Promoting Effect of Ultra-Fine Bubbles on CO2 Hydrate Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and UFB Measurements
2.2. Promoting Effect of CO2 Hydrate Formation Evaluation
3. Results and Discussion
3.1. Characteristics of UFBs in Liquid Samples
3.2. CO2 Hydrate Formation with Various Liquid Samples
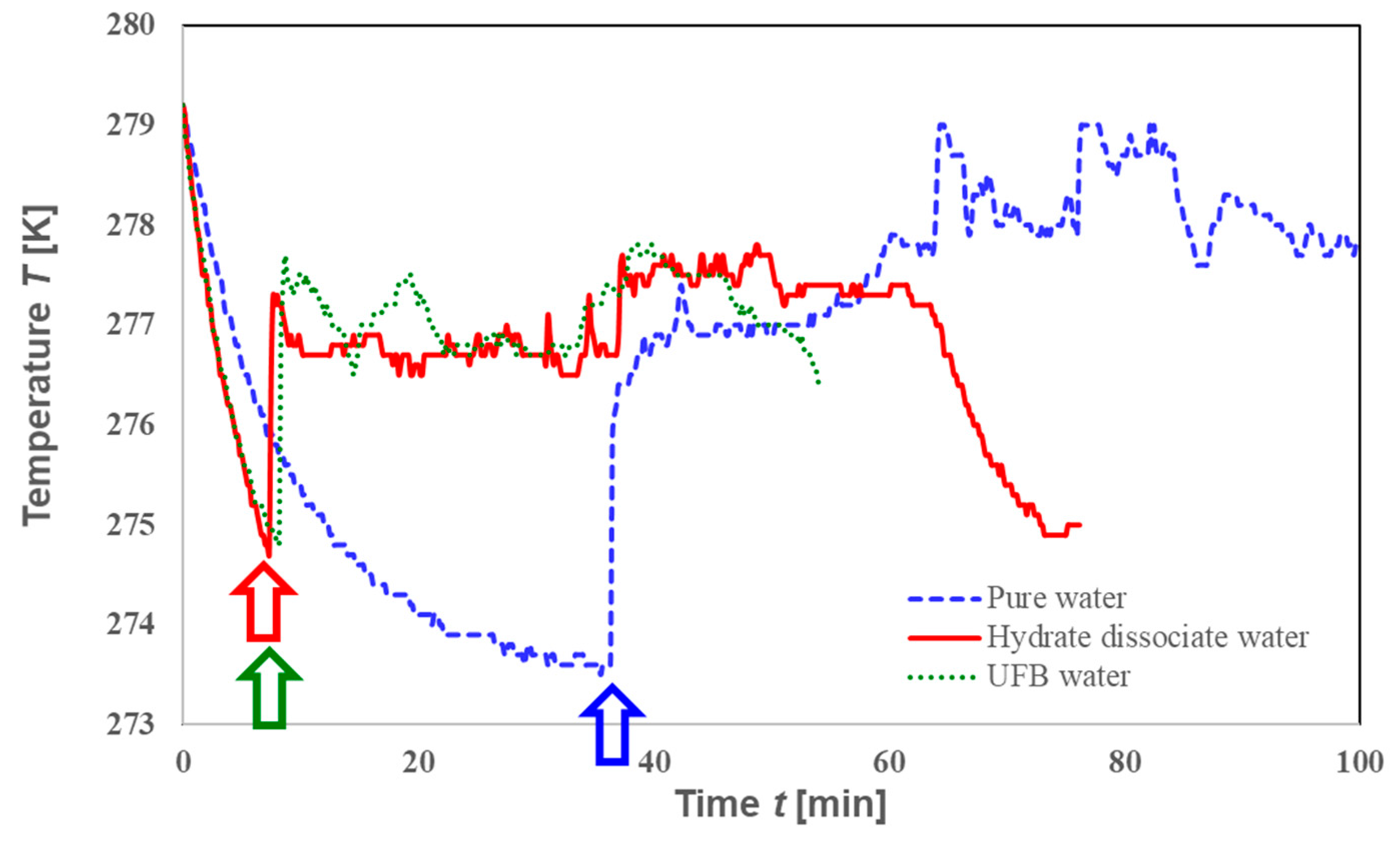
3.3. Comparison of Memory Effects and Roles of UFBs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
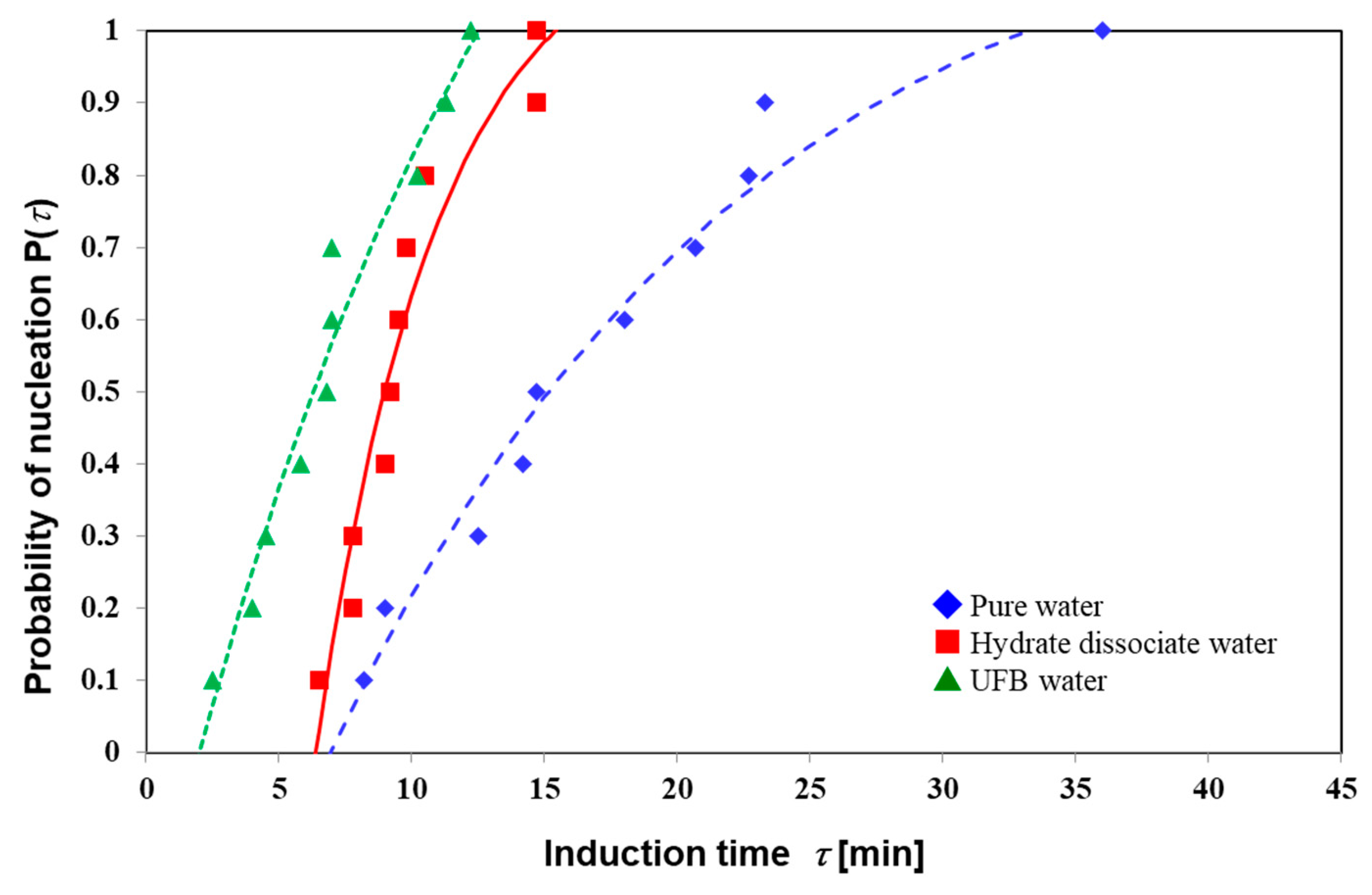
| Nucleation Probability | Induction Time (min) | ||
|---|---|---|---|
| Pure Water | CO2 UFB-Containing Water | CO2 Hydrate-Dissociated Water | |
| 0 | 7 | 6.4 | 2 |
| 0.1 | 8.2 | 6.5 | 2.5 |
| 0.2 | 9 | 7.8 | 4 |
| 0.3 | 12.5 | 7.8 | 4.5 |
| 0.4 | 14.2 | 9 | 5.8 |
| 0.5 | 14.7 | 9.2 | 6.8 |
| 0.6 | 18 | 9.5 | 7 |
| 0.7 | 20.7 | 9.8 | 7 |
| 0.8 | 22.7 | 10.5 | 10.2 |
| 0.9 | 23.3 | 14.7 | 11.3 |
| 1 | 36 | 14.7 | 12.2 |
References
- Kvenvolden, K.A. Methane Hydrate—A major reservoir of carbon in the shallow geosphere? Chem. Geol. 1988, 71, 41–51. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrate of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Masuda, Y.; Uchida, T.; Nagakubo, S.; Satoh, M. Methane hydrates. In Fossil Fuels: Current Status and Future Directions, World Scientific Series in Current Energy Issues; Crawley, G.M., Ed.; World Scientific Pub. Co. Pte. Ltd.: Singapore, 2016; Volume 1, Chapter 10; pp. 289–327. [Google Scholar]
