Robust Superhydrophobic Surface on Polypropylene with Thick Hydrophobic Silica Nanoparticle-Coated Films Prepared by Facile Compression Molding
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
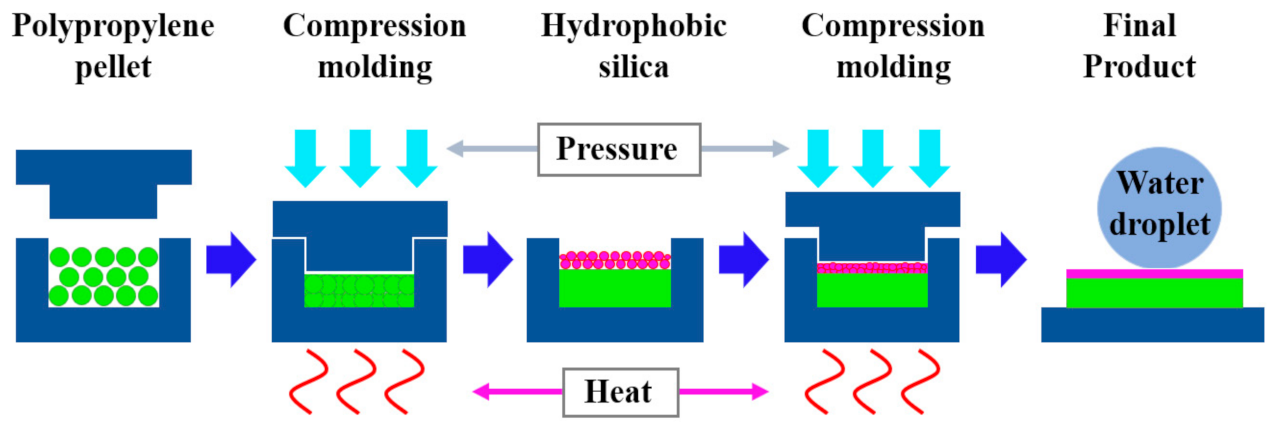
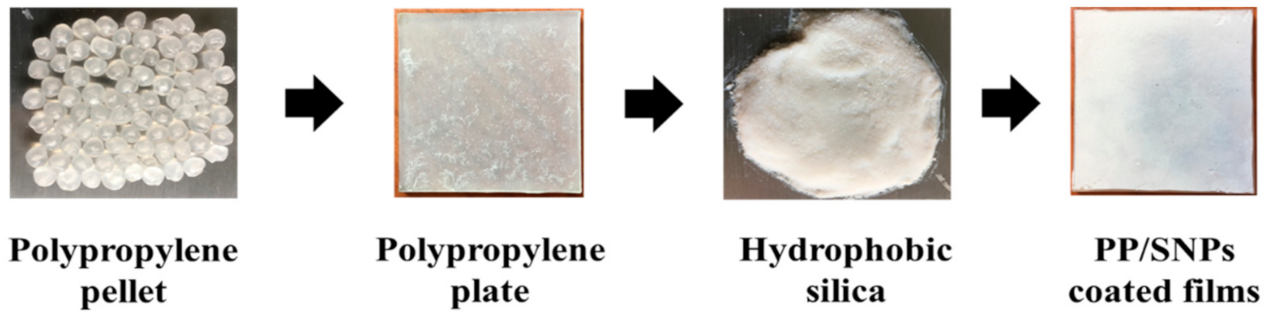
2.2. Fabrication of Superhydrophobic PP/SNPs Coated Films
2.3. Characterization of Surface Morphology and Wettability
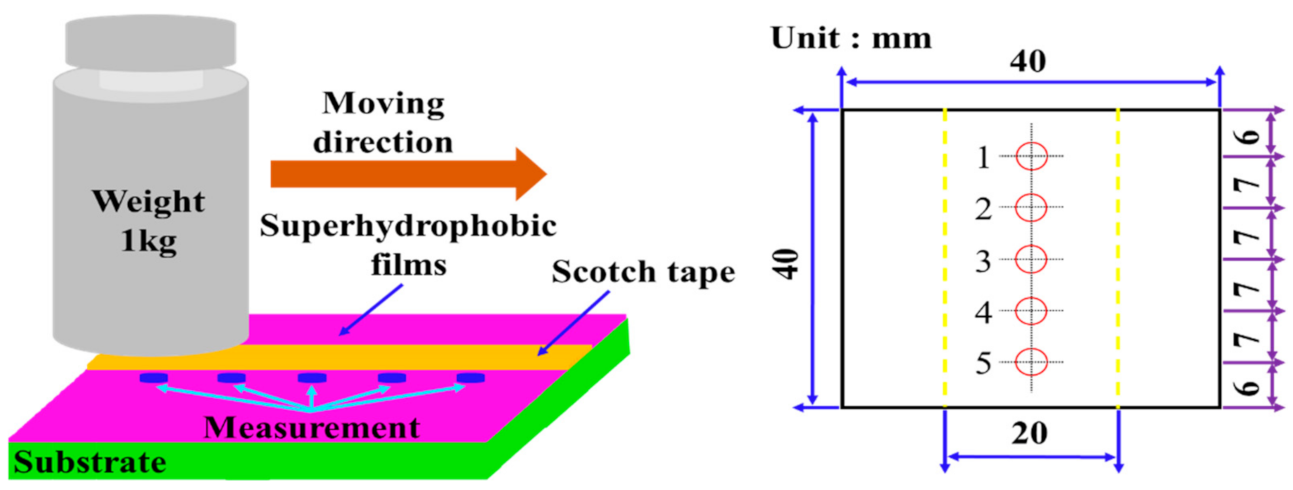
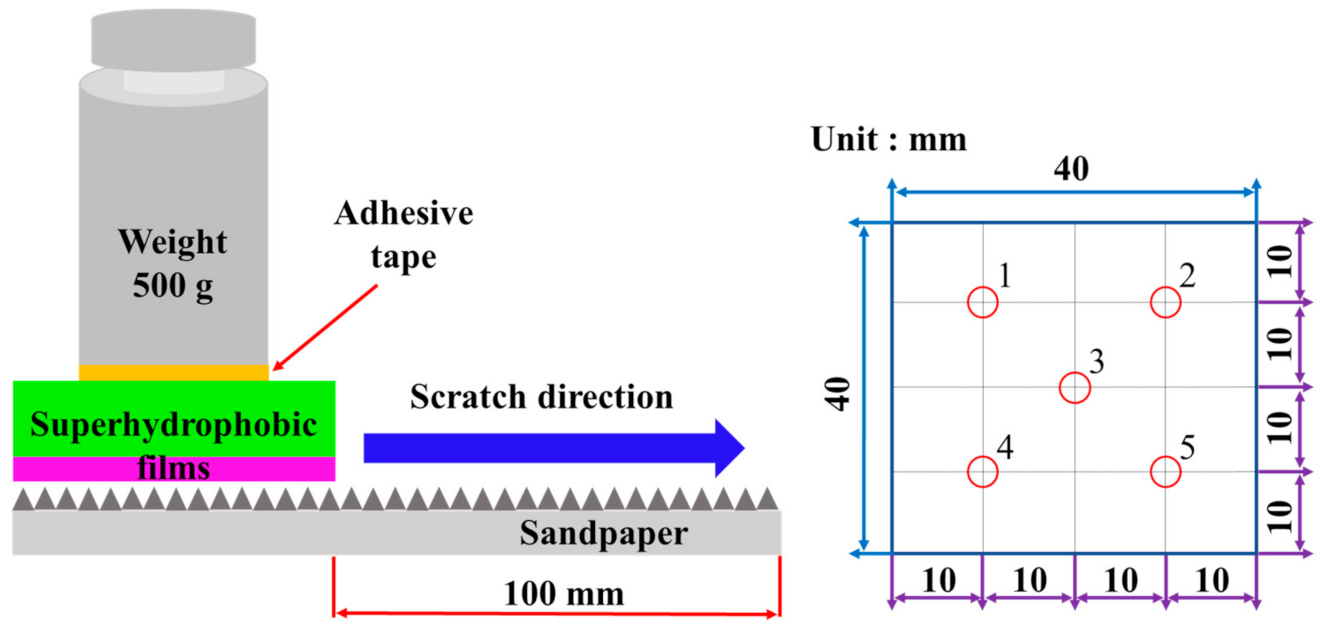
2.4. Mechanical Durability
2.5. Chemical Stability and Aging Stability
3. Results
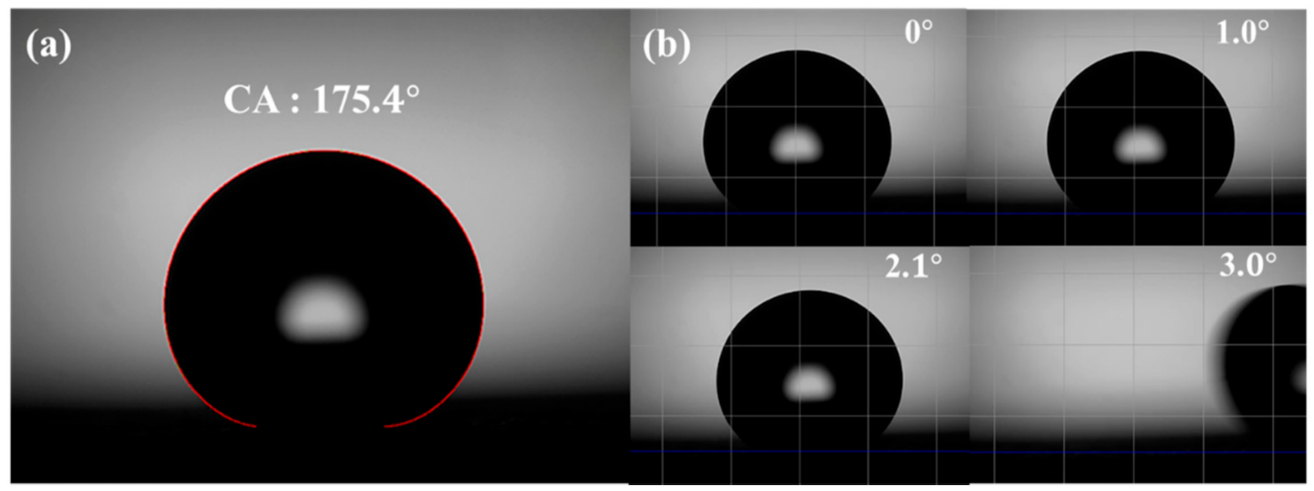
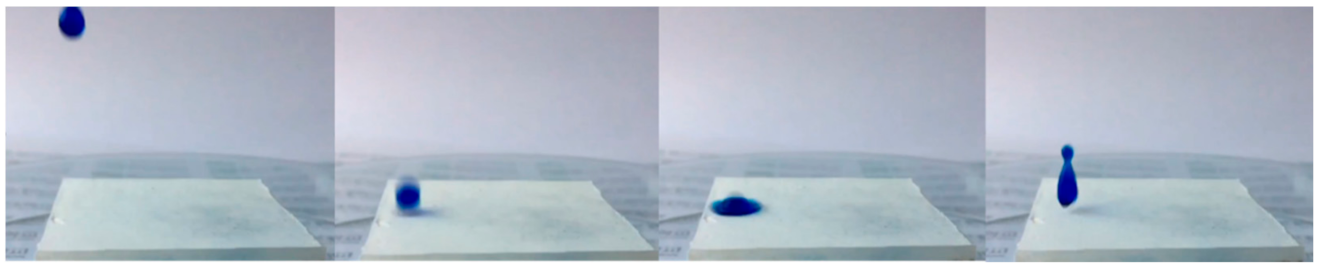
3.1. Wettability
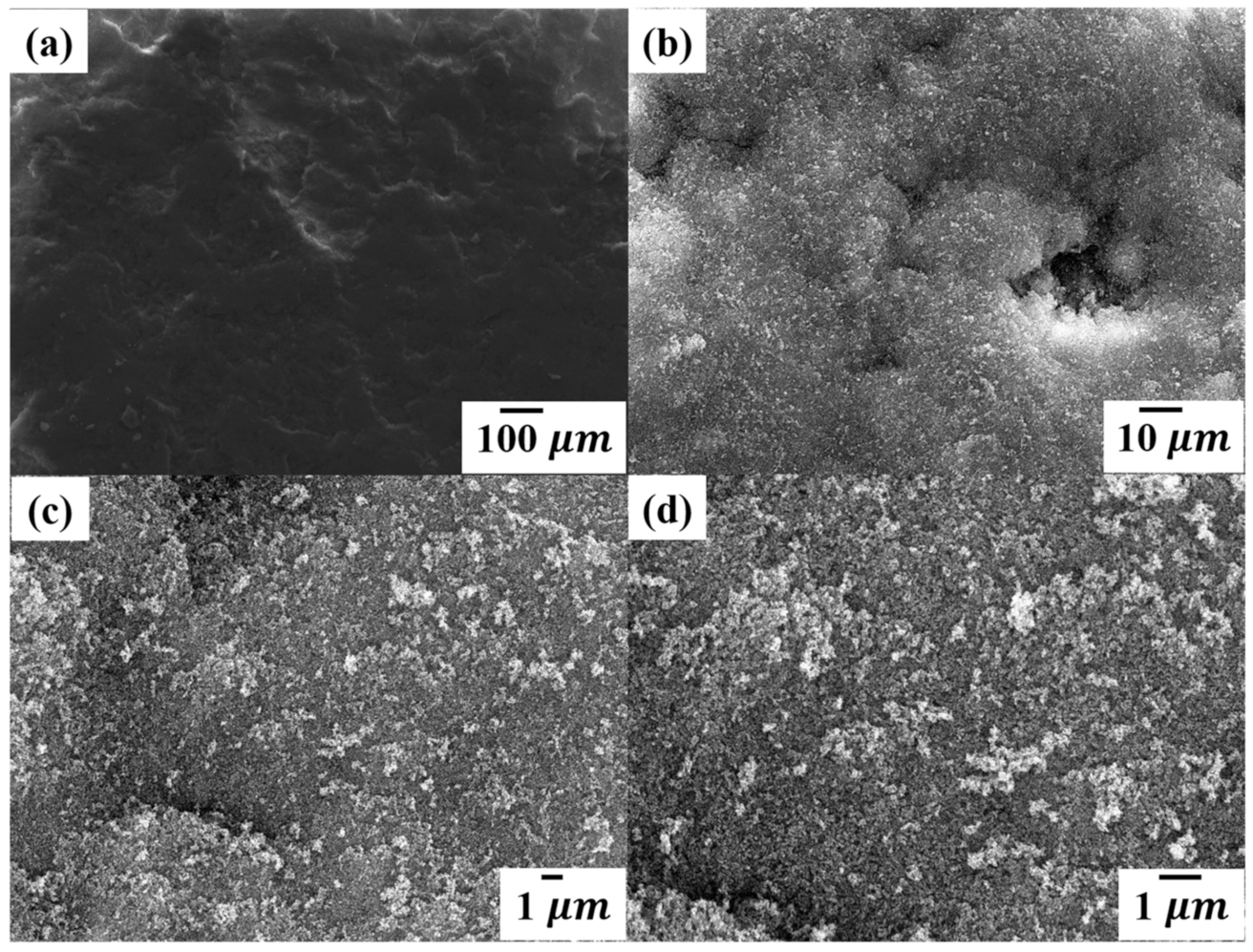
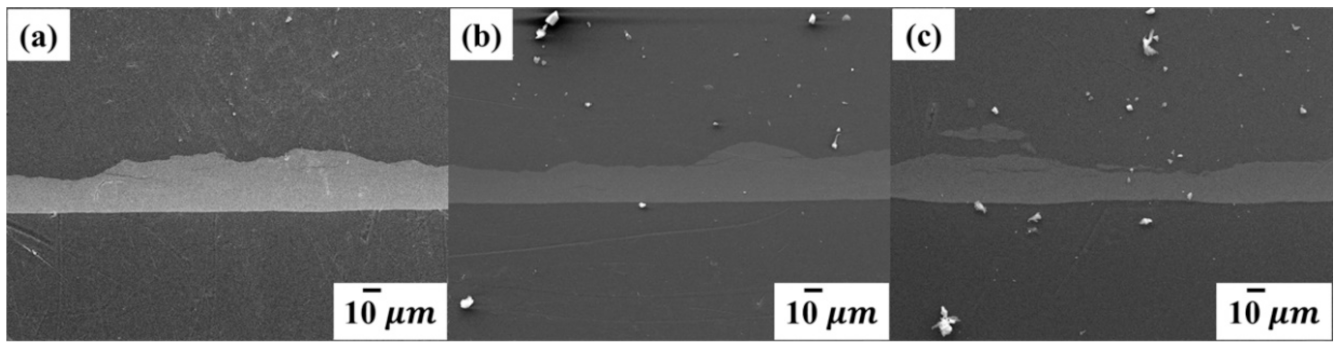
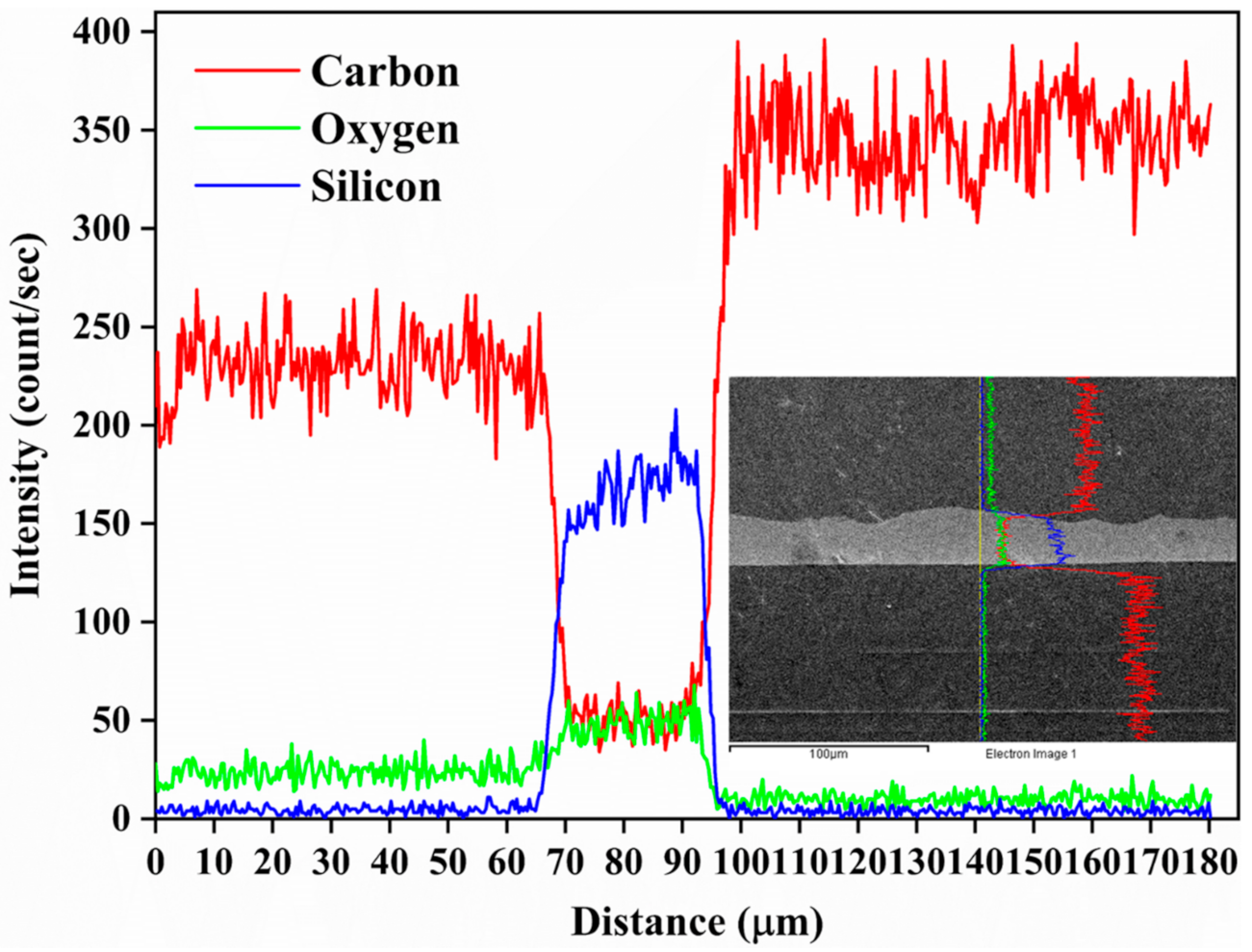
3.2. Surface Morphology
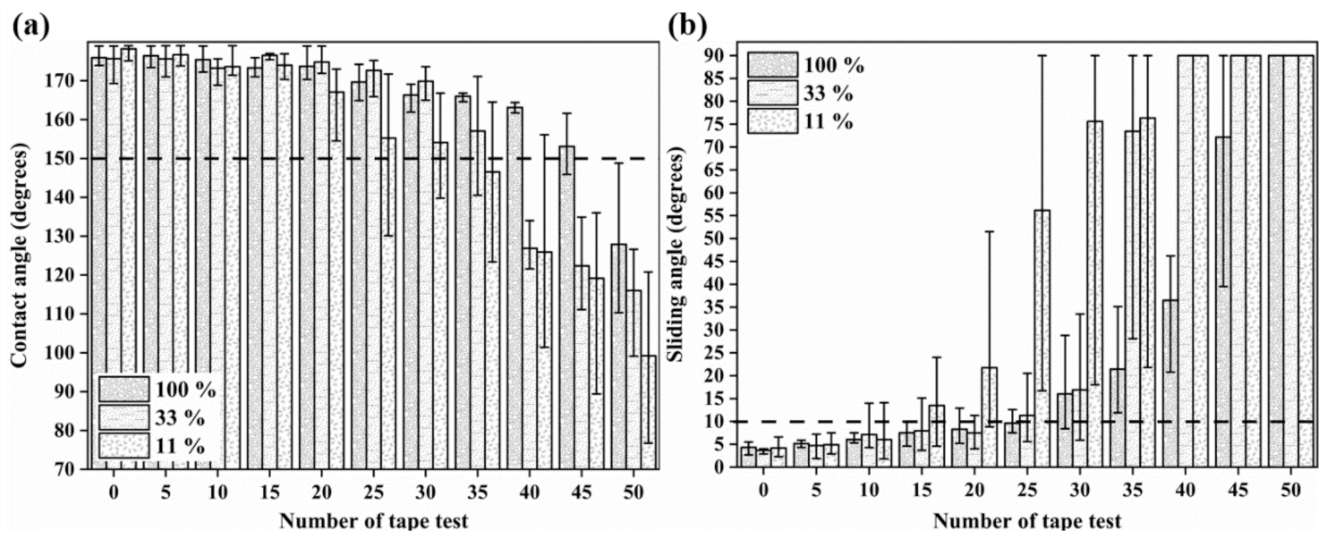
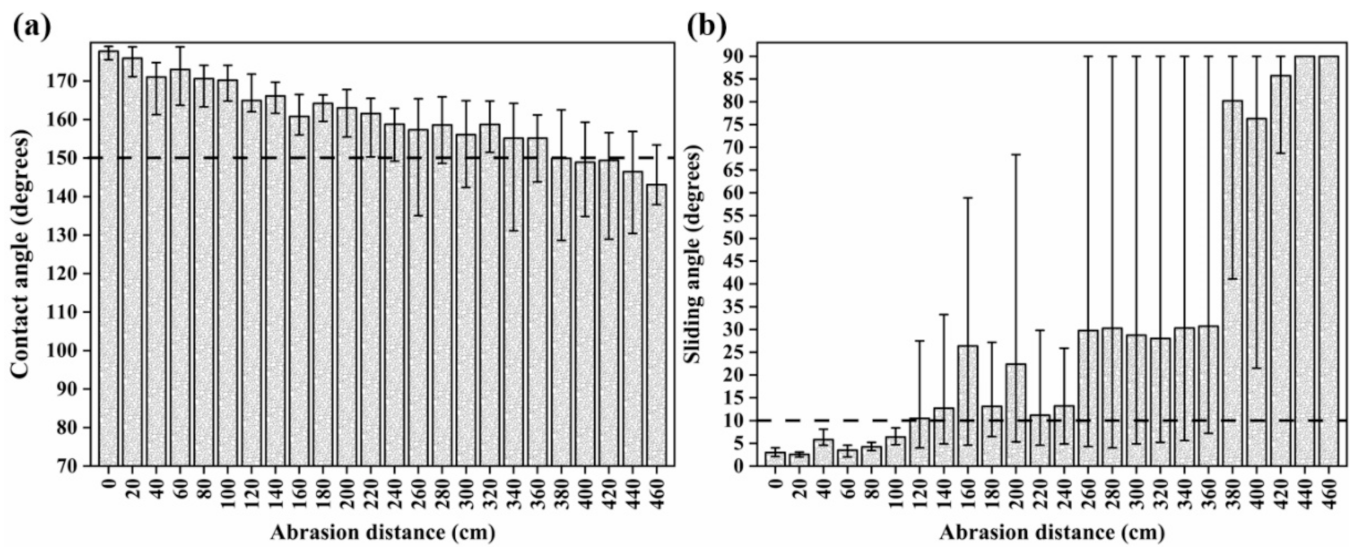
3.3. Mechanical durability
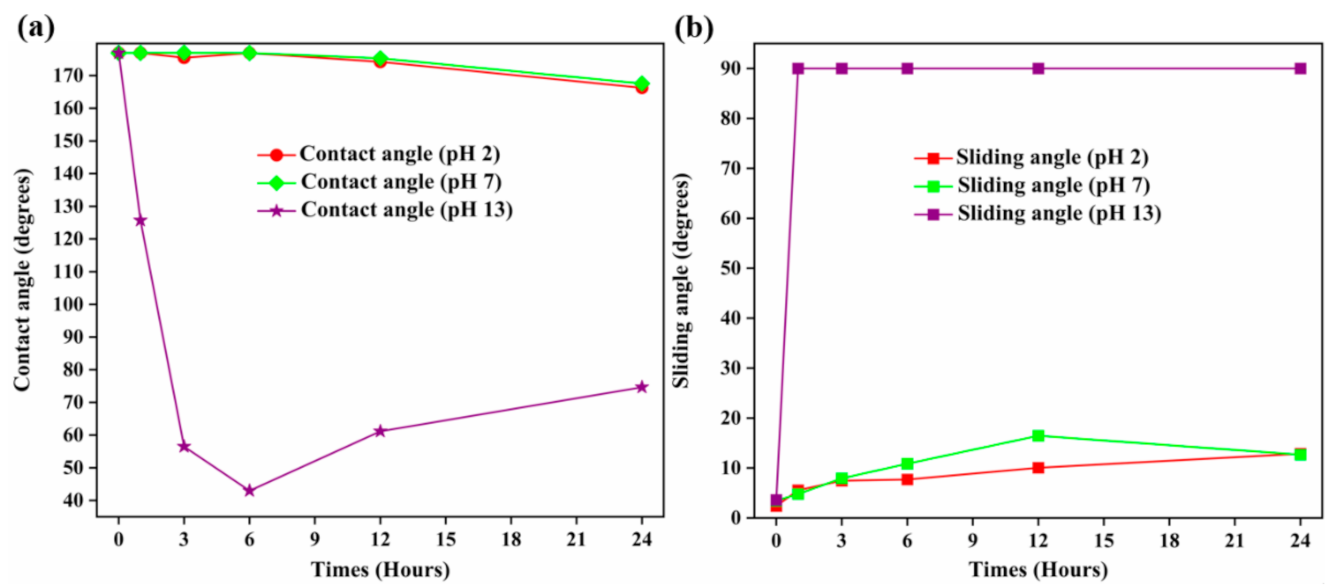
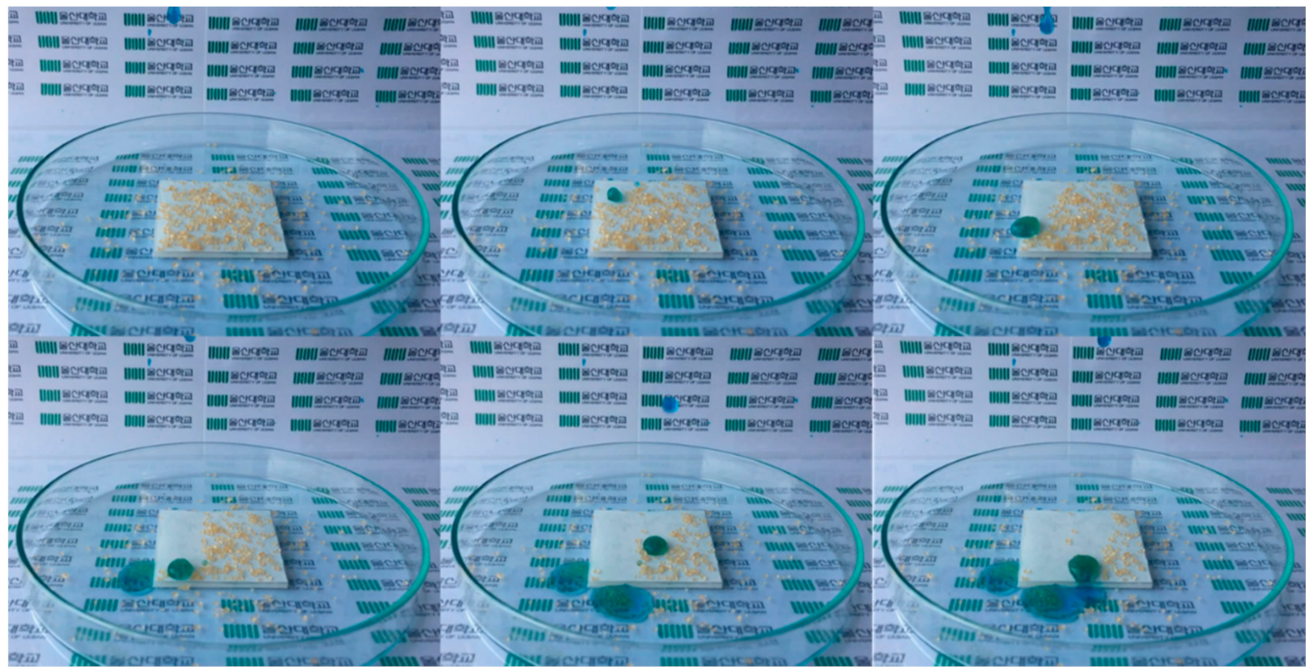
3.4. Stability in Ambient Air and Chemical Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhi, J.-H.; Zhang, L.; Yan, Y.; Zhu, J. Mechanical durability of superhydrophobic surfaces: The role of surface modification technologies. Appl. Surf. Sci. 2017, 392, 286–296. [Google Scholar] [CrossRef]
