A Review of Antiviral and Antioxidant Activity of Bioactive Metabolite of Macroalgae within an Optimized Extraction Method
Abstract
:1. Introduction
2. Methodology
3. Discussion
3.1. Antiviral Activity of Macroalgae
3.2. Antioxidant Activity of Macroalgae
3.3. Macroalgae Active Metabolites and Their Assay Methods
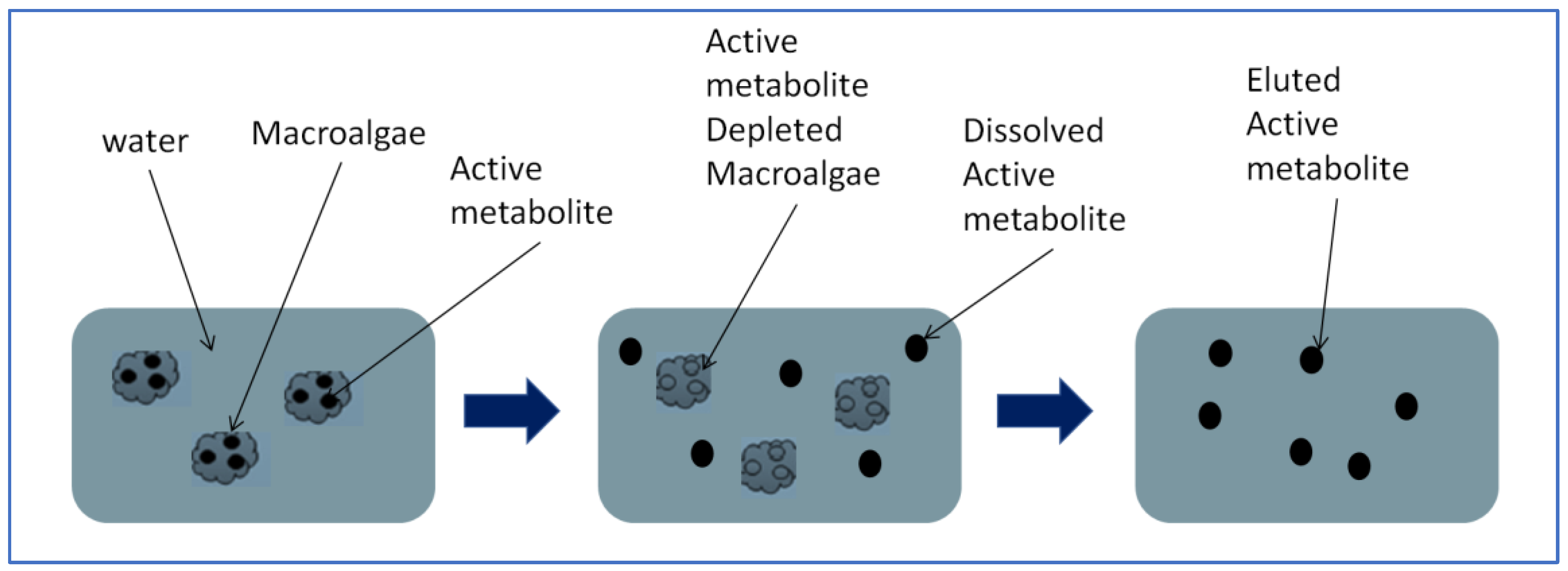
3.4. Extraction Methods of Macroalgae Active Metabolites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, C.-X.; Ho, C.-L.; Phang, S.-M. Trends in seaweed research. Trends Plant Sci. 2006, 11, 165–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, R.K.; Zi-Rong, X. Biomedical Compounds from Marine organisms. Mar. Drugs 2004, 2, 123–146. [Google Scholar] [CrossRef] [Green Version]
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT 2008, 41, 1067–1072. [Google Scholar] [CrossRef]
- Tierney, M.S.; Croft, A.K.; Hayes, M. A review of antihypertensive and antioxidant activities in macroalgae. Bot. Mar. 2010, 53. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Procedia 2017, 128, 504–511. [Google Scholar] [CrossRef]
- Barzkar, N.; Jahromi, S.T.; PoorSaheli, H.B.; Vianello, F. Metabolites from Marine Microorganisms, Micro, and Macroalgae: Immense Scope for Pharmacology. Mar. Drugs 2019, 17, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, P.; Dutcher, J.D.; Adams, E.V.; Sherman, J.H. Protective Effect of Seaweed Extracts for Chicken Embryos Infected with Influenza B or Mumps Virus. Exp. Biol. Med. 1958, 99, 590–593. [Google Scholar] [CrossRef]
- Witvrouw, M.; Desmyter, J.; De Cleroq, E. Antiviral portraitseries: 4. Polysulfates as inhibitors of HIV and other envelopedviruses. Antivir. Chem. Chemother. 1994, 94, 345–359. [Google Scholar] [CrossRef]
- Witvrouw, M.; De Clercq, E. Sulfated Polysaccharides Extracted from Sea Algae as Potential Antiviral Drugs. Gen. Pharmacol. Vasc. Syst. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Carlucci, M.; Scolaro, L.; Damonte, E. Inhibitory Action of Natural Carrageenans on Herpes simplex Virus Infection of Mouse Astrocytes. Chemotherapy 1999, 45, 429–436. [Google Scholar] [CrossRef]
- Cáceres, P.J.; Carlucci, M.J.; Damonte, E.B.; Matsuhiro, B.; Zuniga, E.A. Carrageenans from chileansamples of Stenogramme interrupta (Phyllophoraceae): Structural analysis and biological activity. Phytochemistry 2000, 53, 81–86. [Google Scholar] [CrossRef]
- Thompson, K.D.; Dragar, C. Antiviral activity of Undaria pinnatifida against herpes simplex virus. Phytotherapy Res. 2004, 18, 551–555. [Google Scholar] [CrossRef]
- Ponce, N.M.; Pujol, C.A.; Damonte, E.B.; Flores, M.L.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003, 338, 153–165. [Google Scholar] [CrossRef]
- Pujol, C.; Estevez, J.M.; Carlucci, M.J.; Ciancia, M.; Cerezo, A.S.; Damonte, E.B. Novel DL-Galactan Hybrids from the Red Seaweed Gymnogongrus Torulosusare Potent Inhibitors of Herpes Simplex Virus and Dengue Virus. Antivir. Chem. Chemother. 2002, 13, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Ono, L.; Wollinger, W.; Rocco, I.M.; Coimbra, T.L.; Gorin, P.A.; Sierakowski, M.-R. In vitro and in vivo antiviral properties of sulfated galactomannans against yellow fever virus (BeH111 strain) and dengue 1 virus (Hawaii strain). Antivir. Res. 2003, 60, 201–208. [Google Scholar] [CrossRef]
- Ohta, Y.; Lee, J.-B.; Hayashi, K.; Hayashi, T. Isolation of Sulfated Galactan from Codium fragile and Its Antiviral Effect. Biol. Pharm. Bull. 2009, 32, 892–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Chiu, L.; Ooi, V.; Chan, P.; Ang, P. Antiviral property and mechanisms of a sulphated polysaccharide from the brown alga Sargassum patens against Herpes simplex virus type 1. Phytomedicine 2006, 13, 695–701. [Google Scholar] [CrossRef]
- Vo, T.-S.; Kim, S.-K. Potential Anti-HIV Agents from Marine Resources: An Overview. Mar. Drugs 2010, 8, 2871–2892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pati, M.P.; Das Sharma, S.; Nayak, L.; Panda, C.R. Uses of seaweed and its application to human welfare: A review. Int. J. Pharm. Pharm. Sci. 2016, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Grassauer, A.; Prieschl-Grassauer, E.; Biotech, A.G. Antiviral Composition Comprising a Sulfated Polysaccharide. U.S. Patent No. 10,342,820, 5 March 2009. [Google Scholar]