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2004, 426, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, J.; Borrehaug, A. Frozen hydrate for transport of natural gas. In Proceedings of the 2nd International Conference on Natural Gas Hydrates, Toulouse, France, 2–6 June 1996; pp. 415–422. [Google Scholar]
- Gudmundsson, J.S.; Parlaktuna, M.; Levik, O.I.; Andresson, V. Laboratory for continuous production of natural gas hydrates. In Proceedings of the 5th International Conference on Natural Gas Hydrates, Trondheim, Norway, 13–16 June 2005; Tapir Academic Press: Trondheim, Norway, 2005; Volume 1, pp. 851–858. [Google Scholar]
- Mimachi, H.; Takeya, S.; Yoneyama, A.; Hyodo, K.; Takeda, T.; Gotoh, Y.; Murayama, T. Natural gas storage and transportation within gas hydrate of smaller particle: Size dependence of self-preservation phenomenon of natural gas hydrate. Chem. Eng. Sci. 2014, 118, 208–213. [Google Scholar] [CrossRef]
- Nakajimra, M.; Ohmura, R.; Mori, Y.H. Clathrate hydrate formation from cyclopentane-in-water emulsions. Ind. Eng. Chem. Res. 2008, 47, 8933–8939. [Google Scholar] [CrossRef]