- Chen, K.; Gu, K.; Qiang, S.; Wang, C. Environmental stimuli-responsive self-repairing waterbased superhydrophobic coatings. RSC Adv. 2017, 7, 543–550. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Kodag, V.S.; Bhosale, A.; Kumar, A.M.; Sadasivuni, K.K.; Xing, R.; Liu, S. Self—cleaning superhydrophobic coatings: Potential industrial applications. Prog. Org. Coat. 2019, 128, 52–58. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Li, Y.; Cui, Y.; Wang, H.; Wang, J. Durable superhydrophobic surface prepared by designing ‘micro-eggshell’ and ‘web-like’ structures. Chem. Eng. J. 2020, 392, 123741. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, J.; Li, Z.; Mo, J.; Pan, Y.; Luo, D. Preparation of mechanically durable superhydrophobic aluminum surface by sandblasting and chemical modification. Prog. Org. Coat. 2019, 133, 77–84. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, D.; Deng, Y.; Shi, Y.; Wang, K. Mechanically Robust Superhydrophobic Steel Surface with Anti-Icing, UV-Durability, and Corrosion Resistance Properties. ACS Appl. Mater. Interfaces 2015, 7, 6260–6272. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Lyu, J.; Peng, C.; Liu, J.; Xing, S.; Jiang, D.; Ju, S.; Tiwari, M.K. Compression molding processed superhydrophobic CB/CeO2/PVDF/CF nanocomposites with highly robustness, reusability and multifunction. Colloids Surf. A Physicochem. Eng. Asp. 2020, 590, 124533. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M. Superhydrophobic surfaces. Curr. Opin. Colloid Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Peng, C.; Chen, Z.; Tiwari, M.K. All-organic superhydrophobic coatings with mechanochemical robustness and liquid impalement resistance. Nat. Mater. 2018, 17, 355–360. [Google Scholar] [CrossRef]
- Onda, T.; Shibuichi, S.; Satoh, N.; Tsujii, K. Super-Water-Repellent Fractal Surfaces. Langmuir 1996, 12, 2125–2127. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Liu, Z.; Zhu, Y.; Wu, S.; Wang, C.; Zhu, Y. Fabrication of durable fluorine-free superhydrophobic polyethersulfone (PES) composite coating enhanced by assembled MMT-SiO2 nanoparticles. Appl. Surf. Sci. 2017, 396, 1580–1588. [Google Scholar] [CrossRef]
- Gong, X.; He, S. Highly Durable Superhydrophobic Polydimethylsiloxane/Silica Nanocomposite Surfaces with Good Self-Cleaning Ability. ACS Omega 2020, 5, 4100–4108. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Zhang, P.; Zhang, Z. Facile approach in fabricating superhydrophobic SiO2/polymer nanocomposite coating. Appl. Surf. Sci. 2012, 261, 628–632. [Google Scholar] [CrossRef]
- Jain, R.; Pitchumani, R. Fabrication and characterization of zinc-based superhydrophobic coatings. Surf. Coat. Technol. 2018, 337, 223–231. [Google Scholar] [CrossRef]
- Liu, W.; Luo, Y.; Sun, L.; Wu, R.; Jiang, H.; Liu, Y. Fabrication of the superhydrophobic surface on aluminum alloy by anodizing and polymeric coating. Appl. Surf. Sci. 2013, 264, 872–878. [Google Scholar] [CrossRef]
