3.1. Adsorption Kinetics
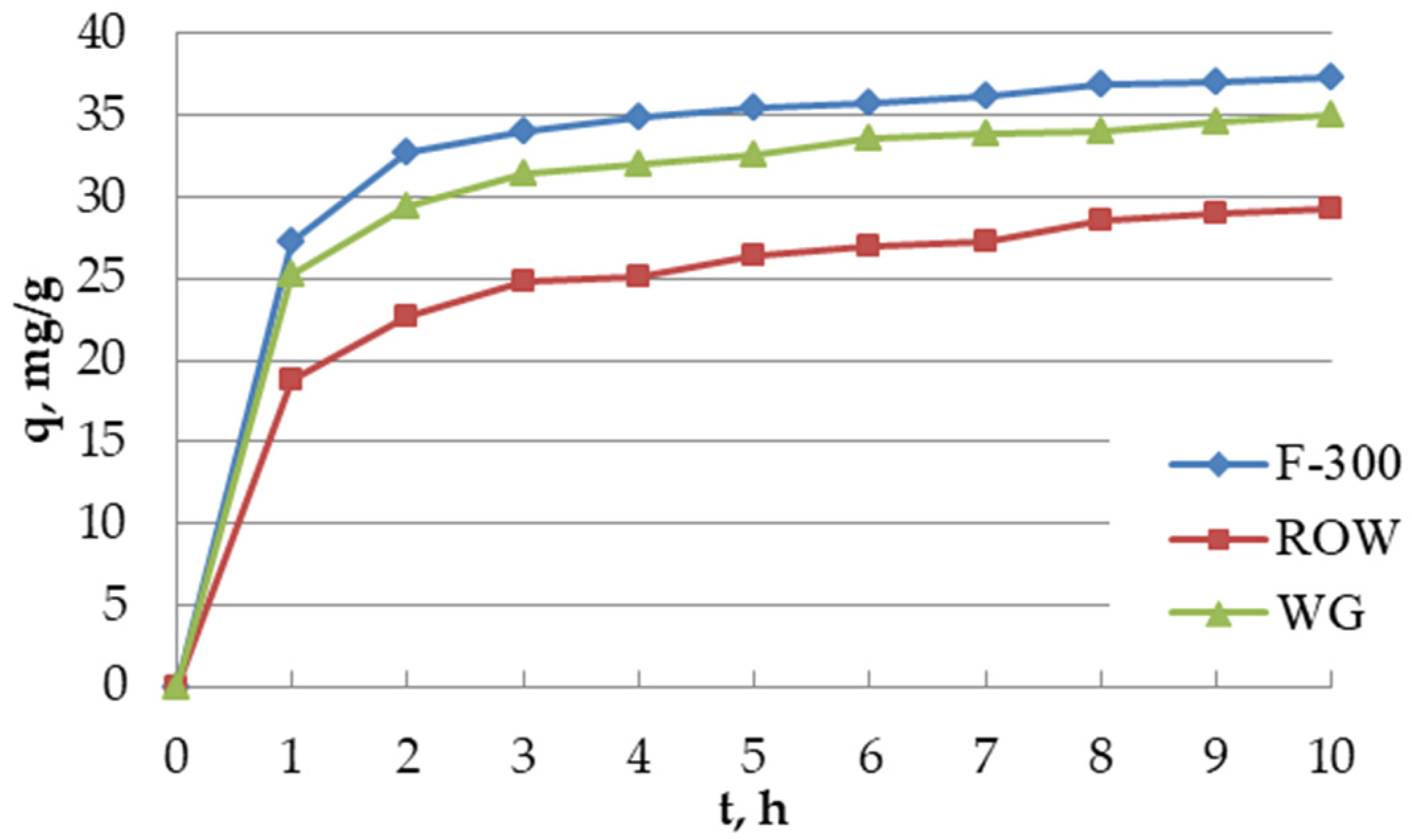
The adsorption kinetics of oxytetracycline on three commercial activated carbons F-300, WG-12 and ROW 08 Supra was analyzed (
Figure 3). The process was conducted with a solution with the oxytetracycline concentration of 100 mg/L, pH = 6, volume = 100 mL, to which 0.2 g of the proper activated carbon was added. The time needed to completely establish the equilibrium on porous sorbents is very long. It was assumed in the study that the time to establish the adsorption equilibrium occurs when the concentration change within 1 h is less than 1% of the initial concentration (i.e., 1 mg/L). It was observed that the adsorption was very fast in the initial stage and then became slower as the adsorption progressed. During the kinetic study, samples of the solutions were taken every 1 h for 10 h. This time was sufficient to establish the adsorption equilibrium on the tested sorbents. For all activated carbons, this time was 8 h. Such similar results are the result of quite a long time between collecting the samples—1 h quite similar porous structure of activated carbons (all carbons are microporous sorbents). The obtained equilibrium time is medium in comparison to the one described in the literature. In the research of Chen et al., concerning the adsorption of tetracycline, the equilibrium time was longer than 200 h while in the work of Yang et al., it was only 2.5 h [
55,
56].
Four models of adsorption kinetics were used to describe the adsorption kinetics: PFO, PSO, intraparticle diffusion model (Weber–Morris), and Elovich (
Table 3 and
Table 6). A very high match of the obtained research results was obtained for the Elovich model (R
2 from 0.9984 to 0.9998) and the intraparticle diffusion model (R
2 from 0.9964 to 0.9993). A good match of the Elovich model would prove the significant importance of chemisorption on the heterogeneous surface of the activated carbon. Copying the experimental values using the Elovich model enabled to estimate the process rate constants α and coefficient β which reflects the number of areas available for adsorption [
57]. Carbons WG-12 and F-300 are characterized by very similar values of coefficient β, higher than the value of this coefficient for carbon ROW 08. Adsorption rate constant α is clearly the highest for activated carbon F-300, and the lowest for ROW 08 Supra.
In the case of the obtained results, a long time to achieve the adsorption equilibrium (approximately 8 h) indicates that internal diffusion is probably dominant over the general adsorption kinetics. Basically, adsorption can be controlled by one or two stages [
58]. By analyzing coefficients of the Weber–Morris equation k′ (intraparticle diffusion rate constant) and b (thickness, layer depth), the effect of the adsorption stages on the adsorption kinetics can be assessed. The greater the b value, the greater the effect of the boundary layer on the adsorption process. If the intraparticle diffusion is the rate limiting factor, then C = 0. For all three activated carbons, quite high C values were obtained, which proves that the factor limiting the reaction rate may be the boundary layer (film) controlled by the diffusion process.
Only slightly lower correlation coefficients (but above 0.93) were obtained for the PSO model. The weakest match was obtained for the PFO model. In many works there are analyzed only two models of kinetics and on this basis the conclusions are drawn. The tetracycline adsorption kinetics (the antibiotics which is the most widely described in the literature from the group of tetracyclines) is the most often better described by means of kinetic equation PSO [
42,
55,
59,
60,
61]., There can also be found better matching results to the kinetic equation PFO than to the second order, although less frequently [
62].