- Zaporozhets, T.S.; Besednova, N.N. Biologically active compounds from marine organisms in the strategies for combating coronaviruses. AIMS Microbiol. 2020, 6, 470–494. [Google Scholar] [CrossRef]
- Ahn, G.; Kim, K.N.; Cha, S.H.; Song, C.B.; Lee, J.; Heo, M.S.; Yeo, I.K.; Lee, N.H.; Jee, Y.H.; Kim, J.S.; et al. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Artan, M.; Li, Y.; Karadeniz, F.; Lee, S.H.; Kim, M.M.; Kim, S.K. Anti-HIV-1 activity of phloroglucinol derivative, 6,6′-bieckol, from Ecklonia cava. Bioorganic Med. Chem. 2008, 16, 7921–7926. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.P.; Pereira, R.C.; Abrantes, J.L.; Cirne dos Santos, C.C.; Rebello, M.A.; Frugulhetti, I.C.; Texeira, V.L. In vitro antiviral diterpenes from the Brazilian brown alga Dictyota pfaffi. Plant Med. 2004, 70, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.B.; Jeong, H.J.; Yoon, S.Y.; Park, J.-Y.; Kim, Y.M.; Park, S.-J.; Rho, M.-C.; Kim, S.-J.; Lee, W.S. Influenza Virus Neuraminidase Inhibitory Activity of Phlorotannins from the Edible Brown Alga Ecklonia cava. J. Agric. Food Chem. 2011, 59, 6467–6473. [Google Scholar] [CrossRef]
- Wang, S.; Bligh, S.; Shi, S.; Wang, Z.; Hu, Z.; Crowder, J.; Branford-White, C.; Vella, C. Structural features and anti-HIV-1 activity of novel polysaccharides from red algae Grateloupia longifolia and Grateloupia filicina. Int. J. Biol. Macromol. 2007, 41, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structural features and antiviral activityof sulphated fucans from the brown seaweed Cystoseira indica. Antivir. Chem. Chemother. 2007, 18, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Queiroz, K.C.S.; Medeiros, V.P.; Queiroz, L.S.; Abreu, L.R.D.; Rocha, H.A.O.; Ferreira, C.V.; Juca, M.B.; Aoyama, H.; Leite, E.L. Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed. Pharmacother. 2008, 62, 303–307. [Google Scholar] [CrossRef]
- Feldman, S.C.; Reynaldi, S.; Stortz, C.A.; Cerezo, A.S.; Damont, E.B. Antiviral properties of fucoidan fractions from Leathesia difformis. Phytomedicine 1999, 6, 335–340. [Google Scholar] [CrossRef]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.S.; Navid, M.H.; Ghosh, T.; Schnitzler, P.; Ray, B. Structural features and in vitro antiviral activities of sulfated polysaccharides from Sphacelaria indica. Phytochemistry 2011, 72, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Harden, E.A.; Falshaw, R.; Carnachan, S.M.; Kern, E.R.; Prichard, M.N. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir. Res. 2009, 83, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, U.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry 2006, 67, 2474–2482. [Google Scholar] [CrossRef]
- Cooper, R.; Dragar, C.; Elliot, K.; Fitton, J.H.; Godwin, J.; Thompson, K. GFS, a preparation of Tasmanian Undaria pinnatifida is associated with healing and inhibition of reactivation of Herpes. BMC Complementary Altern. Med. 2002, 2, 11. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.C.; Merino, E.R.; Pujol, C.A.; Damonte, E.B.; Cerezo, A.S.; Matulewicz, M.C. Galactans from cystocarpic plants of the red seaweed Callophyllis variegata (Kallymeniaceae, Gigartinales). Carbohydr. Res. 2005, 340, 2742–2751. [Google Scholar] [CrossRef] [PubMed]
- Ponce, N.M.A.; Stortz, C.A. A Comprehensive and Comparative Analysis of the Fucoidan Compositional Data across the Phaeophyceae. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Induction of acrosome reaction in human spermatozoa used for subzonal insemination. Hum. Reprod. 1992, 7, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Matsuhiro, B.; Conte, A.F.; Damonte, E.B.; Kolender, A.A.; Matulewicz, M.C.; Mejías, E.G.; Zúñiga, E.A. Structural analysis and antiviral activity of a sulfated galactan from the red seaweed Schizymenia binderi (Gigartinales, Rhodophyta). Carbohydr. Res. 2005, 340, 2392–2402. [Google Scholar] [CrossRef]
- Mazumder, S.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta). Int. J. Biol. Macromol. 2002, 31, 87–95. [Google Scholar] [CrossRef]
- Pérez Recalde, M.; Noseda, M.D.; Pujol, C.A.; Carlucci, M.J.; Matulewicz, M.C. Sulfated mannans from the red seaweed Nemalion helminthoides of the South Atlantic. Phytochemistry 2009, 70, 1062–1068. [Google Scholar] [CrossRef]