- Xu, D.; Wang, M.; Ge, X.; Lam, M.H.-W.; Ge, X. Fabrication of raspberry SiO2/polystyrene particles and superhydrophobic particulate film with high adhesive force. J. Mater. Chem. 2012, 22, 5784–5791. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Liu, F.; Jia, D.; Guo, B. Tailoring the wettability of polypropylene surfaces with halloysite nanotubes. J. Colloid Interface Sci. 2010, 350, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, K.; Momen, G.; Jafari, R.; Farzaneh, M. Direct replication of micro-nanostructures in the fabrication of superhydrophobic silicone rubber surfaces by compression molding. Appl. Surf. Sci. 2018, 458, 619–628. [Google Scholar] [CrossRef]
- Lee, K.-M.; Ngo, C.-V.; Jeong, J.-Y.; Jeon, E.-C.; Je, T.-J.; Chun, D.-M. Fabrication of an Anisotropic Superhydrophobic Polymer Surface Using Compression Molding and Dip Coating. Coatings 2017, 7, 194. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Momen, G.; Jafari, R.; Farzaneh, M.; Carreira, T. Micro-Nanostructured Silicone Rubber Surfaces Using Compression Molding. Mater. Sci. Forum 2018, 941, 1802–1807. [Google Scholar] [CrossRef]
- Chun, D.-M.; Davaasuren, G.; Ngo, C.-V.; Kim, C.-S.; Lee, G.-Y.; Ahn, S.-H. Fabrication of transparent superhydrophobic surface on thermoplastic polymer using laser beam machining and compression molding for mass production. CIRP Ann. 2014, 63, 525–528. [Google Scholar] [CrossRef]
