Use of Bioluminescence for Monitoring Brown Coal Mine Waters from Deep and Surface Drainage
Abstract
1. Introduction
2. Materials and Methods
2.1. The Sampling of the Brown Coal Mine Waters
2.2. Physicochemical Analyses
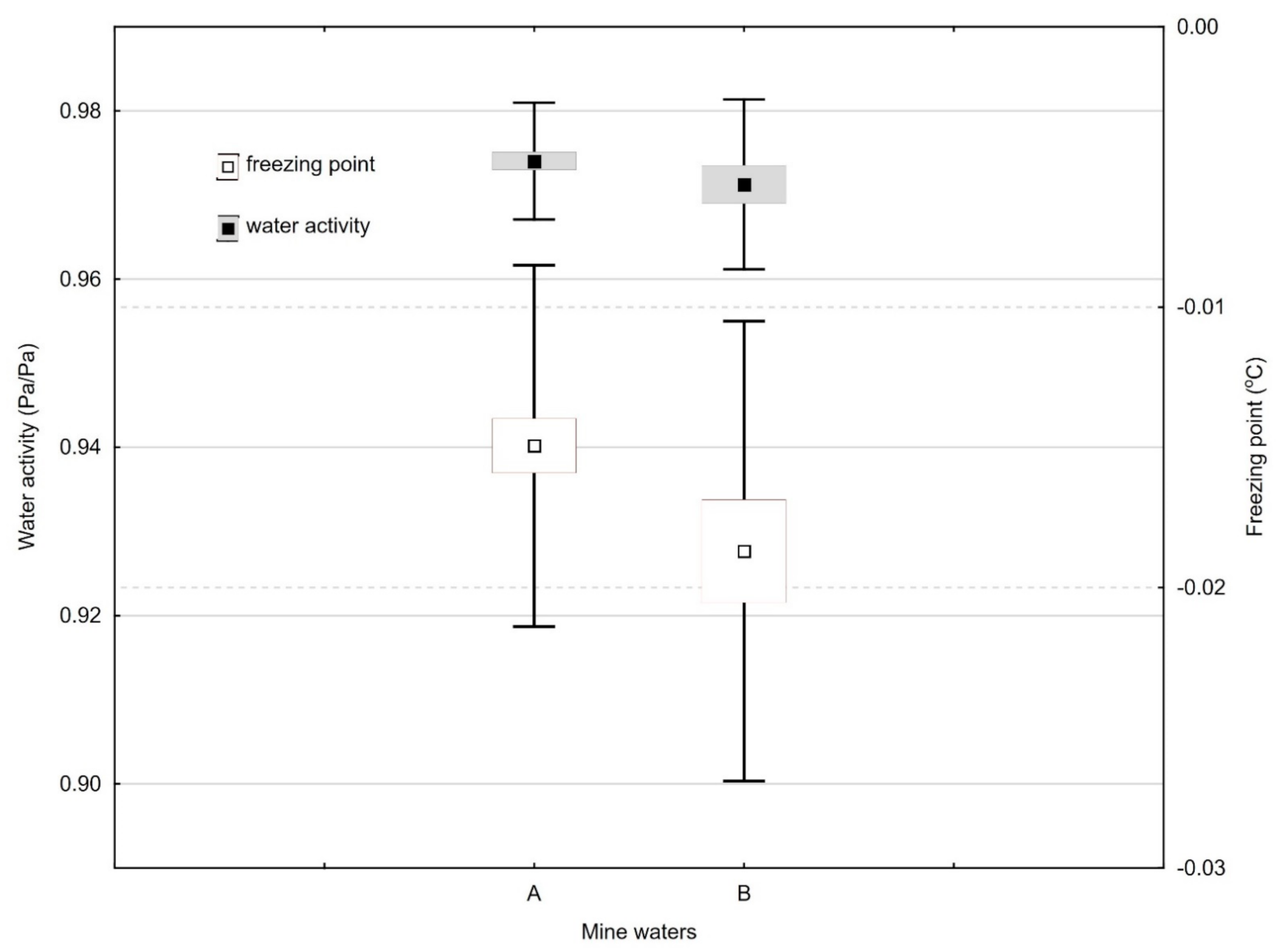
2.3. Water Activity
2.4. Freezing Point
2.5. Bioluminescence
2.6. Color
2.7. Statistical Evaluation
3. Results