- Bouhlal, R.; Haslin, C.; Chermann, J.-C.; Colliec-Jouault, S.; Sinquin, C.; Simon, G.; Cerantola, S.; Riadi, H.; Bourgougnon, N. Antiviral Activities of Sulfated Polysaccharides Isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales). Mar. Drugs 2011, 9, 1187–1209. [Google Scholar] [CrossRef]
- Mandal, P.; Pujol, C.A.; Carlucci, M.J.; Chattopadhyay, K.; Damonte, E.B.; Ray, B. Anti-herpetic activity of a sulfated xylomannan from Scinaia hatei. Phytochemistry 2008, 69, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Talarico, L.B.; Zibetti, R.G.M.; Faria, P.C.S.; Scolaro, L.A.; Duarte, M.E.R.; Noseda, M.D.; Pujol, C.A.; Damonte, E.B. Anti-herpes simplex virus activity of sulfated galactans from the red seaweeds Gymnogongrus griffithsiae and Cryptonemia crenulata. Int. J. Biol. Macromol. 2004, 34, 63–71. [Google Scholar] [CrossRef]
- Huheihel, M.; Ishanub, V.; Talb, J.; Arada, S.M. Activity of Porphyridium sp. polysaccharide against herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Methods 2002, 50, 189–200. [Google Scholar] [CrossRef]
- Haslin, C.; Lahaye, M.; Pellegrini, M.; Chermann, J.-C. In Vitro Anti-HIV Activity of Sulfated Cell-Wall Polysaccharides from Gametic, Carposporic and Tetrasporic Stages of the Mediterranean Red Alga Asparagopsis armata. Planta Med. 2001, 67, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Duarte, K.; Justino, C.; Gomes, A.; Rocha-Santos, T.; Duarte, A.C. Green Analytical Methodologies for Preparation of Extracts and Analysis of Bioactive Compounds. In Comprehensive Analytical Chemistry; Elsevier BV: Amsterdam, The Netherlands, 2014; Volume 65, pp. 59–78. [Google Scholar]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive Proteins, Peptides, and Amino Acids from Macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef]
- Ghosh, P.; Adhikaria, U.; Ghosala, P.K.; Pujolb, C.A.; Carluccib, M.J.; Damonteb, E.B.; Ray, B. In vitro anti-herpetic activity of sulfated polysaccharide fractions from Caulerpa racemosa. Phytochemistry 2004, 65, 3151–3157. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2001, 18, 1–49. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef] [Green Version]
- Jane, P.; Bradford, M. Seaweed: Nature’s Secret for a Long and Healthy Life? Nutr. pract. 2006, 1–21. [Google Scholar]
- Wang, B.; Tong, G.Z.; Le Qu, Y.; Li, L. Microwave-Assisted Extraction and In Vitro Antioxidant Evaluation of Polysaccharides from Enteromorpha prolifera. Appl. Mech. Mater. 2011, 79, 204–209. [Google Scholar] [CrossRef]
- Hans, N.; Malik, A.; Naik, S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Bioresour. Technol. Rep. 2021, 13, 100623. [Google Scholar] [CrossRef]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. Sulfated Seaweed Polysaccharides as Antiviral Agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef]
- Ezeigbo, I.I.; Ezeja, M.; Madubuike, K.; Ifenkwe, D.; Ukweni, I.; Udeh, N.; Akomas, S. Antidiarrhoeal activity of leaf methanolic extract of Rauwolfia serpentina. Asian Pac. J. Trop. Biomed. 2012, 2, 430–432. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.; Castegna, A.; Pocernich, C.B.; Drake, J.; Scapagnini, G.; Calabrese, V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem. 2002, 13, 444–461. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Invited Review: Oxidation of Biological Systems: Oxidative Stress Phenomena, Antioxidants, Redox Reactions, and Methods for Their Quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [Green Version]
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential Antioxidant Capacity of Sulfated Polysaccharides from the Edible Marine Brown SeaweedFucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Zhang, Q.-B.; Yu, P.-Z.; Zhou, G.-F.; Li, Z.-E.; Xu, Z.-H. Studies on antioxidant activities of fucoidan from Laminaria japonica. Chin. Trad. Herbal. Drugs 2003, 34, 824–826. [Google Scholar]
- Kim, S.H.; Choi, D.S.; Athukorala, Y.; Jeon, Y.J.; Senevirathne, M.; Rha, C.K. Antioxidant Activity of Sulphated Polysaccharides Isolated from Sargassum fulvellum. J. Food Sci. Nutr. 2007, 12, 65–73. [Google Scholar]
- De Souza, M.C.R. Antioxidant activity of fucanas and galactans extracted from seaweed. Master’s Thesis, Federal University of Rio Grande do Norte, Natal, Rio Grande do Norte, Brazil, 26 May 2008. [Google Scholar]