- Erbil, H.Y.; Demirel, A.L.; Avcı, Y.; Mert, O. Transformation of a Simple Plastic into a Superhydrophobic Surface. Science 2003, 299, 1377–1380. [Google Scholar] [CrossRef]
- Contreras, C.B.; Chagas, G.; Strumia, M.C.; Weibel, D.E. Permanent superhydrophobic polypropylene nanocomposite coatings by a simple one-step dipping process. Appl. Surf. Sci. 2014, 307, 234–240. [Google Scholar] [CrossRef]
- Kansara, A.M.; Chaudhri, S.G.; Singh, P.S. A facile one-step preparation method of recyclable superhydrophobic polypropylene membrane for oil–water separation. RSC Adv. 2016, 6, 61129–61136. [Google Scholar] [CrossRef]
- Hejazi, I.; Seyfi, J.; Hejazi, E.; Sadeghi, G.M.M.; Jafari, S.H.; Khonakdar, H.A. Investigating the role of surface micro/nano structure in cell adhesion behavior of superhydrophobic polypropylene/nanosilica surfaces. Colloids Surf. B Biointerfaces 2015, 127, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Z.; Men, X.; Yang, J.; Wang, K.; Xu, X.; Zhou, X.; Xue, Q. Robust superhydrophobic surfaces with mechanical durability and easy repairability. J. Mater. Chem. 2011, 21, 15793–15797. [Google Scholar] [CrossRef]
- Kumar, A.; Gogoi, B. Development of durable self-cleaning superhydrophobic coatings for aluminium surfaces via chemical etching method. Tribol. Int. 2018, 122, 114–118. [Google Scholar] [CrossRef]
