MIL-160 as an Adsorbent for Atmospheric Water Harvesting
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Synthesis
2.2. Characterization
2.3. Water-Adsorption Equilibrium
3. Results and Discussions
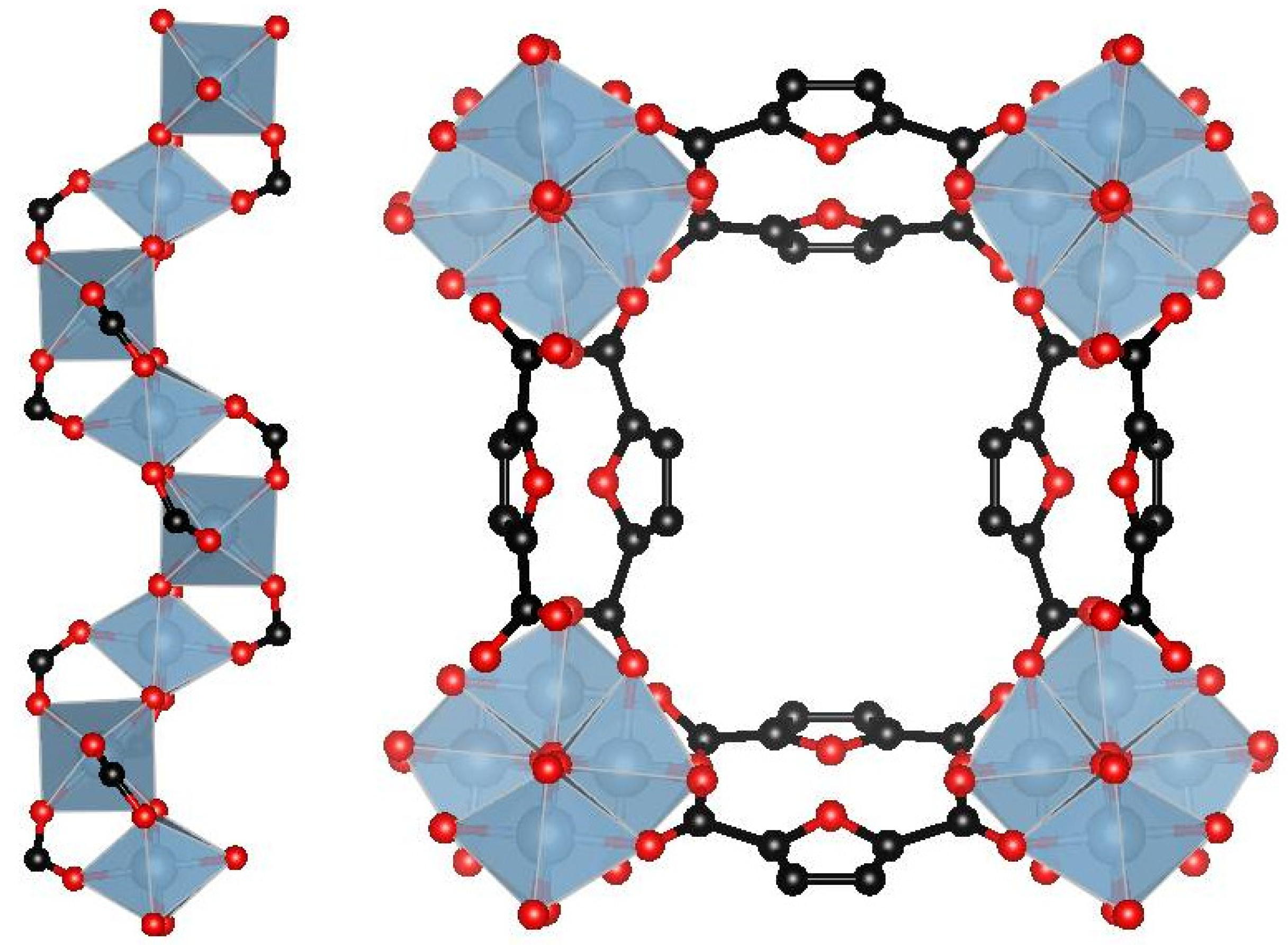
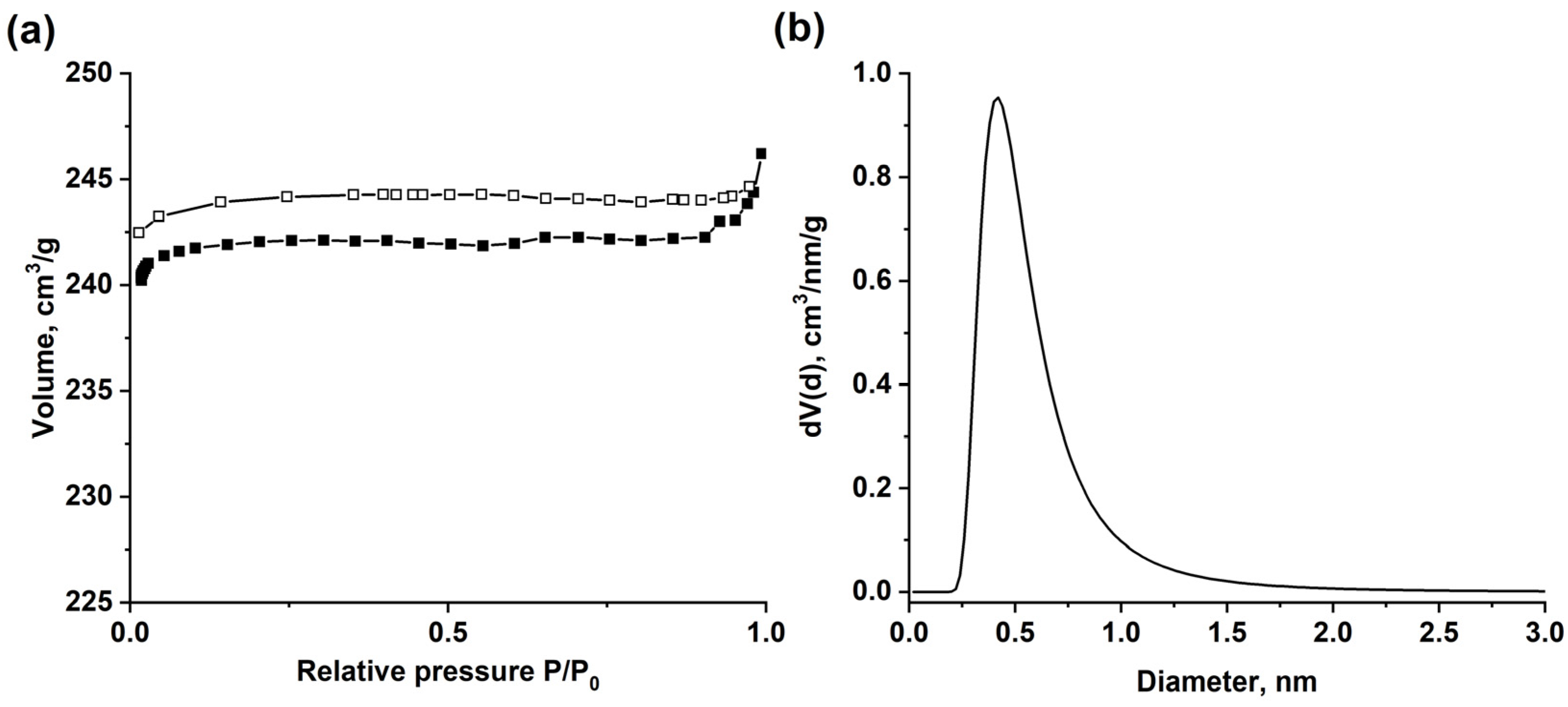
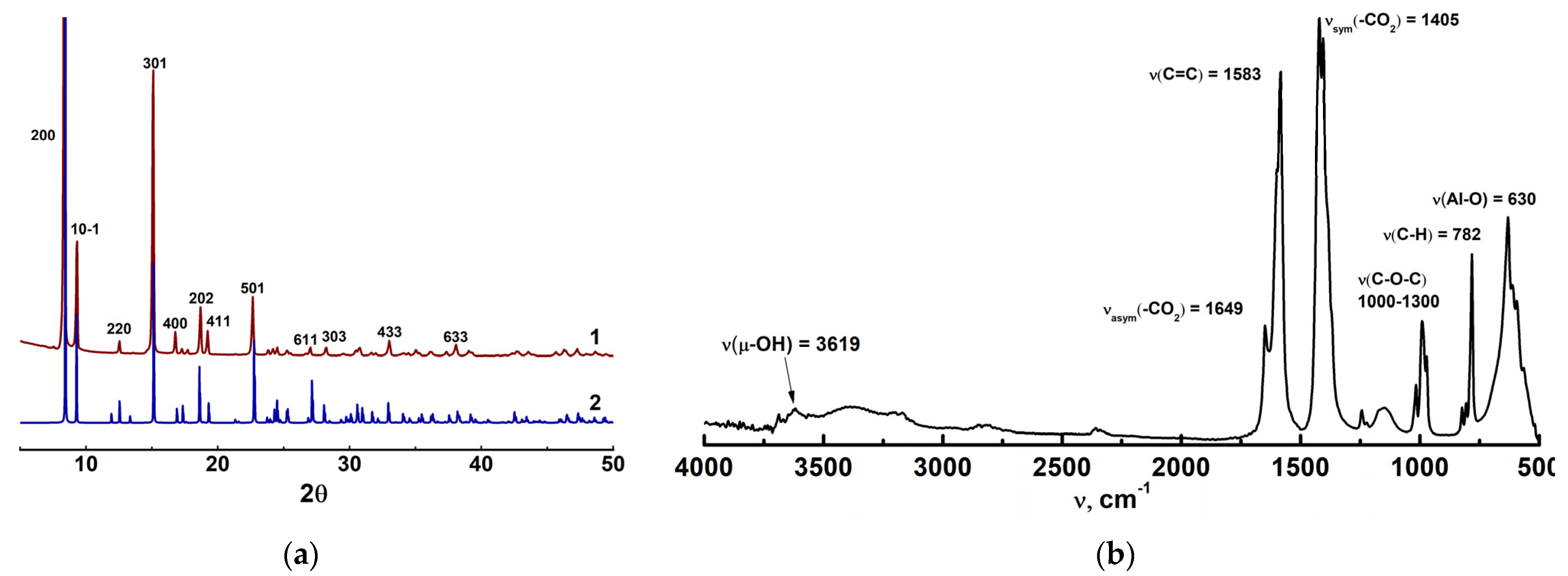
3.1. Structure Characterization of as-Prepared MIL-160
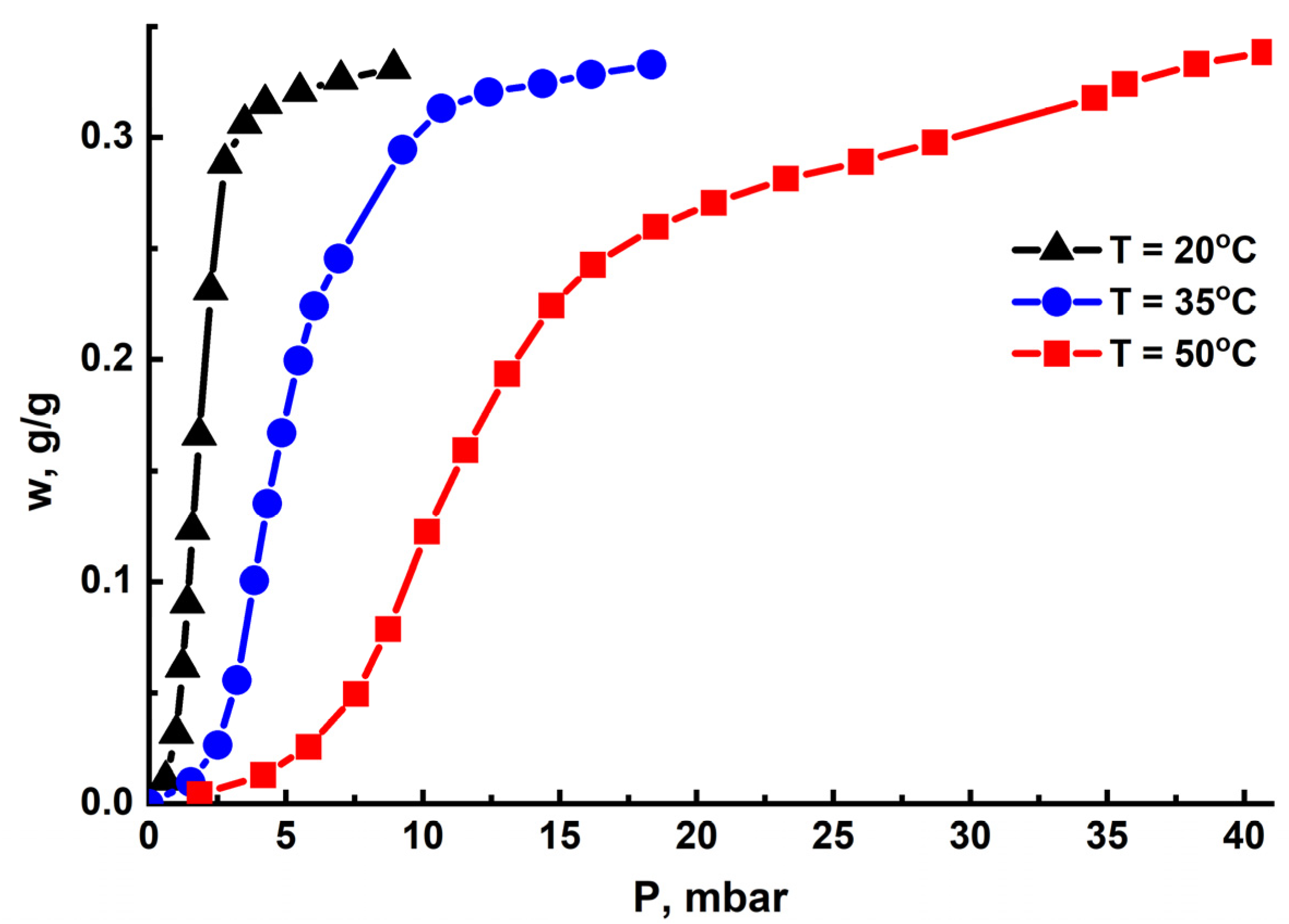
3.2. Water-Vapor Adsorption on MIL-160
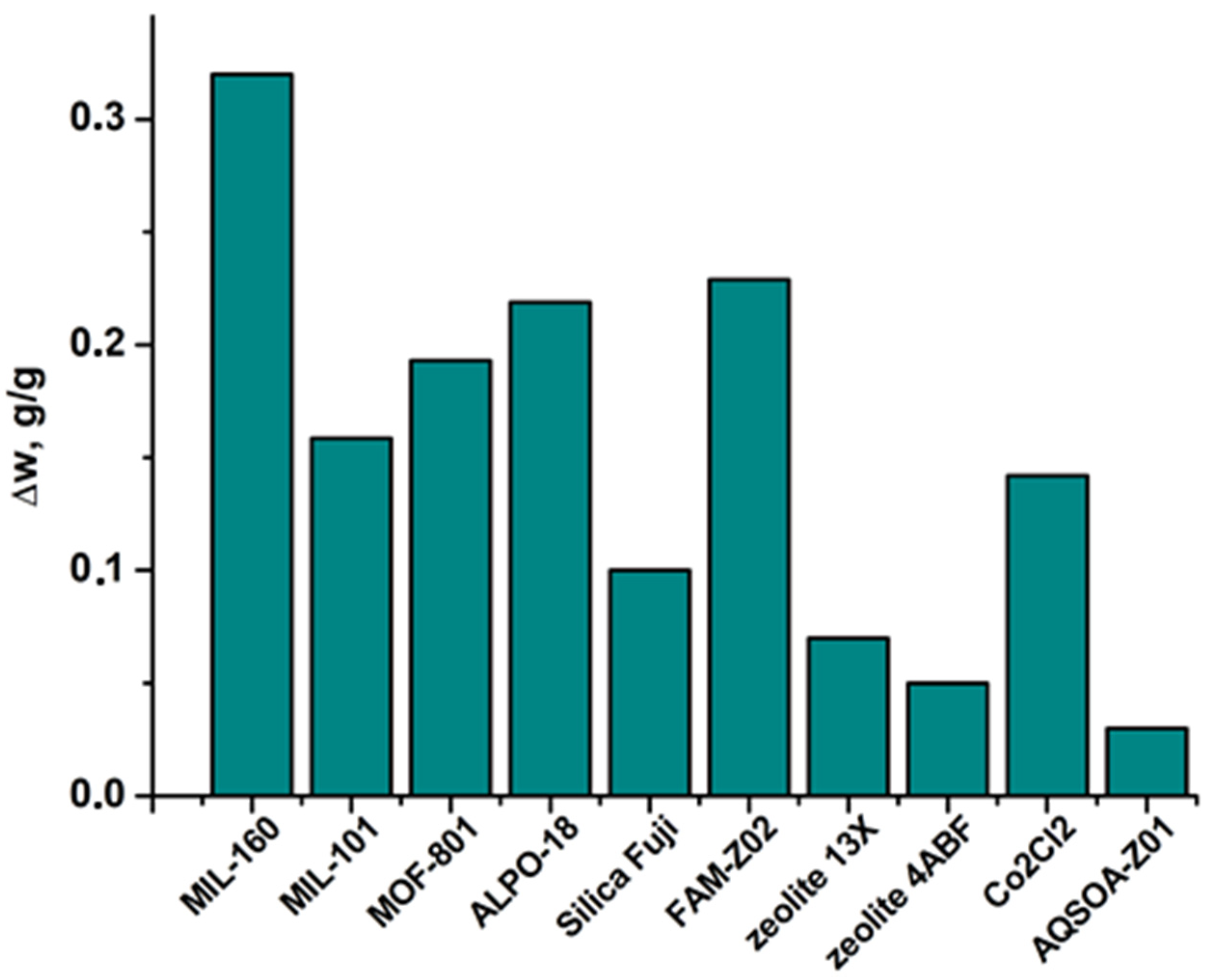
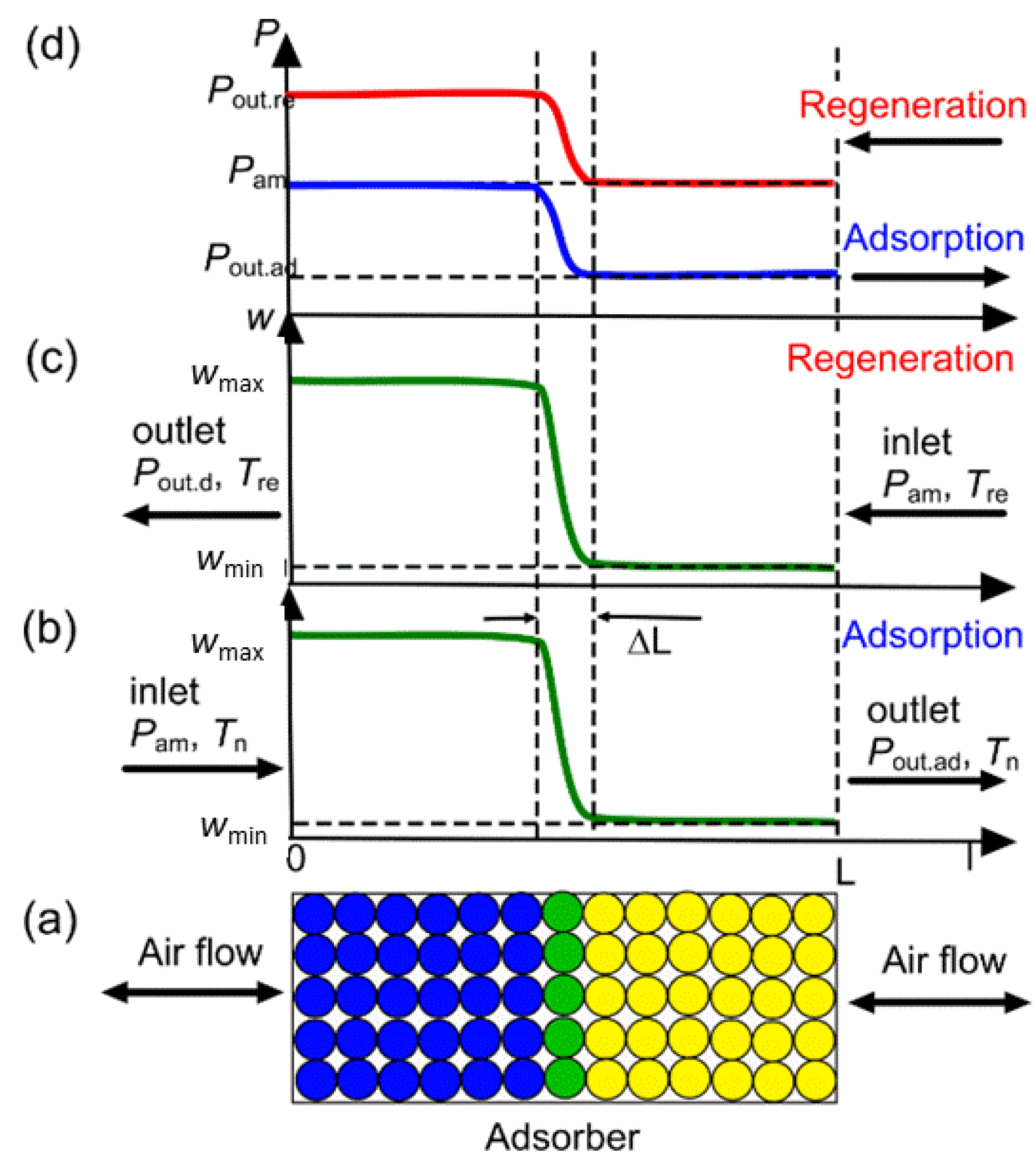
3.3. Performance of AWHA Employing MIL-160
3.3.1. Specific Water Productivity
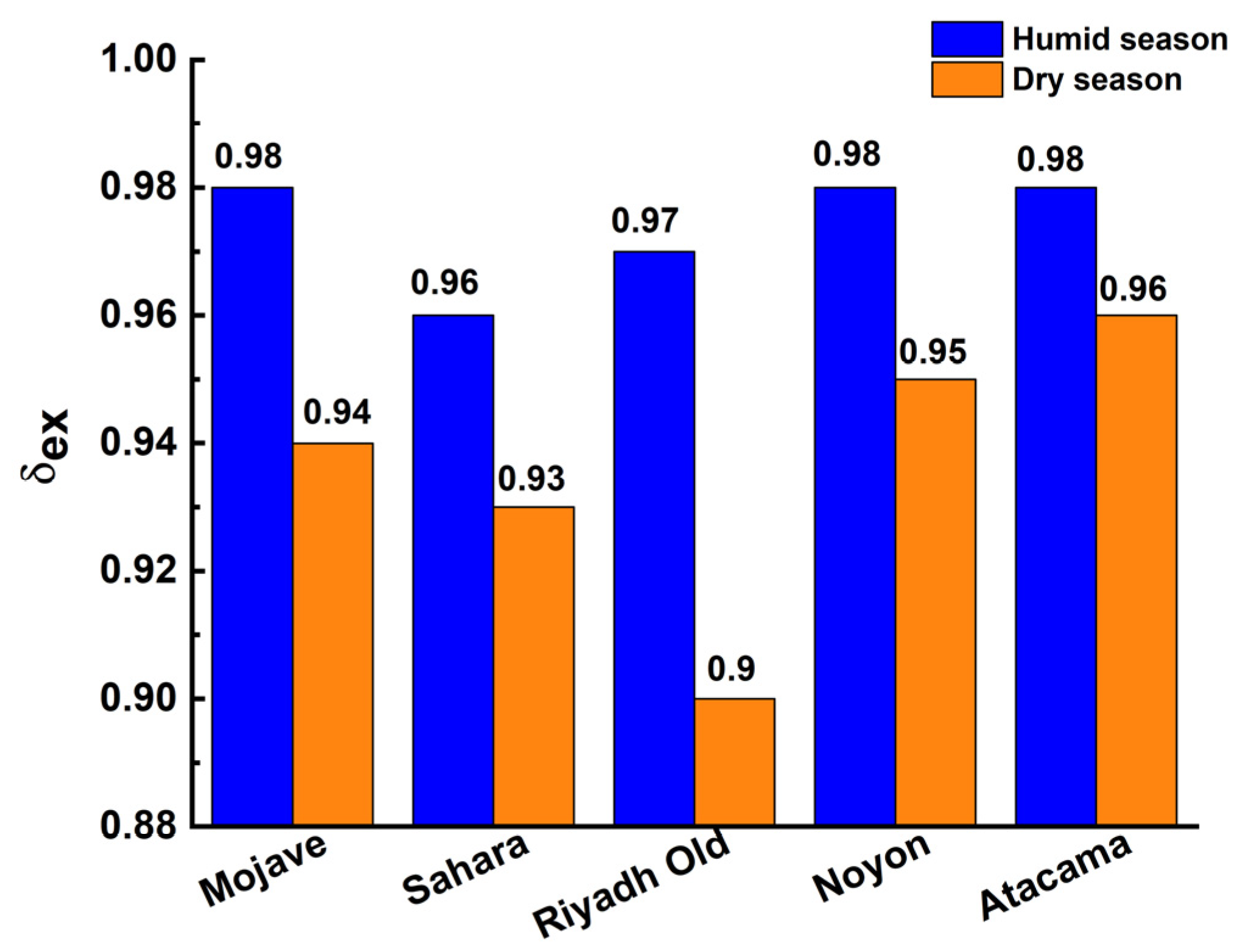
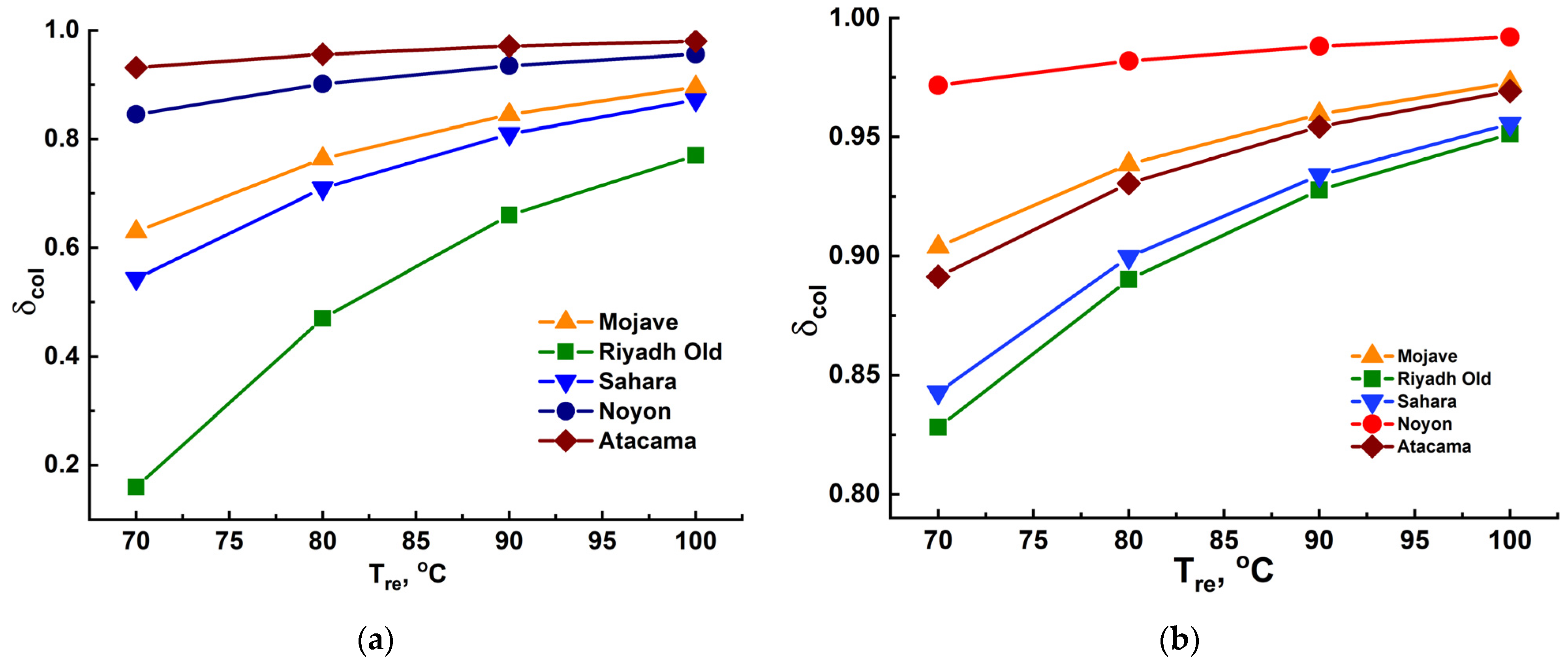
3.3.2. The Fractions of Water Extraction and Collection
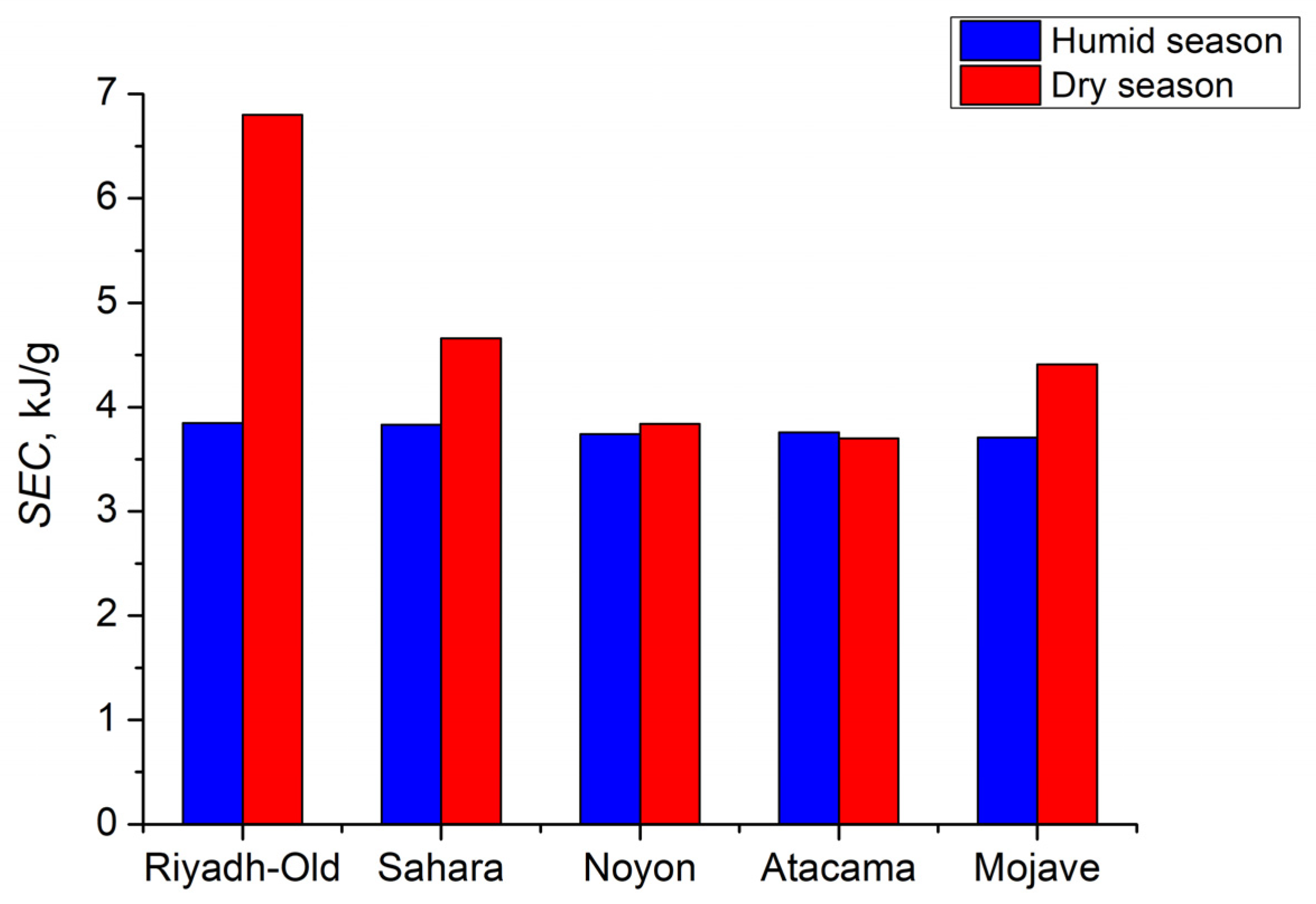
3.3.3. The Specific Energy Consumption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhuang, S.; Qi, H.; Wang, X.; Li, X.; Liu, K.; Liu, J.; Zhang, H. Advances in Solar-Driven Hygroscopic Water Harvesting. Glob. Chall. 2021, 5, 2000085. [Google Scholar] [CrossRef]
- Arnell, N.W. Climate change and global water resources: SRES emissions and socio-economic scenarios. Glob. Environ. Chang. 2004, 14, 31–52. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; Green, P.; Salisbury, J.; Lammers, R.B. Global water resources: Vulnerability from climate change and population growth. Science 2000, 289, 284–288. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Diercks, C.S.; Yaghi, O.M. Metal–Organic Frameworks for Water Harvesting from Air. Adv. Mater. 2018, 30, 1–26. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Distillation vs. membrane filtration: Overview of process evolutions in seawater desalination. Desalination 2002, 143, 207–218. [Google Scholar] [CrossRef]
