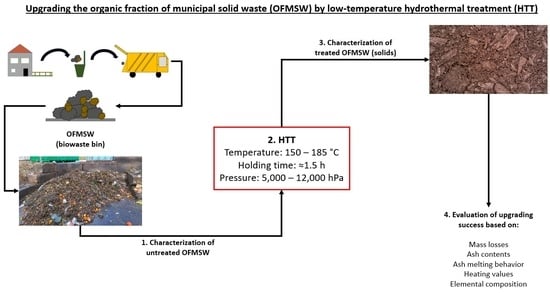
Upgrading the Organic Fraction of Municipal Solid Waste by Low Temperature Hydrothermal Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. OFMSW Sample, Dry Matter, Processing and Hydrothermal Treatment
2.2. Organic Dry Matter
2.3. C, H, N, S, O and Stoichiometric CH4 Potentials
2.4. Calorific Analysis, Cl and Ash Melting Behavior
2.5. Trace Elements
2.6. Statistics
3. Results and Discussion
3.1. Characteristics of Raw and Hydrothermally Treated OFMSW
3.2. Potential Implications of Hydrothermal Treatment on Incineration and Anaerobic Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | anaerobic digestion |
| DM | dry matter |
| DT | deformation temperature |
| DW | distilled water |
| FM | fresh mass |
| FT | flow temperature |
| HHV | higher heating value |
| HTC | hydrothermal carbonization |
| HTT | hydrothermal treatment |
| ICP-OES | inductively coupled plasma-optical emission spectroscopy |
| LHV | lower heating value |
| oDM | organic dry matter |
| OFMSW | organic fraction of municipal solid waste |
| TE | trace element(s) |
| WC | water content |
References
- Federal Ministry of Justice and Consumer Protection. Gesetz zur Förderung der Kreislaufwirtschaft und Sicherung der Umweltverträglichen Bewirtschaftung von Abfällen (Kreislaufwirtschaftsgesetz-KrWG); Bundesministerium der Justiz und für Verbraucherschutz (BMJV): Berlin, Germany, 2020; Available online: https://www.gesetze-im-internet.de/krwg/KrWG.pdf (accessed on 9 May 2021).
- Kern, M.; Raussen, T. Biogas-Atlas: Anlagenhandbuch der Vergärung Biogener Abfälle in Deutschland, 1st ed.; Witzenhausen-Institut für Abfall, Umwelt und Energie GmbH: Witzenhausen, Germany, 2014; ISBN 392867367X. [Google Scholar]
- Birnstengel, B.; Eckhardt, M.; Häusler, A.; Hoffmeister, J.; Labinsky, A.; Lambert, J.; Lühr, O.; Schütz, N.; Simpson, R.; Becker, G.; et al. Statusbericht der Deutschen Kreislaufwirtschaft: Einblicke und Aussichten; BDE—Bundesverband der Deutschen Entsorgungs-, Wasser- und Rohstoffwirtschaft e. V.: Berlin, Germany, 2018; Available online: https://www.bvse.de/images/pdf/Nachrichten_2018/Statusbericht_2018_Ansicht_und_Druck.pdf (accessed on 9 May 2021).
- Federal Ministry of Justice and Consumer Protection. Verordnung Über das Europäische Abfallverzeichnis (Abfallverzeichnis-Verordnung—AVV); Bundesministerium der Justiz und für Verbraucherschutz (BMJV): Berlin, Germany, 2020; Available online: https://www.gesetze-im-internet.de/avv/AVV.pdf (accessed on 9 May 2021).
- Federal Ministry of Justice and Consumer Protection. Verordnung Über die Verwertung von Bioabfällen auf Landwirtschaftlich, Forstwirtschaftlich und Gärtnerisch Genutzten Böden (Bioabfallverordnung—BioAbfV); Bundesministerium der Justiz und für Verbraucherschutz (BMJV): Berlin, Germany, 2017; Available online: https://www.gesetze-im-internet.de/bioabfv/BioAbfV.pdf (accessed on 9 May 2021).
