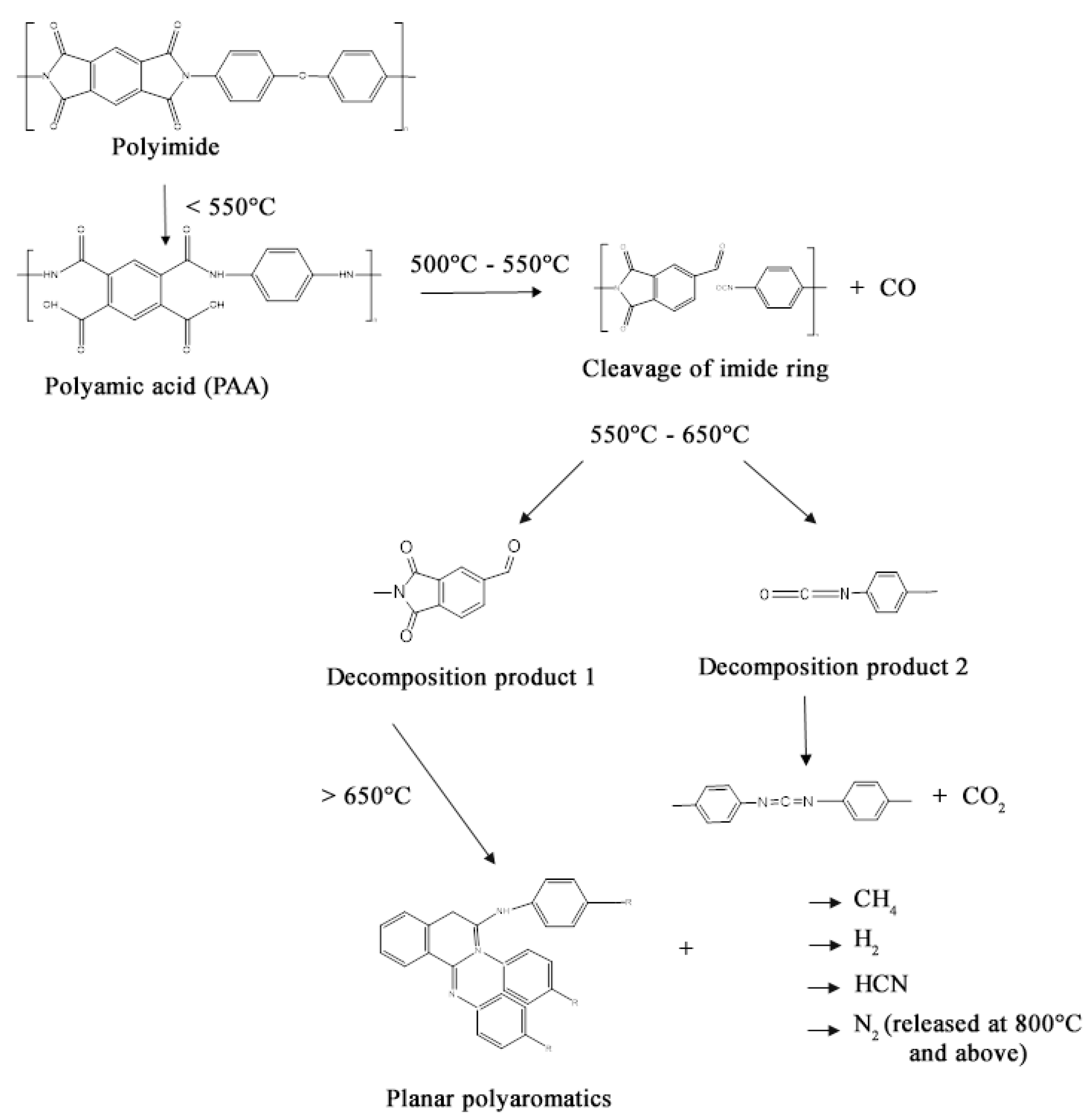
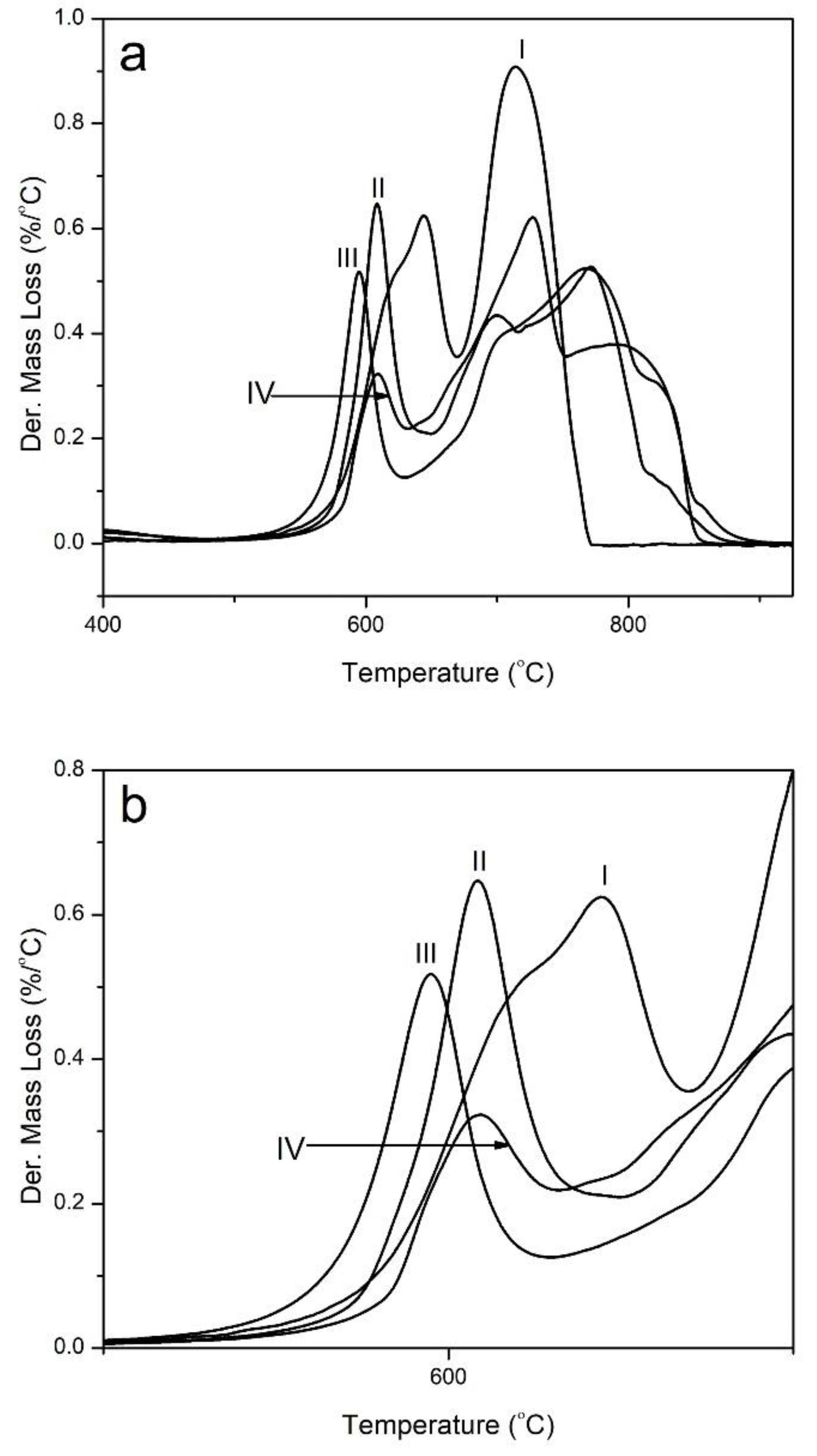
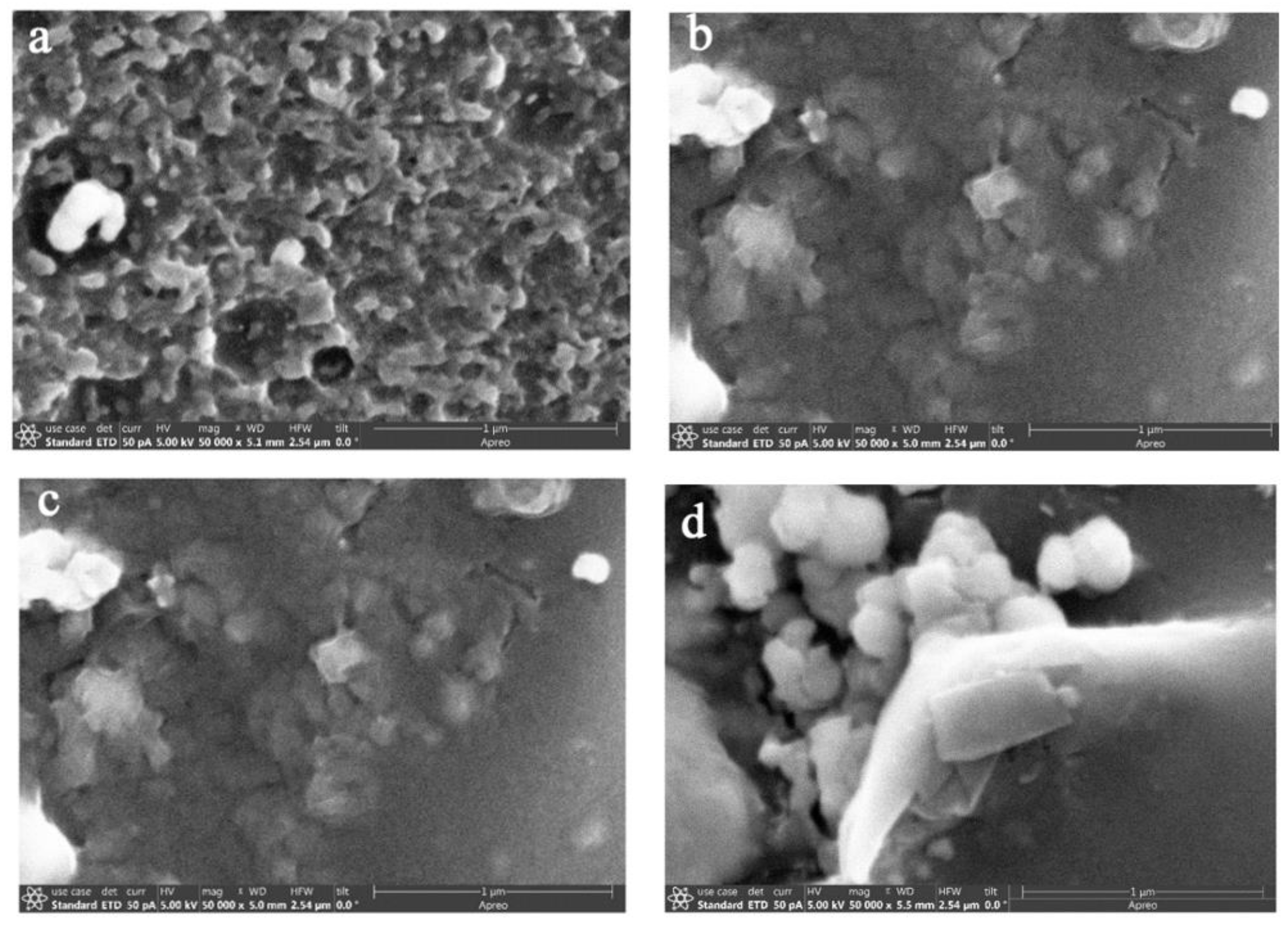
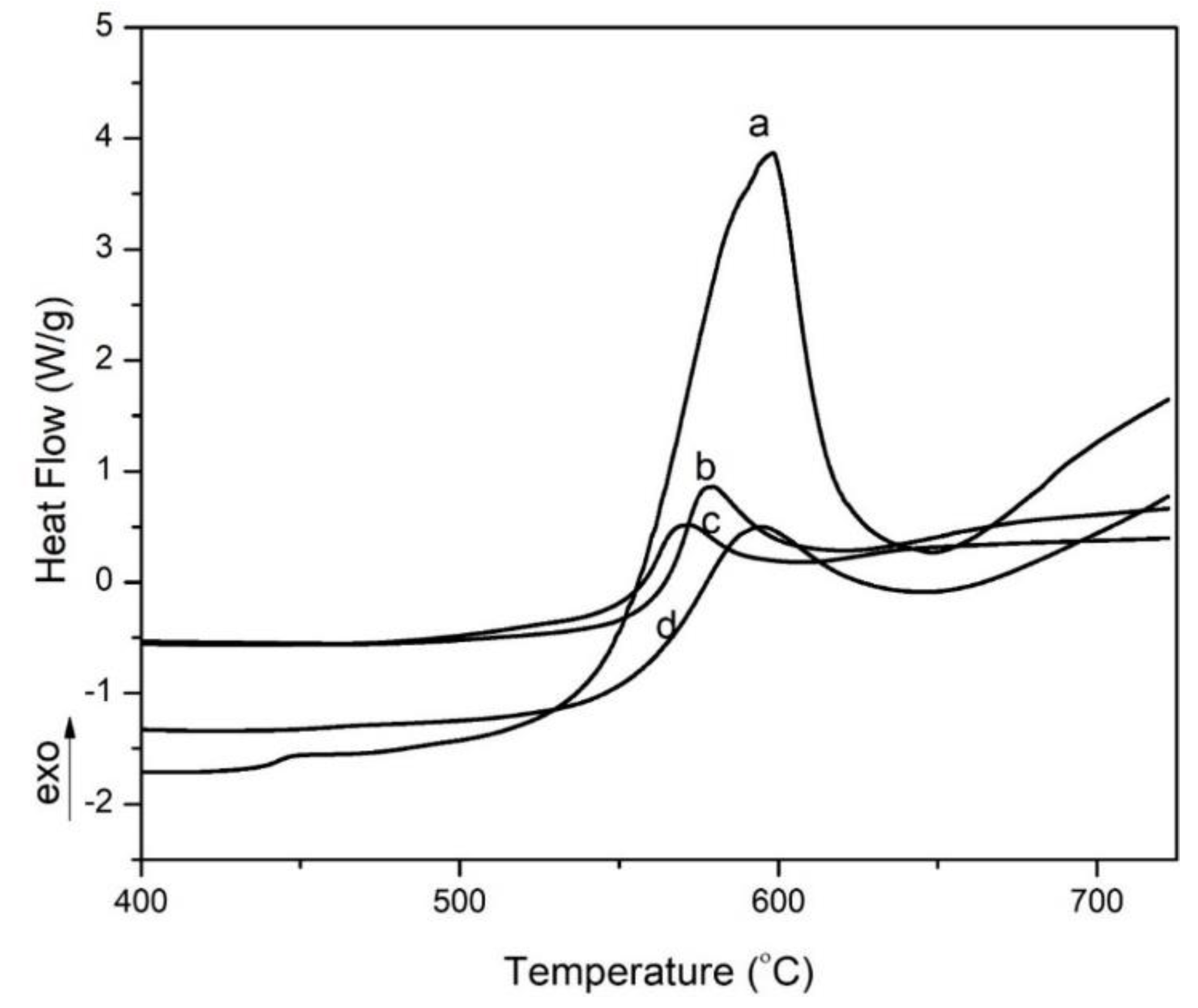
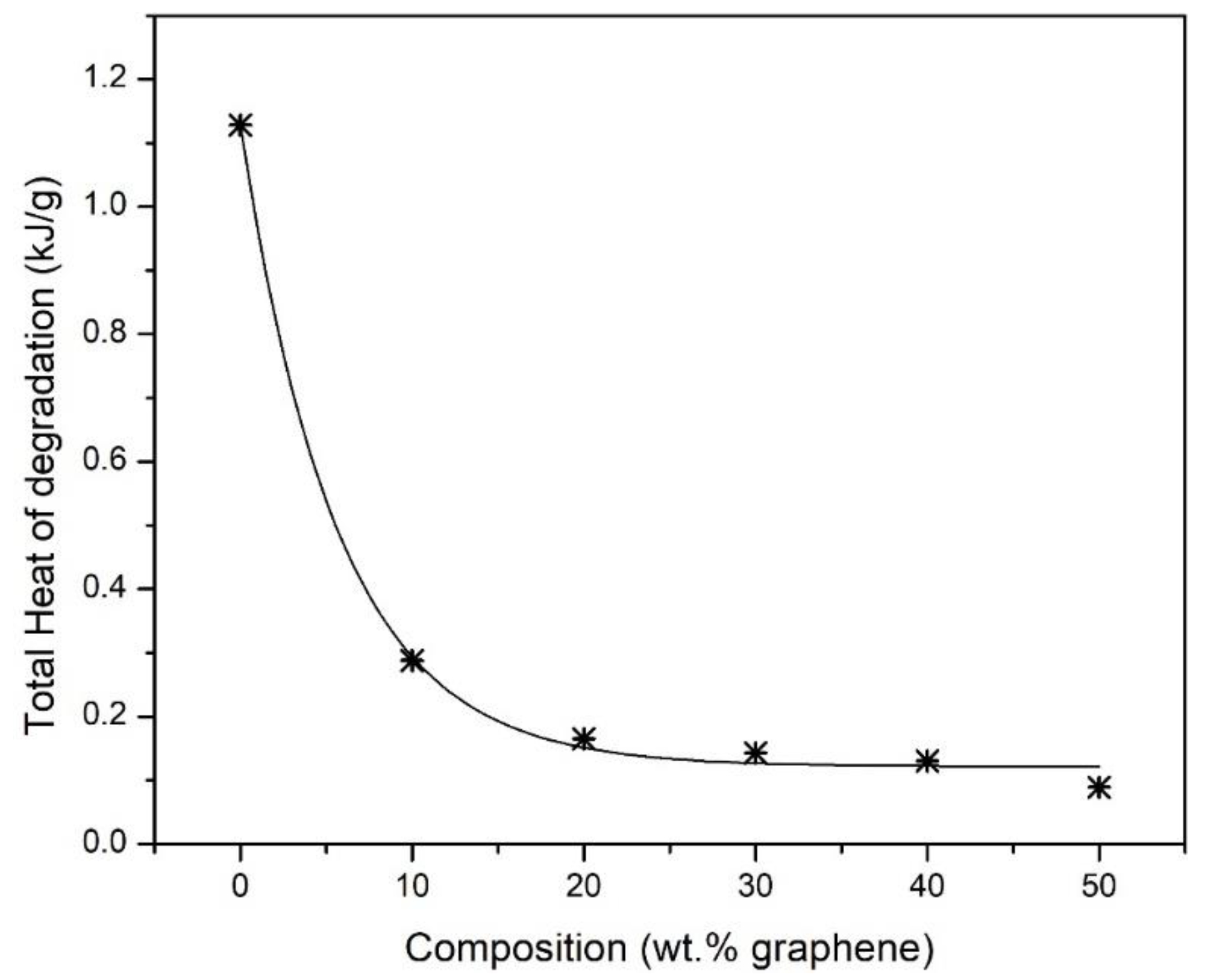
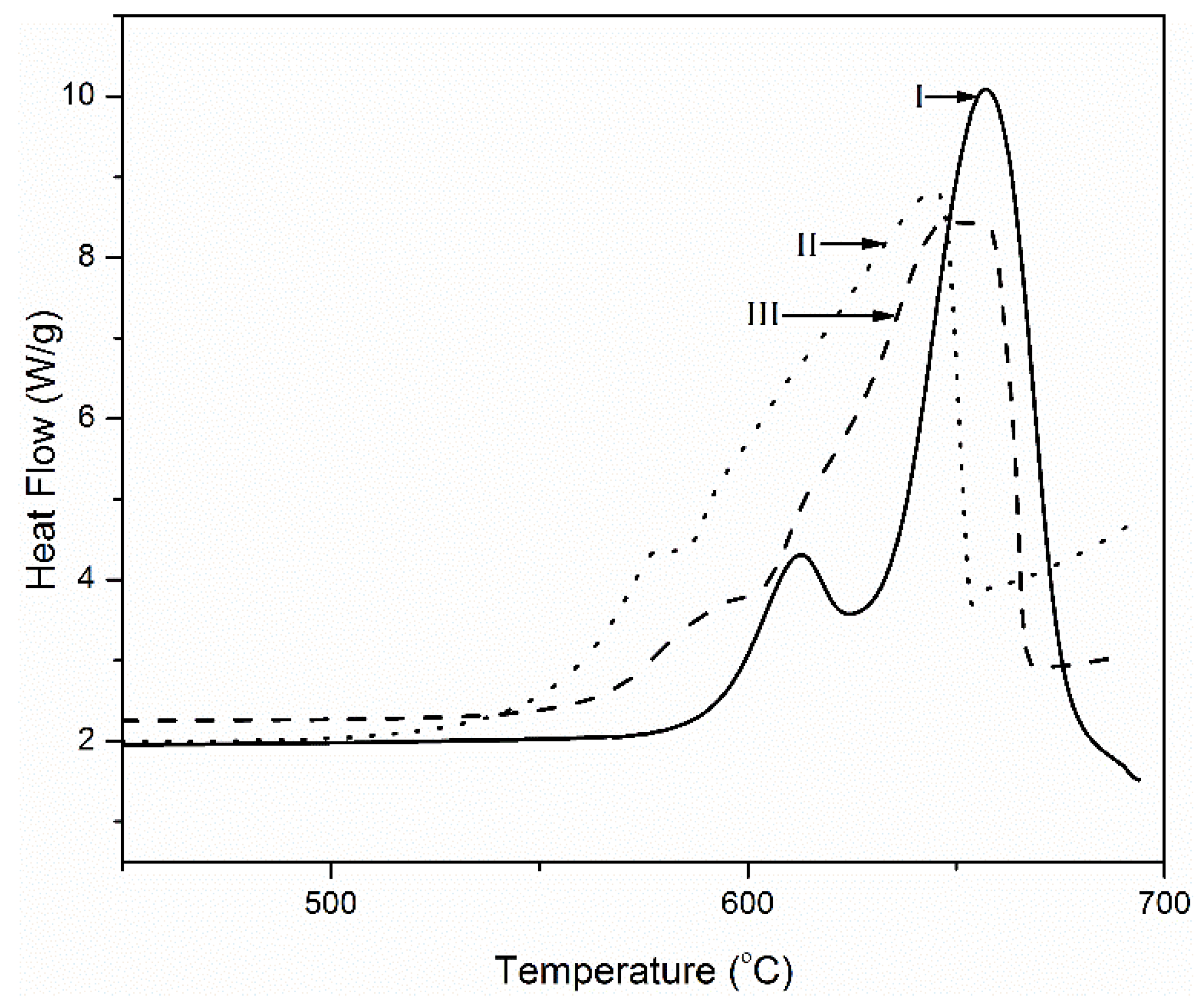
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilman, J.W.; Harris, R.H.; Shields, J.R.; Kashiwagi, T.; Morgan, A.B. A study of the flammability reduction mechanism of polystyrene-layered silicate nanocomposite: Layered silicate reinforced carbonaceous char. Polym. Adv. Technol. 2006, 17, 263–271. [Google Scholar] [CrossRef]
- Bartholmal, M.; Schartel, B. Layered silicate polymer nanocomposites: New approach or illusion for fire retardacy? In-vestigations of the potentials and the tasks using a model system. Polym. Adv. Technol. 2004, 15, 355–364. [Google Scholar] [CrossRef]
- Morgan, A.B. The Future of Flame Retardant Polymers—Unmet Needs and Likely New Approaches. Polym. Rev. 2019, 59, 25–54. [Google Scholar] [CrossRef]
- Morgan, A.B.; Harris, R.H.; Kashiwagi, T.; Chyall, L.J.; Gilman, J.W. Flammability of polystyrene layered silicate (clay) nanocomposites: Carbonaceous char formation. Fire Mater. 2002, 26, 247–253. [Google Scholar] [CrossRef]