- Shimada, W.; Shiro, M.; Kondo, H.; Takeya, S.; Oyama, H.; Ebinuma, T.; Narita, H. Tetra-n-butyl ammonium bromide–water. Acta Crystallogr. C Cryst. Struct. Commun. 2005, 61, o65–o66. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Dalmazzone, D.; Fürst, W.; Delahaye, A.; Fournaison, L.; Clain, P. Accurate DSC measurement of the phase transition temperature in the TBPB—Water system. J. Chem. Thermodyn. 2013, 61, 132–137. [Google Scholar] [CrossRef]
- Oshima, M.; Kida, M.; Nagao, J. Thermal and Crystallographic Properties of Tetra-n-butylammonium bromide + tetra-n-butylammonium chloride mixed semiclathrate hydrates. J. Chem. Eng. Data 2016, 61, 3334–3340. [Google Scholar] [CrossRef]
- Parent, J.S.; Bishnoi, P.R. Investigation into the nucleation behavior of methane gas hydrates. Chem. Eng. Commun. 1996, 144, 51–64. [Google Scholar] [CrossRef]
- Hwang, M.J.; Wright, D.A.; Kapur, A.; Holder, G.D. An experimental study of crystallization and crystal growth of methane hydrates from melting ice. J. Inclusion Phenom. Molecul. Recogn. Chem. 1990, 8, 103–116. [Google Scholar] [CrossRef]
- Ohmura, R.; Ogawa, M.; Yasuoka, K.; Mori, Y.H. Statistical study of clathrate-hydrate nucleation in a water/hydrochlorofluorocarbon system: Search for the nature of the “memory effect”. J. Phys. Chem. B 2003, 107, 5289–5293. [Google Scholar] [CrossRef]
- Sefidroodi, H.; Abrahamsen, E.; Kelland, M.A. Investigation into the strength and source of the memory effect for cyclopentane hydrate. Chem. Eng. Sci. 2013, 87, 133–140. [Google Scholar] [CrossRef]