- Kaltschmitt, M.; Hartmann, H.; Hofbauer, H. Energie aus Biomasse: Grundlagen, Techniken und Verfahren, 3, Aktualisierte und Erweiterte Auflage; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-47437-2. [Google Scholar]
- Kern, M.; Raussen, T.; Fundam, K.; Lootsma, A.; Hofmann, H. Aufwand und Nutzen Einer Optimierten Bioabfallverwertung Hinsichtlich Energieeffizienz, Klima- und Ressourcenschutz; Texte 43/2010; Umweltbundesamt: Dessau-Roßlau, Germany, 2010. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/461/publikationen/4010_0.pdf (accessed on 9 May 2021).
- Sailer, G.; Eichermüller, J.; Poetsch, J.; Paczkowski, S.; Pelz, S.; Oechsner, H.; Müller, J. Datasets on chemical composition and anaerobic digestion of organic fraction of municipal solid waste (OFMSW), digested sewage sludge (inoculum) and ashes from incineration or gasification. Data Brief 2020, 31, 105797. [Google Scholar] [CrossRef] [PubMed]
- Sailer, G.; Silberhorn, M.; Eichermüller, J.; Poetsch, J.; Pelz, S.; Oechsner, H.; Müller, J. Influence of digester temperature on methane yield of Organic Fraction of Municipal Solid Waste (OFMSW). Appl. Sci. 2021, 11, 2907. [Google Scholar] [CrossRef]
- Bergius, F. Holz und Kohle, chemische und wirtschaftliche Betrachtung: Vorgetragen auf der Hauptversammlung des Vereins deutscher Chemiker in Dresden am 31. Mai 1928. Z. Angew. Chem. 1928, 41, 707–711. [Google Scholar] [CrossRef]
- Reza, M.T.; Wirth, B.; Lüder, U.; Werner, M. Behavior of selected hydrolyzed and dehydrated products during hydrothermal carbonization of biomass. Bioresour. Technol. 2014, 169, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.-P.; Shi, Z.-J.; Xu, F.; Sun, R.-C. Hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A. Estimating of structural composition of wood and non-wood biomass samples. Energy Sources 2005, 27, 761–767. [Google Scholar] [CrossRef]
- Duku, M.H.; Gu, S.; Hagan, E.B. A comprehensive review of biomass resources and biofuels potential in Ghana. Renew. Sustain. Energy Rev. 2011, 15, 404–415. [Google Scholar] [CrossRef]
- Ramke, H.-G.; Blöhse, D.; Lehmann, H.-J.; Antonietti, M.; Fettig, J. Machbarkeitsstudie zur Energiegewinnung aus Organischen Siedlungsabfällen Durch Hydrothermale Carbonisierung; Hochschule Ostwestfalen-Lippe: Höxter, Germany, 2010. [Google Scholar]
- Quicker, P.; Weber, K. Biokohle: Herstellung, Eigenschaften und Verwendung von Biomassekarbonisaten; Springer: Wiesbaden, Germany, 2016; ISBN 978-3-658-03689-8. [Google Scholar]
- Aida, T.M.; Sato, Y.; Watanabe, M.; Tajima, K.; Nonaka, T.; Hattori, H.; Arai, K. Dehydration of d-glucose in high temperature water at pressures up to 80 MPa. J. Supercrit. Fluids 2007, 40, 381–388. [Google Scholar] [CrossRef]
- Kabyemela, B.M.; Adschiri, T.; Malaluan, R.M.; Arai, K. Glucose and fructose decomposition in subcritical and supercritical water: Detailed reaction pathway, mechanisms, and kinetics. Ind. Eng. Chem. Res. 1999, 38, 2888–2895. [Google Scholar] [CrossRef]
- Ogihara, Y.; Smith, R.L.; Inomata, H.; Arai, K. Direct observation of cellulose dissolution in subcritical and supercritical water over a wide range of water densities (550–1000 kg/m3). Cellulose 2005, 12, 595–606. [Google Scholar] [CrossRef]
- Knappe, V.; Paczkowski, S.; Tejada, J.; Diaz Robles, L.A.; Gonzales, A.; Pelz, S. Low temperature microwave assisted hydrothermal carbonization (MAHC) reduces combustion emission precursors in short rotation coppice willow wood. J. Anal. Appl. Pyrolysis 2018, 134, 162–166. [Google Scholar] [CrossRef]
- Knappe, V.; Paczkowski, S.; Robles, L.A.D.; Gonzales, A.; Pelz, S. Reducing willow wood fuel emission by low temperature microwave assisted hydrothermal carbonization. J. Vis. Exp. 2019, 147. [Google Scholar] [CrossRef] [PubMed]
- Blöhse, D.; Lehmann, H.-J.; Ramke, H.-G. Verbesserte Energetische Nutzung Organischer Industrieabfälle Durch Hydrothermal Carbonisierung; Hochschule Ostwestfalen-Lippe: Höxter, Germany, 2014. [Google Scholar]
- Tradler, S.B.; Mayr, S.; Himmelsbach, M.; Priewasser, R.; Baumgartner, W.; Stadler, A.T. Hydrothermal carbonization as an all-inclusive process for food-waste conversion. Bioresour. Technol. Rep. 2018, 2, 77–83. [Google Scholar] [CrossRef]
- Blümel, R.; Clemens, A.; Döhling, F.; Kietzmann, F.; Klemm, M.; Meisel, K.; Zeymer, M. Integrierte Verwertungsanlage und Strategie für Kommunale Biomasse—HTC Hallesche Wasser und Stadtwirtschaft: FKZ 03KB049A, FKZ 03KB049B; Deutsches Biomasseforschungszentrum gemeinnützige GmbH (DBFZ): Leipzig, Germany, 2015; Available online: https://www.energetische-biomassenutzung.de/fileadmin/Steckbriefe/dokumente/03KB049_HTC_Endbericht.pdf (accessed on 9 May 2021).