- Beysens, D.; Milimouk, I. The case for alternative fresh water sources. Sécheresse 2000, 11, 11–16. [Google Scholar]
- Tu, Y.D.; Wang, R.Z.; Zhang, Y.N.; Wang, J.Y. Progress and expectation of atmospheric water harvesting. Joule 2018, 2, 1452–1475. [Google Scholar] [CrossRef]
- Al-Farayedhi, A.A.; Ibrahim, N.I.; Gandhidasan, P. Condensate as a water source from vapor compression systems in hot and humid regions. Desalination 2014, 349, 60–67. [Google Scholar] [CrossRef]
- Alayli, Y.; Hadji, N.E.; Leblond, J. A new process for extraction of water from air. Desalination 1987, 67, 227–229. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Tokarev, M.M.; Parmon, B.N.; Aristov, Y.I. Selective water sorbents for multiple applications: 6. Fresh water production from the atmosphere. React. Kinet. Catal. Lett. 1998, 65, 153–160. [Google Scholar] [CrossRef]
- Talaat, M.A.; Awad, M.M.; Zeidan, E.B.; Hamed, A.M. Solar-powered portable apparatus for extracting water from air using desiccant solution. Renew. Energy 2018, 119, 662–674. [Google Scholar] [CrossRef]
- Hamed, A.M. Absorption-regeneration cycle for production of water from air-theoretical approach. Renew. Energy 2000, 19, 625–635. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Solovieva, M.V.; Aristov, Y.I. Potable water extraction from the atmosphere: Potential of MOFs. Renew. Energy 2020, 148, 72–80. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Tokarev, M.M.; Gordeeva, L.G.; Snytnikov, V.N.; Parmon, V.N. New composite sorbents for solar-driven technology of fresh water production from the atmosphere. Sol. Energy 1999, 66, 165–168. [Google Scholar] [CrossRef]
- Arakawa, H. Climates of Northern and Eastern Asia. World Surv. Climatol. 1969, 8, 1–248. [Google Scholar]
- Takahashi, K.; Arakawa, H. Climates of Southern and Western Asia. World Surv. Climatol. 1981, 9, 1–333. [Google Scholar]
- Griffiths, J.F. Climates of Africa. World Surv. Climatol. 1972, 10, 1–604. [Google Scholar]
- Gentilli, J. Climates of Australia and New Zealand. World Surv. Climatol. 1971, 9, 1–405. [Google Scholar]
- Groth, W.; Hussmann, P. Process and system for recovering water from the atmosphere. U.S. Patent 4 146 372, 27 March 1979. [Google Scholar]
- Bennett, C.E. Heat energized vapor adsorbent pump. U.S. Patent 4,377,398, 22 March 1983. [Google Scholar]
- LaPotin, A.; Zhong, Y.; Zhang, L.; Zhao, L.; Leroy, A.; Kim, H.; Rao, S.R.; Wang, E.N. Dual-Stage Atmospheric Water Harvesting Device for Scalable Solar-Driven Water Production. Joule 2021, 5, 166–182. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A. Experimental investigation of solar powered water production from atmospheric air by using composite desiccant material “CaCl2/saw wood”. Desalination 2015, 367, 216–222. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, J.Y.; Wang, R.Z.; Wang, L.W. Experimental investigation on two solar-driven sorption based devices to extract fresh water from atmosphere. Appl. Therm. Eng. 2017, 127, 1608–1616. [Google Scholar] [CrossRef]