3.1. Basic Chemical Composition and Selected Quality Characteristics
3.2. Color Parameters, Color and Whiteness Indices
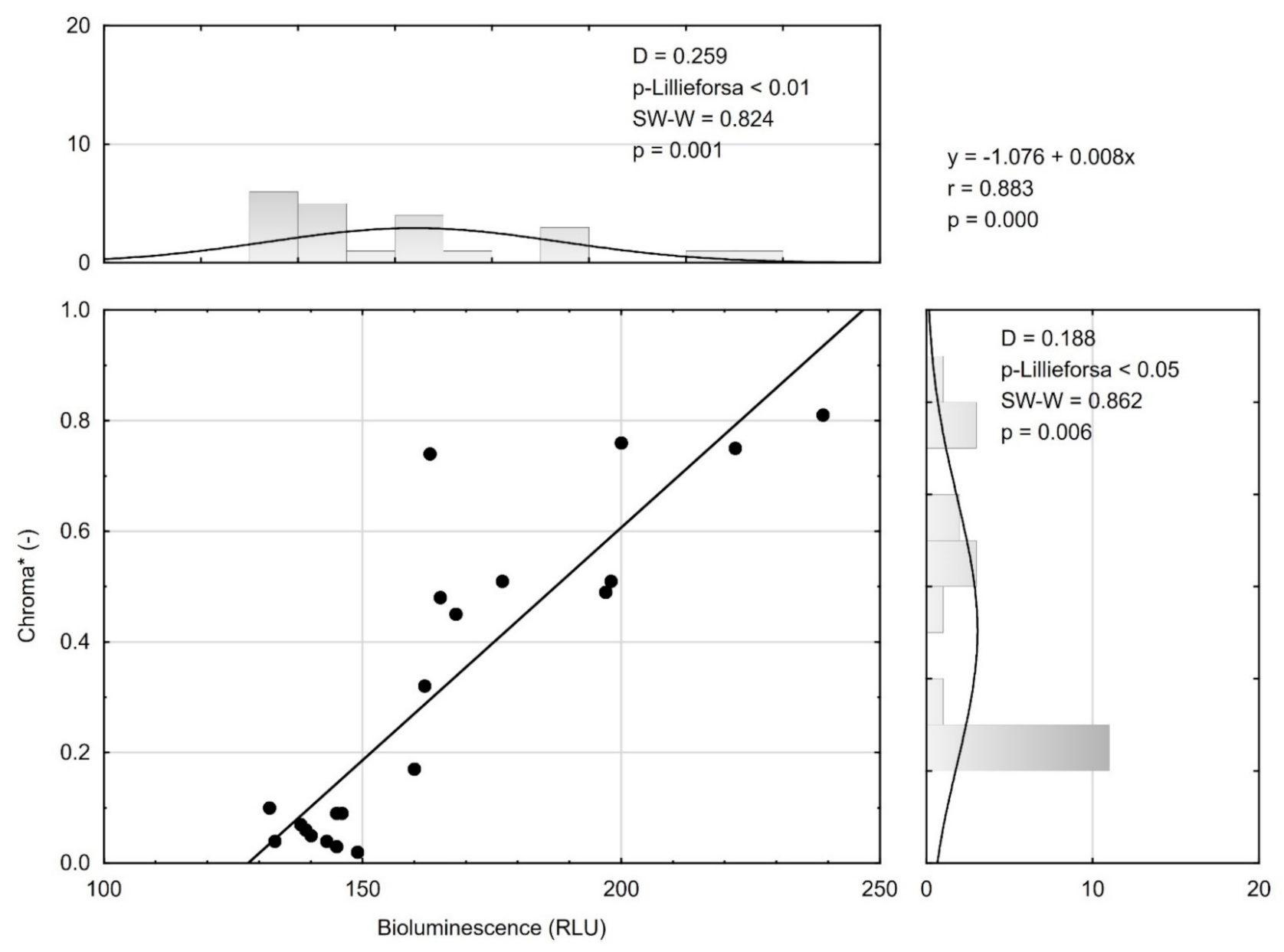
3.3. Bioluminescence and Correlation with Selected Quality Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eurocoal. Coal in Europe. 2019. Available online: https://euracoal.eu/info/euracoal-eu-statistics/ (accessed on 30 October 2020).