- Pack, S.; Si, M.; Koo, J.; Sokolov, J.C.; Koga, T.; Kashiwagi, T.; Rafailovich, M.H. Mode-of-action of self-extinguishing polymer blends containing organoclays. Polym. Degrad. Stab. 2009, 94, 306–326. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Zammarano, M.; Gilman, J.W.; Davis, R.D.; Kashiwagi, T. Effect of montmorillonite dispersion on flammability properties of poly(styrene-co-acrylonitrile) nanocomposites. Polymer 2011, 52, 3092–3103. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Harris, R.H.; Zhang, X.; Briber, R.; Cipriano, B.H.; Raghavan, S.R.; Awad, W.H.; Shields, J.R. Flame retardant mechanism of polyamide 6–clay nanocomposites. Polymer 2004, 45, 881–891. [Google Scholar] [CrossRef]
- Manias, E.; Touny, A.; Wu, L.; Strawhecker, K.; Lu, A.B.; Chung, T.C. Polypropylene/Montmorillonite Nanocomposites. Review of the Synthetic Routes and Materials Properties. Chem. Mater. 2001, 13, 3516–3523. [Google Scholar] [CrossRef] [Green Version]
- Strawhecker, K.E.; Manias, E. Structure and Properties of Poly(vinyl alcohol)/Na+Montmorillonite Nanocomposites. Chem. Mater. 2000, 12, 2943–2949. [Google Scholar] [CrossRef]
- Cai, D.; Song, M. Recent advance in functionalized graphene/polymer nanocomposites. J. Mater. Chem. 2010, 20, 7906–7915. [Google Scholar] [CrossRef]
- Gilman, J. Flammability and thermal stability studies of polymer layered-silicate (clay) nanocomposites. Appl. Clay Sci. 1999, 15, 31–49. [Google Scholar] [CrossRef]
- Pradhan, B.; Setyowati, K.; Liu, H.; Waldeck, D.H.; Chen, J. Carbon Nanotube−Polymer Nanocomposite Infrared Sensor. Nano Lett. 2008, 8, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Calizo, I.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Temperature Dependence of the Raman Spectra of Graphene and Graphene Multilayers. Nano Lett. 2007, 7, 2645–2649. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer gra-phene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y. Approaching ballistic transport in suspended grapheme. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; van der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable Atomic Membranes from Graphene Sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A.B.; Putthanarat, S. Use of inorganic materials to enhance thermal stability and flammability behavior of a polyimide. Polym. Degrad. Stab. 2011, 96, 23–32. [Google Scholar] [CrossRef]
- Tandon, G.; Pochiraju, K.; Schoeppner, G. Thermo-oxidative behavior of high-temperature PMR-15 resin and composites. Mater. Sci. Eng. A 2008, 498, 150–161. [Google Scholar] [CrossRef]
- Putthanarat, S.; Tandon, G.P.; Schoeppner, G.A. Influence of aging temperature, time, and environment on ther-mo-oxidative behavior of PMR-15: Nanomechanical characterization. J. Mater. Sci. 2008, 43, 6714–6723. [Google Scholar] [CrossRef]
- Pochiraju, K.V.; Tandon, G.P.; Schoeppner, G.A. Evolution of stress and deformations in high-temperature polymer matrix composites during thermo-oxidative aging. Mech. Time Depend. Mater. 2007, 12, 45–68. [Google Scholar] [CrossRef]
- Lu, Y.C.; Tandon, G.P.; Jones, D.C.; Schoeppner, G.A. Elastic and viscoelastic characterization of thermally-oxidized polymer resin using nanoindentation. Mech. Time Depend. Mater. 2009, 13, 245–260. [Google Scholar] [CrossRef]