The results obtained during the study of the tetracycline adsorption kinetics equations carried out by Jang and Kan were similar to the results obtained in this article [
63]. The best match was obtained for the Elovich equation, a slightly weaker one for the intraparticle diffusion model, then for PSO and the weakest one for the PFO. The similar results obtained Zhu et al., while examining the adsorption kinetics [
64]. The best matching results were obtained for the Elovich model, next for PSO and the weakest for PFO. Additionally, Sun et al., while examining the kinetics of oxytetracycline on activated carbons made of chemically activated cotton fibers, obtained better results for pseudo-second-order kinetics models than for pseudo-first-order one [
44]. According to Ahmed et al., a higher R
2 of the pseudo-second order kinetic model indicates that chemical adsorption is an important factor deciding about the rate of this process, and the adsorption capacity is proportional to the active areas [
65]. According to Płaziński and Rudziński, the PFO and PSO models do not represent any physical model because they are empirical in nature [
66]. It is therefore wrong to draw conclusions about the nature of the process on their basis.
Based on coefficient k2 characterizing the adsorption rate, activated carbons can be classified as follows: F-300 > WG-12 > ROW08.
3.2. Adsorption Statics—The Effect of the Type of Activated Carbon
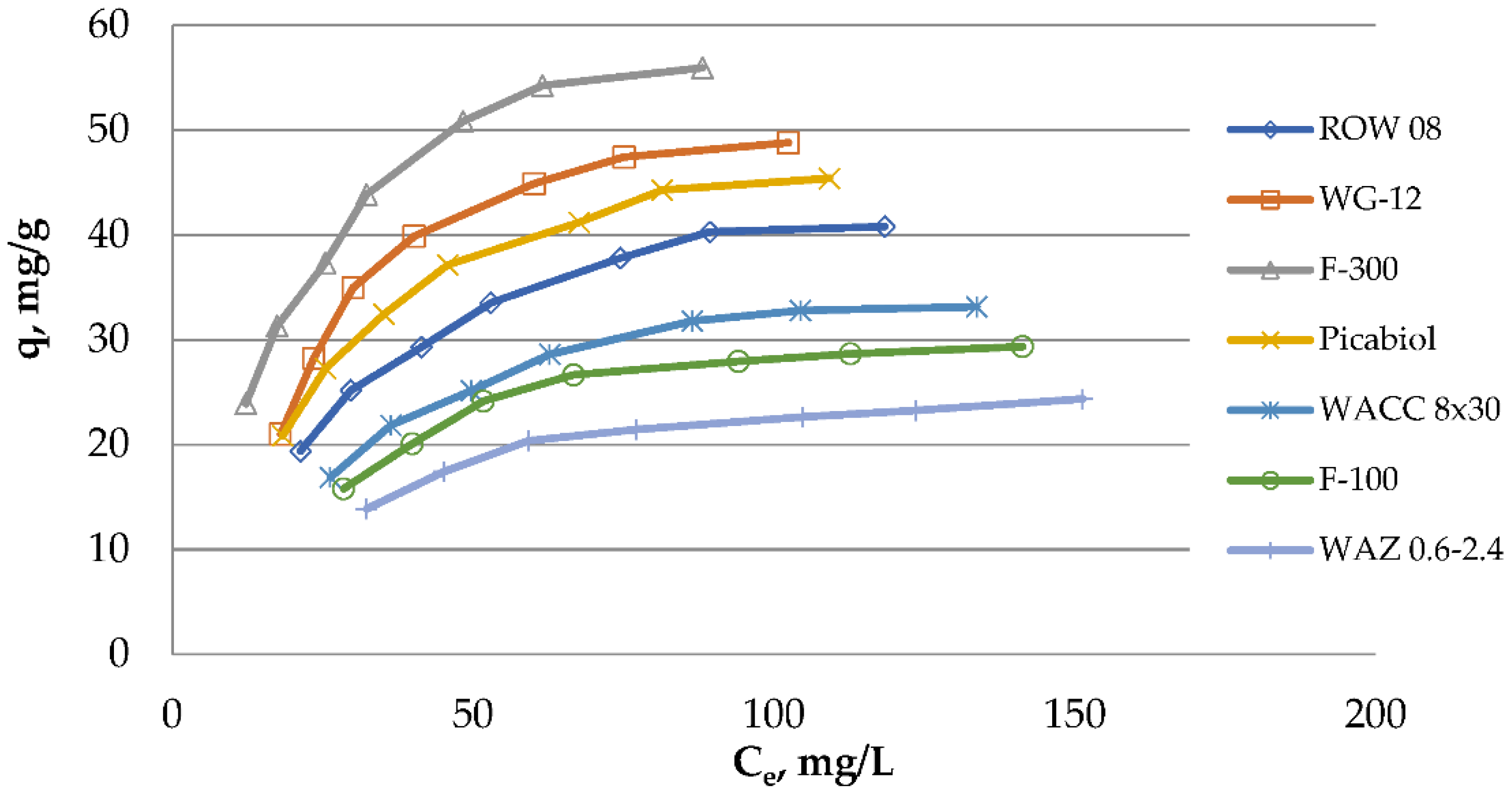
Seven commercial activated carbons used in water treatment plants were assessed. For this purpose, oxytetracycline adsorption isotherms from solutions at room temperature and pH = 6 ± 0.2 were carried out (
Figure 4). The activated carbons used in the studies differ significantly in the amount of oxytetracycline adsorption. For the most effective of them, the activated carbon F-300 (q
m Langmuir isotherm), the adsorption capacity obtained in the studies was 55.9 mg/g. The smallest adsorption capacity 24.3 mg/g had activated carbon WAZ 0.6–2.4. Taking the possibility of removing oxytetracycline into account, the carbons used in the tests can be ranked as follows: F-300 > WG-12 > Picabiol > ROW 08 > WACC 8 × 30 > F-100 > WAZ 0.6–2.4. Such a rank order does not coincide with the rank order of activated carbons due to the size of the specific surface area: Picabiol > WG-12 > ROW 08 > F-300 > WACC 8 × 30 > WAZ 0.6–2.4 > F-100. However, there are some common features between these ranks. The first four activated carbons in the rank order for oxytetracycline adsorption are also in the top four of the rank orders for specific surface areas, but in a slightly different configuration. The specific surface area is therefore an important parameter, but not the only one that determines the amount of oxytetracycline adsorption. Additionally, in the research of Huang et. al., a greater adsorption of oxytetracycline on activated carbon with a higher specific surface area was observed [
67]. The essential influence of the size of the specific surface area on the efficiency of tetracycline adsorption is underlined by many researchers [
59,
68,
69]. Undoubtedly, the porous structure and accessibility, or rather the lack of access to the pores of the smallest diameter, for oxytetracycline particles and the chemical structure of the surface of activated carbons also have the effect. The specific surface area is not always the deciding parameter. Ken et al. while examining three different activated carbons, the greatest oxytetracycline adsorption obtained on the activated carbon which had the smallest specific surface area [
70]. The presence of a great number of functional groups on the surface of activated carbons in the case of adsorption from a solution with pH = 6, may be disadvantageous due to the possibility of blocking access to the pores.