- She, Z.; Li, Q.; Wang, Z.; Li, L.; Chen, F.; Zhou, J. Researching the fabrication of anticorrosion superhydrophobic surface on magnesium alloy and its mechanical stability and durability. Chem. Eng. J. 2013, 228, 415–424. [Google Scholar] [CrossRef]
- Wang, H.; He, M.; Liu, H.; Guan, Y. One-Step Fabrication of Robust Superhydrophobic Steel Surfaces with Mechanical Durability, Thermal Stability, and Anti-icing Function. ACS Appl. Mater. Interfaces 2019, 11, 25586–25594. [Google Scholar] [CrossRef]
- Xue, C.-H.; Tian, Q.-Q.; Jia, S.-T.; Zhao, L.-L.; Ding, Y.-R.; Li, H.-G.; An, Q.-F. The fabrication of mechanically durable and stretchable superhydrophobic PDMS/SiO2 composite film. RSC Adv. 2020, 10, 19466–19473. [Google Scholar] [CrossRef]
- Liu, M.; Luo, Y.; Jia, D. Synthesis of mechanically durable superhydrophobic polymer materials with roughness-regeneration performance. Compos. Part A Appl. Sci. Manuf. 2020, 133, 105861. [Google Scholar] [CrossRef]
- Zhang, X.; Zhi, D.; Sun, L.; Zhao, Y.; Tiwari, M.K.; Carmalt, C.J.; Parkin, I.P.; Lu, Y. Super-durable, non-fluorinated superhydrophobic free-standing items. J. Mater. Chem. A 2017, 6, 357–362. [Google Scholar] [CrossRef]
- Zimmermann, J.; Artus, G.; Seeger, S. Long term studies on the chemical stability of a superhydrophobic silicone nanofilament coating. Appl. Surf. Sci. 2007, 253, 5972–5979. [Google Scholar] [CrossRef]