- Rocha de Souza, M.C.; Marques, C.T.; Dore, C.M.G.; da Silva, F.R.F.; Rocha, H.A.O.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, C.; Shi, F.; Hu, X. Purification and Characterization of Lipopolysaccharides. In Alzheimer’s Disease; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2010; Volume 53, pp. 27–51. [Google Scholar]
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Solanki, H.K. RETRACTED: Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014, 105, 97–112. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, T.; Zhang, Q.; Li, Z.; Zhao, Z.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Ghosh, T.; Sinha, S.; Chattopadhyay, K.; Karmakar, P.; Ray, B. Polysaccharides from Turbinaria conoides: Structural features and antioxidant capacity. Food Chem. 2010, 118, 823–829. [Google Scholar] [CrossRef]
- Heo, S.-J.; Cha, S.-H.; Lee, K.-W.; Jeon, Y.-J. Antioxidant Activities of Red Algae from Jeju Island. ALGAE 2006, 21, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, C.C.R.; Paixão, I.C.N.D.P.; Teixeira, V.L. Antioxidant Activity of Natural Products Isolated from Red Seaweeds. Nat. Prod. Commun. 2014, 9, 1031–1036. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Zubia, M.; Robledo, D.; Freile-Pelegrin, Y. Antioxidant activities in tropical marine macroalgae from the Yucatan Peninsula, Mexico. J. Appl. Phycol. 2007, 19, 449–458. [Google Scholar] [CrossRef]
- Kim, A.R.; Shin, T.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; Park, N.K.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with anti-oxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef]
- Srivastava, N.; Saurav, K.; Mohanasrinivasan, V.; Kannabiran, K.; Singh, M. Antibacterial Potential of Macroalgae Collected from the Mandapam Coast. India Br. J. Pharmacol. Toxicol. 2010, 1, 72–76. [Google Scholar]
- Kosanić, M.; Ranković, B.; Stanojković, T. Biological activities of two macroalgae from Adriatic coast of Montenegro. Saudi J. Biol. Sci. 2015, 22, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Karim, O.H.; Gheda, S.F.; Ismail, G.A.; Abo-Shady, A.M. Phytochemical Screening and antioxidant activity of Chlorella vulgaris. Delta J. Sci. 2020, 41, 81–91. [Google Scholar] [CrossRef]
- Shibata, T.; Iimuro, Y.; Yamamoto, Y.; Maetani, Y.; Ametani, F.; Itoh, K.; Konishi, J. Small Hepatocellular Carcinoma: Comparison of Radio-frequency Ablation and Percutaneous Microwave Coagulation Therapy. Radiology 2002, 223, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, Y.; Kishimoto, Y.; Ohsako, S. Immunohistochemical study of Ca2+/calmodulin-dependent protein kinase II in the Drosophila brain using a specific monoclonal antibody. Brain Res. 2003, 974, 99–116. [Google Scholar] [CrossRef]
- Connan, F.; Murphy, F.; Connor, S.E.J.; Rich, P.; Murphy, T.; Bara-Carill, N.; Landau, S.; Krljes, S.; Ng, V.; Williams, S.; et al. Hippocampal volume and cognitive function in anorexia nervosa. Psychiatry Res. 2006, 146, 117–125. [Google Scholar] [CrossRef]
- Kang, M.C.; Wijesinghe, W.A.; Lee, S.H.; Kang, S.M.; Ko, S.C.; Yang, X.; Kang, N.; Jeon, B.T.; Kim, J.; Lee, D.H.; et al. Dieckol isolated from brown seaweed Ecklonia cava attenuates type capital I, Ukrainiancapital I, Ukrainian diabetes in db/db mouse model. Food Chem. Toxicol. 2013, 53, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Shin, T.; Utsuki, T.; Choi, J.-S.; Byun, D.-S.; Kim, H.-R. Isolation and Identification of Phlorotannins from Ecklonia stolonifera with Antioxidant and Hepatoprotective Properties in Tacrine-Treated HepG2 Cells. J. Agric. Food Chem. 2012, 60, 5340–5349. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, L. Phlorotannins from Brown Algae (Fucus vesiculosus) Inhibited the Formation of Advanced Glycation Endproducts by Scavenging Reactive Carbonyls. J. Agric. Food Chem. 2012, 60, 1326–1334. [Google Scholar] [CrossRef]
- Shibata, Y.; Hu, J.; Kozlov, M.M.; Rapoport, T.A. Mechanisms Shaping the Membranes of Cellular Organelles. Annu. Rev. Cell Dev. Biol. 2009, 25, 329–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, S.J.; Kim, J.P.; Jung, W.K.; Lee, N.H.; Kang, H.S.; Jun, E.M.; Park, S.H.; Kang, S.M.; Lee, Y.J.; Park, P.J.; et al. Identification of chemical structure and free radical scavenging activity of diphlorethohydroxycarmalol isolated from a brown alga, Ishige okamurae. J. Microbiol. Biotechnol. 2008, 18, 676–681. [Google Scholar]