The obtained results were described by two-parameter equations: Freundlich, Langmuir, Temkin, Jovanovic, Halsey and three-parameter equations: Redlich–Peterson and Thot (
Table 4 and
Table 7). The investigators use these models to describe the adsorption of organic compounds, including antibiotics, on activated carbons [
65,
71,
72]. All the used models describe the obtained for oxytetracycline adsorption results well. The lowest R
2 obtained is 0.9917. Nevertheless, the best match of the results was obtained for the following models: Redlich–Peterson, Thot and Jovanovic. These are isotherms used to describe adsorption from aqueous solutions, but these isotherms have different assumptions (e.g., for surface heterogeneity and homogeneity). The Jovanovic isotherm assumes surface homogeneity, the Thoth isotherm—heterogeneity, and the Redlich–Peterson isotherm, as the compilation of the Freundlich and Langmuir isotherms, is applicable to both types of sorbent surfaces. Nevertheless, these different oxytetracycline absorption isotherms used have a similar correlation coefficient R
2. Such results may indicate slight differences in the adsorption potential of the surfaces of the analyzed activated carbons. Other researchers describing their research with different models also obtained similar correlation coefficients, despite their different assumptions [
73,
74,
75]. Most likely, commercial activated carbons have such a diverse surface that it is difficult to talk about a single adsorption mechanism.
The most commonly used isotherms of Langmuir and Freundlich are marked by high (though not the highest) coefficients R
2 (from 0.993 to 0.999). Constant n in the Freundlich equation characterizes the adsorption intensity. For all activated carbons, the values of this coefficient range from 1 to 10, which proves the favorable adsorption of oxytetracycline on all adsorbents. Other authors who examined both the adsorption of oxytetracycline and tetracycline on the different carbon sorbents also claimed that the adsorption is beneficial [
44,
55,
56,
60]. The reciprocal of the coefficient n (1/n) indicates the degree of diversity of active areas on the surface of activated carbon. The obtained values of 1/n (from 0.307 to 0.396) are similar for the tested adsorbents. Since they are closer to zero than to one, it proves that the surface of the activated carbons is not very heterogeneous. Capacitive coefficient K
F ranges from 5.38 (WAZ 0.6–2.4) to 10.62 mg/g (F-300).
Higher correlation coefficients were obtained for the Langmuir isotherm (0.9973 < R
2 < 0.9993). In a great number of scientific studies, the investigators use the description of the tetracycline adsorption from the model of Langmuir and Freundlich. However, on the basis of the literature review, it clearly cannot be claimed which of the aforementioned models describes the tetracycline adsorption in a better way, which is related to the different construction of the activated carbons. In some articles greater R2 was obtained for Langmuir isotherm [
42,
45,
59,