- Shimooka, S.; Oshima, K.; Hidaka, H.; Takewaki, T.; Kakkiuchi, H.; Kodama, A.; Kubota, M.; Matsuda, H. The evaluation of direct cooling and heating desiccant devices coated with FAM. J. Chem. Eng. Jpn. 2007, 40, 1330–1334. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Aristov, Y.I. Composites “salt inside porous matrix” for adsorption heat transformation: A current state of the art and new trends. Int. J. Low-Carbon Technol. 2012, 7, 288–302. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwing, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasne, B.; Farrusseng, D. Water adsorption in MOFs: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef]
- De Lange, M.F.; Verouden, K.J.F.M.; Vlugt, T.J.H.; Gascon, J.; Kapteijn, F. Adsorption driven heat pumps—The potential of Metal-Organic Frameworks. Langmuir 2015, 31, 12783–12796. [Google Scholar] [CrossRef]
- Song, D.; Bae, J.; Ji, H.; Kim, M.-B.; Bae, Y.-S.; Park, K.S.; Moon, D.; Jeong, N.C. Coordinative Reduction of Metal Nodes Enhances the Hydrolytic Stability of a Paddlewheel Metal−Organic Framework. J. Am. Chem. Soc. 2019, 141, 7853–7864. [Google Scholar] [CrossRef]
- Bae, J.; Lee, C.Y.; Jeong, N.C. Weak Coordination Bond of Chloromethane: A Unique Way to Activate Metal Node Within an Unstable Metal–Organic Framework DUT-34. Korean Chem. Soc. 2021, 42, 658–666. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-K.; Yoon, J.W.; Lee, J.S.; Hwang, Y.K.; Jun, C.-H.; Chang, J.-S.; Wuttkee, S.; Bazin, P.; Vimont, M.; Daturi, M.; et al. Energy-efficient dehumidification over hierarchically porous metal-organic frameworks as advanced water adsorbents. Adv. Mater. 2012, 24, 806–810. [Google Scholar] [CrossRef] [PubMed]