- Wachowiak, G.; Galiniak, G.; Jończyk, W.; Martyniuk, R. Evaluation of outflow changes in the Widawka river basin in the hydrological year 2010 under the impact of a mining and energetics project in the Bełchatów region. Górnictwo i Geoinżynieria 2011, 35, 381–395. (In Polish) [Google Scholar]
- Pepliński, B.; Czubak, W. The influence of opencast lignite mining dehydration on plant production—a methodological study. Energies 2021, 14, 1917. [Google Scholar] [CrossRef]
- Domska, D.; Raczkowski, M. Effect of strip mine activity on changes of some physicochemical properties of soil. Acta Agroph. 2008, 12, 73–77. [Google Scholar]
- Staniszewski, R. Heavy metals in waters and sediments of rivers affected by brown coal mine waters. Pol. J. Environ. Stud. 2014, 23, 2217–2222. [Google Scholar] [CrossRef]
- Hildmann, E.; Wonsche, M. Lignite mining and its after-effects on the Central German landscape. Water Air Soil Pollut. 1996, 91, 79–87. [Google Scholar] [CrossRef]
- Kavouridis, K. Lignite industry in Greece within a world context: Mining, energy supply and environment. Energy Policy 2008, 36, 1257–1272. [Google Scholar] [CrossRef]
- Hancock, S.; Wolkersdorfer, C. Renewed demands for mine water management. Mine Water Environ. 2012, 31, 147–158. [Google Scholar] [CrossRef]
- Luo, H.; Zhou, W.; Jiskani, I.M.; Wang, Z. Analyzing characteristics of particulate matter pollution in open-pit coal mines: Implications for Green Mining. Energies 2021, 14, 2680. [Google Scholar] [CrossRef]
- Friese, K.; Hupfer, M.; Schultze, M. Chemical characteristics of water and sediment in acid mining lakes of the lusatian lignite district. In Acidic Mining Lakes; Environmental Science; Geller, W., Klapper, H., Salomons, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar] [CrossRef]
- Hammes, F.; Goldschmidt, F.; Vital, M.; Wang, Y.; Egli, T. Measurement and interpretation of microbial adenosine tri-phosphate (ATP) in aquatic environments. Water Res. 2010, 44, 3915–3923. [Google Scholar] [CrossRef]
- Yang, N.C.; Ho, W.M.; Chen, Y.H.; Hu, M.L. A convenient one-step extraction of cellular ATP using boiling water for the luciferin–luciferase assay of ATP. Biochem. Anal. 2002, 306, 323–327. [Google Scholar] [CrossRef]
- Cais-Sokolińska, D.; Pikul, J.; Wójtowski, J. Use of bioluminescence in the assessment of the degree of cleanliness of milk tanks in goat milk processing plants. Arch. Lebensmittelhyg. 2010, 61, 75–79. [Google Scholar] [CrossRef]
- Cais-Sokolińska, D.; Wójtowski, J.; Pikul, J.; Dobrzańska, A. An attempt at the specification of common cleanliness limits for abiotic surfaces in dairy processing plants based on ATP bioluminescence. Arch. Lebensmittelhyg. 2016, 67, 12–16. [Google Scholar] [CrossRef]
- Montanez, G.; Passman, F.; Machtiger, N.; Montemayor, R.; Whalen, P. Bioluminescence testing for rapid detection of microbial contamination in aqueous polymer emulsions. Int. Biodeterior. Biodegradation 2016, 114, 216–221. [Google Scholar] [CrossRef]
- Deininger, R.A.; Lee, J. Rapid determination of bacteria in drinking water using an ATP assay. Field Anal. Chem. Technol. 2001, 5, 185–189. [Google Scholar] [CrossRef]
- European Commission. Technical Background Document on Identification of Mixing Zones, EU Common Implementation Strategy Document “Guidelines for the Identification of Mixing Zones under the EQS Directive (2008/105/EC); European Commission: Brussels, Belgium, 2010; p. 34. [Google Scholar]
- Kaszyński, P.; Kamiński, J. Coal demand and environmental regulations: A case study of the Polish power sector. Energies 2020, 13, 1521. [Google Scholar] [CrossRef]
- HACH Company. Hach Methods Approved/Accepted by the USEPA, Colorado, USA. 1999, p. 12. Available online: https://www.hach.com/cms/documents/pdf/EPA (accessed on 30 October 2020).
- HACH. Water Analysis Guide. DOC316.53.01336. 2013. Available online: https://www.hach.com/WAH (accessed on 30 October 2020).