- Ghosh, A.; Subrahmanyam, K.S.; Krishna, K.S.; Datta, S.; Govindaraj, A.; Pati, S.K.; Rao, C.N.R. Uptake of H2 and CO2 by Graphene. J. Phys. Chem. C 2008, 112, 15704–15707. [Google Scholar] [CrossRef]
- Fornes, T.; Yoon, P.; Hunter, D.; Keskkula, H.; Paul, D. Effect of organoclay structure on nylon 6 nanocomposite morphology and properties. Polymer 2002, 43, 5915–5933. [Google Scholar] [CrossRef]
- Cullis, C. The role of pyrolysis in polymer combustion and flame retardance. J. Anal. Appl. Pyrolysis 1987, 11, 451–463. [Google Scholar] [CrossRef]
- Lua, A.C.; Su, J. Isothermal and non-isothermal pyrolysis kinetics of Kapton® polyimide. Polym. Degrad. Stab. 2006, 91, 144–153. [Google Scholar] [CrossRef]
- Hatori, H.; Yamada, Y.; Shiraishi, M.; Yoshihara, M.; Kimura, T. The mechanism of polyimide pyrolysis in the early stage. Carbon 1996, 34, 201–208. [Google Scholar] [CrossRef]
- Cella, J.A. Degradation and stability of polyimides. Polym. Degrad. Stab. 1992, 36, 99–110. [Google Scholar] [CrossRef]
