Flow Configurations of Membraneless Microfluidic Fuel Cells: A Review
Abstract
1. Introduction
2. Fundamentals of MMFCs
3. Architecture and Fabrication
4. Design and Flow Configurations
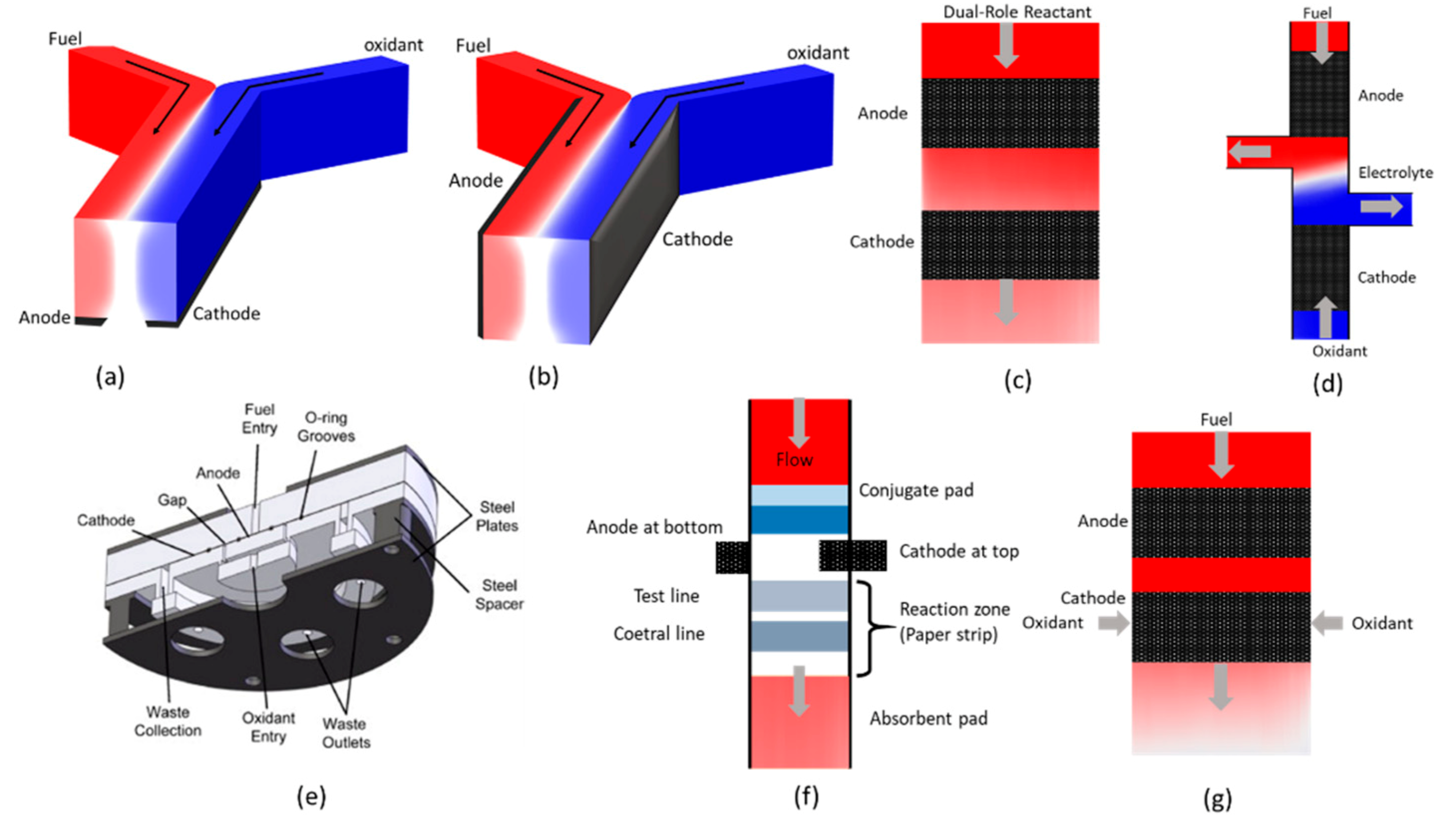
4.1. CLFMMFCs
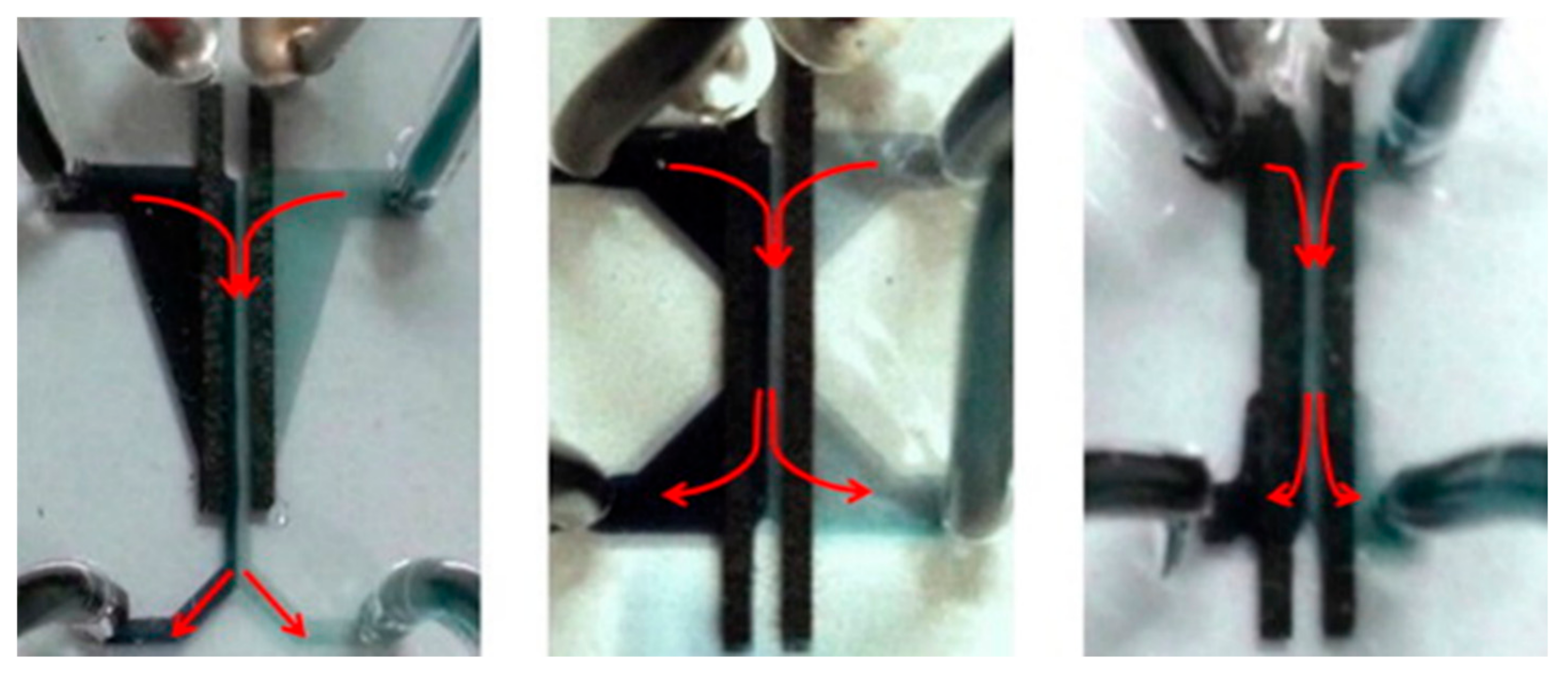
4.1.1. CLFMMFCs with Side-By-Side Streaming
4.1.2. CLFMMFCs with Vertical-Layered Streaming
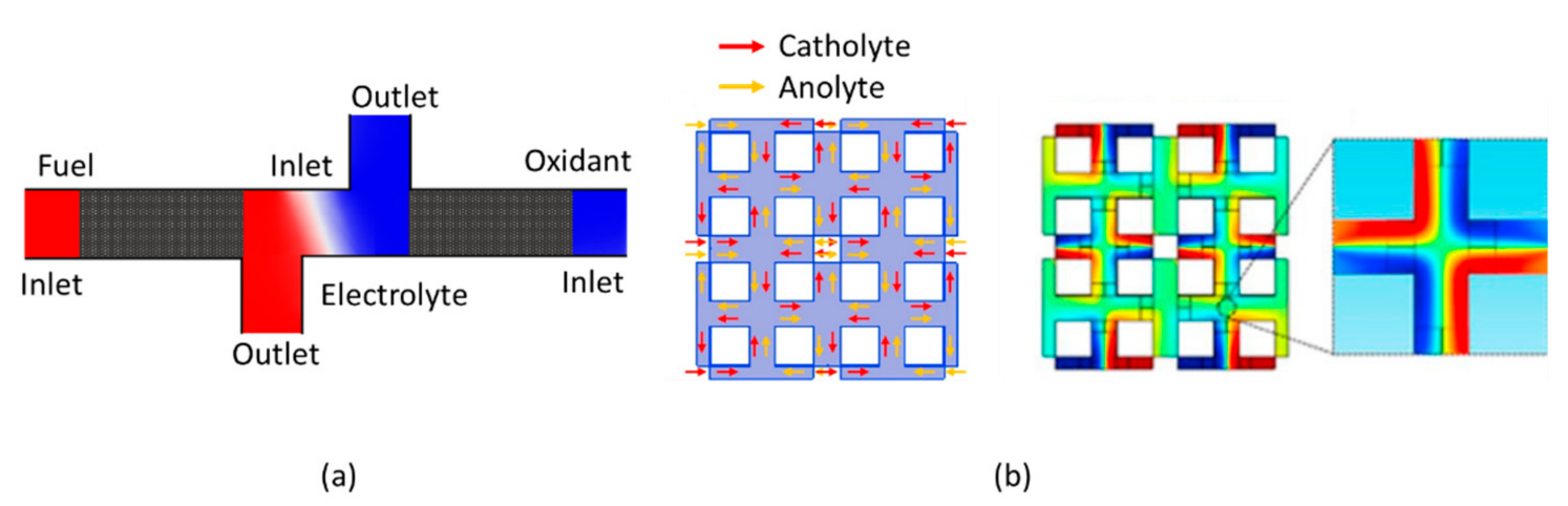
4.1.3. Multi-Stream CLFMMFCs
4.1.4. Dual-Pass CLFMMFCs
4.1.5. CLFMMFCs with Recirculation
4.2. SSMMFCs
4.3. CFMMFCs
4.4. RFMMFCs
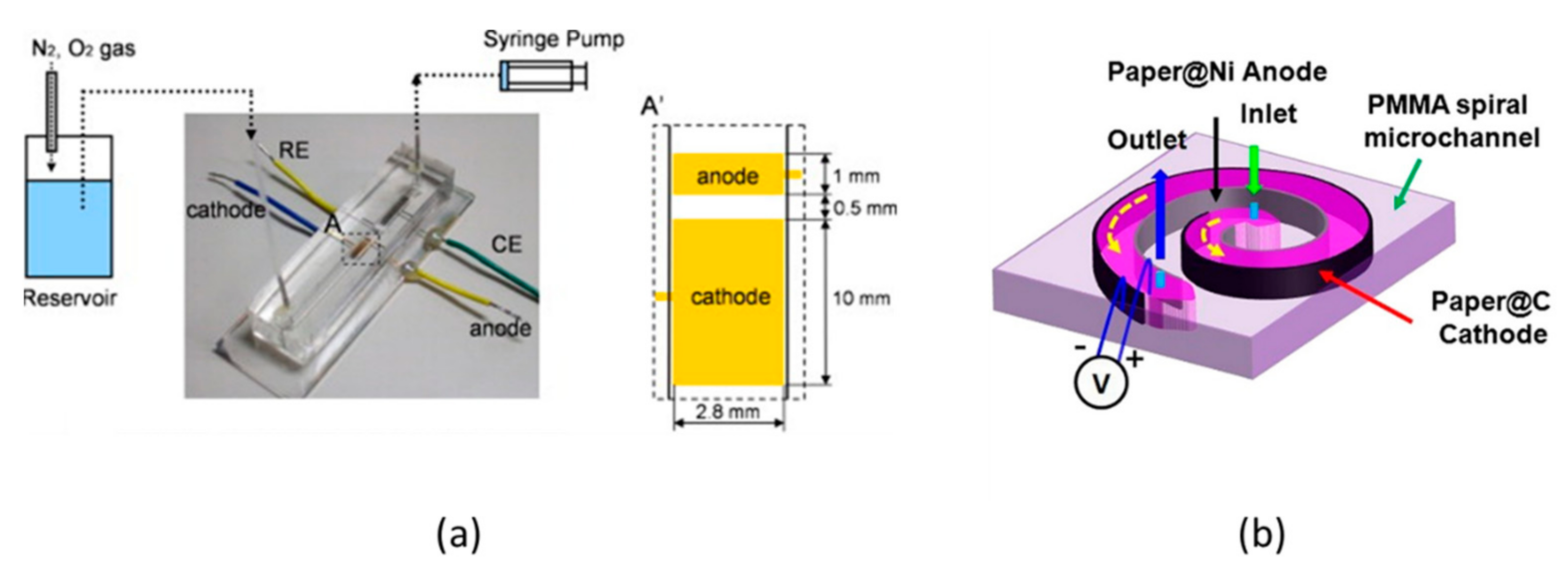
4.5. LFMMFCs
4.6. OFMMFCs
5. Stacked MMFCs
6. Fuels and Oxidants
6.1. Fuels
| Methanol/O2 in acidic media | |
| 2CH3OH + 2H2O → 2CO2 + 12H+ + 12e− | anode (E0 = 0.02 V) |
| 12H+ + 12e− +3O2 → 6H2O | cathode (E0 = 1.23 V) |
| Methanol/O2 in alkaline media | |
| 2CH3OH + 12OH− → 2CO2 + 10H2O + 12e− | anode (E0 = −0.81 V) |
| 3O2 + 6H2O +12e− →12OH− | cathode (E0 = 0.4 V) |
| Overall reaction for fully acidic or fully alkaline working media | |
| 2CH3OH + 3O2 → 2CO2 + 10H2O | (∆E = 1.2 V) |
| Methanol/O2 in mixed media | |
| 2CH3OH + 2H2O → 2CO2 + 12H+ + 12e− | anode (E0 = 0.02 V) |
| 3O2 + 6H2O +12e− →12OH− | cathode (E0 = 0.4 V) |
| Overall reaction for mixed working media | |
| 2CH3OH + 3O2 + 8H2O → 2CO2 + 12OH− + 12H+ | (∆E = 0.38 V) |
6.2. Oxidants
7. Performance Comparison
8. Challenges and Prospective Studies
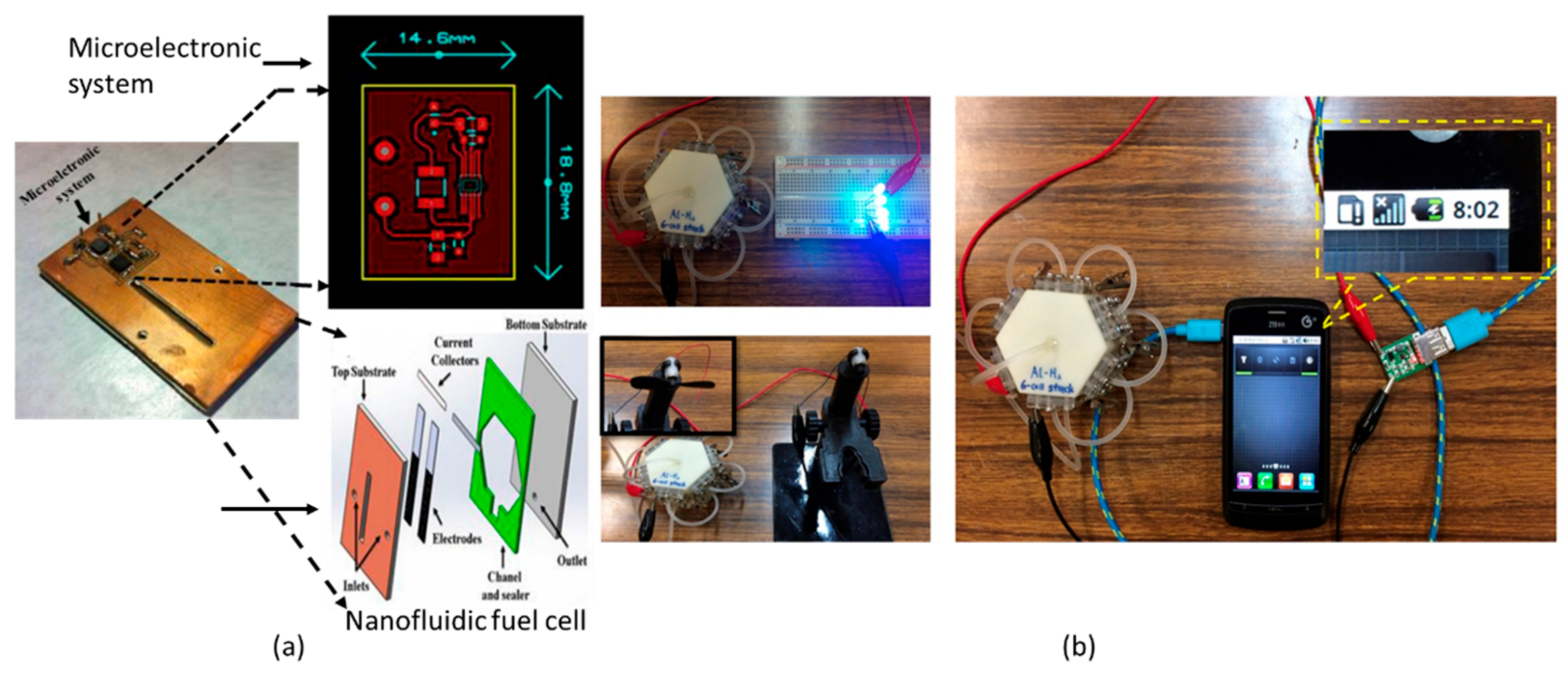
9. Applications
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rugolo, J.; Aziz, M.J. Electricity storage for intermittent renewable sources. Energy Environ. Sci. 2012, 5, 7151–7160. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Battery Technologies for Large-Scale Stationary Energy Storage. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Kosek, J.A.; Laconti, A.B. Advanced hydrogen electrode for a hydrogen-bromine battery. J. Power Sources 1988, 22, 293–300. [Google Scholar] [CrossRef]
- Liu, Q.; Shinkle, A.A.; Li, Y.; Monroe, C.W.; Thompson, L.T.; Sleightholme, A.E.S. Non-aqueous chromium acetylacetonate electrolyte for redox flow batteries. Electrochem. Commun. 2010, 12, 1634–1637. [Google Scholar] [CrossRef]
- Duduta, M.; Ho, B.; Wood, V.C.; Limthongkul, P.; Brunini, V.E.; Carter, W.C.; Chiang, Y.M. Semi-solid lithium rechargeable flow battery. Adv. Energy Mater. 2011, 1, 511–516. [Google Scholar] [CrossRef]
- Kurzweil, P.; Garche, J. Overview of batteries for future automobiles. In Lead-Acid Batteries for Future Automobiles; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 27–96. ISBN 9780444637031. [Google Scholar]
- Dyer, C.K. Fuel cells for portable applications. J. Power Sources 2002, 106, 31–34. [Google Scholar] [CrossRef]
- Kundu, A.; Jang, J.; Gil, J.; Jung, C.; Lee, H.R.; Kim, S.H.; Ku, B.; Oh, Y.S. Micro-fuel cells—Current development and applications. J. Power Sources 2007, 170. [Google Scholar] [CrossRef]
- Bloch, D. Miniature fuel cells for portable applications. In Technologies and Devices; Low Power Electronic Design; CRC Press: Boca Raton, FL, USA, 2005; Volume 106, pp. 44–1–44–20. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.C.; Xuan, J.; Wang, H. A review on unitized regenerative fuel cell technologies, part-A: Unitized regenerative proton exchange membrane fuel cells. Renew. Sustain. Energy Rev. 2016, 65, 961–977. [Google Scholar] [CrossRef]
- Larminie, J.; Dicks, A. Fuel Cell Systems Explained, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2003. [Google Scholar]
- Kelley, S.C.; Deluga, G.A.; Smyrl, W.H. Miniature methanol/air polymer electrolyte fuel cell. Electrochem. Solid State Lett. 2000, 3, 407–409. [Google Scholar] [CrossRef]
- Ferrigno, R.; Stroock, A.D.; Clark, T.D.; Mayer, M.; Whitesides, G.M. Membraneless vanadium redox fuel cell using laminar flow. J. Am. Chem. Soc. 2002, 124, 12930–12931. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Merida, W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184, 104–119. [Google Scholar] [CrossRef]
- Choban, E.R.; Markoski, L.J.; Wieckowski, A.; Kenis, P.J.A. Microfluidic fuel cell based on laminar flow. J. Power Sources 2004, 128, 54–60. [Google Scholar] [CrossRef]
- Jayashree, R.S.; Gancs, L.; Choban, E.R.; Primak, A.; Natarajan, D.; Markoski, L.J.; Kenis, P.J.A. Air-breathing laminar low-based microfluidic fuel cell. J. Am. Chem. Soc. 2005, 127, 16758–16759. [Google Scholar] [CrossRef]