- Staniszewski, R.; Szoszkiewicz, K.; Zbierska, J.; Leśny, J.; Jusik, S.; Clarke, R.T. Assessment of sources of uncertainty in macrophyte surveys and the consequences for river classification. Hydrobiologia 2006, 566, 235–246. [Google Scholar] [CrossRef]
- Lewicki, P.P. Water as the determinant of food engineering properties. A review. J. Food Eng. 2004, 61, 483–495. [Google Scholar] [CrossRef]
- ISO. Milk-Determination of Freezing Point—Thermistor Cryoscope Method Reference Method; ISO 5764, IDF bulletin N° 42; International Dairy Federation: Brussels, Belgium, 2009. [Google Scholar]
- Regulation of the Minister of Maritime Economy and Inland Navigation of July 12, 2019 on substances particularly harmful to the aquatic environment and the conditions that must be met when discharging sewage into waters or soil, as well as draining rainwater or snowmelt to water or water devices. J. Laws 2019, 1311, 1–48. (In Polish)
- Ming, F.; Chen, L.; Li, D.; Du, C. Investigation into Freezing Point Depression in Soil Caused by NaCl Solution. Water 2020, 12, 2232. [Google Scholar] [CrossRef]
- Martuzzi, F.; Summer, A.; Formaggioni, P.; Mariani, P. Milk of Italian Saddle and Haflinger nursing mares: Physico–chemical characteristics, nitrogen composition and mineral elements at the end of lactation. Ital. J. Anim. Sci. 2004, 3, 293–299. [Google Scholar] [CrossRef]
- Navratilova, P.; Janstova, B.; Glossova, P.; Vorlova, L. Freezing point of heat-treated drinking milk in the Czech Republic. Czech J. Food Sci. 2006, 24, 156–163. [Google Scholar] [CrossRef]
- Staniszewski, R.; Jusik, S. Impact of mine waters discharge from open-pit lignite mine on river water quality. Rocznik Ochrona Środowiska (Annual Set Environ. Protect.) 2013, 15, 2652–2665. [Google Scholar]
- Watanabe, A.; Tamaki, N.; Yokota, K.; Matsuyama, M.; Kokeguchi, S. Monitoring of bacterial contamination of dental unit water lines using adenosine triphosphate bioluminescence. J. Infect. Hosp. 2016, 94, 393–396. [Google Scholar] [CrossRef]
- Magic-Knezev, A.; Van Der Kooij, D. Optimisation and significance of ATP analysis for measuring active biomass in granular activated carbon filters used in water treatment. Water Res. 2004, 38, 3971–3979. [Google Scholar] [CrossRef]
- Vang, Ó.K.; Corfitzen, C.B.; Smith, C.; Albrechtsen, H.J. Evaluation of ATP measurements to detect microbial ingress by wastewater and surface water in drinking water. Water Res. 2014, 64, 309–320. [Google Scholar] [CrossRef]
- Li, G.Q.; Yu, T.; Wu, Q.Y.; Lu, Y.; Hu, H.Y. Development of an ATP luminescence-based method for assimilable organic carbon determination in reclaimed water. Water Res. 2017, 123, 345–352. [Google Scholar] [CrossRef]
- Okibe, N.; Johnson, D.B. A rapid ATP-based method for determining active microbial populations in mineral leach liquors. Hydrometallurg 2011, 108, 195–198. [Google Scholar] [CrossRef]
- Greenstein, K.E.; Wert, E.C. Using rapid quantification of adenosine triphosphate (ATP) as an indicator for early detection and treatment of cyanobacterial blooms. Water Res. 2019, 154, 171–179. [Google Scholar] [CrossRef]
- Zamyadi, A.; Choo, F.; Newcombe, G.; Stuetz, R.; Henderson, R.K. A review of monitoring technologies for real-time management of cyanobacteria: Recent advances and future direction. Trac. Trends Anal. Chem. 2016, 85, 83–96. [Google Scholar] [CrossRef]