- Ye, H.; Wanga, K.; Zhoub, C.; Liua, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xue, C.-H.; Li, B.-F. Study of antioxidant activities of sulfated polysaccharides from Laminaria japonica. J. Appl. Phycol. 2007, 20, 431–436. [Google Scholar] [CrossRef]
- Ananthi, S.; Raghavendran, H.R.B.; Sunil, A.G.; Gayathri, V.; Ramakrishnan, G.; Vasanthi, H.R. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (Marine Brown Alga). Food Chem. Toxicol. 2010, 48, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Barahona, T.; Encinas, M.V.; Mansilla, A.; Matsuhiro, B.; Zúñiga, E.A. A sulfated galactan with antioxidant capacity from the green variant of tetrasporic Gigartina skottsbergii (Gigartinales, Rhodophyta). Carbohydr. Res. 2012, 347, 114–120. [Google Scholar] [CrossRef]
- Yoshizawa, Y.; Tsunehiro, J.; Nomura, K.; Itoh, M.; Fukui, F.; Ametani, A.; Kaminogawa, S. In Vivo Macrophage-stimulation Activity of the Enzyme-degraded Water-soluble Polysaccharide Fraction from a Marine Alga (Gracilaria verrucosa). Biosci. Biotechnol. Biochem. 1996, 60, 1667–1671. [Google Scholar] [CrossRef] [Green Version]
- Makkar, F.; Chakraborty, K. Highly oxygenated antioxidative 2H-chromen derivative from the red seaweed Gracilaria opuntia with pro-inflammatory cyclooxygenase and lipoxygenase inhibitory properties. Nat. Prod. Res. 2017, 32, 2756–2765. [Google Scholar] [CrossRef]
- Hickey, R.M. Extraction and Characterization of Bioactive Carbohydrates with Health Benefits from Marine Resources: Macro- and Microalgae, Cyanobacteria, and Invertebrates. In Marine Bioactive Compounds; Springer: New York, NY, USA, 2011; pp. 159–172. [Google Scholar]
- Wang, R.; Paul, V.J.; Luesch, H. Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2–ARE pathway. Free Radic. Biol. Med. 2013, 57, 141–153. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef]
- Barbot, Y.N.; Al-Ghaili, H.; Benz, R. A Review on the Valorization of Macroalgal Wastes for Biomethane Production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, M.; You, S. Sulfated Polysaccharides from Green Seaweeds. In Hb25_Springer Handbook of Marine Biotechnology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2015; pp. 941–953. [Google Scholar]
- Glasson, C.R.; Sims, I.M.; Carnachan, S.M.; de Nys, R.; Magnusson, M. A cascading biorefinery process targeting sulfated polysaccharides (ulvan) from Ulva ohnoi. Algal Res. 2017, 27, 383–391. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Sankaranarayanan, S.; Gajaria, T.K.; Li, G.; Kujawski, W.; Kujawa, J.; Navia, R. A Short Review on the Valorization of Green Seaweeds and Ulvan: FEEDSTOCK for Chemicals and Biomaterials. Biomolecules 2020, 10, 991. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Magnusson, M.; De Nys, R.; Glasson, C.R. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Lahaye, A.M.; Robic, A. Structure and Functional Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Robic, J.F.; Sassi, M. Lahaye, Impact of stabilization treatments of the green seaweed Ulva rotundata (Chlorophyta) on the extraction yield, the physico-chemical and rheological properties of ulvan. Carbohydr. Polym. 2008, 74, 344–352. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. A practical perspective on ulvan extracted from green algae. J. Appl. Phycol. 2012, 25, 407–424. [Google Scholar] [CrossRef] [Green Version]
- Roselló-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N.; et al. Application of Non-conventional Extraction Methods: Toward a Sustainable and Green Production of Valuable Compounds from Mushrooms. Food Eng. Rev. 2015, 8, 214–234. [Google Scholar] [CrossRef]
- Jessop, P.G.; Al, E.; And, P.G.J. ChemInform Abstract: Opportunities for Greener Alternatives in Chemical Formulations. ChemInform 2015, 46, 2664–2678. [Google Scholar] [CrossRef]