- Mousavi Shaegh, S.A.; Nguyen, N.T.; Chan, S.H. A review on membraneless laminar flow-based fuel cells. Int. J. Hydrogen Energy 2011, 36, 5675–5694. [Google Scholar] [CrossRef]
- Cohen, J.L.; Volpe, D.J.; Westly, D.A.; Pechenik, A.; Abruña, H.D. A dual electrolyte H2/O2 planar membraneless microchannel fuel cell system with open circuit potentials in excess of 1.4 V. Langmuir 2005, 21, 3544–3550. [Google Scholar] [CrossRef]
- Chen, F.; Chang, M.H.; Lin, M.K. Analysis of membraneless formic acid microfuel cell using a planar microchannel. Electrochim. Acta 2007, 52, 2506–2514. [Google Scholar] [CrossRef]
- Kjeang, E.; Proctor, B.T.; Brolo, A.G.; Harrington, D.A.; Djilali, N.; Sinton, D. High-performance microfluidic vanadium redox fuel cell. Electrochim. Acta 2007, 52, 4942–4946. [Google Scholar] [CrossRef]
- Goulet, M.A.; Kjeang, E. Co-laminar flow cells for electrochemical energy conversion. J. Power Sources 2014, 260, 186–196. [Google Scholar] [CrossRef]
- Tanveer, M.; Kim, K.-Y. Effects of geometric configuration of the channel and electrodes on the performance of a membraneless micro-fuel cell. Energy Convers. Manag. 2017, 136. [Google Scholar] [CrossRef]
- Ho, B.; Kjeang, E. Microfluidic fuel cell systems. Cent. Eur. J. Eng. 2011, 1. [Google Scholar] [CrossRef]
- Kjeang, E.; Djilali, N.; Sinton, D. Microfluidic fuel cells: A review. J. Power Sources 2009, 186, 353–369. [Google Scholar] [CrossRef]
- Goel, S. From waste to watts in micro-devices: Review on development of Membraned and Membraneless Microfluidic Microbial Fuel Cell. Appl. Mater. Today 2018, 11, 270–279. [Google Scholar] [CrossRef]
- Hristovski, K.D.; Dhanasekaran, B.; Tibaquirá, J.E.; Posner, J.D.; Westerhoff, P.K. Producing drinking water from hydrogen fuel cells. J. Water Supply Res. Technol. AQUA 2009, 58, 327–335. [Google Scholar] [CrossRef]
- Tanveer, M.; Kim, K. A membraneless microfluidic fuel cell with a hollow flow channel and porous flow-through electrodes. Int. J. Energy Res. 2021, 45, 8536–8550. [Google Scholar] [CrossRef]
- Tanveer, M.; Lim, E.S.; Kim, K.Y. Effects of channel geometry and electrode architecture on reactant transportation in membraneless microfluidic fuel cells: A review. Fuel 2021, 298, 120818. [Google Scholar] [CrossRef]
- Hanapi, I.H.; Kamarudin, S.K.; Zainoodin, A.M.; Hasran, U.A. Membrane-less micro fuel cell system design and performance: An overview. Int. J. Energy Res. 2019, 43, 8956–8972. [Google Scholar] [CrossRef]
- Novak, J.; Gowin, D. Learning How to Learn; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Bird, R.B. Transport phenomena. Appl. Mech. Rev. 2002, 55, R1–R4. [Google Scholar] [CrossRef]
- Bazylak, A.; Sinton, D.; Djilali, N. Improved fuel utilization in microfluidic fuel cells: A computational study. J. Power Sources 2005, 143, 57–66. [Google Scholar] [CrossRef]
- Chang, M.H.; Chen, F.; Fang, N.S. Analysis of membraneless fuel cell using laminar flow in a Y-shaped microchannel. J. Power Sources 2006, 159, 810–816. [Google Scholar] [CrossRef]
- Hollinger, A.S.; Kenis, P.J.A. Manufacturing all-polymer laminar flow-based fuel cells. J. Power Sources 2013, 240, 486–493. [Google Scholar] [CrossRef]
- Salloum, K.S.; Hayes, J.R.; Friesen, C.A.; Posner, J.D. Sequential flow membraneless microfluidic fuel cell with porous electrodes. J. Power Sources 2008, 180, 243–252. [Google Scholar] [CrossRef]
- Tominaka, S.; Obata, H.; Osaka, T. On-chip direct methanol fuel cells of a monolithic design: Consideration on validity of active-type system. Energy Environ. Sci. 2009, 2, 845–848. [Google Scholar] [CrossRef]
- Arun, R.K.; Bekele, W.; Ghatak, A. Self oscillating potential generated in patterned micro-fluidic fuel cell. Electrochim. Acta 2013, 87, 489–496. [Google Scholar] [CrossRef]
- Cuevas-Muñiz, F.M.; Guerra-Balcázar, M.; Castaneda, F.; Ledesma-García, J.; Arriaga, L.G. Performance of Au and AuAg nanoparticles supported on Vulcan in a glucose laminar membraneless microfuel cell. J. Power Sources 2011, 196, 5853–5857. [Google Scholar] [CrossRef]
- Hasegawa, S.; Shimotani, K.; Kishi, K.; Watanabe, H. Electricity generation from decomposition of hydrogen peroxide. Electrochem. Solid State Lett. 2005, 8, A119. [Google Scholar] [CrossRef]