62], and in other ones for Freundlich model [
55,
60,
64,
76]. In order to establish whether the adsorption is favorable, it is necessary to determine distribution coefficient R
L. The ranges of parameter R
L values for all activated carbons fitted in range from 0 to 1 (precisely from 0.833 to 0.971), which means that the adsorption in the tested concentration range was favorable. This is consistent with the conclusions drawn from the analysis of the Freundlich equation. A good match of Langmuir isotherms can indicate the monolayer adsorption of oxytetracycline on active centers. The obtained monolayer capacities which were calculated with the usage of the Langmuir equation range from 29.87 (WAZ) to 73.31 mg/g (F-300).
Slightly lower correlation coefficients were obtained for the Temkin isotherm assuming that the adsorption mechanism is chemisorption, and for the Halsey model assuming the formation of a liquid adsorbate film on mesoporous adsorbents.
3.3. Effect of Process Conditions on Oxytetracycline Adsorption
The influence of the adsorbent dose and pH value of the solution on the amount of oxytetracycline adsorption was analyzed for three of the four most effective commercial adsorbents that are already used in Polish water treatment plants: F-300; WG-12 and ROW 08 Supra. Picabiol activated carbon was not included since it is not used in any water treatment plant in Poland.
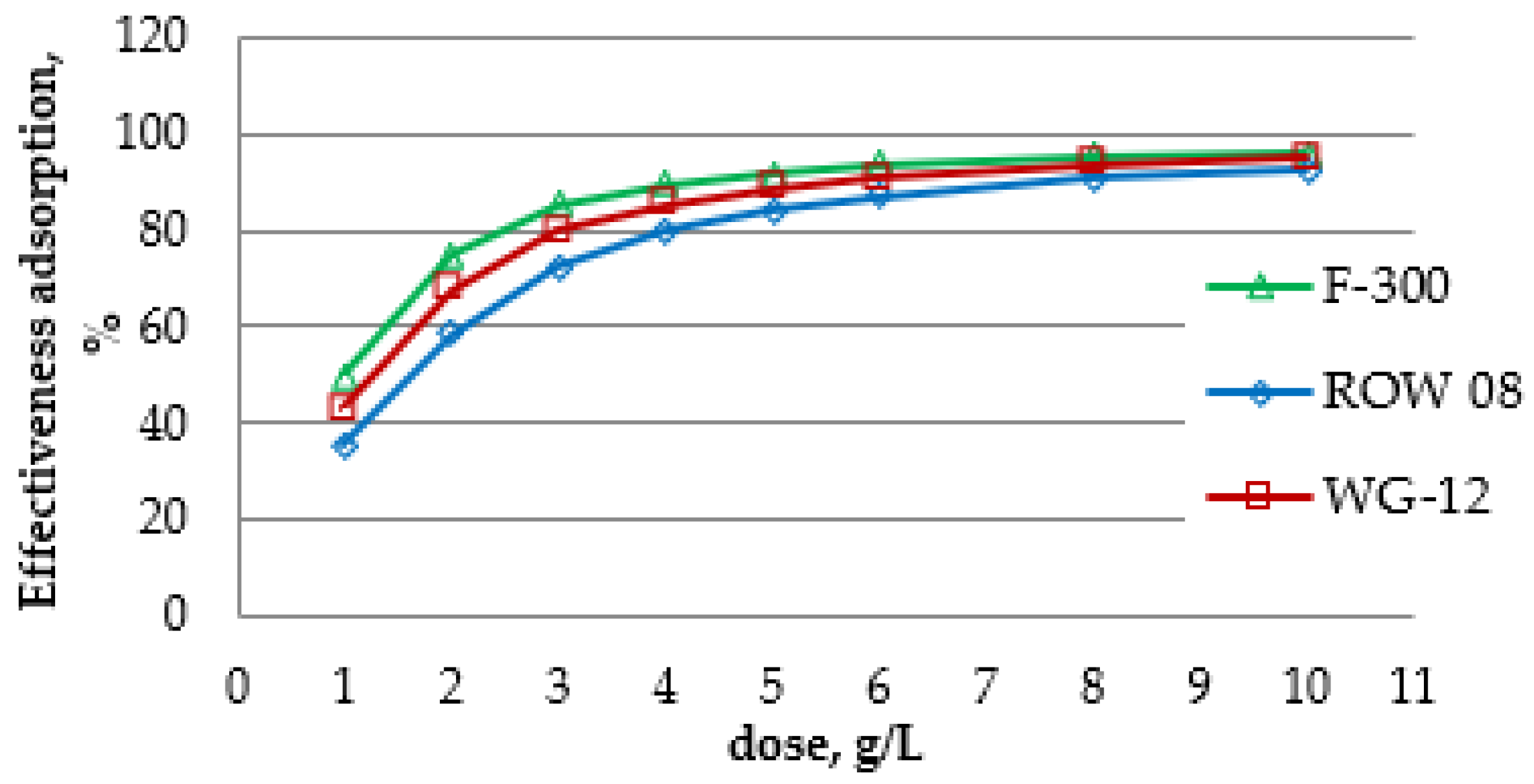
The influence of the adsorbent dose was assessed for the concentration of oxytetracycline 100 mg/L (
Figure 5). The following doses of activated carbon were used: 1, 2, 3, 4, 5, 6, 8 and 10 g/L. Similar doses during the adsorption of various antibiotics were also used by other researchers, e.g., Fen et al., −8 g/L during the adsorption of chloramphenicol or Yazidi et al. 2020 from 0.9 to 14 g/l during the adsorption of amoxicillin and tetracycline [
43,
77]. The amounts of activated carbon F-300 enabled the removal of oxytetracycline from 50% (dose of 1 g/L) to over 96% (dose of 10 g/L), analogously from 43 to almost 95% for activated carbon WG-12 and from 35% to almost 93% for ROW 08 Supra. The differences in the efficiency of tetracycline removal with the dose of 6 and 8 g/L for carbon F-300 are small and amount to less than 2%, and for the other two activated carbons, such small differences were obtained between the doses of 8 and 10 g/L.
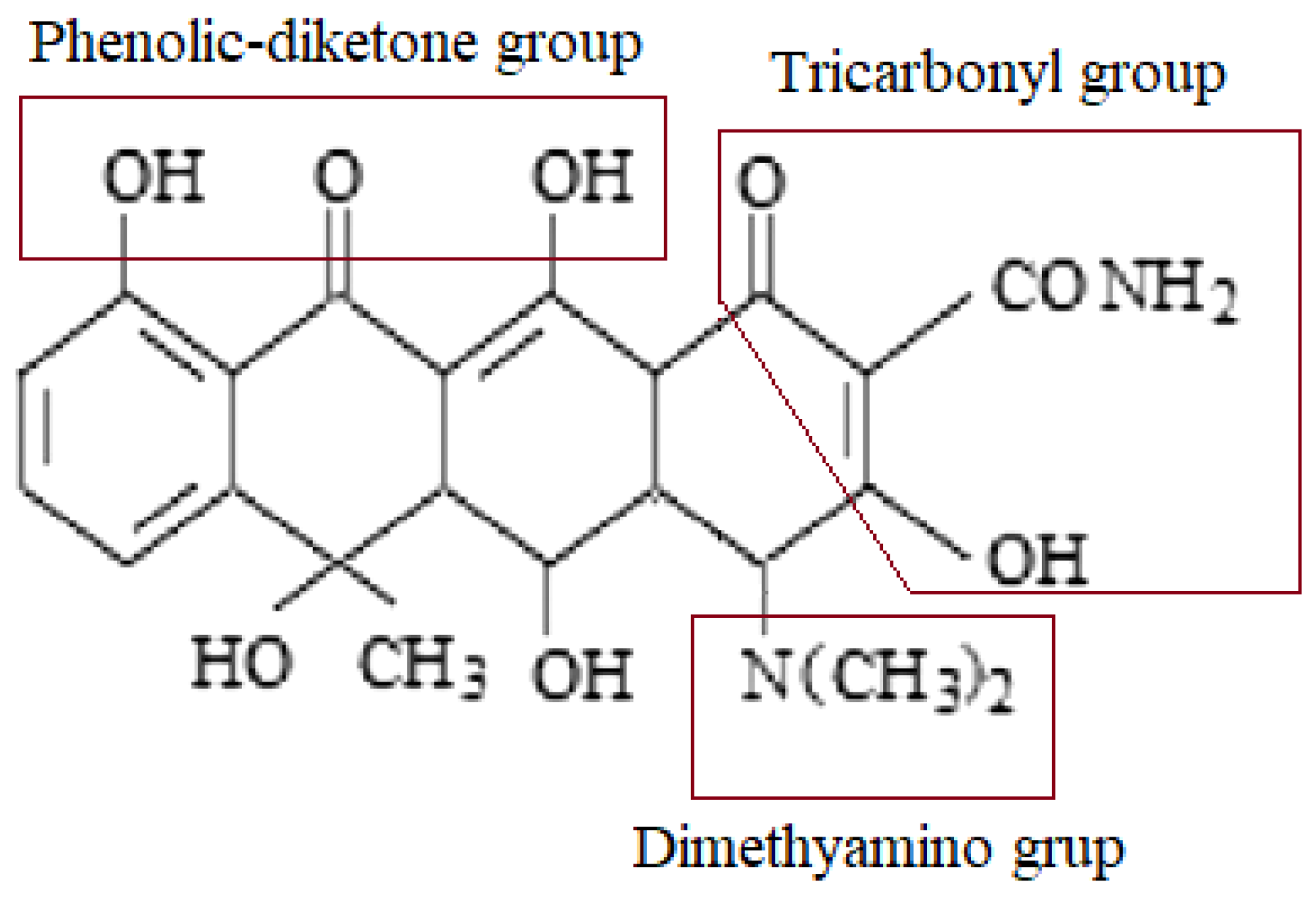
The solution pH may affect the size of the adsorption process due to changes in the surface properties of adsorbents and adsorbates. This parameter often determines the adsorption mechanism. Oxytetracycline, depending on the solution pH, can appear in the following forms: H
3L
+, H
2L
±, HL
−, and L
2− (L means OTC) [
78]. In the range of pH 2 to 3.8, there is a transformation of H
3L
+ to H
2L
± (pK
a1 = 3.22—deprotonating OH
3—tricarbonyl group), from pH 5.5 to 12.0, H
2L
± transforms to HL
− and L
2− (pK
a2 = 7.46 deprotonation phenolic-diketone group, p
Ka3 = 8.94 deprotonation of the dimethylamine group NH4) (
Figure 6).