- Buchanan, P.; Soper, A.K.; Thompson, H.; Westacott, R.E.; Creek, J.L.; Hubson, G.; Koh, C.A. Search for memory effects in methane hydrate: Structure of water before hydrate formation and after hydrate decomposition. J. Chem. Phys. 2005, 123, 164507. [Google Scholar] [CrossRef]
- Rodger, P.M. Methane hydrate, melting and memory. Ann. N. Y. Acad. Sci. 2000, 912, 474–482. [Google Scholar] [CrossRef]
- Uchida, T.; Yamazaki, K.; Gohara, K. Generation of micro- and nano-bubbles in water by dissociation of gas hydrates. Korean J. Chem. Eng. 2016, 33, 1749–1755. [Google Scholar] [CrossRef] [Green Version]
- Uchida, T.; Yamazaki, K.; Gohara, K. Gas nano-bubbles as nucleation promoting in the gas-hydrate memory effect. J. Phys. Chem. C 2016, 120, 26620–26629. [Google Scholar] [CrossRef]
- Uchida, T.; Miyoshi, H.; Sugibuchi, R.; Suzuta, A.; Yamazaki, K.; Gohara, K. Contribution of ultra-fine bubbles to promoting effect on propane hydrate formation. Front. Chem. 2020, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- ISO 20480-1: 2017 Fine Bubble Technology—General Principles for Usage and Measurement of Fine Bubbles—Part 1: Terminology. Available online: https://www.iso.org/obp/ui/#iso:std:iso:20480:-1:ed-1:v1:en (accessed on 14 April 2021).
- Seddon, J.R.T.; Lohse, D.; Ducker, W.A.; Craig, V.S.J. A deliberation on nanobubbles at surfaces and in bulk. ChemPhysChem 2012, 13, 2179–2187. [Google Scholar] [CrossRef]