- Bürger, A.; Fitzer, E.; Heym, M.; Terwiesch, B. Polyimides as precursors for artificial carbon. Carbon 1975, 13, 149–157. [Google Scholar] [CrossRef]
- Akinyi, C.; Longun, J.; Chen, S.; Iroh, J.O. Decomposition and Flammability of Polyimide Graphene Composites. Minerals 2021, 11, 168. [Google Scholar] [CrossRef]
- Arnold, C.; Borgman, L.K. Chemistry and Kinetics of Polyimide Degradation. Ind. Eng. Chem. Product Res. Dev. 1972, 11, 322–325. [Google Scholar] [CrossRef]
- Wunderlich, B. The heat capacity of polymers. Thermochim. Acta 1997, 300, 43–65. [Google Scholar] [CrossRef]
- Yuan, B.; Sheng, H.; Mu, X.; Song, L.; Tai, Q.; Shi, Y.; Liew, K.M.; Hu, Y. Enhanced flame retardancy of polypropylene by melamine-modified graphene oxide. J. Mater. Sci. 2015, 50, 5389–5401. [Google Scholar] [CrossRef]
- Song, Y.; Yu, J.; Yu, L.; Alam, F.E.; Dai, W.; Li, C.; Jiang, N. Enhancing the thermal, electrical, and mechanical properties of silicone rubber by addition of graphene nanoplatelets. Mater. Des. 2015, 88, 950–957. [Google Scholar] [CrossRef]