Oxytetracycline in solution at pH = 3 is present approximately in 80% as an OTC
+ cation and in 20% as bipolar OTC
±, in solution at pH = 6, the whole compound is in the bipolar form (OTC
±). In alkaline solution at pH = 10, it is present as a di- and monovalent anion in equal amounts (about 50% OTC
− and 50% OTC
−2) [
44].
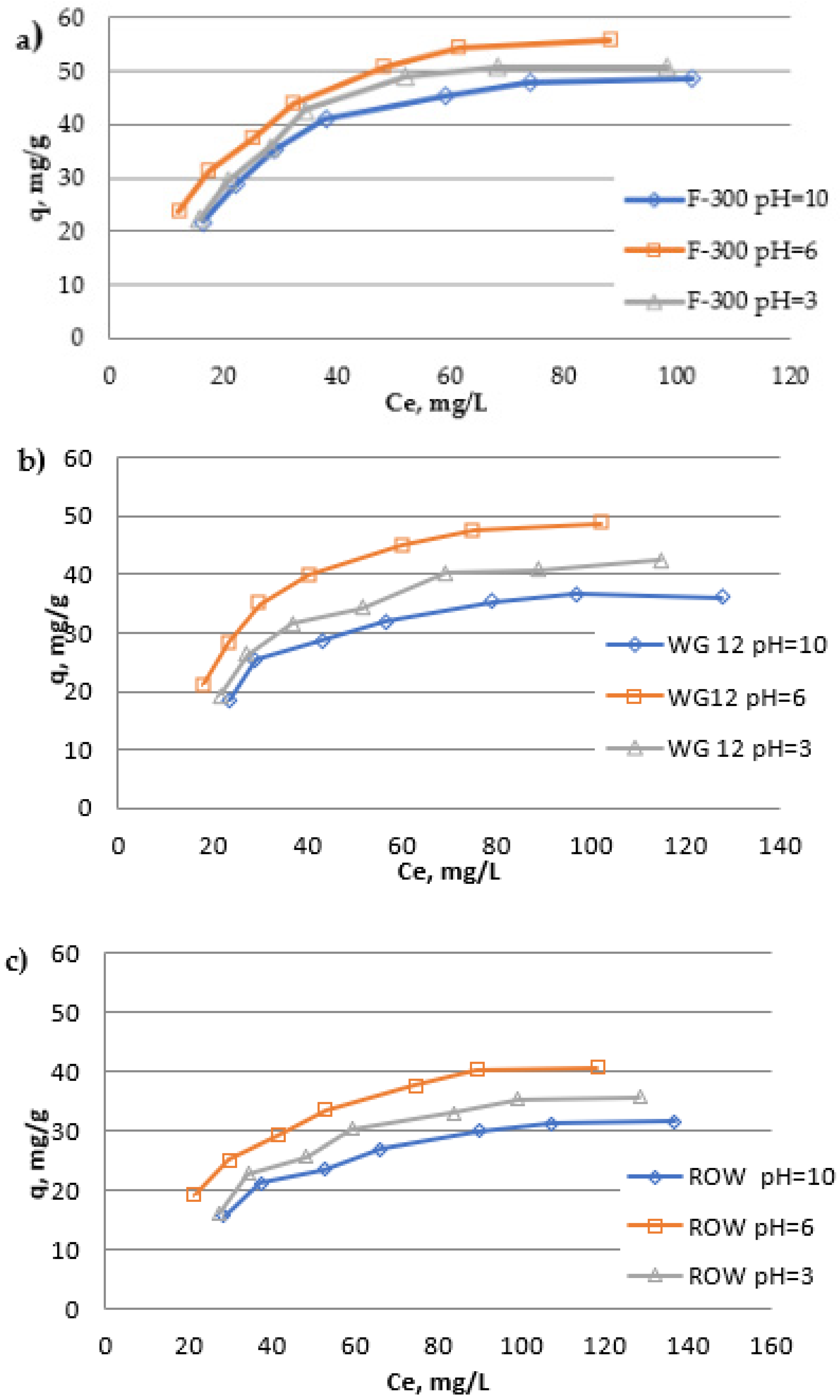
While assessing the effect of the pH of the solution on the amount of oxytetracycline adsorption, a significant importance of this parameter was found (
Figure 7 and
Table 8). For all the activated carbons used in the research, the same tendencies of the influence of pH on the adsorption of oxytetracycline were observed. The highest adsorption efficiency was obtained when the process was carried out from a solution with pH = 6, and the lowest with pH = 10. The similar influence of pH on the tetracycline adsorption obtained Wand et al., and Liu et al., (the maximum adsorption at pH = 5 and the minimum adsorption in the strongly alkaline solutions) [
59,
60]. The influence of pH on the tetracycline adsorption is, however, depended on the used sorbent. In the research [
55,
56] there was observed that the higher pH, the better TC adsorption. Wang et al. in their research observed high and similar adsorption within the range of pH from 4 to 8 [
45]. Differences between q
m monolayer capacities calculated with the Langmuir isotherm are significant. The largest differences in adsorption capacities q
m were observed for activated carbon WG-12, for which q
m for pH = 10 is 46.29 and the pH = 6 q
m = 65.94 mg/g. The smallest differences were noted in the case of activated carbon F-300: for pH = 10 q
m = 63,59 mg/g and for pH = 6 q
m = 73.31 mg/g. It is clear that oxytetracycline in the OTC
± (pH = 6) form is better adsorbed than in the anionic or cationic form. A similar effect of the pH of the solution on the results of oxytetracycline adsorption was obtained inter alia by Sun et al. [
44]. Both basic and acid functional groups are present on the surface of activated carbons produced by the steam-gas method. Their presence was determined by the Bohem method, and the results are presented in
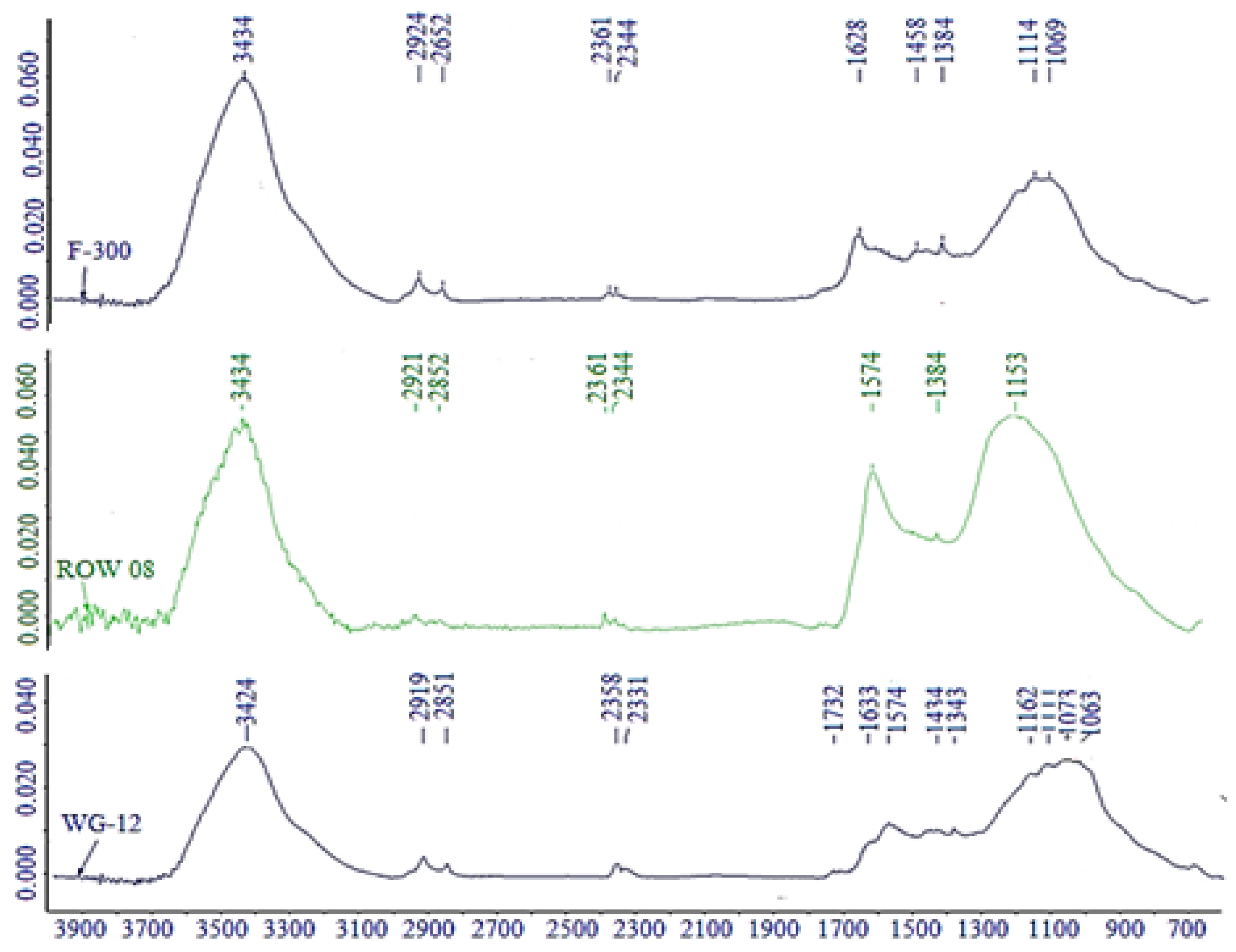
Table 9. The tested activated carbons do not differ significantly in the chemical nature of the surface, which was also found on the basis of FTIR tests (
Figure 8). Commercial activated carbons used in the research were not additionally oxidized, therefore their surface has relatively few acid-base groups. It can be observed that these carbons have similar amounts of acidic (from 0.434 to 0.586 mmol/g) and basic groups (from 0.467 to 0.592 mmol/g). The simultaneous presence of acidic and basic groups on the surface of activated carbons is characteristic for sorbents produced by the steam-gas method. Carbon ROW 08 Supra, adsorbing the smallest amounts of oxytetracycline, has the lowest amount of acidic groups capable of anion exchange, but the highest amounts of basic groups among the tested sorbents. Activated carbon F-300 with the best oxytetracycline adsorption has moderate amounts of both acidic and basic groups. However, while analyzing the chemical structure of the activated carbons, it is not so obvious to determine simple dependencies between the adsorption capacity and the number of groups on the surface of activated carbons determined by the Boehm method. With such small differences in the chemical structure of the surface of activated carbons, the porous structure and the accessibility of active areas may also be important.