- Hernández-Garibay, E.; Zertuche-González, J.A.; Pacheco-Ruíz, I. Isolation and chemical characterization of algal polysaccharides from the green seaweed Ulva clathrata (Roth) C. Agardh. J. Appl. Phycol. 2010, 23, 537–542. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Herrero, M.; Mendiola, J.A.; Ibanez, E. Subcritical water extraction of bioactive components from algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing: Sawston, UK, 2013; pp. 534–560. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical Water Extraction of Bioactive Compounds from Plants and Algae: Applications in Pharmaceutical and Food Ingredients. Food Eng. Rev. 2015, 8, 23–34. [Google Scholar] [CrossRef]
- Zollmann, M.; Robin, A.; Prabhu, M.; Polikovsky, M.; Gillis, A.; Greiserman, S.; Golberg, A. Green technology in green macroalgal biorefineries. Phycologia 2019, 58, 516–534. [Google Scholar] [CrossRef]
- Rocha, C.M.; Genisheva, Z.; Ferreira-Santos, P.; Rodrigues, R.; Vicente, A.A.; Teixeira, J.A.; Pereira, R.N. Electric field-based technologies for valorization of bioresources. Bioresour. Technol. 2018, 254, 325–339. [Google Scholar] [CrossRef] [Green Version]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of Novel Extraction Technologies for Bioactives from Marine Algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Hachemi, I.; Murzin, D.Y. Comparative study of the extraction methods for recovery of carotenoids from algae: Extraction kinetics and effect of different extraction parameters. J. Chem. Technol. Biotechnol. 2014, 89, 1607–1626. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Muñoz, M.J.G. Ultrasound-Assisted Extraction of Bioactive Carbohydrates. In Water Extraction of Bioactive Compounds; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 317–331. [Google Scholar]
- Wu, S.-C. Antioxidant Activity of Sulfated Seaweeds Polysaccharides by Novel Assisted Extraction. In Solubility Polysacch.; IntechOpen: London, UK, 2017; pp. 89–108. [Google Scholar] [CrossRef] [Green Version]
- Navya, P.; Khora, S.S. In vitro cytotoxicity analysis of sulfated polysaccharides from green seaweed Codium tomentosum Stackhouse, 1797. J. Appl. Pharm. Sci. 2017, 7, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Camargo, A.D.P.; Ibáñez, E.; Cifuentes, A.; Herrero, M. Bioactives Obtained from Plants, Seaweeds, Microalgae and Food By-Products Using Pressurized Liquid Extraction and Supercritical Fluid Extraction. Compr. Anal. Chem. 2017, 27–51. [Google Scholar] [CrossRef]
- Ibañez, E.; Herrero, M.; Mendiola, J.A.; Castro-Puyana, M. Extraction and Characterization of Bioactive Compounds with Health Benefits from Marine Resources: Macro and Micro Algae, Cyanobacteria, and Invertebrates. In Marine Bioactive Compounds; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2012; pp. 55–98. [Google Scholar]
- Turner, C.; Ibañez, E. Pressurized Hot Water Extraction and Processing. Light Scatt. Technol. Food Prop. Qual. Safety Assess. 2011, 223–254. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; del Castillo, M.D.; Ibáñez, E.; Herrero, M. Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res. Int. 2010, 43, 2341–2348. [Google Scholar] [CrossRef] [Green Version]
- Santoyo, S.; Jaime, L.; Plaza, M.; Herrero, M.; Rodriguez-Meizoso, I.; Ibañez, E.; Reglero, G. Antiviral compounds obtained from microalgae commonly used as carotenoid sources. J. Appl. Phycol. 2011, 24, 731–741. [Google Scholar] [CrossRef] [Green Version]
- Santoyo, S.; Plaza, M.; Jaime, L.; Ibáñez, E.; Reglero, G.; Señorans, J. Pressurized liquids as an alternative green process to extract antiviral agents from the edible seaweed Himanthalia elongata. J. Appl. Phycol. 2010, 23, 909–917. [Google Scholar] [CrossRef] [Green Version]
- Santoyo, S.; Ramírez Anguiano, A.; García, L.; Reglero, G.; Rivas, C. Antiviral Activities of Boletus Edulis, Pleurotus Ostreatus and Lentinus Edodes Extracts and Polysaccharide Fractions Against Herpes Simplex Virus Type 1. 2012. Available online: https://www.researchgate.net/ (accessed on 12 December 2020).