- LaPotin, A.; Kim, H.; Rao, S.R.; Wang, E.N. Adsorption-based atmospheric water harveisting: Impact of material and component properties on system-level performance. Acc. Chem. Res. 2019, 52, 1588–1597. [Google Scholar] [CrossRef]
- Trapani, F.; Polyzoidis, A.; Loebbecke, S.; Piscopo, C.G. On the general water harvesting capability of metal-organic frameworks under well-defined climatic conditions. Microporous Mesoporous Mater. 2016, 230, 20–24. [Google Scholar] [CrossRef]
- Kim, S.-I.; Yoon, T.-U.; Kim, M.-B.; Lee, S.-J.; Hwang, Y.K.; Chang, J.-S.; Kim, H.-J.; Lee, H.-N.; Lee, U.-H.; Bae, Y.-S. Metal–organic frameworks with high working capacities and cyclic hydrothermal stabilities for fresh water production. Chem. Eng. J. 2016, 286, 467–475. [Google Scholar] [CrossRef]
- Rieth, A.J.; Yang, S.; Wang, E.N.; Dincǎ, M. Record Atmospheric Fresh Water Capture and Heat Transfer with a Material Operating at the Water Uptake Reversibility Limit. ACS Cent. Sci. 2017, 3, 668–672. [Google Scholar] [CrossRef]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef]
- Hanikel, N.; Prevot, M.S.; Fathieh, F.; Kapustin, E.A.; Lyu, H.; Wang, H.; Diercks, N.J.; Glover, T.G.; Yaghi, O.M. Rapid cycling and exceptional yield in a metal-organic framework water harvester. ACS Cent. Sci. 2019, 5, 1699–1706. [Google Scholar] [CrossRef]