- Pramoda, K.P.; Chung, T.S.; Liu, S.L.; Oikawa, H.; Yamaguchi, A. Characterization and thermal degradation of polyimide and polyamide liquid crystalline polymers. Polym. Degrad. Stab. 2000, 67, 365–374. [Google Scholar] [CrossRef]
- Price, A.R.H.D. Combustion processes of textile fibres. In Handbook of Fire Resistant Textiles; Kilinc, F.S., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 3–25. [Google Scholar]
- Biteau, H.; Steinhaus, T.; Schemel, C.; Simeoni, A.; Marlair, G.; Bal, N.; Torero, J. Calculation Methods for the Heat Release Rate of Materials of Unknown Composition. Fire Saf. Sci. 2008, 9, 1165–1176. [Google Scholar] [CrossRef] [Green Version]
- Walters, R.N.; Hackett, S.M.; Lyon, R.E. Heats of combustion of high temperature polymers. Fire Mater. 2000, 24, 245–252. [Google Scholar] [CrossRef]
- Jessup, R.S. Heats of combustion of diamond and of graphite. J. Res. Natl. Inst. Stand. Technol. 1938, 21, 475. [Google Scholar] [CrossRef]




| Average Lateral Dimension (mm) | Thickness (nm) | Oxygen Content (%) | Specific Surface Area m2/g | Density (g/cm3) | Carbon (wt.%) | Hydrogen (wt.%) | Nitrogen (wt.%) | Oxygen (wt.%) |
|---|---|---|---|---|---|---|---|---|