- VDI 4630. Fermentation of Organic Materials: Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; 13.030.30, 27.190; Beuth: Berlin, Germany, 2016. [Google Scholar]
- DIN EN 12579:2013. Soil Improvers and Growing Media—Sampling; 65.080; Beuth Verlag GmbH: Berlin, Germany, 2013. [Google Scholar]
- DIN EN 13040:2007. Soil Improvers and Growing Media—Sample Preparation for Chemical and Physical Tests, Determination of Dry Matter Content, Moisture Content and Laboratory Compacted Bulk Density; 65.080; Beuth Verlag GmbH: Berlin, Germany, 2008. [Google Scholar]
- DIN EN 14775:2009. Solid Biofuels—Determination of Ash Content; 75.160.10; Beuth Verlag GmbH: Berlin, Germany, 2012. [Google Scholar]
- DIN EN 13039:2011. Soil Improvers and Growing Media—Determination of Organic Matter Content and Ash; 65.080; Beuth Verlag GmbH: Berlin, Germany, 2012. [Google Scholar]
- DIN EN ISO 16948:2015. Solid Biofuels—Determination of Total Content of Carbon, Hydrogen and Nitrogen; 75.160.10; Beuth Verlag GmbH: Berlin, Germany, 2015. [Google Scholar]
- Boyle, W.C. Energy recovery from sanitary landfills: A review. In Proceedings of the Seminar Sponsored by the UN Institute for Training and Research (UNITAR) and the Ministry for Research and Technology of the Federal Republic of Germany, Göttingen, Germany, 4–8 October 1976; pp. 119–138. [Google Scholar] [CrossRef]
- Buswell, A.M.; Hatfield, W.D. Anaerobic Fermentations; Bulletin No. 32; State of Illinois, Department of Registration and Education, Division of the State Water Survey: Chicago, IL, USA, 1936; Available online: https://www.ideals.illinois.edu/bitstream/handle/2142/94555/ISWSB-32.pdf?sequence=1 (accessed on 9 May 2021).