- Liu, Y.; Xi, N.; Fu, S.; Yang, G.; Fu, H.; Chen, H.; Zhang, X.; Liu, N.; Gao, W. Mechanical and chemical stability of super-hydrophobic coatings on SMA490BW substrate prepared by HVOF spraying. Mater. Res. Express 2018, 5, 115030. [Google Scholar] [CrossRef]
- Yin, L.; Yang, J.; Tang, Y.; Chen, L.; Liu, C.; Tang, H.; Li, C. Mechanical durability of superhydrophobic and oleophobic copper meshes. Appl. Surf. Sci. 2014, 316, 259–263. [Google Scholar] [CrossRef]
- Company Website of Hydrophobic Silica. Available online: https://www.oci.co.kr/ (accessed on 8 May 2021).
- Ducom, G.; Laubie, B.; Ohannessian, A.; Chottier, C.; Germain, P.; Chatain, V. Hydrolysis of polydimethylsiloxane fluids in controlled aqueous solutions. Water Sci. Technol. 2013, 68, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Zhi, J.; Zhang, L.-Z. Durable superhydrophobic surface with highly antireflective and self-cleaning properties for the glass covers of solar cells. Appl. Surf. Sci. 2018, 454, 239–248. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Dynamic Effects of Bouncing Water Droplets on Superhydrophobic Surfaces. Langmuir 2008, 24, 6262–6269. [Google Scholar] [CrossRef] [PubMed]
- Crick, C.R.; Parkin, I.P. Water droplet bouncing—A definition for superhydrophobic surfaces. Chem. Commun. 2011, 47, 12059–12061. [Google Scholar] [CrossRef]

| Parameter | Unit | Value |
|---|---|---|
| Compression molding pressure | MPa | 7.5–8 |