- Lim, K.G.; Palmore, G.T.R. Microfluidic biofuel cells: The influence of electrode diffusion layer on performance. Biosens. Bioelectron. 2007, 22, 941–947. [Google Scholar] [CrossRef]
- Morales-Acosta, D.; Morales-Acosta, M.D.; Godinez, L.A.; Álvarez-Contreras, L.; Duron-Torres, S.M.; Ledesma-García, J.; Arriaga, L.G. PdCo supported on multiwalled carbon nanotubes as an anode catalyst in a microfluidic formic acid fuel cell. J. Power Sources 2011, 196, 9270–9275. [Google Scholar] [CrossRef]
- Shyu, J.C.; Wei, C.S.; Lee, C.J.; Wang, C.C. Investigation of bubble effect in microfluidic fuel cells by a simplified microfluidic reactor. Appl. Therm. Eng. 2010, 30, 1863–1871. [Google Scholar] [CrossRef]
- López-Montesinos, P.O.; Yossakda, N.; Schmidt, A.; Brushett, F.R.; Pelton, W.E.; Kenis, P.J.A. Design, fabrication, and characterization of a planar, silicon-based, monolithically integrated micro laminar flow fuel cell with a bridge-shaped microchannel cross-section. J. Power Sources 2011, 196, 4638–4645. [Google Scholar] [CrossRef]
- Sun, M.H.; Velve Casquillas, G.; Guo, S.S.; Shi, J.; Ji, H.; Ouyang, Q.; Chen, Y. Characterization of microfluidic fuel cell based on multiple laminar flow. Microelectron. Eng. 2007, 84, 1182–1185. [Google Scholar] [CrossRef]
- Shyu, J.C.; Huang, C.L. Characterization of bubble formation in microfluidic fuel cells employing hydrogen peroxide. J. Power Sources 2011, 196, 3233–3238. [Google Scholar] [CrossRef]
- Kjeang, E.; Brolo, A.G.; Harrington, D.A.; Djilali, N.; Sinton, D. Hydrogen Peroxide as an Oxidant for Microfluidic Fuel Cells. J. Electrochem. Soc. 2007, 154, B1220–B1226. [Google Scholar] [CrossRef]
- Wang, H.Y.; Su, J.Y. Membraneless microfluidic microbial fuel cell for rapid detection of electrochemical activity of microorganism. Bioresour. Technol. 2013, 145, 271–274. [Google Scholar] [CrossRef]
- Zebda, A.; Renaud, L.; Cretin, M.; Pichot, F.; Innocent, C.; Ferrigno, R.; Tingry, S. A microfluidic glucose biofuel cell to generate micropower from enzymes at ambient temperature. Electrochem. Commun. 2009, 11, 592–595. [Google Scholar] [CrossRef]
- Zebda, A.; Renaud, L.; Cretin, M.; Innocent, C.; Ferrigno, R.; Tingry, S. Membraneless microchannel glucose biofuel cell with improved electrical performances. Sens. Actuators B Chem. 2010, 149, 44–50. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; LeDuc, P.R.; Gregory, K.B. Microbial electricity generation via microfluidic flow control. Biotechnol. Bioeng. 2011, 108, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.L.; Westly, D.A.; Pechenik, A.; Abruña, H.D. Fabrication and preliminary testing of a planar membraneless microchannel fuel cell. J. Power Sources 2005, 139, 96–105. [Google Scholar] [CrossRef]
- Déctor, A.; Esquivel, J.P.; González, M.J.; Guerra-Balcázar, M.; Ledesma-García, J.; Sabaté, N.; Arriaga, L.G. Formic acid microfluidic fuel cell evaluation in different oxidant conditions. Electrochim. Acta 2013, 92, 31–35. [Google Scholar] [CrossRef]
- Morales-Acosta, D.; Rodríguez, G.H.; Godinez, L.A.; Arriaga, L.G. Performance increase of microfluidic formic acid fuel cell using Pd/MWCNTs as catalyst. J. Power Sources 2010, 195, 1862–1865. [Google Scholar] [CrossRef]
- Yoon, S.K.; Fichtl, G.W.; Kenis, P.J.A. Active control of the depletion boundary layers in microfluidic electrochemical reactors. Lab Chip 2006, 6, 1516–1524. [Google Scholar] [CrossRef]
- Sprague, I.B.; Dutta, P.; Ha, S. Characterization of a membraneless direct-methanol micro fuel cell. Proc. Inst. Mech. Eng. Part A J. Power Energy 2009, 223, 799–808. [Google Scholar] [CrossRef]
- Choban, E.R.; Waszczuk, P.; Kenis, P.J.A. Characterization of limiting factors in laminar flow-based membraneless microfuel cells. Electrochem. Solid State Lett. 2005, 8. [Google Scholar] [CrossRef]
- Choban, E.R.; Spendelow, J.S.; Gancs, L.; Wieckowski, A.; Kenis, P.J.A. Membraneless laminar flow-based micro fuel cells operating in alkaline, acidic, and acidic/alkaline media. Electrochim. Acta 2005, 50, 5390–5398. [Google Scholar] [CrossRef]
- Galindo, R.; Dector, A.; Arriaga, L.G.; Gutiérrez, S.; Herrasti, P. Maghemite as a catalyst for glucose oxidation in a microfluidic fuel cell. J. Electroanal. Chem. 2012, 671, 38–43. [Google Scholar] [CrossRef]
- A Laser-Micromachined Polymeric Membraneless Fuel Cell—IOPscience. Available online: https://iopscience.iop.org/article/10.1088/0960-1317/17/6/002 (accessed on 5 April 2020).