| 7 | 70–100 | <1 | 10–15 | 0.1–0.3 | ≥97 | ≤1 | ≤0.5 | ≤2 |
| Sample | Carbon (%) | Oxygen (%) | Nitrogen (%) | Silicon (%) |
|---|---|---|---|---|
| Neat PI | 98.2 | 1.8 | - | - |
| PI-10 | 96.2 | 1.6 | 2.2 | - |
| PI-30 | 96.7 | 1.5 | 1.9 | - |
| PI-50 | 96.7 | 1.1 | 1.6 | 0.7 |
| Graphene | 96.7 | 2.3 | 0.3 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinyi, C.J.; Iroh, J.O. Heat of Decomposition and Fire Retardant Behavior of Polyimide-Graphene Nanocomposites. Energies 2021, 14, 3948. https://doi.org/10.3390/en14133948
Akinyi CJ, Iroh JO. Heat of Decomposition and Fire Retardant Behavior of Polyimide-Graphene Nanocomposites. Energies. 2021; 14(13):3948. https://doi.org/10.3390/en14133948
Chicago/Turabian StyleAkinyi, Caroline J., and Jude O. Iroh. 2021. "Heat of Decomposition and Fire Retardant Behavior of Polyimide-Graphene Nanocomposites" Energies 14, no. 13: 3948. https://doi.org/10.3390/en14133948
APA StyleAkinyi, C. J., & Iroh, J. O. (2021). Heat of Decomposition and Fire Retardant Behavior of Polyimide-Graphene Nanocomposites. Energies, 14(13), 3948. https://doi.org/10.3390/en14133948