Measurements of the FTIR spectrum of the analyzed activated carbons were also carried out. It is the measurement which is often carried out in order to explain the mechanisms of the tetracycline adsorption [
42,
60]. Carbon F-300, adsorbing the largest amounts of oxytetracycline, is marked by the highest intensity of phenolic O–H groups forming the hydrogen bond (3200–3500 cm
−1). A lower intensity of carbonyl groups > C=O (1740–1500 cm
–1) and ester groups C–O–C (1300–1000 cm
–1) was observed on carbon F-300 compared to carbon ROW 08 Supra, which absorbs the lowest degree of oxytetracycline. A small peak of carboxyl groups (1732 cm
−1) was observed only for activated carbon WG-12. There is a possibility of creating hydrogen bonds between oxytetracycline and phenolic groups on the surface of activated carbon.
In a solution with an acidic pH = 3, dissociated functional groups present on the surface of activated carbons have a repulsive effect on oxytetracycline in the form of OTC+. However, ion exchange (H+ OTC+ functional groups) and electrostatic attraction may occur since some of the oxytetracycline is in the OTC± form. Since oxytetracycline is present in the form of an anion (monovalent and bivalent) in a solution with pH = 10, then the increase of the amount of competing OH– ions and the dissociation of weak acid groups (COO− and O−) on the surface of activated carbons will reduce the adsorption efficiency compared to adsorption from a solution with pH = 6. Therefore, the repulsive interactions between the negatively charged surface and the adsorbate anion may be significant forces, which explains the lowest value of adsorption from such solutions.
Lower adsorption, but not of zero, from solutions with pH = 3 and 10 may indicate the effect of van der Waals forces, ion exchange and electrostatic interactions. In solutions with low pH values, the π–π EDA interaction between the aromatic rings of tetracycline and activated carbons is possible [
72,
79]. Hydrophobic interactions are also possible [
63]. Therefore, oxytetracycline adsorption is most likely the result of various interactions [
44,
45,
80].
The Freundlich, Langmuir, Jovanovic, Redlich–Peterson and Thot isotherms used to describe the results of the research on the influence of the solution pH on the adsorption volume of oxytetracycline describe the obtained results with very high accuracy. The first two isotherms—Freundlich and Langmuir, which are the most widely used, have slightly lower correlation coefficients than other ones (R
2 for Freundlich from 0.9903–0.9970, Langmuir from 0.8959 to 0.9993). The isotherms with the highest correlation coefficient (from 0.9953 to 0.9998) are the Jovanovic, Redlich–Peterson and Thot models, whose common feature is that they are a modification, compilation or are based on the assumptions of the Langmuir isotherm (in the case of Redlich–Peterson also the Freundlich isotherms). These isotherms in many cases enable a better match of the results than the Langmuir isotherm [
81]. Moreover, the Thot and Redlich–Peterson isotherms are three-parameter equations.