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Señoráns, F.; Cifuentes, A.; Ibáñez, E. Subcritical water extraction and characterization of bioactive compounds from Haematococcus pluvialis microalga. J. Pharm. Biomed. Anal. 2010, 51, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Li, L.; Sun, H.; Zhang, Z. Optimization of Subcritical Water Extraction of Polysaccharides from Inonotus Obliquus and their Antioxidant Activities. Int. J. Biol. 2017, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.; Cifuentes, A.; Ibanez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Anaëlle, T.; Leon, E.S.; Laurent, V.; Elena, I.; Mendiola, J.A.; Stéphane, C.; Nelly, K.; Stéphane, L.B.; Luc, M.; Valérie, S.-P. Green improved processes to extract bioactive phenolic compounds from brown macroalgae using Sargassum muticum as model. Talanta 2013, 104, 44–52. [Google Scholar] [CrossRef]
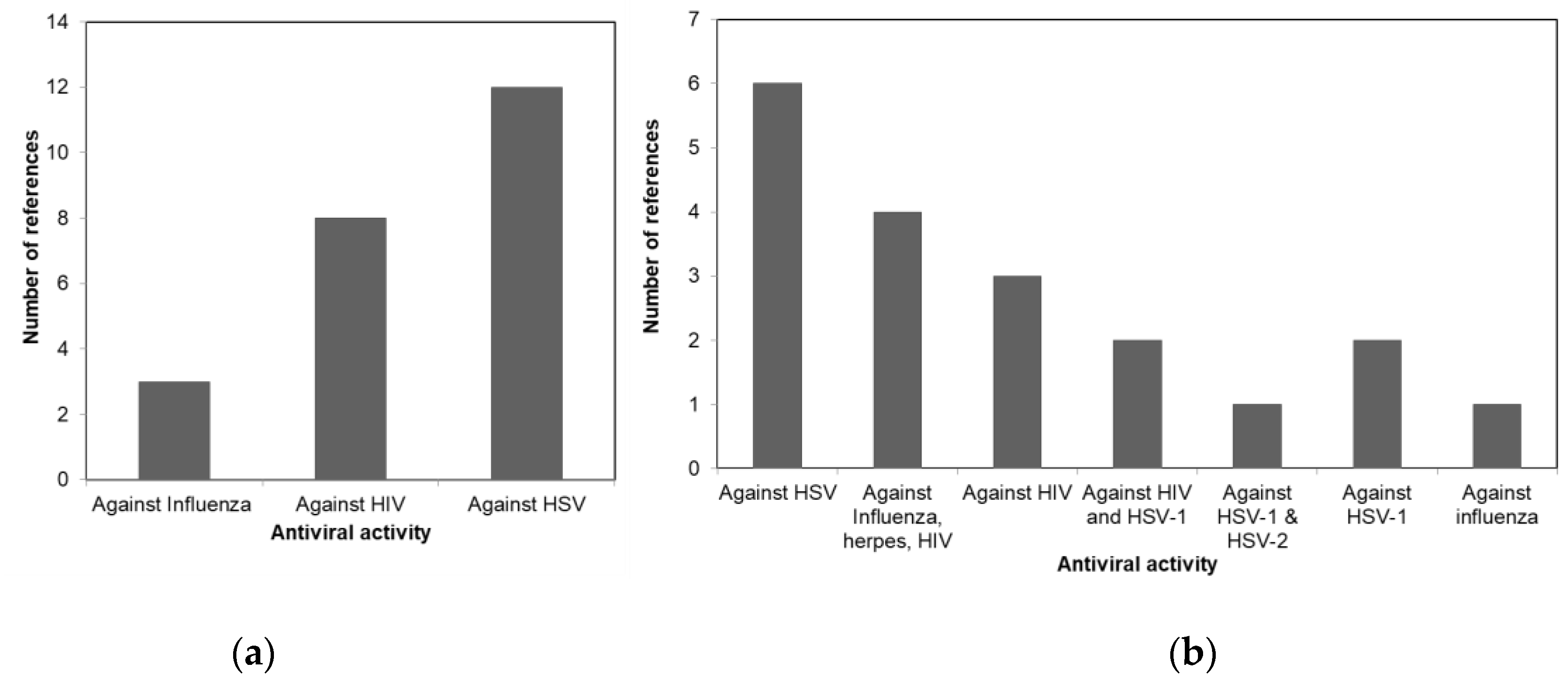
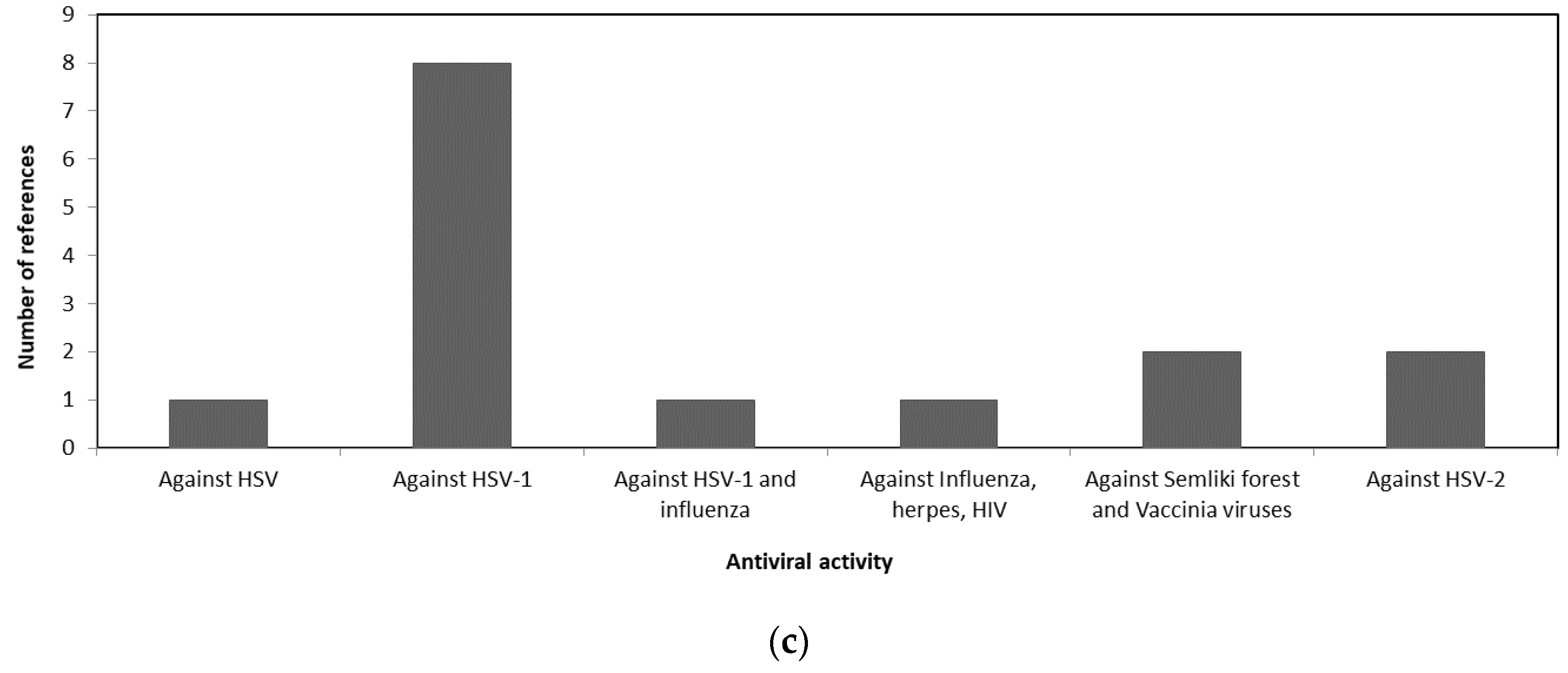
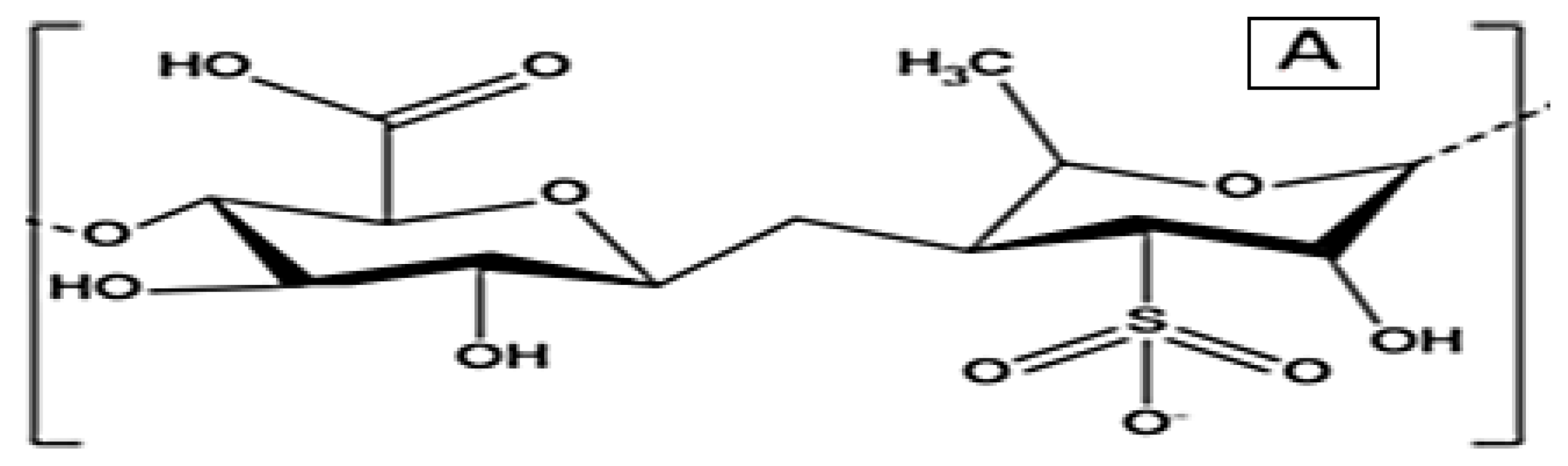
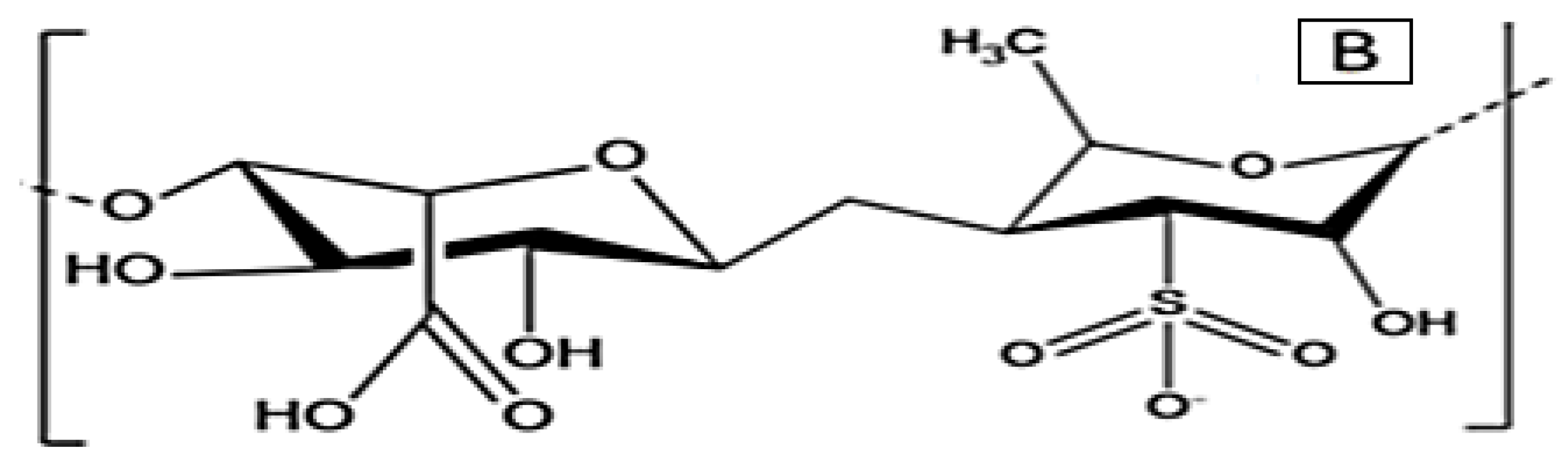
| Macroalgae Taxa | Macroalgae Species | Bioactive Metabolites | Antiviral Activity | Reference |
|---|---|---|---|---|
| Phaeophyceae | Ecklonia cava | Phlorotannin (6,6′-Bieckol, 8,8′-bieckol) | Against HIV | [23,24] |
| Dictyota caribaea horning & schnetter | Sulphated Fucans | Against HIV | [25] | |
| Ecklonia cava | Phlorotannin (Phloroglucinol, eckol, 7-Phloroeckol, phlorofucofuroeckol, dieckol) | Against Influenza | [26] | |
| Grateloupia filicina | Sulphated polysaccharides | Against HSV | [27] | |
| Grateloupia longifolia | Sulphated polysaccharides | Against HIV | [27] | |
| Adenocystis utricularis | Sulphated polysaccharides | Against HSV | [13] | |
| Cystoseira indica | Sulphated polysaccharides | Against HSV | [28] | |
| Dictyota mertensii | Sulphated polysaccharides | Against HIV | [29] | |
| Fucus vesiculosus | Sulphated polysaccharides | Against HIV | [29] | |
| Hydroclathrus clathratus | Sulphated polysaccharides | Against HSV | [27] | |
| Leathesia difformis | Sulphated polysaccharides | Against Influenza | [30] | |
| Lobophora variegate | Sulphated fucans | Against HIV | [29] | |
| Padina tetrastromatica | Sulphated polysaccharides | Against HSV | [31] | |
| Sphacelaria indica | Sulphated polysaccharides | Against HSV | [32] | |
| Spachnidium rugosum | Sulphated polysaccharides | Against HSV | [33] | |
| Spatoglossum schroederi | Sulphated polysaccharides | Against HIV | [29] | |
| Stoechodperumum magiatum | Sulphated polysaccharides | Against HSV | [34] | |
| Undaria pinnatifida | Sulphated polysaccharides | Against HSV | [29,35] | |
| Sargassum patens | Sulphated polysaccharides | Against HSV | [17] | |
| Undaria pinnatifida | Sulphated polysaccharides | Against HSV | [12] | |
| Callophyllis variegate | Sulphated galactans | Against HSV | [36] | |
| Undaria pinnatifida | Sulphated polysaccharides | Against HIV | [33] | |
| Adenocystis utricularis | Fucoidans | Against HSV | [37] |
| Macroalgae Taxa | Macroalgae Species | Bioactive Metabolites | Antiviral Activity | Reference |
|---|---|---|---|---|
| Rhodophyceae | Gigartina atropupurea | Sulphated Polysaccharides | Against HSV | [33] |
| Chondria sulphated polysaccharides | Peptides (Condriamide A) | Against HSV | [38] | |
| Schizymenia binderi | Sulphated Galactan | Against HSV | [39] | |
| Plocamium cartilagineum | Sulphated Polysaccharides | Against HSV | [33] | |
| Gracilaria corticate | Sulphated Polysaccharides (Galactan Sulphates) | Against HSV | [40] | |
| Sebdeniia polydactyla | Sulphated Polysaccharides | Against Influenza, Herpes, HIV | [31] | |
| Nemalion helminthoides | Sulphated Polysaccharides | Against Influenza, Herpes, HIV | [41] | |
| Sphaerococcus coronopifolius | Sulphated Polysaccharides | Against Influenza, Herpes, HIV | [42] | |
| Boergeseniella thuyoides | Sulphated Polysaccharides | Against Influenza, Herpes, HIV | [42] | |
| _ | Sulfated Xylomannan | Against HSV-1 & HSV-2 | [43] | |
| Bryopsis sulphated polysaccharides | Cyclic Depsipeptide (Kahalalide F) | Against HIV | [44] | |
| Cryptonemia crenulate | Sulphated Polysaccharides | Against HSV-1 | [45] | |
| Gelidium cartilagenium | Sulphated Polysaccharides | Against Influenza. | [46] | |
| Grateloupia filicina | Sulfated GA lactones | Against HIV | [27] | |
| Stenogramme interrupta | Carrageenans | Against HSV-1 & HSV-2 | [11] | |
| Asparagopsis armata | Sulfated agaran | Against HSV-1 | [47] | |
| Bostrychia montagnei | Sulfated agarans | Against HSV-1 & HSV-2 | [48] | |
| Gymnogongrus torulosus | DL- hybrid galactans | Against HSV-2, dengue virus 2 | [14] | |
| Gracilaria corticata | Sulfated agarans | Against HSV-1 & HSV-2 | [40] | |