- Korak, J.A.; Wert, E.C.; Rosario-Ortiz, F.L. Evaluating fluorescence spectroscopy as a tool to characterize cyanobacteria intracellular organic matter upon simulated release and oxidation in natural water. Water Res. 2015, 68, 432–443. [Google Scholar] [CrossRef]
| Parameter | Mine Waters | MSE | |||
|---|---|---|---|---|---|
| A | B | ||||
| Mean | P5–P95 | Mean | P5–P95 | ||
| Nitrates (mg N-NO3/L) | 0.09 b | 0.07–0.09 | 0.01 a | 0.01–0.02 | 0.000 |
| SRP (mg PO4/L) | 0.35 | 0.30–0.39 | 0.30 | 0.09–0.51 | 0.012 |
| TP (mg P/L) | 0.26 | 0.23–0.29 | 0.22 | 0.12–0.33 | 0.004 |
| Alkalinity (mg CaCO3/L) | 304.46 a | 290.02–318.88 | 347.80 b | 336.20–359.39 | 354.540 |
| pH reaction (-) * | 7.96 a | 7.89–8.05 | 8.13 b | 7.86–8.54 | 0.032 |
| Conductivity (mS/cm) | 0.60 a | 0.58–0.63 | 0.69 b | 0.67–0.72 | 0.001 |
| Item | Mine Waters | MSE | |||
|---|---|---|---|---|---|
| A | B | ||||
| Mean | P5–P95 | Mean | P5–P95 | ||
| Platinum-Cobalt Standard | |||||
| Apparent color | 7.55 a | 5.63–9.45 | 39.00 b | 18.56–59.44 | 83.20 |
| Platinum-Cobalt Standard after filtration | |||||
| True color | 4.27 a | 2.65–5.89 | 21.80 b | 20.18–23.42 | 4.642 |
| CIELAB color space | |||||
| L* (%) | 26.01 | 25.65–26.38 | 25.31 | 24.09–26.53 | 0.486 |
| a* | −0.06 | −0.08–−0.05 | −0.04 | −0.08–0.00 | 0.001 |
| b* | −0.54 a | −0.68–−0.40 | −0.03 b | −0.08–0.01 | 0.029 |
| C* | 0.54 b | 0.41–0.68 | 0.06 a | 0.02–0.10 | 0.029 |
| WI (%) | 73.99 | 73.62–74.35 | 74.69 | 73.47–75.91 | 0.487 |
| Quality Parameter (y) | Mine Waters | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | |||||||
| WD | a | b | p | WD | a | b | p | |
| Nitrates (mg N-NO3/L) | 0.57 | −5.53 × 105 | 0.09 | 0.826 | 0.01 | −8.43 × 106 | 0.02 | 0.985 |
| Alkalinity (mg CaCO3/L) | 9.24 | −0.24 | 349.86 | 0.363 | 16.38 | −0.49 | 416.80 | 0.499 |
| pH reaction (-) | 0.92 | −0.01 | 8.05 | 0.779 | 9.50 | 0.01 | 6.66 | 0.614 |
| Conductivity (mS/cm) | 0.05 | 2.80 | 0.60 | 0.947 | 9.44 | −0.01 | 0.78 | 0.615 |
| Apparent color | 5.41 | 0.03 | 2.95 | 0.491 | 2.25 | −0.32 | 84.05 | 0.810 |
| C* | 48.61 | 0.01 | −0.43 | 0.017 | 77.31 | 0.00 | −0.50 | 0.049 |
| aw (Pa/Pa) | 0.02 | 1.85 | 0.97 | 0.967 | 35.88 | 0.00 | 0.92 | 0.268 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staniszewski, R.; Cais-Sokolińska, D.; Kaczyński, Ł.K.; Bielska, P. Use of Bioluminescence for Monitoring Brown Coal Mine Waters from Deep and Surface Drainage. Energies 2021, 14, 3558. https://doi.org/10.3390/en14123558
Staniszewski R, Cais-Sokolińska D, Kaczyński ŁK, Bielska P. Use of Bioluminescence for Monitoring Brown Coal Mine Waters from Deep and Surface Drainage. Energies. 2021; 14(12):3558. https://doi.org/10.3390/en14123558
Chicago/Turabian StyleStaniszewski, Ryszard, Dorota Cais-Sokolińska, Łukasz K. Kaczyński, and Paulina Bielska. 2021. "Use of Bioluminescence for Monitoring Brown Coal Mine Waters from Deep and Surface Drainage" Energies 14, no. 12: 3558. https://doi.org/10.3390/en14123558
APA StyleStaniszewski, R., Cais-Sokolińska, D., Kaczyński, Ł. K., & Bielska, P. (2021). Use of Bioluminescence for Monitoring Brown Coal Mine Waters from Deep and Surface Drainage. Energies, 14(12), 3558. https://doi.org/10.3390/en14123558