3.4. Oxytetracycline Adsorption on Modified Activated Carbons
The two methods of activated carbon WG-12 modification were compared. One of them is the modification with steam, carbon dioxide or air in a rotary kiln at a temperature of 400 °C. The second method is to use activated carbon as a semiconductor and heat it while the electric current flows to a temperature of 400 °C with the flow of carbon dioxide or air. The carbon used for the modification had quite high values of the adsorption capacity among the sorbents used in the research. The size of the specific surface area, the porous structure and the number of acidic and basic groups for commercial and modified carbon are presented in
Table 10. The sorbents formed as a result of the modification of commercial carbon, regardless of its method, were characterized by an increased specific surface area and an increased amount of basic and acidic functional groups. However, the differences are not large and reach a maximum of 10% in the case of the specific surface, up to 15% for basic groups, and up to 50% for acidic groups (for carbon modified with air in a rotary kiln).
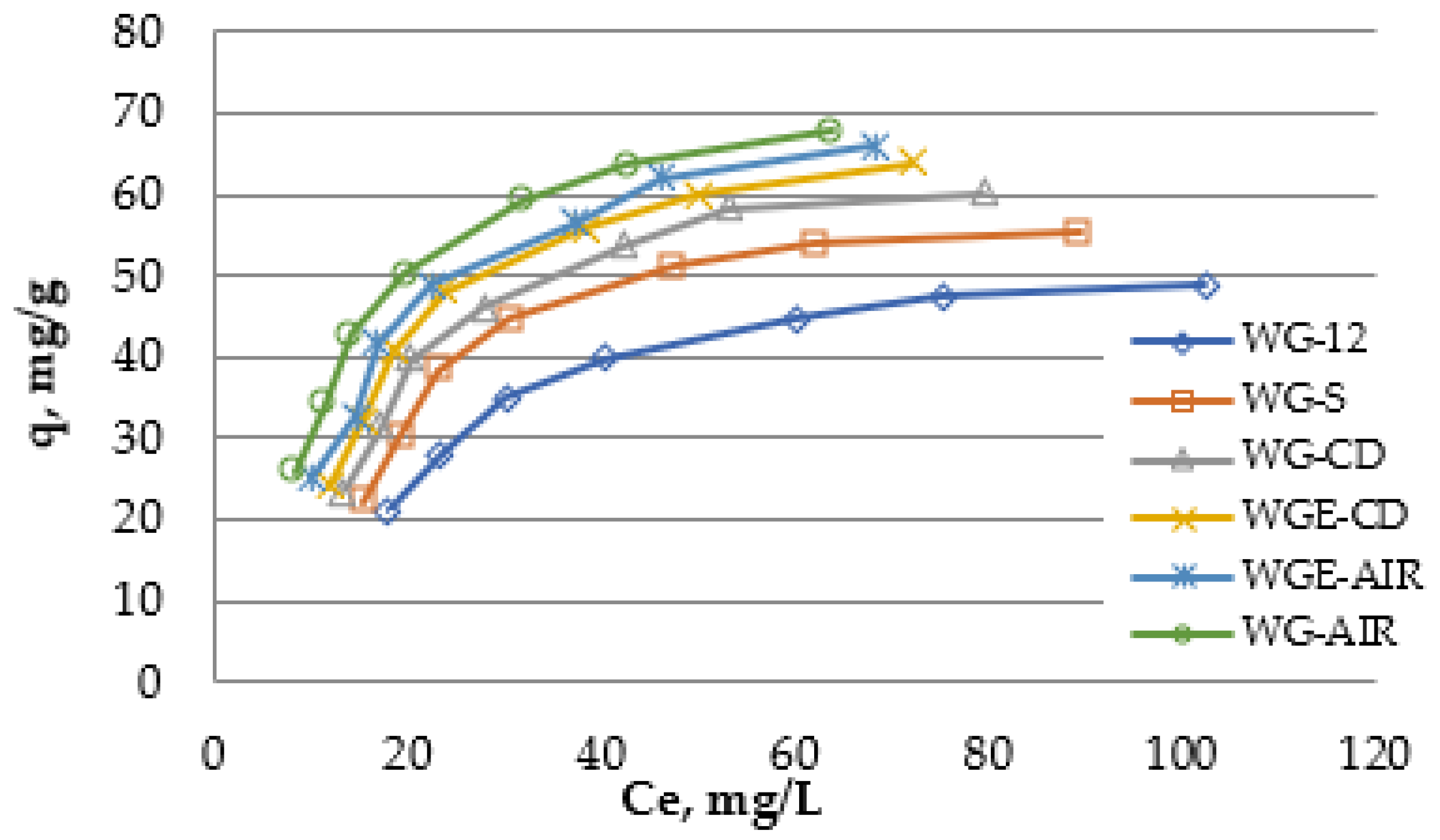
Oxytetracycline isotherms were carried out for all modified activated carbons and increased removal of the antibiotic was obtained (
Figure 9 and
Table 11). Due to the monolayer capacity calculated from the Langmuir isotherm (q
m), the tested activated carbons can be arranged in the following order: WG-12 < WG-S < WG-CDWGE-CDWG-AIR ≈ WGE-AIR. A similar, but not identical, order of the activated carbons was obtained by ranking them on the basis of q
max calculated from the Thot and Jovanovic isotherm. According to these models, activated carbon WG-AIR adsorbs more oxytetracycline than carbon WGE-AIR. Such calculated maximum adsorption capacities are consistent with the isothermal diagrams, because the WG-AIR curve lies above the WGE-AIR curve. These differences are not large, but it seems that these isotherms reflect reality better than the Langmuir isotherm. However, the obtained q
max from the Thot and Jovanovic isotherms are closer to the maximum values obtained in the tests, and the Langmuir isotherm determines the capacity of the monolayer.