- Sung, W.; Choi, J.W. A membraneless microscale fuel cell using non-noble catalysts in alkaline solution. J. Power Sources 2007, 172, 198–208. [Google Scholar] [CrossRef]
- Salloum, K.S.; Posner, J.D. Counter flow membraneless microfluidic fuel cell. J. Power Sources 2010, 195, 6941–6944. [Google Scholar] [CrossRef]
- Wang, H.; Gu, S.; Leung, D.Y.C.; Xu, H.; Leung, M.K.H.; Zhang, L.; Xuan, J. Development and characteristics of a membraneless microfluidic fuel cell array. Electrochim. Acta 2014, 135, 467–477. [Google Scholar] [CrossRef]
- Xuan, J.; Leung, D.Y.C.; Leung, M.K.H.; Wang, H.; Ni, M. Chaotic flow-based fuel cell built on counter-flow microfluidic network: Predicting the over-limiting current behavior. J. Power Sources 2011, 196, 9391–9397. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Zhang, L.; Leung, D.Y.C.; Wang, H.; Xuan, J. A Counter-flow Microfluidic Fuel Cell Achieving Concentrated Fuel Operation. Energy Procedia 2015, 75, 1990–1995. [Google Scholar] [CrossRef]
- Li, L.; Zheng, K.; Ni, M.; Leung, M.K.H.; Xuan, J. Partial modification of flow-through porous electrodes in microfluidic fuel cell. Energy 2015, 88, 563–571. [Google Scholar] [CrossRef]
- Tanveer, M.; Kim, K.-Y. Performance analysis of microfluidic fuel cells with various inlet locations and multiple compartments. Energy Convers. Manag. 2018, 166. [Google Scholar] [CrossRef]
- Cinti, S.; Minotti, C.; Moscone, D.; Palleschi, G.; Arduini, F. Fully integrated ready-to-use paper-based electrochemical biosensor to detect nerve agents. Biosens. Bioelectron. 2017, 93, 46–51. [Google Scholar] [CrossRef]
- Nie, Z.; Nijhuis, C.A.; Gong, J.; Chen, X.; Kumachev, A.; Martinez, A.W.; Narovlyansky, M.; Whitesides, G.M. Electrochemical sensing in paper-based microfluidic devices. Lab Chip 2010, 10, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ge, L.; Ge, S.; Yan, M.; Yu, J.; Huang, J.; Liu, S. Three-dimensional paper-based electrochemiluminescence device for simultaneous detection of Pb2+ and Hg2+ based on potential-control technique. Biosens. Bioelectron. 2013, 41, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, C.; Liu, X. A paper-based microfluidic biosensor integrating zinc oxide nanowires for electrochemical glucose detection. Microsyst. Nanoeng. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Liu, H.; Xiang, Y.; Lu, Y.; Crooks, R.M. Aptamer-Based Origami Paper Analytical Device for Electrochemical Detection of Adenosine. Angew. Chem. 2012, 51, 6925–6928. [Google Scholar] [CrossRef] [PubMed]
- Zang, D.; Ge, L.; Yan, M.; Song, X.; Yu, J. Electrochemical immunoassay on a 3D microfluidic paper-based device. Chem. Commun. 2012, 48, 4683–4685. [Google Scholar] [CrossRef]
- Shen, L.L.; Zhang, G.R.; Li, W.; Biesalski, M.; Etzold, B.J.M. Modifier-Free Microfluidic Electrochemical Sensor for Heavy-Metal Detection. ACS Omega 2017, 2, 4593–4603. [Google Scholar] [CrossRef]
- Sun, G.; Wang, P.; Ge, S.; Ge, L.; Yu, J.; Yan, M. Photoelectrochemical sensor for pentachlorophenol on microfluidic paper-based analytical device based on the molecular imprinting technique. Biosens. Bioelectron. 2014, 56, 97–103. [Google Scholar] [CrossRef]
- Dossi, N.; Toniolo, R.; Pizzariello, A.; Impellizzieri, F.; Piccin, E.; Bontempelli, G. Pencil-drawn paper supported electrodes as simple electrochemical detectors for paper-based fluidic devices. Electrophoresis 2013, 34, 2085–2091. [Google Scholar] [CrossRef]
- Li, Z.; Li, F.; Hu, J.; Wee, W.H.; Han, Y.L.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Direct writing electrodes using a ball pen for paper-based point-of-care testing. Analyst 2015, 140, 5526–5535. [Google Scholar] [CrossRef]
- Hayes, J.R.; Engstrom, A.M.; Friesen, C. Orthogonal flow membraneless fuel cell. J. Power Sources 2008, 183, 257–259. [Google Scholar] [CrossRef]
- Kjeang, E.; McKechnie, J.; Sinton, D.; Djilali, N. Planar and three-dimensional microfluidic fuel cell architectures based on graphite rod electrodes. J. Power Sources 2007, 168, 379–390. [Google Scholar] [CrossRef]
- Tominaka, S.; Ohta, S.; Obata, H.; Momma, T.; Osaka, T. On-chip fuel cell: Micro direct methanol fuel cell of an air-breathing, membraneless, and monolithic design. J. Am. Chem. Soc. 2008, 130, 10456–10457. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, R.; Wang, W.; Yang, G.; Leung, M.K.H.; Liu, F.; Feng, S.-P. Dual-electrolyte aluminum/air microfluidic fuel cell with electrolyte-recirculation. Electrochim. Acta 2021, 388, 138584. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Y.; Ye, D.-D.; Chen, R.; Zhang, T.; Liao, Q. Discrete-holes film fueling anode heads for high performance air-breathing microfluidic fuel cell. J. Power Sources 2021, 482, 228966. [Google Scholar] [CrossRef]
- Kjeang, E.; Michel, R.; Harrington, D.A.; Djilali, N.; Sinton, D. A microfluidic fuel cell with flow-through porous electrodes. J. Am. Chem. Soc. 2008, 130. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.C. A high-performance aluminum-feed microfluidic fuel cell stack. J. Power Sources 2016, 336, 427–436. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.; Go, M.; Park, J.Y. Stand-alone external power-free microfluidic fuel cell system harnessing osmotic pump for long-term operation. J. Micromech. Microeng. 2018, 28, 125005. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Liu, X.; Zhao, W.; Zong, C.; Gai, H. A self-driven miniaturized liquid fuel cell. Chem. Commun. 2016, 52, 12068–12071. [Google Scholar] [CrossRef]
- Hur, J.I.; Meng, D.D.; Kim, C.J. Self-Pumping membraneless miniature fuel cell with an air-breathing cathode. J. Microelectromech. Syst. 2012, 21, 476–483. [Google Scholar] [CrossRef]
- Esquivel, J.P.; Del Campo, F.J.; Gómez De La Fuente, J.L.; Rojas, S.; Sabaté, N. Microfluidic fuel cells on paper: Meeting the power needs of next generation lateral flow devices. Energy Environ. Sci. 2014, 7, 1744–1749. [Google Scholar] [CrossRef]
- Tanveer, M.; Kim, K.-Y. Effects of bridge-shaped microchannel geometry on the performance of a micro laminar flow fuel cell. Micromachines 2019, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, H.N.; Zhu, X.; Ye, D.D.; Liao, Q.; Sui, P.C.; Djilali, N.; Jiang, L.; Fu, Y.L. Effect of geometrical configurations on alkaline air-breathing membraneless microfluidic fuel cells with cylinder anodes. Sci. China Technol. Sci. 2019, 62, 388–396. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, B.; Ye, D.D.; Li, J.; Liao, Q. Air-breathing direct formic acid microfluidic fuel cell with an array of cylinder anodes. J. Power Sources 2014, 247, 346–353. [Google Scholar] [CrossRef]
- Ye, D.D.; Zhang, B.; Zhu, X.; Sui, P.C.; Djilali, N.; Liao, Q. Computational modeling of alkaline air-breathing microfluidic fuel cells with an array of cylinder anodes. J. Power Sources 2015, 288, 150–159. [Google Scholar] [CrossRef]
- Ha, S.M.; Ahn, Y. Laminar flow-based micro fuel cell utilizing grooved electrode surface. J. Power Sources 2014, 267, 731–738. [Google Scholar] [CrossRef]
- Influence of Electrode Groove Geometry on the Passive Control of the Depletion Layer in Microfluidic Fuel Cells—IOPscience. Available online: https://iopscience.iop.org/article/10.1088/0960-1317/25/12/127001 (accessed on 5 April 2020).
- Ebrahimi Khabbazi, A.; Richards, A.J.; Hoorfar, M. Numerical study of the effect of the channel and electrode geometry on the performance of microfluidic fuel cells. J. Power Sources 2010, 195, 8141–8151. [Google Scholar] [CrossRef]
- Thorson, M.R.; Brushett, F.R.; Timberg, C.J.; Kenis, P.J.A. Design rules for electrode arrangement in an air-breathing alkaline direct methanol laminar flow fuel cell. J. Power Sources 2012, 218, 28–33. [Google Scholar] [CrossRef]
- Xuan, J.; Leung, D.Y.C.; Leung, M.K.H.; Ni, M.; Wang, H. A computational study of bifunctional oxygen electrode in air-breathing reversible microfluidic fuel cells. Int. J. Hydrogen Energy 2011, 36, 9231–9241. [Google Scholar] [CrossRef]
- Armenta-González, A.J.; Carrera-Cerritos, R.; Moreno-Zuria, A.; Álvarez-Contreras, L.; Ledesma-García, J.; Cuevas-Muñiz, F.M.; Arriaga, L.G. An improved ethanol microfluidic fuel cell based on a PdAg/MWCNT catalyst synthesized by the reverse micelles method. Fuel 2016, 167, 240–247. [Google Scholar] [CrossRef]
- Escalona-Villalpando, R.A.; Dector, A.; Dector, D.; Moreno-Zuria, A.; Durón-Torres, S.M.; Galván-Valencia, M.; Arriaga, L.G.; Ledesma-García, J. Glucose microfluidic fuel cell using air as oxidant. Int. J. Hydrogen Energy 2016, 41, 23394–23400. [Google Scholar] [CrossRef]
- Shaegh, S.A.M.; Nguyen, N.T.; Chan, S.H. An air-breathing microfluidic formic acid fuel cell with a porous planar anode: Experimental and numerical investigations. J. Micromech. Microeng. 2010, 20. [Google Scholar] [CrossRef]