- Silva, M.P.; Ribeiro, A.M.; Silva, C.G.; Nogueira, I.B.R.; Cho, K.-H.; Lee, U.-H.; Faria, J.L.; Loureiro, J.L.; Chang, J.-S.; Rodrigues, A.E.; et al. MIL-160(Al) MOF’s potential in adsorptive water harvesting. Adsorption 2021, 27, 213–226. [Google Scholar] [CrossRef]
- Cadiau, A.; Lee, J.S.; Borges, D.D.; Fabry, P.; Devic, T.; Wharmby, M.T.; Martineau, C.; Foucher, D.; Taulelle, F.; Jun, C.-H.; et al. Design of Hydrophilic Metal-Organic Framework Water Adsorbents for Heat Reallocation. Adv. Mater. 2015, 27, 4775–4780. [Google Scholar] [CrossRef]
- Permyakova, A.; Skrylnyk, O.; Courbon, E.; Affram, M.; Wang, S.; Lee, U.-H.; Valekar, A.H.; Nouar, F.; Mouchaham, G.; Devic, T.; et al. Synthesis Optimization, Shaping, and Heat Reallocation Evaluation of the Hydrophilic Metal–Organic Framework MIL-160(Al). ChemSusChem 2017, 10, 1419–1426. [Google Scholar] [CrossRef]
- Wahiduzzaman, M.; Lenzen, D.; Maurin, G.; Stock, N.; Wharmby, M.T. Rietveld refinement of MIL-160 and its structural flexibility upon H2O and N2 adsorption. Eur. J. Inorg. Chem. 2018, 32, 3626–3632. [Google Scholar] [CrossRef]
- Liu, Q.; Chapman, J.; Huang, A.; Williams, K.C.; Wagner, A.; Garapati, N.; Sierros, K.A.; Dinu, C.Z. User-Tailored Metal–Organic Frameworks as Supports for Carbonic Anhydrase. ACS Appl. Mater. Interfaces 2018, 10, 41326–41337. [Google Scholar] [CrossRef]
- Polanyi, M. Theories of the adsorption of gas. Trans. Faraday Soc. 1932, 28, 316–333. [Google Scholar] [CrossRef]
- Food and Agriculture Organisation of the United Nations. Available online: http://www.fao.org/fileadmin/user_upload/newsroom/docs/full-map.png (accessed on 2 April 2021).