- DIN EN 14918:2009. Solid Biofuels—Determination of Calorific Value; 75.160.10; Beuth Verlag GmbH: Berlin, Germany, 2014. [Google Scholar]
- DIN EN ISO 16994:2015. Solid Biofuels—Determination of Total Content of Sulfur and Chlorine; 75.160.10; Beuth Verlag GmbH: Berlin, Germany, 2015. [Google Scholar]
- DIN CEN/TS 15370-1:2006. Solid Biofuels—Method for the Determination of Ash Melting Behaviour; 75.160.10; Beuth Verlag GmbH: Berlin, Germany, 2006. [Google Scholar]
- Kaltschmitt, M.; Hartmann, H.; Hofbauer, H. Energie aus Biomasse: Grundlagen, Techniken und Verfahren, 2; Neu Bearb. und Erweiterte Aufl.; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-85094-6. [Google Scholar]
- DIN EN ISO 11885:2009. Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES); 13.060.50; Beuth Verlag GmbH: Berlin, Germany, 2009. [Google Scholar]
- DIN EN 13650:2001. Soil Improvers and Growing Media—Extraction of Aqua Regia Soluble Elements; 65.080; Beuth Verlag GmbH: Berlin, Germany, 2002. [Google Scholar]
- DIN EN ISO 16967:2015-07. Biogene Festbrennstoffe—Bestimmung von Hauptelementen—Al, Ca, Fe, Mg, P, K, Si, Na und Ti (ISO_16967:2015); Deutsche Fassung EN_ISO_16967:2015; Beuth Verlag GmbH: Berlin, Germany, 2015. [Google Scholar]
- DIN EN ISO 16968:2015. Biogene Festbrennstoffe—Bestimmung von Spurenelementen (ISO_); Deutsche Fassung EN_ISO_16968:2015; Beuth Verlag GmbH: Berlin, Germany, 2015. [Google Scholar]
- Lu, X.; Jordan, B.; Berge, N.D. Thermal conversion of municipal solid waste via hydrothermal carbonization: Comparison of carbonization products to products from current waste management techniques. Waste Manag. 2012, 32, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Diederick, R.; Flora, J.R.V.; Berge, N.D. Hydrothermal carbonization of food waste and associated packaging materials for energy source generation. Waste Manag. 2013, 33, 2478–2492. [Google Scholar] [CrossRef] [PubMed]
- Idowu, I.; Li, L.; Flora, J.R.V.; Pellechia, P.J.; Darko, S.A.; Ro, K.S.; Berge, N.D. Hydrothermal carbonization of food waste for nutrient recovery and reuse. Waste Manag. 2017, 69, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Danso-Boateng, E.; Shama, G.; Wheatley, A.D.; Martin, S.J.; Holdich, R.G. Hydrothermal carbonisation of sewage sludge: Effect of process conditions on product characteristics and methane production. Bioresour. Technol. 2015, 177, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Gai, C.; Guo, Y.; Liu, T.; Peng, N.; Liu, Z. Hydrogen-Rich gas production by steam gasification of hydrochar derived from sewage sludge. Int. J. Hydrog. Energy 2016, 41, 3363–3372. [Google Scholar] [CrossRef]
- prEN ISO 17225-8:2016. Biogene Festbrennstoffe—Brennstoffspezifikationen und Klassen. Teil 8 Klassifizierung von Thermisch Behandelten und Gepressten Brennstoffen aus Biomasse/Solid Biofuels—Fuel Specifications and Classes, Graded Thermally Treated and Densified Biomass Fuels; 75.160.10; Beuth Verlag GmbH: Berlin, Germany, 2016. [Google Scholar]
- Lenz, V. Feinstaubminderung im Betrieb von Scheitholzkaminöfen unter Berücksichtigung der Toxikologischen Relevanz (DBFZ Report Nr. 3); Deutsches Biomasseforschungszentrum Gemeinnützige GmbH (DBFZ), Leipzig. Ph.D. Thesis, Technischen Universität Hamburg, Harburg, Germany, 2010. Available online: https://d-nb.info/1066883084/34 (accessed on 9 May 2021).
- Obernberger, I.; Brunner, T.; Barnthaler, G. Chemical properties of solid biofuels—Significance and impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Federal Ministry of Justice and Consumer Protection. Erste Verordnung zur Durchführung des Bundes-Immissionsschutzgesetzes (Verordnung Über Kleine und Mittlere Feuerungsanlagen—1. BImSchV); Bundesministerium der Justiz und für Verbraucherschutz (BMJV): Berlin, Germany, 2020; Available online: https://www.gesetze-im-internet.de/bimschv_1_2010/1._BImSchV.pdf (accessed on 9 May 2021).