- Ortiz-Ortega, E.; Goulet, M.A.; Lee, J.W.; Guerra-Balcázar, M.; Arjona, N.; Kjeang, E.; Ledesma-García, J.; Arriaga, L.G. A nanofluidic direct formic acid fuel cell with a combined flow-through and air-breathing electrode for high performance. Lab Chip 2014, 14, 4596–4598. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, B.; Zhu, X.; Ye, D.D.; Chen, R.; Zhang, T.; Gong, X.L.; Liao, Q. Enhancing fuel transport in air-breathing microfluidic fuel cells by immersed fuel micro-jet. J. Power Sources 2020, 445. [Google Scholar] [CrossRef]
- Shyu, J.C.; Wang, P.Y.; Lee, C.L.; Chang, S.C.; Sheu, T.S.; Kuo, C.H.; Huang, K.L.; Yang, Z.Y. Fabrication and test of an air-breathing microfluidic fuel cell. Energies 2015, 8, 2082–2096. [Google Scholar] [CrossRef]
- Mousavi Shaegh, S.A.; Nguyen, N.T.; Chan, S.H. Air-breathing microfluidic fuel cell with fuel reservoir. J. Power Sources 2012, 209, 312–317. [Google Scholar] [CrossRef][Green Version]
- Zhang, B.; Ye, D.D.; Sui, P.C.; Djilali, N.; Zhu, X. Computational modeling of air-breathing microfluidic fuel cells with flow-over and flow-through anodes. J. Power Sources 2014, 259, 15–24. [Google Scholar] [CrossRef]
- Xuan, J.; Leung, D.Y.C.; Wang, H.; Leung, M.K.H.; Wang, B.; Ni, M. Air-breathing membraneless laminar flow-based fuel cells: Do they breathe enough oxygen? Appl. Energy 2013, 104, 400–407. [Google Scholar] [CrossRef]
- Jayashree, R.S.; Yoon, S.K.; Brushett, F.R.; Lopez-Montesinos, P.O.; Natarajan, D.; Markoski, L.J.; Kenis, P.J.A. On the performance of membraneless laminar flow-based fuel cells. J. Power Sources 2010, 195, 3569–3578. [Google Scholar] [CrossRef]
- Whipple, D.T.; Jayashree, R.S.; Egas, D.; Alonso-Vante, N.; Kenis, P.J.A. Ruthenium cluster-like chalcogenide as a methanol tolerant cathode catalyst in air-breathing laminar flow fuel cells. Electrochim. Acta 2009, 54, 4384–4388. [Google Scholar] [CrossRef]
- López-González, B.; Jiménez-Valdés, R.J.; Moreno-Zuria, A.; Cuevas-Muñiz, F.M.; Ledesma-García, J.; García-Cordero, J.L.; Arriaga, L.G. Waste-to-energy conversion from a microfluidic device. J. Power Sources 2017, 360, 80–86. [Google Scholar] [CrossRef]
- Jayashree, R.S.; Egas, D.; Spendelow, J.S.; Natarajan, D.; Markoski, L.J.; Kenis, P.J.A. Air-breathing laminar flow-based direct methanol fuel cell with alkaline electrolyte. Electrochem. Solid State Lett. 2006, 9. [Google Scholar] [CrossRef]
- Brushett, F.R.; Jayashree, R.S.; Zhou, W.P.; Kenis, P.J.A. Investigation of fuel and media flexible laminar flow-based fuel cells. Electrochim. Acta 2009, 54, 7099–7105. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yang, P. Performance of an air-breathing direct methanol fuel cell. J. Power Sources 2003, 123, 37–42. [Google Scholar] [CrossRef]
- Goulet, M.A.; Ibrahim, O.A.; Kim, W.H.J.; Kjeang, E. Maximizing the power density of aqueous electrochemical flow cells with in operando deposition. J. Power Sources 2017, 339, 80–85. [Google Scholar] [CrossRef]
- Kjeang, E.; Michel, R.; Harrington, D.A.; Sinton, D.; Djilali, N. An alkaline microfluidic fuel cell based on formate and hypochlorite bleach. Electrochim. Acta 2008, 54, 698–705. [Google Scholar] [CrossRef]
- Fuerth, D.; Bazylak, A. Up-scaled microfluidic fuel cells with porous flow-through electrodes. J. Fluids Eng. Trans. ASME 2013, 135. [Google Scholar] [CrossRef]
- Lee, J.W.; Kjeang, E. Nanofluidic fuel cell. J. Power Sources 2013, 242, 472–477. [Google Scholar] [CrossRef]
- Lee, J.W.; Kjeang, E. Chip-embedded thin film current collector for microfluidic fuel cells. Int. J. Hydrogen Energy 2012, 37, 9359–9367. [Google Scholar] [CrossRef]
- Mousavi Shaegh, S.A.; Nguyen, N.T.; Mousavi Ehteshami, S.M.; Chan, S.H. A membraneless hydrogen peroxide fuel cell using Prussian Blue as cathode material. Energy Environ. Sci. 2012, 5, 8225–8228. [Google Scholar] [CrossRef]
- Mousavi Shaegh, S.A.; Nguyen, N.T.; Chan, S.H.; Zhou, W. Air-breathing membraneless laminar flow-based fuel cell with flow-through anode. Int. J. Hydrogen Energy 2012, 37, 3466–3476. [Google Scholar] [CrossRef]
- Moore, S.; Sinton, D.; Erickson, D. A plate-frame flow-through microfluidic fuel cell stack. J. Power Sources 2011, 196, 9481–9487. [Google Scholar] [CrossRef]
- Li, L.; Nikiforidis, G.; Leung, M.K.H.; Daoud, W.A. Vanadium microfluidic fuel cell with novel multi-layer flow-through porous electrodes: Model, simulations and experiments. Appl. Energy 2016, 177, 729–739. [Google Scholar] [CrossRef]
- Lee, S.H.; Ahn, Y. Upscaling of microfluidic fuel cell using planar single stacks. Int. J. Energy Res. 2019, 43, 5027–5037. [Google Scholar] [CrossRef]
- Goulet, M.A.; Kjeang, E. Reactant recirculation in electrochemical co-laminar flow cells. Electrochim. Acta 2014, 140, 217–224. [Google Scholar] [CrossRef]
- Lee, J.W.; Goulet, M.A.; Kjeang, E. Microfluidic redox battery. Lab Chip 2013, 13, 2504–2507. [Google Scholar] [CrossRef]
- Jayashree, R.S.; Mitchell, M.; Natarajan, D.; Markoski, L.J.; Kenis, P.J.A. Microfluidic hydrogen fuel cell with a liquid electrolyte. Langmuir 2007, 23, 6871–6874. [Google Scholar] [CrossRef]
- Lee, S.H.; Ahn, Y. A laminar flow-based single stack of flow-over planar microfluidic fuel cells. J. Power Sources 2017, 351, 67–73. [Google Scholar] [CrossRef]
- Ahmed, D.H.; Park, H.B.; Sung, H.J. Optimum geometrical design for improved fuel utilization in membraneless micro fuel cell. J. Power Sources 2008, 185, 143–152. [Google Scholar] [CrossRef]
- Wu, R.; Ye, D.; Chen, R.; Zhang, B.; Zhu, X.; Guo, H.; Liu, Z. A membraneless microfluidic fuel cell with continuous multistream flow through cotton threads. Int. J. Energy Res. 2020, 44, 2243–2251. [Google Scholar] [CrossRef]
- Kwok, Y.H.; Wang, Y.; Wu, M.; Li, F.; Zhang, Y.; Zhang, H.; Leung, D.Y.C. A dual fuel microfluidic fuel cell utilizing solar energy and methanol. J. Power Sources 2019, 409, 58–65. [Google Scholar] [CrossRef]
- Lu, X.; Xuan, J.; Leung, D.Y.C.; Zou, H.; Li, J.; Wang, H.; Wang, H. A switchable pH-differential unitized regenerative fuel cell with high performance. J. Power Sources 2016, 314, 76–84. [Google Scholar] [CrossRef]
- Ibrahim, O.A.; Goulet, M.A.; Kjeang, E. In-situ characterization of symmetric dual-pass architecture of microfluidic co-laminar flow cells. Electrochim. Acta 2016, 187, 277–285. [Google Scholar] [CrossRef]
- Ibrahim, O.A.; Kjeang, E. Leveraging co-laminar flow cells for non-aqueous electrochemical systems. J. Power Sources 2018, 402, 7–14. [Google Scholar] [CrossRef]
- Ho, B.; Kjeang, E. Planar multiplexing of microfluidic fuel cells. J. Fluids Eng. Trans. ASME 2013, 135. [Google Scholar] [CrossRef]
- An, L.; Zhao, T.; Yan, X.; Zhou, X.; Tan, P. The dual role of hydrogen peroxide in fuel cells. Sci. Bull. 2015, 60, 55–64. [Google Scholar] [CrossRef]
- Togo, M.; Takamura, A.; Asai, T.; Kaji, H.; Nishizawa, M. An enzyme-based microfluidic biofuel cell using vitamin K3-mediated glucose oxidation. Electrochim. Acta 2007, 52, 4669–4674. [Google Scholar] [CrossRef]
- Togo, M.; Takamura, A.; Asai, T.; Kaji, H.; Nishizawa, M. Structural studies of enzyme-based microfluidic biofuel cells. J. Power Sources 2008, 178, 53–58. [Google Scholar] [CrossRef]
- Arun, R.K.; Sardar, M.; Singh, P.; Jha, B.M.; Chanda, N. A spiral shaped regenerative microfluidic fuel cell with Ni-C based porous electrodes. Int. J. Energy Res. 2019, 43, 8834–8840. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Leung, D.Y.C.; Xuan, J.; Wang, H. A counter-flow-based dual-electrolyte protocol for multiple electrochemical applications. Appl. Energy 2018, 217, 241–248. [Google Scholar] [CrossRef]
- Lu, X.; Leung, D.Y.C.; Wang, Y.; Wang, H.; Xuan, J. An Up-scaling Strategy for Counter-flow Based Microfluidic Network: A Numerical Study. Energy Procedia 2017, 142, 661–666. [Google Scholar] [CrossRef]
- Wang, H.; Leung, D.Y.C.; Xuan, J. Modeling of an air cathode for microfluidic fuel cells: Transport and polarization behaviors. Int. J. Hydrogen Energy 2011, 36, 14704–14718. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, H.; Wang, H.; Leung, D.Y.C.; Zhang, L.; Cao, J.; Jiao, K.; Xuan, J. Counter-flow formic acid microfluidic fuel cell with high fuel utilization exceeding 90%. Appl. Energy 2015, 160, 930–936. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.C.; Zhang, H.; Xuan, J.; Wang, H. Numerical and experimental comparative study of microfluidic fuel cells with different flow configurations: Co-flow vs. counter-flow cell. Appl. Energy 2017, 203, 535–548. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.C. Toward the scaling up of microfluidic fuel cells, investigation and optimization of the aggravated cathode flooding problem. Electrochim. Acta 2016, 222, 312–322. [Google Scholar] [CrossRef]