- Oshita, S.; Uchida, T. Basic Characterization of nanobubbles and its potential applications. In Bio-Nanotechnology: A Revolution in Biomedical Sciences, & Human Health; Bagchi, D., Bagchi, M., Moriyama, H., Shahidi, F., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2013; Chapter 29; pp. 506–516. [Google Scholar]
- Takahashi, M. ζ potential of microbubbles in aqueous solutions: Electrical properties of the gas-water interface. J. Phys. Chem. B 2005, 109, 21858–21864. [Google Scholar] [CrossRef]
- Yagasaki, T.; Matsumoto, M.; Andoh, Y.; Okazaki, S.; Tanaka, H. Effect of bubble formation on the dissociation of methane hydrate in water: A molecular dynamic study. J. Phys. Chem. B 2014, 118, 1900–1906. [Google Scholar] [CrossRef]
- Bagherzadeh, S.A.; Alavi, S.; Ripmeester, J.; Englezos, P. Formation of methane nano-bubbles during hydrate decomposition and their effect on hydrate growth. J. Chem. Phys. 2015, 142, 214701. [Google Scholar] [CrossRef]
- Kondori, J.; James, L.; Zendehboudi, S. Molecular scale modeling approach to evaluate stability and dissociation of methane and carbon dioxide hydrates. J. Mol. Liq. 2020, 297, 111503. [Google Scholar] [CrossRef]
- Zheng, J.; Chong, Z.R.; Qureshi, M.F.; Linga, P. Carbon dioxide sequestration via gas hydrates: A potential pathway toward decarbonization. Energy Fuels 2020, 34, 10529–10546. [Google Scholar] [CrossRef]