- Bar, E. Extraction of water from air—An alternative solution for water supply. Desalination 2004, 165, 335. [Google Scholar] [CrossRef]
- Habeebullah, B.A. Potential use of evaporator coils for water extraction in hot and humid areas. Desalination 2009, 237, 330–345. [Google Scholar] [CrossRef]
- Cui, S.; Marandi, A.; Lebourleux, G.; Thimon, M.; Bourdon, M.; Chen, C.; Severino, M.I.; Steggles, V.; Nouar, F.; Serre, C. Heat properties of a hydrophilic carboxylate-based MOF for water adsorption applications. Appl. Therm. Eng. 2019, 161, 114135. [Google Scholar] [CrossRef]
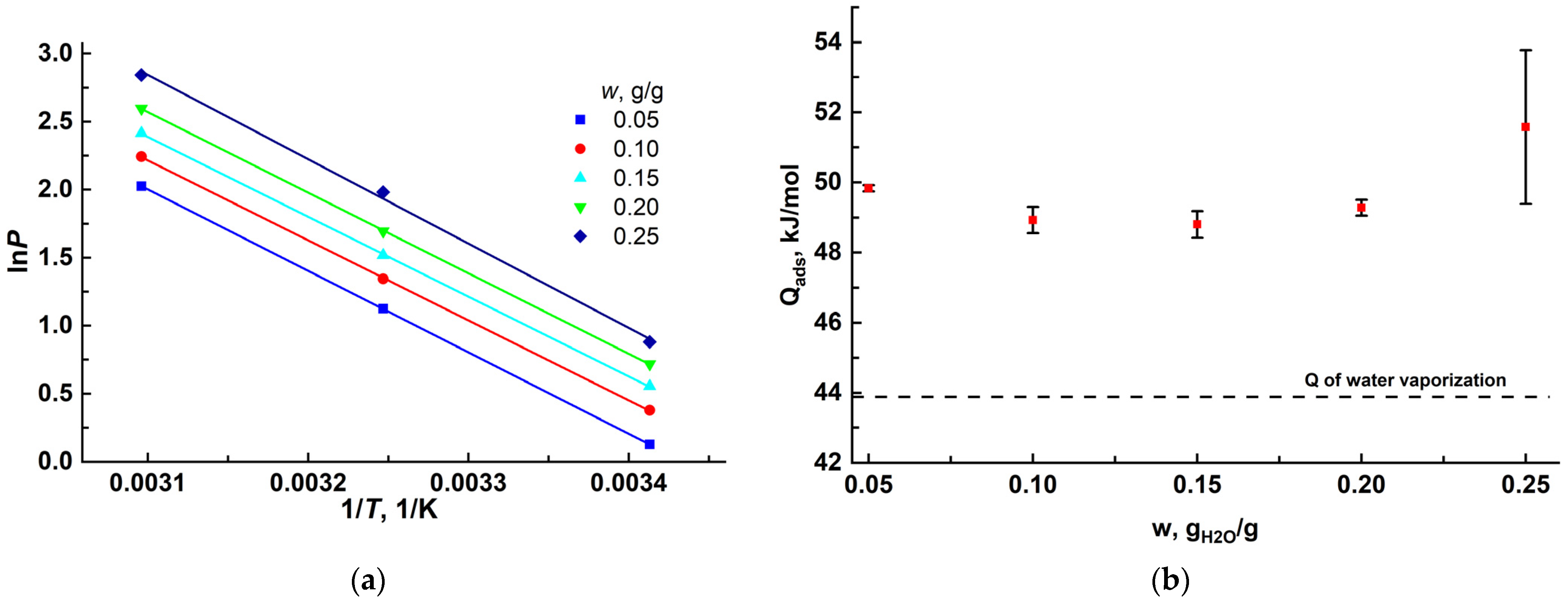
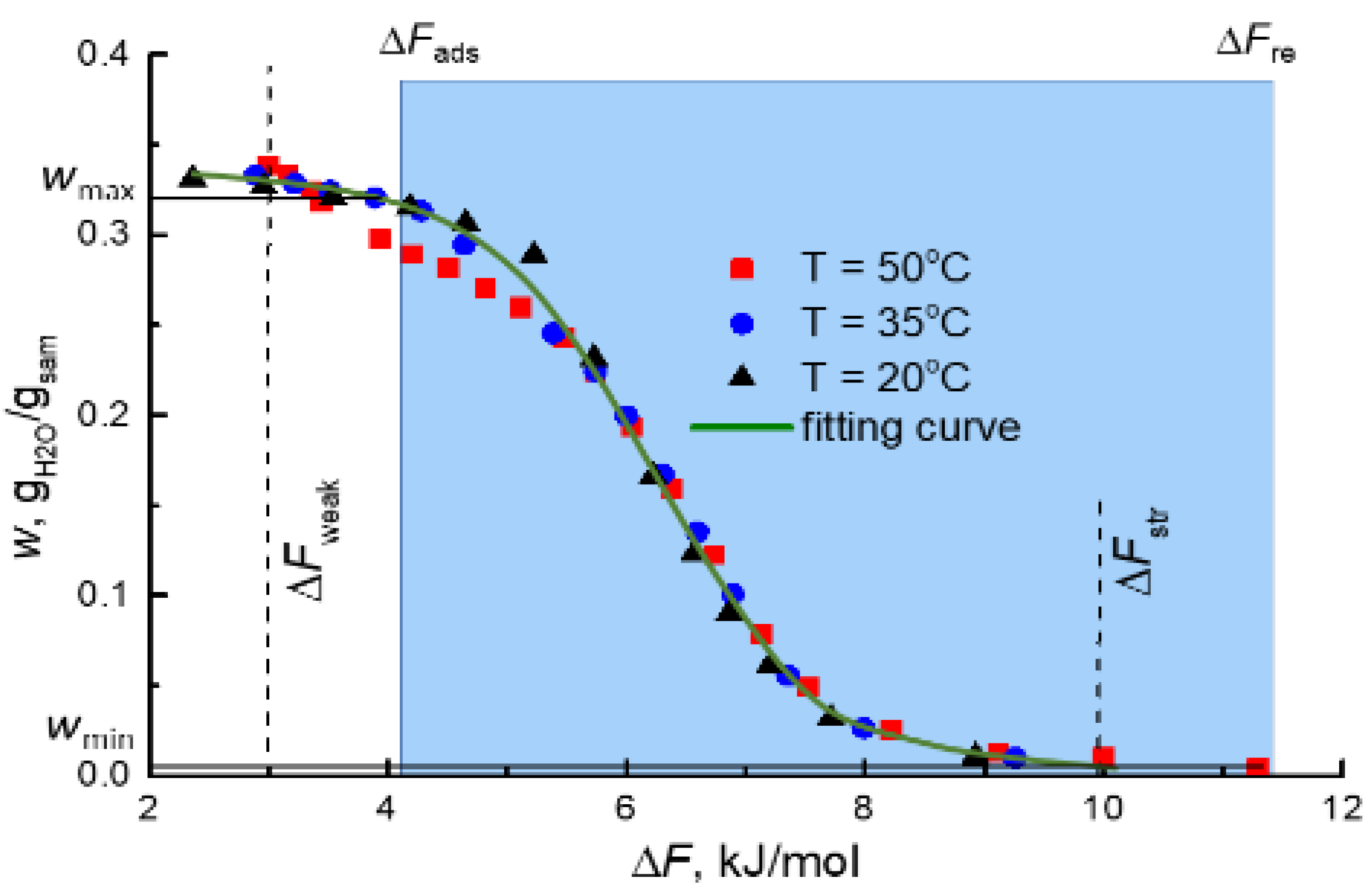
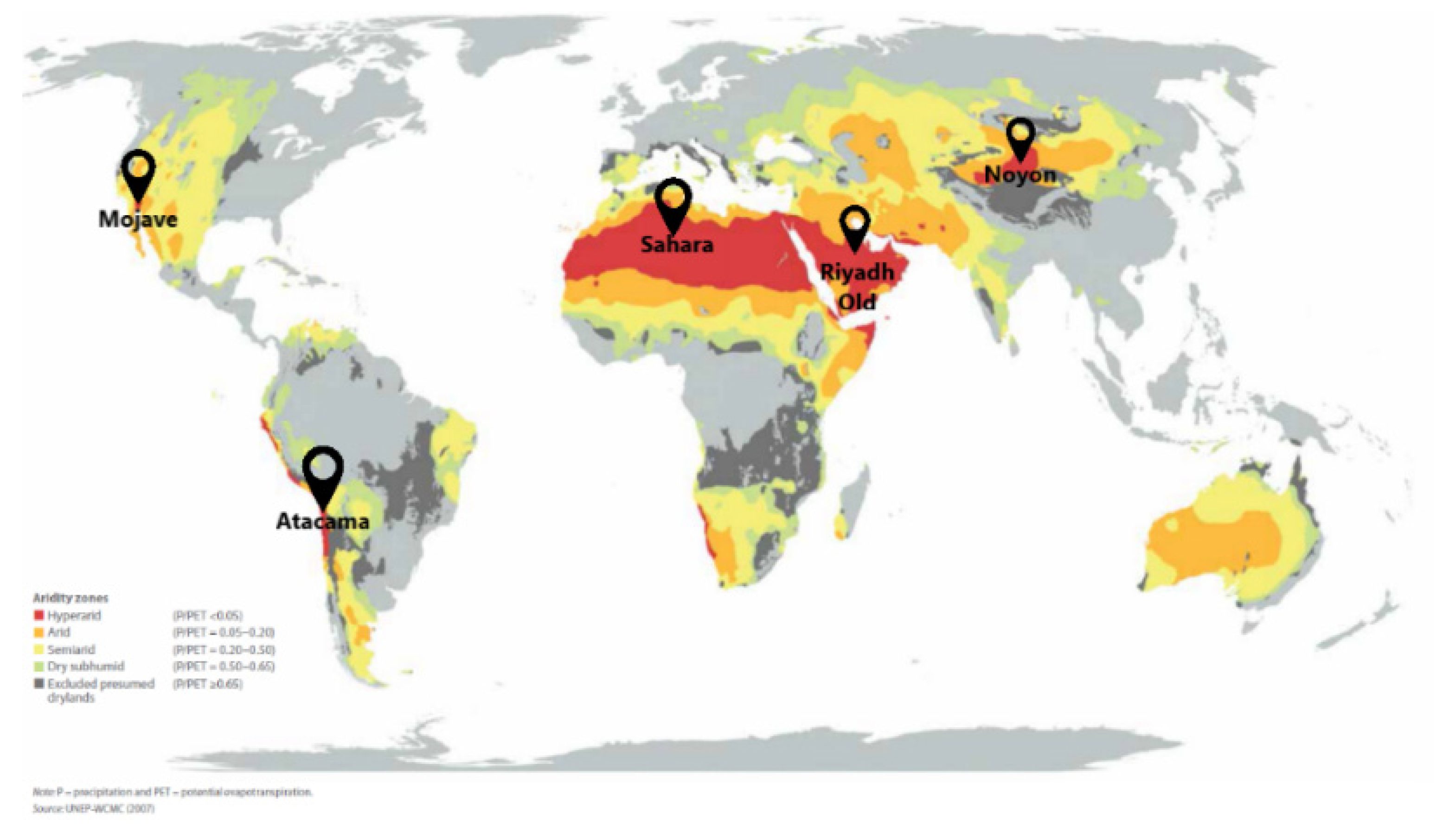

| Region | Tn, °C | RHn, % | Td, °C | RHd, % | Pam, mbar | ΔFad, kJ/mol | ΔFre, kJ/mol (Tre = 80 °C) |
|---|---|---|---|---|---|---|---|
| January | |||||||
| Riyadh-Old | 12.5 | 55.9 | 16.3 | 45.1 | 8.3 | 1.3 | 11.9 |
| Sahara | 11.1 | 33.0 | 14.9 | 27.2 | 4.5 | 2.5 | 13.7 |
| Noyon | −13.0 | 55.6 | −9.1 | 41.7 | 1.3 | 1.3 | 17.4 |
| Atacama | 5.3 | 73.0 | 9.4 | 55.5 | 6.5 | 0.7 | 12.6 |
| Mojave | 3.0 | 26.3 | 7.6 | 17.9 | 6.8 | 1.5 | 14.1 |
| June/July | |||||||