- Tanveer, M.; Kim, K.-Y. Performance analysis of a micro laminar flow fuel cell with multiple inlets of a bridge-shaped microchannel. J. Power Sources 2018, 399. [Google Scholar] [CrossRef]
- Li, L.; Bei, S.; Liu, R.; Xu, Q.; Zheng, K.; She, Y.; He, Y. Design of a radial vanadium redox microfluidic fuel cell: A new way to break the size limitation. Int. J. Energy Res. 2019, 43, 3028–3037. [Google Scholar] [CrossRef]
- Osborn, J.L.; Lutz, B.; Fu, E.; Kauffman, P.; Stevens, D.Y.; Yager, P. Microfluidics without pumps: Reinventing the T-sensor and H-filter in paper networks. Lab Chip 2010, 10, 2659–2665. [Google Scholar] [CrossRef]
- Tata Rao, L.; Rewatkar, P.; Dubey, S.K.; Javed, A.; Goel, S. Performance optimization of microfluidic paper fuel-cell with varying cellulose fiber papers as absorbent pad. Int. J. Energy Res. 2020, 44, 3893–3904. [Google Scholar] [CrossRef]
- Arun, R.K.; Gupta, V.; Singh, P.; Biswas, G.; Chanda, N. Selection of Graphite Pencil Grades for the Design of Suitable Electrodes for Stacking Multiple Single-Inlet Paper-Pencil Fuel Cells. ChemistrySelect 2019, 4, 152–159. [Google Scholar] [CrossRef]
- Yan, X.; Xu, A.; Zeng, L.; Gao, P.; Zhao, T. A Paper-Based Microfluidic Fuel Cell with Hydrogen Peroxide as Fuel and Oxidant. Energy Technol. 2018, 6, 140–143. [Google Scholar] [CrossRef]
- del Torno-de Román, L.; Navarro, M.; Hughes, G.; Esquivel, J.P.; Milton, R.D.; Minteer, S.D.; Sabaté, N. Improved performance of a paper-based glucose fuel cell by capillary induced flow. Electrochim. Acta 2018, 282, 336–342. [Google Scholar] [CrossRef]
- Shen, L.L.; Zhang, G.R.; Venter, T.; Biesalski, M.; Etzold, B.J.M. Towards best practices for improving paper-based microfluidic fuel cells. Electrochim. Acta 2019, 298, 389–399. [Google Scholar] [CrossRef]
- Jung, D.G.; Ahn, Y. Microfabricated paper-based vanadium co-laminar flow fuel cell. J. Power Sources 2020, 451. [Google Scholar] [CrossRef]
- Pasala, V.; Ramanujam, K. Flexible paper-based borohydride-vanadium fuel cell for powering micro-nanosystems. Ionics 2017, 23, 1811–1817. [Google Scholar] [CrossRef]
- Chino, I.; Muneeb, O.; Do, E.; Ho, V.; Haan, J.L. A paper microfluidic fuel cell powered by urea. J. Power Sources 2018, 396, 710–714. [Google Scholar] [CrossRef]
- Galvan, V.; Domalaon, K.; Tang, C.; Sotez, S.; Mendez, A.; Jalali-Heravi, M.; Purohit, K.; Pham, L.; Haan, J.; Gomez, F.A. An improved alkaline direct formate paper microfluidic fuel cell. Electrophoresis 2016, 37, 504–510. [Google Scholar] [CrossRef]
- Esquivel, J.P.; Buser, J.R.; Lim, C.W.; Domínguez, C.; Rojas, S.; Yager, P.; Sabaté, N. Single-use paper-based hydrogen fuel cells for point-of-care diagnostic applications. J. Power Sources 2017, 342, 442–451. [Google Scholar] [CrossRef]
- Chandra, S.; Lal, S.; Janardhanan, V.M.; Sahu, K.C.; Deepa, M. Ethanol based fuel cell on paper support. J. Power Sources 2018, 396, 725–733. [Google Scholar] [CrossRef]
- Lal, S.; Deepa, M.; Janardhanan, V.M.; Sahu, K.C. Paper based hydrazine monohydrate fuel cells with Cu and C composite catalysts. Electrochim. Acta 2017, 232, 262–270. [Google Scholar] [CrossRef]
- Lal, S.; Janardhanan, V.M.; Deepa, M.; Sagar, A.; Sahu, K.C. Low Cost Environmentally Benign Porous Paper Based Fuel Cells for Micro-Nano Systems. J. Electrochem. Soc. 2015, 162, F1402–F1407. [Google Scholar] [CrossRef]
- González-Guerrero, M.J.; del Campo, F.J.; Esquivel, J.P.; Giroud, F.; Minteer, S.D.; Sabaté, N. Paper-based enzymatic microfluidic fuel cell: From a two-stream flow device to a single-stream lateral flow strip. J. Power Sources 2016, 326, 410–416. [Google Scholar] [CrossRef]
- Purohit, K.H.; Emrani, S.; Rodriguez, S.; Liaw, S.S.; Pham, L.; Galvan, V.; Domalaon, K.; Gomez, F.A.; Haan, J.L. A microfluidic galvanic cell on a single layer of paper. J. Power Sources 2016, 318, 163–169. [Google Scholar] [CrossRef]
- Arun, R.K.; Halder, S.; Chanda, N.; Chakraborty, S. A paper based self-pumping and self-breathing fuel cell using pencil stroked graphite electrodes. Lab Chip 2014, 14, 1661–1664. [Google Scholar] [CrossRef]
- Copenhaver, T.S.; Purohit, K.H.; Domalaon, K.; Pham, L.; Burgess, B.J.; Manorothkul, N.; Galvan, V.; Sotez, S.; Gomez, F.A.; Haan, J.L. A microfluidic direct formate fuel cell on paper. Electrophoresis 2015, 36, 1825–1829. [Google Scholar] [CrossRef]
- Mousavi Ehteshami, S.M.; Asadnia, M.; Tan, S.N.; Chan, S.H. Paper-based membraneless hydrogen peroxide fuel cell prepared by micro-fabrication. J. Power Sources 2016, 301, 392–395. [Google Scholar] [CrossRef]
- Wang, Y.; Kwok, H.Y.H.; Zhang, Y.; Pan, W.; Zhang, H.; Lu, X.; Leung, D.Y.C. A flexible paper-based hydrogen fuel cell for small power applications. Int. J. Hydrogen Energy 2019, 44, 29680–29691. [Google Scholar] [CrossRef]
- Dector, A.; Galindo-de-la-Rosa, J.; Amaya-Cruz, D.M.; Ortíz-Verdín, A.; Guerra-Balcázar, M.; Olivares-Ramírez, J.M.; Arriaga, L.G.; Ledesma-García, J. Towards autonomous lateral flow assays: Paper-based microfluidic fuel cell inside an HIV-test using a blood sample as fuel. Int. J. Hydrogen Energy 2017, 42, 27979–27986. [Google Scholar] [CrossRef]
- Domalaon, K.; Tang, C.; Mendez, A.; Bernal, F.; Purohit, K.; Pham, L.; Haan, J.; Gomez, F.A. Fabric-based alkaline direct formate microfluidic fuel cells. Electrophoresis 2017, 38, 1224–1231. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, D.; Chen, R.; Zhang, B.; Zhu, X.; Li, J.; Liao, Q. A woven thread-based microfluidic fuel cell with graphite rod electrodes. Int. J. Hydrogen Energy 2018, 43, 22467–22473. [Google Scholar] [CrossRef]
- Pasala, V.; Ramanujam, K. Paper-Based Disposable Zinc-Vanadium Fuel Cell for Micropower Applications. ChemistrySelect 2019, 4, 8398–8403. [Google Scholar] [CrossRef]
- Salloum, K.S.; Posner, J.D. A membraneless microfluidic fuel cell stack. J. Power Sources 2011, 196, 1229–1234. [Google Scholar] [CrossRef]
- Abrego-Martínez, J.C.; Moreno-Zuria, A.; Cuevas-Muñiz, F.M.; Arriaga, L.G.; Sun, S.; Mohamedi, M. Design, fabrication and performance of a mixed-reactant membraneless micro direct methanol fuel cell stack. J. Power Sources 2017, 371, 10–17. [Google Scholar] [CrossRef]
- Moreno-Zuria, A.; Ortiz-Ortega, E.; Gurrola, M.P.; Chávez-Ramírez, A.U.; Ledesma-García, J.; Arriaga, L.G. Evolution of microfluidic fuel stack design as an innovative alternative to energy production. Int. J. Hydrogen Energy 2017, 42, 27929–27939. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.C.; Xuan, J.; Wang, H. A review on unitized regenerative fuel cell technologies, part B: Unitized regenerative alkaline fuel cell, solid oxide fuel cell, and microfluidic fuel cell. Renew. Sustain. Energy Rev. 2017, 75, 775–795. [Google Scholar] [CrossRef]
- Bamgbopa, M.O.; Almheiri, S.; Sun, H. Prospects of recently developed membraneless cell designs for redox flow batteries. Renew. Sustain. Energy Rev. 2017, 70, 506–518. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.C. A circular stacking strategy for microfluidic fuel cells with volatile methanol fuel. Appl. Energy 2016, 184, 659–669. [Google Scholar] [CrossRef]
- Ibrahim, O.A.; Goulet, M.-A.; Kjeang, E. Microfluidic Electrochemical Cell Array in Series: Effect of Shunt Current. J. Electrochem. Soc. 2015, 162, F639–F644. [Google Scholar] [CrossRef]
- Galindo-de-la-Rosa, J.; Arjona, N.; Moreno-Zuria, A.; Ortiz-Ortega, E.; Guerra-Balcázar, M.; Ledesma-García, J.; Arriaga, L.G. Evaluation of single and stack membraneless enzymatic fuel cells based on ethanol in simulated body fluids. Biosens. Bioelectron. 2017, 92, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Escalona-Villalpando, R.A.; Hasan, K.; Milton, R.D.; Moreno-Zuria, A.; Arriaga, L.G.; Minteer, S.D.; Ledesma-García, J. Performance comparison of different configurations of Glucose/O2 microfluidic biofuel cell stack. J. Power Sources 2019, 414, 150–157. [Google Scholar] [CrossRef]
- Yang, W.; Lee, K.K.; Choi, S. A laminar-flow based microbial fuel cell array. Sens. Actuators B Chem. 2017, 243, 292–297. [Google Scholar] [CrossRef]
- Brushett, F.R.; Zhou, W.P.; Jayashree, R.S.; Kenis, P.J. Alkaline Microfluidic Hydrogen-Oxygen Fuel Cell as a Cathode Characterization Platform. J. Electrochem. Soc. 2009, 156, B565–B571. [Google Scholar] [CrossRef]
- Hollinger, A.S.; Maloney, R.J.; Jayashree, R.S.; Natarajan, D.; Markoski, L.J.; Kenis, P.J.A. Nanoporous separator and low fuel concentration to minimize crossover in direct methanol laminar flow fuel cells. J. Power Sources 2010, 195, 3523–3528. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.C.; Xuan, J.; Wang, H. A vapor feed methanol microfluidic fuel cell with high fuel and energy efficiency. Appl. Energy 2015, 147, 456–465. [Google Scholar] [CrossRef]