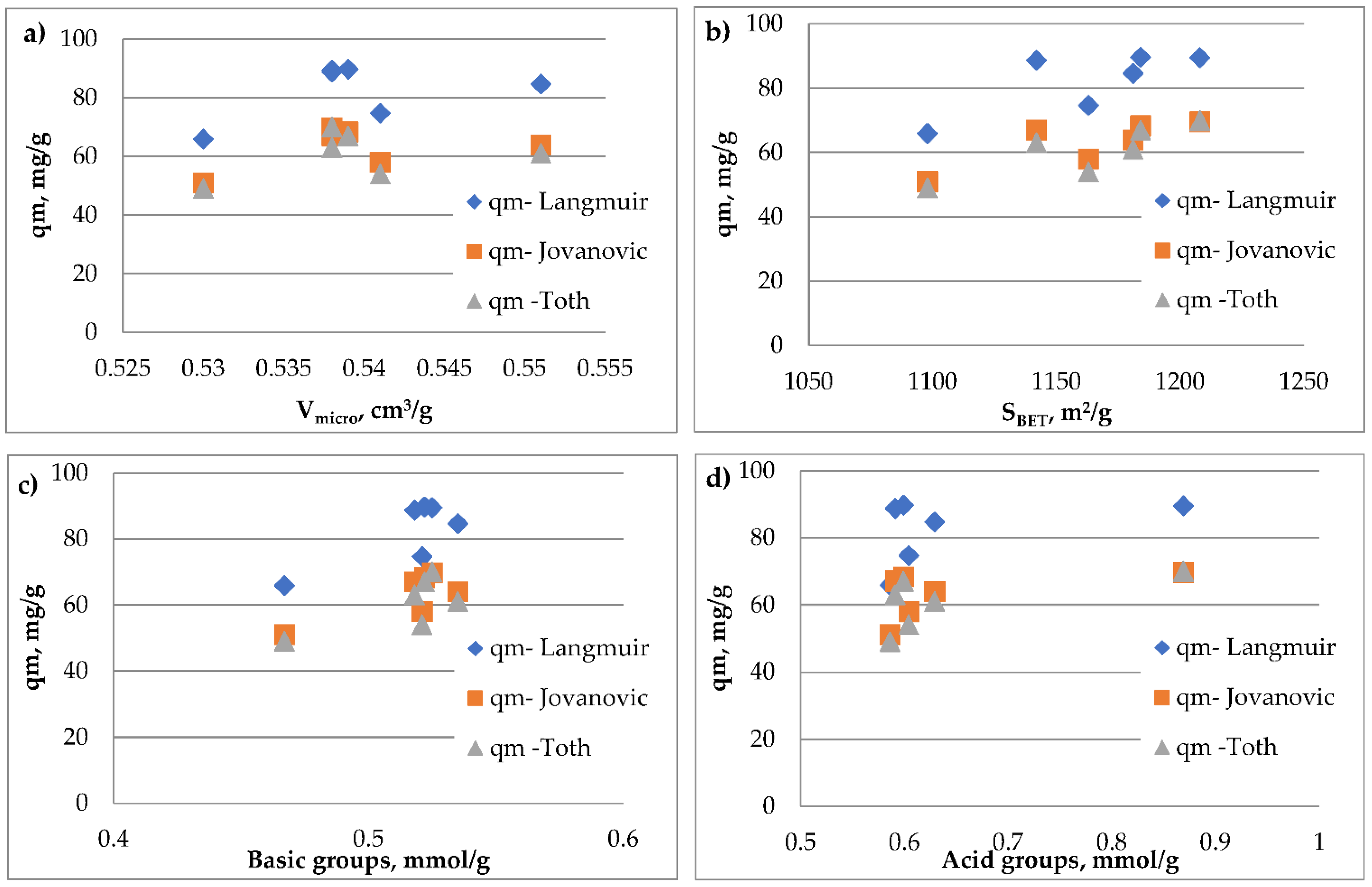
The Pearson correlation between qm/qmax determined from the Langmuir/Jovanovic/Thot isotherm and the specific surface area/volume of micropores/number of acid groups/number of basic groups was analyzed (
Table 12). The strength of the correlation depending on the Pearson coefficient is defined as: weak correlation below 0.2, 0.2–0.4 low correlation, 0.4–0.6 moderate correlation (significant dependence), 0.6–0.8 high correlation, 0.8–0.9 very high correlation, 0.9–1 practically full correlation. A high or very high correlation was found between the maximum adsorption capacity and the specific surface area. A slightly higher Pearson correlation coefficient was obtained when the maximum capacity read from the Thot and Jovanovic isotherm was analyzed compared to q
m obtained from the Langmuir isotherm. A high correlation was obtained while analyzing the effect of the number of basic groups on q
max and moderate correlation when the effect of the number of acid groups and value of micropore was analyzed. The dependence of the adsorption capacity counted on the basis of the Langmuir/Jovanovic/Thot isotherm on the specific surface area/volume of micropores/number of acid groups/number of basic groups there was presented in
Figure 10.
Comparing both methods of modification, it can be seen that higher adsorption occurred on air-modified carbons compared to modification with carbon dioxide or steam (qmax 66 mg/g for WG-12 and 90 mg/g for WG-AIR and WGE-AIR). The modification on the proprietary SEOW stand gives similar results to the modification in a rotary kiln at the same temperature. However, this method is much less energy-consuming, and this parameter is the major disadvantage of high-temperature modification.
The paper attempts to calculate the amount of energy consumed for both methods of activated carbon modification in an approximate manner. This is an approximate comparison because the rotary kiln is made in a fractional-technical scale, and the SEOW stand is a laboratory scale. 1 dm3 of activated carbon was modified within an hour in the rotary kiln, and 0.38 dm3 on the SEOW. The calculations did not take the amount of current required to heat the rotary kiln into account, because if the kiln were to run continuously, this value would have little effect on the final result.
Calculation of energy consumption for the modification of 1 dm3 activated carbon in a rotary kiln:
Work within 1 h
Total time of turning on reactor heaters—20 min—power 7.2 kW; 2.4 kWh
Continuous operation of receiving chamber heating element—1 h-power 0.5 kW; 0.5 kWh
Continuous operation of reactor and feeder drive unit—1 h—power 0.2 kW; 0.2 kWh
Total time of turning on gas superheater heaters—25 min—power 4.5 kW; 1.9 kWh
Total energy consumption: 5 kWh
In the case of modification on the SEOW stand within 24 min (during that time the activated carbon was heated to 400 °C), the energy consumption was 0.2 kWh. However, since there was 0.38 dm3 of activated carbon in the reactor, the energy consumption per 1 dm3 was about 0.6 kWh.
The above calculations prove a large disproportion in electricity consumption for the modification of activated carbon.
The adsorption capacities of commercial activated carbons obtained in the study are lower than those presented in the literature (
Table 13). It should be emphasized that activated carbons are often prepared in a special way due to the adsorbed contamination; therefore, the monolayer capacities q
m are higher for such carbons than for the commercial sorbents used in the paper. By analyzing the match of the results to the applied kinetic equations, it can be concluded that the PSO equation describes the adsorption kinetics of tetracyclines better than the PFO. The use of other kinetic equations is not common, but sometimes gives a better match to the results than the PSO. However, it depends on the used adsorbent. It is similar in the case of describing the results of adsorption statics with isotherms. The most popular and widely used Langmuir and Freundlich isotherms are often characterized by a lower correlation coefficient R
2 than, for example, the Redlich–Peterson equation.
By analyzing the literature reports on the adsorption of tetracyclines (
Table 13), it can be noticed that regardless of the used adsorbent, its production and modification method, the physical adsorption is a very important mechanism, but chemical adsorption may occur as well. In the literature, the forces responsible for the adsorption of tetracyclines are mainly described as van der Waals forces, π–π EDA interactions, hydrogen bonds and electrostatic interaction, depending on the used adsorbent. The dominating mechanisms are different depending on the used adsorbent [
45,
59,
76].