| Compression heating time | min | 5 |
| Compression pressure time | min | 5 |
| Molding temperature | °C | 160 |
| Mold cavity size | mm | 40 × 40 × 10 |
| Cooling time | min | 40 |
| Polypropylene weight | g | 3.6 |
| Relative weight ratio of hydrophobic silica (Maximum weight for molding cavity: 0.53 g) | 100% | |
| 33% | ||
| 11% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdene-Ochir, O.; Chun, D.-M. Robust Superhydrophobic Surface on Polypropylene with Thick Hydrophobic Silica Nanoparticle-Coated Films Prepared by Facile Compression Molding. Energies 2021, 14, 3155. https://doi.org/10.3390/en14113155
Erdene-Ochir O, Chun D-M. Robust Superhydrophobic Surface on Polypropylene with Thick Hydrophobic Silica Nanoparticle-Coated Films Prepared by Facile Compression Molding. Energies. 2021; 14(11):3155. https://doi.org/10.3390/en14113155
Chicago/Turabian StyleErdene-Ochir, Oyunchimeg, and Doo-Man Chun. 2021. "Robust Superhydrophobic Surface on Polypropylene with Thick Hydrophobic Silica Nanoparticle-Coated Films Prepared by Facile Compression Molding" Energies 14, no. 11: 3155. https://doi.org/10.3390/en14113155
APA StyleErdene-Ochir, O., & Chun, D.-M. (2021). Robust Superhydrophobic Surface on Polypropylene with Thick Hydrophobic Silica Nanoparticle-Coated Films Prepared by Facile Compression Molding. Energies, 14(11), 3155. https://doi.org/10.3390/en14113155