- Brushett, F.; Mitchell, M.; Jayashree, R.; Zhou, W.-P.; Kenis, P. Vapor Feed Direct Methanol Fuel Cell with Flowing Electrolyte. ECS Trans. 2007, 11, 1419–1424. [Google Scholar] [CrossRef]
- Yang, Y.; Xue, Y.; Huang, F.; Zhang, H.; Tao, K.; Zhang, R.; Shen, Q.; Chang, H. A Facile Microfluidic Hydrogen Peroxide Fuel Cell with High Performance: Electrode Interface and Power-Generation Properties. ACS Appl. Energy Mater. 2018, 1, 5328–5335. [Google Scholar] [CrossRef]
- Shyu, J.C.; Huang, C.L.; Sheu, T.S.; Ay, H. Experimental study of direct hydrogen peroxide microfluidic fuel cells. Micro Nano Lett. 2012, 7, 740–743. [Google Scholar] [CrossRef]
- Chen, F.; Chang, M.H.; Hsu, C.W. Analysis of membraneless microfuel cell using decomposition of hydrogen peroxide in a Y-shaped microchannel. Electrochim. Acta 2007, 52, 7270–7277. [Google Scholar] [CrossRef]
- Ye, D.; Yang, Y.; Li, J.; Zhu, X.; Liao, Q.; Zhang, B. A laminar flow microfluidic fuel cell for detection of hexavalent chromium concentration. Biomicrofluidics 2015, 9, 064110. [Google Scholar] [CrossRef]
- Moore, C.M.; Minteer, S.B.; Martin, R.S. Microchip-based ethanol/oxygen biofuel cell. Lab Chip 2005, 5, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Da Mota, N.; Finkelstein, D.A.; Kirtland, J.D.; Rodriguez, C.A.; Stroock, A.D.; Abruña, H.D. Membraneless, room-temperature, direct borohydride/cerium fuel cell with power density of over 0.25 W/cm2. J. Am. Chem. Soc. 2012, 134, 6076–6079. [Google Scholar] [CrossRef] [PubMed]
- Arjona, N.; Palacios, A.; Moreno Zuria, A.; Guerra-Balcázar, M.; Ledesma García, J.; Arriaga, L.G. AuPd/polyaniline as the anode in an ethylene glycol microfluidic fuel cell operated at room temperature. Chem. Commun. 2014, 50, 8151–8153. [Google Scholar] [CrossRef] [PubMed]
- Dector, A.; Cuevas-Muñiz, F.M.; Guerra-Balcázar, M.; Godínez, L.A.; Ledesma-García, J.; Arriaga, L.G. Glycerol oxidation in a microfluidic fuel cell using Pd/C and Pd/MWCNT anodes electrodes. Int. J. Hydrogen Energy 2013, 38, 12617–12622. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wu, Z.; Leung, D.Y.C. A direct urea microfluidic fuel cell with flow-through Ni-supported-carbon-nanotube-coated sponge as porous electrode. J. Power Sources 2017, 363, 61–69. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, Y.F.; Kwok, Y.H.; Wu, Z.C.; Xia, D.H.; Leung, D.Y.C. A Direct Ammonia Microfluidic Fuel Cell using NiCu Nanoparticles Supported on Carbon Nanotubes as an Electrocatalyst. ChemSusChem 2018, 11, 2889–2897. [Google Scholar] [CrossRef]
- Braff, W.A.; Bazant, M.Z.; Buie, C.R. Membrane-less hydrogen bromine flow battery. Nat. Commun. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Martins, C.A.; Ibrahim, O.A.; Pei, P.; Kjeang, E. “Bleaching” glycerol in a microfluidic fuel cell to produce high power density at minimal cost. Chem. Commun. 2017, 54, 192–195. [Google Scholar] [CrossRef]
- Martins, C.A.; Ibrahim, O.A.; Pei, P.; Kjeang, E. Towards a fuel-flexible direct alcohol microfluidic fuel cell with flow-through porous electrodes: Assessment of methanol, ethylene glycol and glycerol fuels. Electrochim. Acta 2018, 271, 537–543. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, L.; Liu, Y.; Manthiram, A. A Membraneless Direct Isopropanol Fuel Cell (DIPAFC) Operated with a Catalyst-Selective Principle. J. Phys. Chem. C 2018, 122, 13558–13563. [Google Scholar] [CrossRef]
- Maya-Cornejo, J.; Ortiz-Ortega, E.; Álvarez-Contreras, L.; Arjona, N.; Guerra-Balcázar, M.; Ledesma-García, J.; Arriaga, L.G. Copper-palladium core-shell as an anode in a multi-fuel membraneless nanofluidic fuel cell: Toward a new era of small energy conversion devices. Chem. Commun. 2015, 51, 2536–2539. [Google Scholar] [CrossRef] [PubMed]
- Kwok, Y.H.; Tsang, A.C.H.; Wang, Y.; Leung, D.Y.C. Ultra-fine Pt nanoparticles on graphene aerogel as a porous electrode with high stability for microfluidic methanol fuel cell. J. Power Sources 2017, 349, 75–83. [Google Scholar] [CrossRef]
- Kwok, Y.H.; Wang, Y.F.; Tsang, A.C.H.; Leung, D.Y.C. Graphene-carbon nanotube composite aerogel with Ru@Pt nanoparticle as a porous electrode for direct methanol microfluidic fuel cell. Appl. Energy 2018, 217, 258–265. [Google Scholar] [CrossRef]
- Guo, S.; Sun, J.; Zhang, Z.; Sheng, A.; Gao, M.; Wang, Z.; Zhao, B.; Ding, W. Study of the electrooxidation of borohydride on a directly formed CoB/Ni-foam electrode and its application in membraneless direct borohydride fuel cells. J. Mater. Chem. A 2017, 5, 15879–15890. [Google Scholar] [CrossRef]
- Zhong, H.; Tian, R.; Gong, X.; Li, D.; Tang, P.; Alonso-Vante, N.; Feng, Y. Advanced bifunctional electrocatalyst generated through cobalt phthalocyanine tetrasulfonate intercalated Ni2Fe-layered double hydroxides for a laminar flow unitized regenerative micro-cell. J. Power Sources 2017, 361, 21–30. [Google Scholar] [CrossRef]
- Lee, J.; Keng, G.L.; Palmore, G.T.R.; Tripathi, A. Optimization of microfluidic fuel cells using transport principles. Anal. Chem. 2007, 79, 7301–7307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, D.; Chen, R.; Zhang, B.; Zhu, X.; Liao, Q. A dual-functional three-dimensional herringbone-like electrode for a membraneless microfluidic fuel cell. J. Power Sources 2019, 438, 227058. [Google Scholar] [CrossRef]
- López-González, B.; Dector, A.; Cuevas-Muñiz, F.M.; Arjona, N.; Cruz-Madrid, C.; Arana-Cuenca, A.; Guerra-Balcázar, M.; Arriaga, L.G.; Ledesma-García, J. Hybrid microfluidic fuel cell based on Laccase/C and AuAg/C electrodes. Biosens. Bioelectron. 2014, 62, 221–226. [Google Scholar] [CrossRef]
- Ye, D.; Yang, Y.; Li, J.; Zhu, X.; Liao, Q.; Deng, B.; Chen, R. Performance of a microfluidic microbial fuel cell based on graphite electrodes. Int. J. Hydrogen Energy 2013, 38, 15710–15715. [Google Scholar] [CrossRef]
- Brushett, F.R.; Duong, H.T.; Ng, D.; Behrens, R.L.; Wieckowski, A.; Kenis, P.J.A. Investigation of Pt, Pt3Co, and Pt3Co/Mo Cathodes for the ORR in a Microfluidic H2/O2 Fuel Cell. J. Electrochem. Soc. 2010, 157, B837. [Google Scholar] [CrossRef]
- Park, H.B.; Lee, K.H.; Sung, H.J. Performance of H-shaped membraneless micro fuel cells. J. Power Sources 2013, 226, 266–271. [Google Scholar] [CrossRef]
- Xu, Q.; She, Y.; Li, L. Model-based analysis of geometrical effects in microfluidic fuel cell with flow-through porous electrodes. Int. J. Mod. Phys. B 2019. [Google Scholar] [CrossRef]
- Marschewski, J.; Ruch, P.; Ebejer, N.; Huerta Kanan, O.; Lhermitte, G.; Cabrol, Q.; Michel, B.; Poulikakos, D. On the mass transfer performance enhancement of membraneless redox flow cells with mixing promoters. Int. J. Heat Mass Transf. 2017, 106, 884–894. [Google Scholar] [CrossRef]
- Miao, S.; He, S.; Liang, M.; Lin, G.; Cai, B.; Schmidt, O.G. Microtubular Fuel Cell with Ultrahigh Power Output per Footprint. Adv. Mater. 2017, 29, 1607046. [Google Scholar] [CrossRef]
- Yeh, E.C.; Fu, C.C.; Hu, L.; Thakur, R.; Feng, J.; Lee, L.P. Self-powered integrated microfluidic point-of-care low-cost enabling (SIMPLE) chip. Sci. Adv. 2017, 3, e1501645. [Google Scholar] [CrossRef]
- Gurrola, M.P.; Ortiz-Ortega, E.; Farias-Zuñiga, C.; Chávez-Ramírez, A.U.; Ledesma-García, J.; Arriaga, L.G. Evaluation and coupling of a membraneless nanofluidic device for low-power applications. J. Power Sources 2016, 307, 244–250. [Google Scholar] [CrossRef]









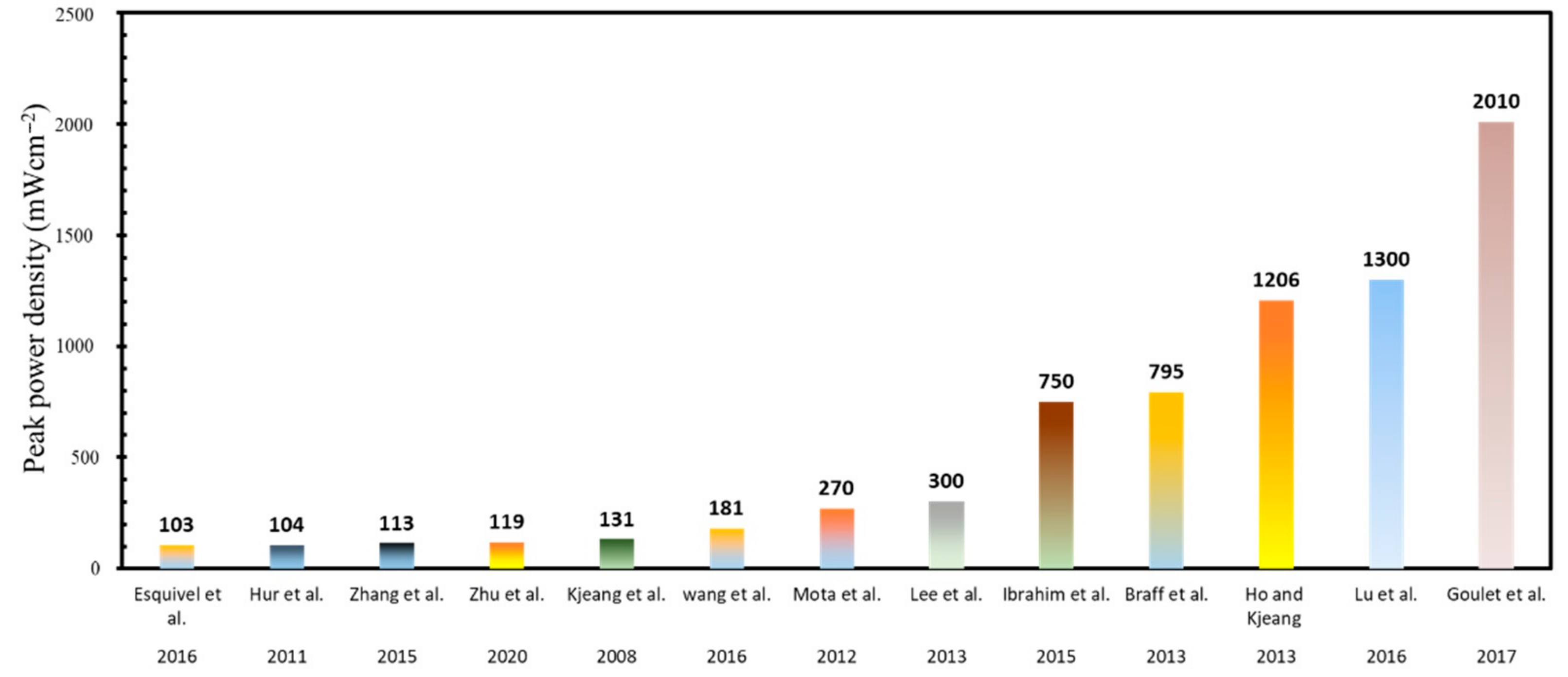
| Reference | Fuel/Oxidant | Electrodes Type | OCV | Current Density | Power Density |
|---|---|---|---|---|---|
| Imax (mAcm−2) | Pmax (mWcm−2) | ||||
| Rao et al. [146] | HCOOH/Air | Graphite pencil stroked | 1.51 | 0.174 | |
| Arun et al. [147] | HCOOH/Air | Graphite pencil stroked | 82 | 100 | |
| Yan et al. [148] | H2O2/H2O2 | Silver nanowires and carbon nanotubes | 1.09 | 10 | 0.88 |
| Roman et al. [149] | Glucose/O2 | Carbon | 0.0375 | ||
| Shen et al. [150] | KCOOH/KOH | Graphite Foils | 0.86 | 7.10 | |
| Esquivel et al. [87] | MeOH/KOH | Gold/Carbon Paper | 0.55 | 22.5 | 4.4 |
| Jung and Ahn [151] | V2+/ | Thin Graphene layer | 30 | 15 | |
| Pasala et al. [152] | NaBH4/ | 2.1 | 4.5 | ||
| Chino et al. [153] | Urea/AgNO3 | Pt/Carbon | 1.54 | 4.3 | 0.91 |
| Galvan et al. [154] | HCOOK/H2O2 | Pd/Carbon | 1.1 | 11.5 | 2.53 |
| Esquivel et al. [155] | H2/O2 | Pt | 276 | 103.2 | |
| Chandra et al. [156] | C2H5OH/Cr2O72− | Stainless Steel/Carbon | 1.42 | 12 | 6.32 |
| Lal et al. [157] | N2H4·H2O/KMnO4 | Multi walled carbon nanotubes | 1.85 | 7.5 | 3.58 |
| Lal et al. [158] | HCOOH/KMnO4 | Graphite | 1.3 | 1.4 | 0.57 |
| González-Guerrero [159] | Glucose/Air | Glucose Oxidase/Laccase | 0.55 | 0.225 | 0.024 |
| Purohit et al. [160] | HCOOK/AgNO3 | Pd/Carbon | 1.14 | 16 | 4.1 |