- Castellani, B.; Rossetti, G.; Tupsakhare, S.; Rossi, F.; Nicolini, A.; Castaldi, M.J. Simulation of CO2 storage and methane gas production from gas hydrates in a large scale laboratory reactor. J. Petro. Sci. Eng. 2016, 147, 515–527. [Google Scholar] [CrossRef]
- The Chemical Society of Japan. Kagaku-Binran (Handbook of Chemistry), 5th ed.; fundamental II-144-149; Maruzen Co. Ltd.: Tokyo, Japan, 2004. [Google Scholar]
- Takeya, S.; Hori, A.; Hondoh, T.; Uchida, T. Freezing-memory effect of water on nucleation of CO2 hydrate crystals. J. Phys. Chem. B 2000, 104, 4164–4168. [Google Scholar] [CrossRef]
- May, E.F.; Wu, R.; Kelland, M.A.; Aman, Z.M.; Kozielski, K.A.; Hartley, P.G.; Maeda, N. Quantitative kinetic inhibitor comparisons and memory effect measurements from hydrate formation probability distributions. Chem. Eng. Sci. 2014, 107, 1–12. [Google Scholar] [CrossRef]
- Huang, X.; Li, Z.; Deng, Y.; Cai, W.; Gu, L.; Lu, H. Effect of micro- and nanobubbles on the crystallization of THF hydrate based on the observation by atomic force microscopy. J. Phys. Chem. C 2020, 124, 13966–13975. [Google Scholar] [CrossRef]
- Uchida, T.; Liu, S.; Enari, M.; Oshita, S.; Yamazaki, K.; Gohara, K. Effect of NaCl on the lifetime of micro- and nanobubbles. Nanomaterials 2016, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Ebinuma, T.; Narita, H. Observations of CO2-hydrate decomposition and reformation processes. J. Cryst. Growth 2000, 217, 189–200. [Google Scholar] [CrossRef]