| Riyadh-Old | 32.6 | 20.2 | 36.7 | 16.5 | 10.1 | 4.1 | 11.3 |
| Sahara | 26.9 | 25.8 | 30.4 | 21.2 | 9.3 | 3.4 | 11.5 |
| Noyon | 8.6 | 25.8 | 13.7 | 16.4 | 2.9 | 3.2 | 15.0 |
| Atacama | −1.7 | 33.1 | 5.1 | 20.0 | 1.8 | 2.5 | 16.4 |
| Mojave | 21.6 | 26.3 | 27.5 | 17.9 | 6.9 | 3.3 | 12.6 |
| Tcon = Td = 33.1 °C | Tcon = 20 °C | Tcon = 10 °C |
|---|---|---|
| 6.8 | 4.1 | 3.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solovyeva, M.; Krivosheeva, I.; Gordeeva, L.; Aristov, Y. MIL-160 as an Adsorbent for Atmospheric Water Harvesting. Energies 2021, 14, 3586. https://doi.org/10.3390/en14123586
Solovyeva M, Krivosheeva I, Gordeeva L, Aristov Y. MIL-160 as an Adsorbent for Atmospheric Water Harvesting. Energies. 2021; 14(12):3586. https://doi.org/10.3390/en14123586
Chicago/Turabian StyleSolovyeva, Marina, Irina Krivosheeva, Larisa Gordeeva, and Yuri Aristov. 2021. "MIL-160 as an Adsorbent for Atmospheric Water Harvesting" Energies 14, no. 12: 3586. https://doi.org/10.3390/en14123586
APA StyleSolovyeva, M., Krivosheeva, I., Gordeeva, L., & Aristov, Y. (2021). MIL-160 as an Adsorbent for Atmospheric Water Harvesting. Energies, 14(12), 3586. https://doi.org/10.3390/en14123586