| Arun et al. [161] | HCOOH/O2 | Graphite pencil stroked | 0.33 | 650 | 32 |
| Copenhaver et al. [162] | HCOOK/KMnO4 | Carbon/Graphite | 0.6 | 3.6 | 0.5 |
| Ehteshami et al. [163] | H2O2/H2O2 | Al/Prussian Blue | 0.61 | 0.81 | |
| Wang et al. [164] | H2/Air | Carbon/Carbon Nanotubes | 0.93 | 8 | 4 |
| Dector [165] | Glucose/Air | Glucose Oxidase/Carbon | 0.8 | 0.16 | |
| Domalaon et al. [166] | HCOOK/H2O2 | Stainless steel/Silver epoxy | 0.92 | 22.83 | 4.4 |
| Liu et al. [167] | HCOONa/H2O2 | Graphite rod | 1.44 | 56.6 | 20.7 |
| Wu et al. [127] | HCOONa/O2 | Pt/Carbon | 0.91 | 111.2 | 19.9 |
| Zhou et al. [101] | HCOONa/O2 | Pd/Carbon | 1.04 | 325 | 119.3 |
| Reference | Design Feature | OCV | Fuel/Oxidant | Current Density | Power Density | Efficiency |
|---|---|---|---|---|---|---|
| (V) | (Imax) | (Pmax) | ||||
| Cohen et al. [51] | 2 cells connected in parallel | 0.59 | HCOOH/O2 | 1.16 mA | 118 μW | 89% |
| Kjeang et al. [78] | 12 Pair of electrode rods in parallel | 1.33 | / | 92 mAcm−2 | 35 mWcm−2 | 78% |
| Salloum and Posner [169] | 2 cells connected in parallel | 1.1 | / | 16.5 mWcm−2 | 11% | |
| Moore et al. [119] | 2 cells connected in parallel | 1.4 | / | 15.7 mAcm−2 | 5.8 mWcm−2 | 66% |
| Zhu et al. [90] | Electrode rods in parallel | 0.8 | HCOOH/Air | 118.3 mAcm−3 | 21.5 mWcm−3 | 87.6% |
| Ho and Kjeang [132] | 2 cells connected in series | 3.0 | / | 1206 mWcm−2 | 64% | |
| Wang et al. [62] | 4 cells connected in series/parallel | 2.89 | HCOOH/H2O2 | 1133.6 μA | 265.8 μW | 93% |
| Wang and Leung [174] | 6 cells connected in series | 5.5 | MEOH/Air | 80 mAcm−2 | 108.7 mWcm−2 | 98.4% |
| Ibrahim et al. [175] | 2 cells connected in series | 2.83 | / | 13.5 mA | 9 mW | |
| Abrego-Martínez et al. [170] | 2 cells connected in series | 0.89 | MeOH/KOH | 25 mAcm−2 | 1.42 mWcm−2 | 75% |
| Moreno-Zuria et al. [171] | 2 cells connected in parallel | 0.75 | HCOOH/Air | 345 mAcm−2 | 83 mWcm−2 | 95% |
| Lee and Ahn [121] | 4 cells in parallel | 2.07 | HCOOH/KMnO4 | 12.78 mAcm−3 | 15.27 mWcm−3 | 8.3% |
| Wang et al. [164] | 4-cell stack Paper based | 3.8 | H2/Air | 8 mAcm−2 | 12.5 mWcm−2 | 90.6% |
| Lee and Ahn [125] | Planar single stack in parallel | 2.0 | HCOOH/KMnO4 | 26 μA | 15.3 μW | |
| Galindo-de-la-Rosa et al. [176] | Hybrid microfluidic and enzymatic | 1.03 | C2H5OH/KOH+ O2 | 11.42 mAcm−2 | 3.154 mWcm−2 | |
| Escalona-Villalpando et al. [177] | 4 cells connected in series-parallel | 0.76 | Glucose/O2 | 2007 μAcm−2 | 579 μWcm−2 | 76.9% |
| Lu et al. [137] | Up-scaled network | 1.0 | HCOOH/O2 | 180 mAcm−2 | 7 mWcm−2 | 85% |
| Yang et al. [178] | 4 cells connected in series | 3.0 | 56 μAcm−2 | 60.5 μWcm−2 | ||
| Wang and Leung [83] | 6 cells in series | 6 | Aluminum/H2+Air | 550 mAcm−2 | 180.6 mWcm−2 |
| References | Flow-Configuration | Electrodes Type | OCV | Current Density | Power Density | Fuel Utilization |
|---|---|---|---|---|---|---|
| (V) | Imax (mAcm−2) | Pmax (mWcm−2) | ||||
| Choban et al. [57] | Side-by-side streaming | Flow-over | 1.40 | 40 | 12 | 10% |
| Montesinos et al. [43] | Side-by-side streaming | Flow-over | 1.35 | 49 | 15.4% | |
| Jayashree et al. [16] | Vertical-layered streaming | Air-breathing | 0.92 | 130 | 26 | 8% |
| Ortega et al. [100] | Vertical-layered streaming | Flow-through anode air-breathing cathode | 0.9 | 490 | 100 | |
| Shaegh et al. [103] | Vertical-layered streaming | Air-breathing with fuel reservoir | 0.8 | 140 | 29 | 22% |
| Kjeang et al. [82] | Vertical-layered streaming | Flow-through | 1.55 | 326 | 131 | 100% |
| Kjeang et al. [113] | Vertical-layered streaming | Flow-through | 1.42 | 230 | 52 | 100% |
| Goulet et al. [112] | Vertical-layered streaming | Flow-through | 1.5 | 4600 | 2010 | 90% |
| Lee and Ahn [121] | Multi-stream | Flow-through (single cell) | 1.04 | 15.25 mAcm−3 | 7.42 mWcm−3 | |
| Sun et al. [44] | Multi-stream | Flow-over | 1.0 | 2.6 | 0.7 | |
| Jayashree et al. [124] | Multi-stream | Flow-through | 0.94 | 960 | 191 | |
| Kwok et al. [128] | Multi-stream | Air-breathing | 0.95 | 450 | 210 | |
| Lu et al. [129] | Multi-stream | Air-breathing | 1.89 | 3600 | 1300 | |
| Ibrahim et al. [130] | Dual Pass | Flow-through | 1.554 | 1000 | 750 | 52% |
| Ho and Kjeang [132] | Dual Pass | Flow-through | 3 | 2000 | 1206 | 64% |
| Goulet et al. [122] | Dual Pass | Flow-through | 1.53 | 5.6 mA | ||
| Esquivel et al. [155] | Lateral-flow | 276 | 103.2 | |||
| Arun et al. [161] | Lateral-flow | 0.33 | 650 | 32 | ||
| Wu et al. [127] | Lateral-flow multi-stream | Flow-through | 0.91 | 111.2 | 19.9 | 20.7% |
| Zhou et al. [101] | Lateral-flow multi-stream | Flow-over anode air-breathing cathode | 1.04 | 325 mAcm−3 | 119.3 mWcm−3 | 6.5% |
| Salloum and Posner [61] | Counter-flow | Flow-through | 1.21 | 19 | 5 | 24.9% |
| Lu et al. [137] | Counter-flow | Flow-through | 1.0 | 180 | 7 | |
| Xuan et al. [63] | Counter-flow | Flow-over | 0.8 | 181 | 35 | |
| Salloum et al. [35] | Radial flow | Flow-through | 1.2 | 5 | 2.8 | 58% |
| Li et al. [144] | Radial flow | Flow-through | 1.51 | 0.35 mW | 53.33% | |
| Hayes et al. [77] | Orthogonal flow | Flow-through | 0.9 | 46 | 42% |
| References | Fuel/Oxidant | Electrode Type | Electrolyte | OCV | Current Density | Power Density |
|---|---|---|---|---|---|---|
| (V) | Imax (mAcm−2) | Pmax (mAcm−2) | ||||
| Jayashree et al. [16] | HCOOH/Air | Air-breathing | 0.5 M H2SO4 | 0.92 | 130 | 26 |
| Ortega et al. [100] | HCOOH/Air | Flow-through anode air-breathing cathode | 0.5 M H2SO4 | 0.9 | 490 | 100 |
| Shaegh et al. [103] | HCOOH/Air | Air-breathing with fuel reservoir | 2 M H2SO4 | 0.8 | 140 | 29 |
| Shaegh et al. [118] | HCOOH/Air | Air-breathing cathode and flow through anode | 0.5 M H2SO4 | 0.9 | 120 | 26.5 |
| Brushett et al. [110] | HCOOH/Air | Air-breathing | 0.5 M H2SO4 | 130 | 26 | |
| Kjeang et al. [113] | HCOOH/NaOCl | Flow-through | 2.8 M NaOH | 1.42 | 230 | 52 |
| Choban et al. [57] | CH3OH/O2 | Flow-over | 1.40 | 40 | 12 | |
| Whipple et al. [107] | CH3OH/Air | Air-breathing | 0.5 M H2SO4 | 0.7 | 62 | 4 |
| Jayashree et al. [109] | CH3OH/KOH | Air-breathing | 0.5 M H2SO4 | 1.05 | 120 | 17 |
| Thorson et al. [95] | CH3OH/Air | Electrode arrangement in Air-breathing MFC | 1 M KOH | 0.7 | 121 | 24.9 |
| Hollinger et al. [180] | CH3OH/Air | Air-breathing | 1 M H2SO4 | 0.64 | 655 | 90 |
| Ferrigno et al. [13] | / | Flow-over | 0.25 M H2SO4 | 38 | ||
| Lee et al. [115] | / | Nano-foam flow-through | 1.8 M H2SO4 | 1.5 | 80 | |
| Lee et al. [116] | / | Chip embedded flow-through | 1.8 M H2SO4 | 1.5 | 93 | |
| Kjeang et al. [82] | / | Flow-through | 1 M H2SO4 | 1.55 | 326 | 131 |
| Ibrahim et al. [130] | / | Dual Pass Flow-through | 1 M H2SO4 | 1.55 | 1000 | 750 |
| Ho and Kjeang [132] | / | Dual Pass Flow-through | 1 M H2SO4 | 3 | 2000 | 1206 |
| Goulet et al. [112] | / | Flow-through | 4 M H2SO4 | 1.5 | 4600 | 2010 |
| Hayes et al. [77] | H2/O2 | Flow-through | 1.8 M H2SO4 | 0.9 | 46 | |
| Brushett et al. [179] | H2/O2 | Flow-through | 3 M KOH | 1 | 600 | 108 |
| Jayashree et al. [124] | H2/O2 | Flow-through | 2 M H2SO4 | 0.94 | 191 | |
| Lu et al. [129] | H2/O2 | Air-breathing | 3 M H2SO4 & 3 M KOH | 1.89 | 3600 | 1300 |
| Braff et al. [193] | H2/Br2 | Flow-over and H2-breathing | 1 M HBr | 0.95 | 795 | |
| Martins et al. [194] | Glycerorl/bleach | Flow-through | 1 M H2SO4 & 2 M KOH | 2 | 638 | 315 |
| Mota et al. [188] | NaBH4/CAN | Chaotic flow-over | 2.2 | 270 |
| Flow configuration/Electrode Architecture/Technique | Attributed Feature |
|---|---|
| Counter-flow/Radial-flow | Minimize redundant convective mixing |
| Multi-stream (additional electrolytic stream) | Minimize cross-over issues |
| Dual Pass/Recirculation | To add up OCV and enhance maximum power density |
| Flow-through anode | Enhance fuel utilization |
| Air-breathing cathode | Ensures uniform oxidant concentration |
| Fuel/Oxidant (Vanadium redox/Aluminum) | To have higher theoretical OCV |
| Stacking | To enhance overall OCV and maximize power density |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanveer, M.; Kim, K.-Y. Flow Configurations of Membraneless Microfluidic Fuel Cells: A Review. Energies 2021, 14, 3381. https://doi.org/10.3390/en14123381
Tanveer M, Kim K-Y. Flow Configurations of Membraneless Microfluidic Fuel Cells: A Review. Energies. 2021; 14(12):3381. https://doi.org/10.3390/en14123381
Chicago/Turabian StyleTanveer, Muhammad, and Kwang-Yong Kim. 2021. "Flow Configurations of Membraneless Microfluidic Fuel Cells: A Review" Energies 14, no. 12: 3381. https://doi.org/10.3390/en14123381
APA StyleTanveer, M., & Kim, K.-Y. (2021). Flow Configurations of Membraneless Microfluidic Fuel Cells: A Review. Energies, 14(12), 3381. https://doi.org/10.3390/en14123381