| Grateloupia longifolia | Sulfated Galactones | Against HIV | [27] | |
| Sphaerococcus coronopifolius | Sulphated Polysaccharides | Against HIV & HSV-1 | [42] | |
| Boergeseniella boergesen | Sulphated Polysaccharides | Against HIV & HSV-1 | [42] | |
| Schizymenia binderi | Sulfated Galactan | Against HSV | [39] |
| Macroalgae Taxa | Macroalgae Species | Bioactive Metabolites | Antiviral Activity | Reference |
|---|---|---|---|---|
| Chlorophyceae | Codium fragile | Polysaccharides | Against HSV-2 | [16] |
| Ulva sulphated polysaccharides | Peptides (Hexapeptide) | Against HSV | [49] | |
| Caulerpa racemose | Sulphated Polysaccharides | Against HSV-2 | [50] | |
| Ulva fasciata | Sulphated Polysaccharides | Against Semliki Forest & Vaccinia Viruses | [51] | |
| Codium elongatum | Sulphated Polysaccharides | Against Semliki Forest & Vaccinia Viruses | [51] | |
| Caulerpa brachypus | Sulphated Polysaccharides | Against HSV-1 | [52] | |
| Caulerpa scapelliformis | Sulphated Polysaccharides | Against HSV-1 | ||
| Caulerpa okamurai | Sulphated Polysaccharides | Against HSV-1 | ||
| Chaetomorpha crassa | Sulphated Polysaccharides | Against HSV-1 | ||
| Chaetomorpha spiralis | Sulphated Polysaccharides | Against HSV-1 | ||
| Monostroma nitidum, | Sulphated Polysaccharides | Against HSV-1 | ||
| Codium adhaerens | Sulphated Polysaccharides | Against HSV-1 | ||
| Codium latum | Sulphated Polysaccharides | Against HSV-1 |
| Macroalgae Taxa | Macroalgae Species | Bioactive Metabolites | Reference |
|---|---|---|---|
| Phaeophyceae | Eisenia bicyclis | Polyphenols | [71,79] |
| Rhodophyceae | Martensia fragilis | Alkaloids | [80] |
| Phaeophyceae | Laminaria species | Phenolic compounds | [81] |
| Phaeophyceae | Ecklonia cava | Phlorotannin (2,7-Phloroglucinol, 6,6′-bieckol) | [23,82] |
| Phaeophyceae | E. kurome | Phlortotannin (dieckol) | [23] |
| Phaeophyceae | Padina perindusiata Thivy | Sulphated Fucans | [65] |
| Phaeophyceae | Ecklonia stolonifera | Phlorotannin (Phlorofucofuroeckol A, dieckol, dioxinodehydroeckol) | [75] |
| Phaeophyceae | Ecklonia stolonifera | Phlorotannin (Phloroglucinol) | [23] |
| Phaeophyceae | Lobophora | bromophenols and phenols | [74] |
| Phaeophyceae | Ecklonia stolonifera | Phlorotannin (2 Phloroeckol, eckol, phlorofucofuroeckol B, 6,6′-bieckol) | [83] |
| Phaeophyceae | Fucus vesiculosus | Phlorotannin (Fucophlorethol A, tetrafucol A, trifucodiphlorethol A) | [84] |
| Phaeophyceae | Eisenia bicyclis | Phlorotannin (Triphlorethol A, 8,8′-Bieckol, phlorofucofuroeckol A, eckol, dieckol) | [85] |
| Phaeophyceae | Ishige okamurae | Phlorotannin (Diphloroethohydroxycarmalol | [86] |
| Phaeophyceae | Sargassum pallidum | Sulphated Polysaccharides | [87] |
| Phaeophyceae | Laminaria japonica | Sulphated Polysaccharides | [62,88] |
| Phaeophyceae | Turbinaria ornata | Sulphated Polysaccharides | [89] |
| Rhodophyceae | Gigartina skottsbergi | Sulphated Polysaccharides | [90] |
| Rhodophyceae | Gracilaria verrucose | Sulphated Polysaccharides | [91] |
| Rhodophyceae | Gracilaria opuntia | Azocinylmorpholinone | [92] |
| Chlorophyceae | Ulva pertusa | Sulphated Polysaccharides (ulvans) | [93] |
| Chlorophyceae | Ulva lactuca | Monounsaturated fatty acids (MUFA) derivatives | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Shafei, R.; Hegazy, H.; Acharya, B. A Review of Antiviral and Antioxidant Activity of Bioactive Metabolite of Macroalgae within an Optimized Extraction Method. Energies 2021, 14, 3092. https://doi.org/10.3390/en14113092
El-Shafei R, Hegazy H, Acharya B. A Review of Antiviral and Antioxidant Activity of Bioactive Metabolite of Macroalgae within an Optimized Extraction Method. Energies. 2021; 14(11):3092. https://doi.org/10.3390/en14113092
Chicago/Turabian StyleEl-Shafei, Rasha, Hala Hegazy, and Bishnu Acharya. 2021. "A Review of Antiviral and Antioxidant Activity of Bioactive Metabolite of Macroalgae within an Optimized Extraction Method" Energies 14, no. 11: 3092. https://doi.org/10.3390/en14113092
APA StyleEl-Shafei, R., Hegazy, H., & Acharya, B. (2021). A Review of Antiviral and Antioxidant Activity of Bioactive Metabolite of Macroalgae within an Optimized Extraction Method. Energies, 14(11), 3092. https://doi.org/10.3390/en14113092