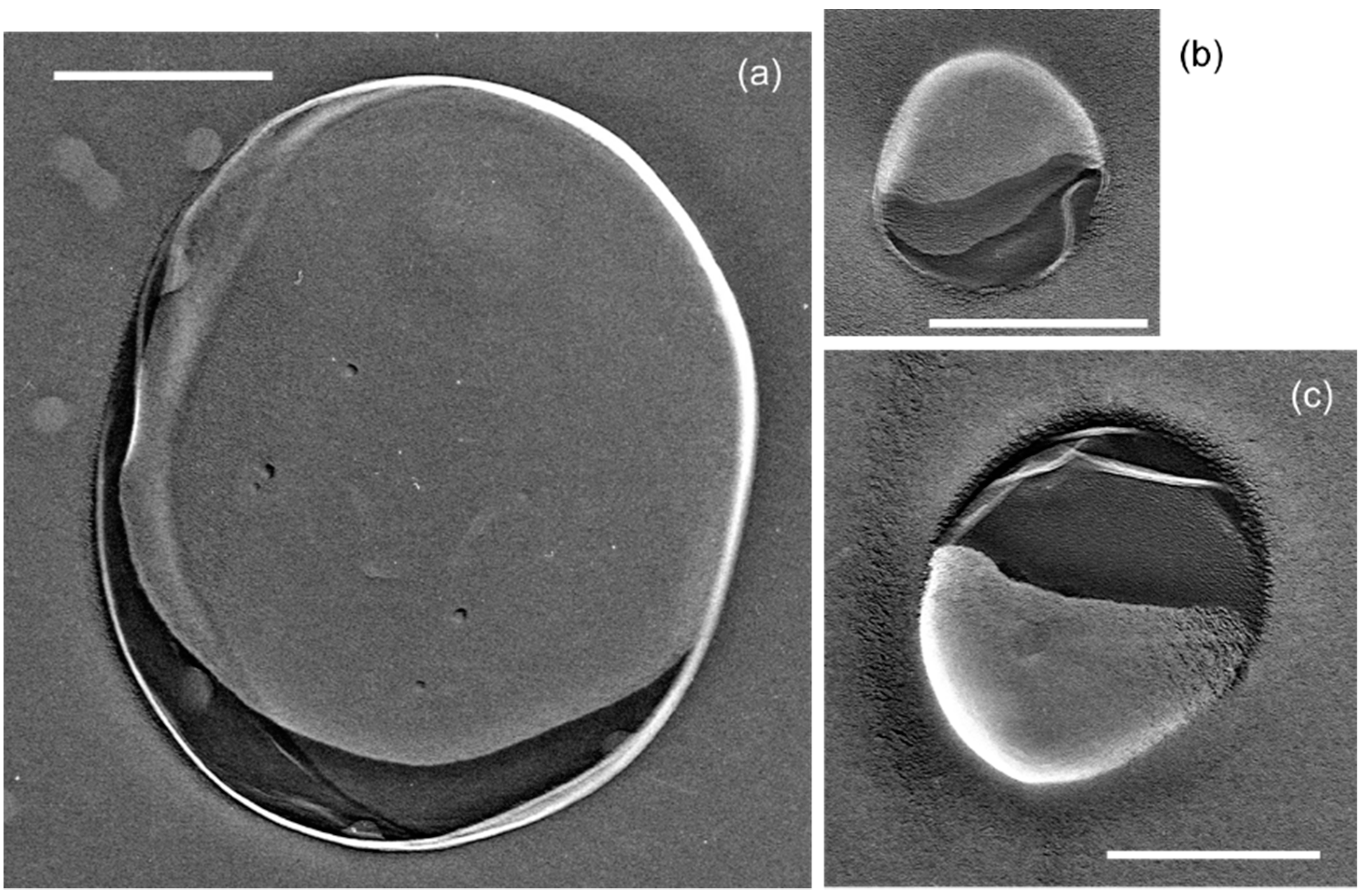
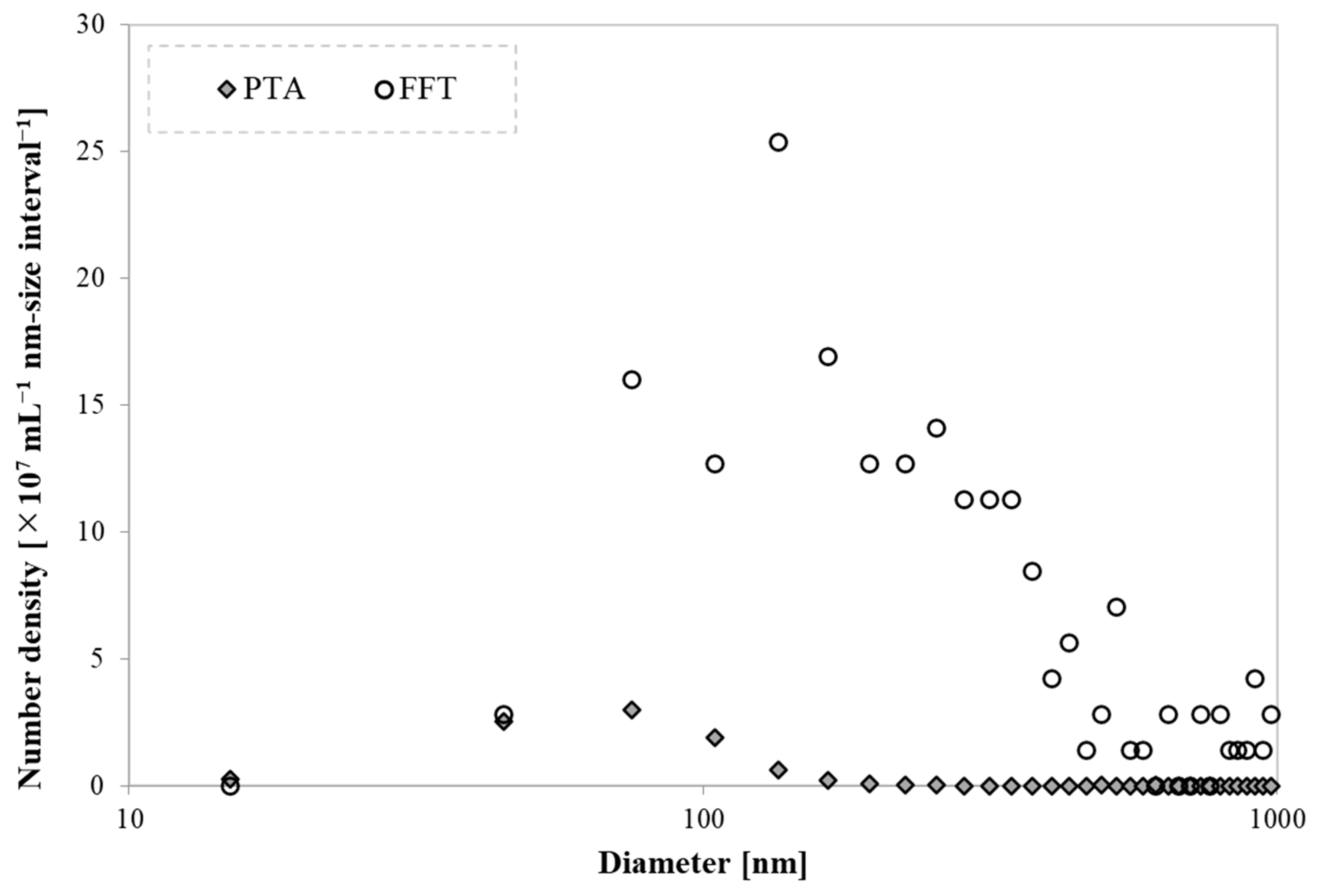
| Sample | D (nm) | N (×108 mL−1) |
|---|---|---|
| CO2-UFB-containing water | 917 ± 443 (FFT) | 8.1 ± 4.1 (FFT) |
| >300 (LS) | 2.1 ± 0.7 (LS) | |
| 114 ± 21 (PTA) | 0.04 ± 0.01 (PTA) | |
| CO2-hydrate-dissociated water | 672 ± 528 (FFT) | 22.8 ± 7.1 (FFT) |
| >300 (LS) | 2.0 ± 0.6 (LS) | |
| 81.7 ± 4.0 (PTA) | 0.087 (PTA) | |
| pH: 4.0 | ||
| ζ-potential: −10.26 ± 5.3 [mV] | ||
| pure water | N.A. | N.A. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, T.; Miyoshi, H.; Yamazaki, K.; Gohara, K. Promoting Effect of Ultra-Fine Bubbles on CO2 Hydrate Formation. Energies 2021, 14, 3386. https://doi.org/10.3390/en14123386
Uchida T, Miyoshi H, Yamazaki K, Gohara K. Promoting Effect of Ultra-Fine Bubbles on CO2 Hydrate Formation. Energies. 2021; 14(12):3386. https://doi.org/10.3390/en14123386
Chicago/Turabian StyleUchida, Tsutomu, Hiroshi Miyoshi, Kenji Yamazaki, and Kazutoshi Gohara. 2021. "Promoting Effect of Ultra-Fine Bubbles on CO2 Hydrate Formation" Energies 14, no. 12: 3386. https://doi.org/10.3390/en14123386
APA StyleUchida, T., Miyoshi, H., Yamazaki, K., & Gohara, K. (2021). Promoting Effect of Ultra-Fine Bubbles on CO2 Hydrate Formation. Energies, 14(12), 3386. https://doi.org/10.3390/en14123386