| HTT Variant | Feedstock | Heating Program | Output | Replicates |
|---|---|---|---|---|
| 150 °C | OFMSW: 140 gDM DW: 1.4 L | Ramp 40–150 °C: 2.7 °C/min Hold: 5 min Ramp 150–40 °C: −4.8 °C/min Mixing: 200 rpm | Solids | 3 |
| 170 °C | OFMSW: 140 gDM DW: 1.4 L | Ramp 40–170 °C: 3.8 °C/min Hold: 5 min Ramp 170–40 °C: −3.9 °C/min Mixing: 200 rpm | Solids | 3 |
| 185 °C | OFMSW: 140 gDM DW: 1.4 L | Ramp 40–185 °C: 3.8 °C/min Hold: 5 min Ramp 185–40 °C: −3.6 °C/min Mixing: 200 rpm | Solids | 3 |
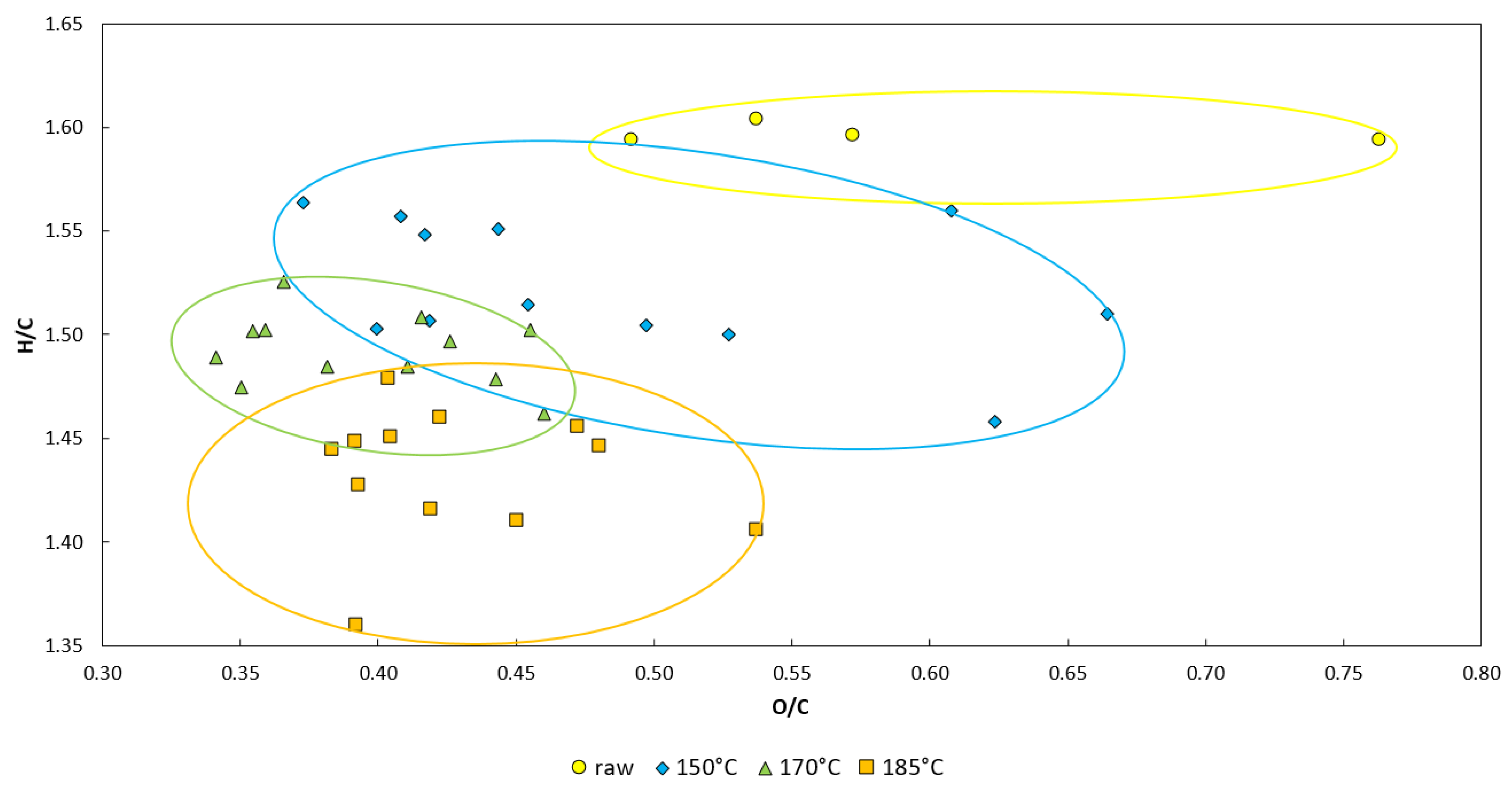
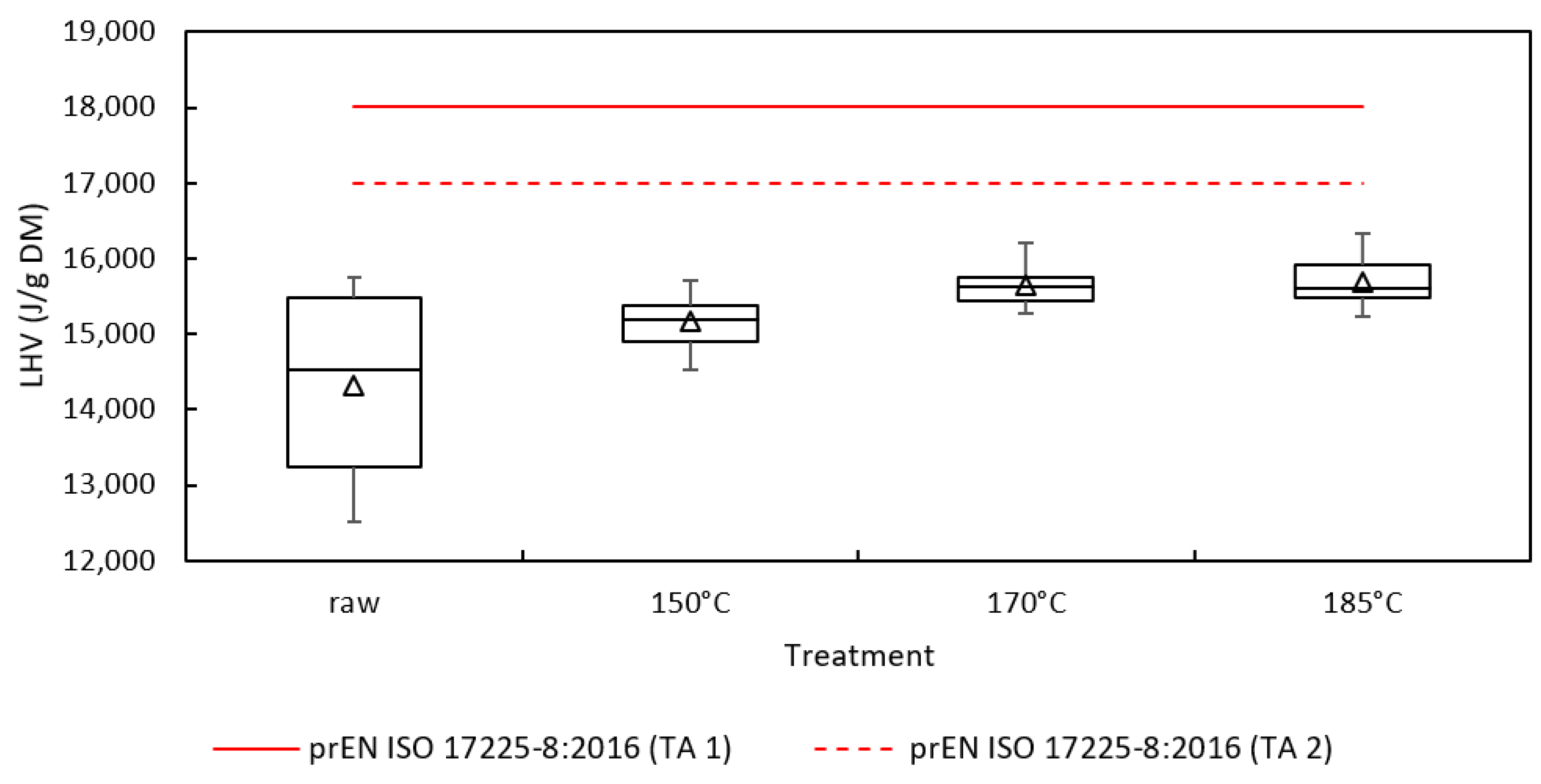
| Sample | Mass Loss (%DM) | HHV (J/gDM) | DT (°C) | FT (°C) | oDM (%DM) | C (%DM) | H (%DM) | N (%DM) | S (%DM) | O (%DM) | H/C (-) | O/C (-) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | - | 15,417 ± 1258 | 1162 M ± 7 1621 C | 1263 M ± 21 1681 C | 77.88 ± 1.37 | 39.49 ± 2.55 | 5.29 ± 0.35 | 2.13 ± 0.32 | 0.21 ± 0.01 | 30.76 ± 3.14 | 1.60 | 0.58 |
| 150 °C | 31.16 ± 2.74 | 16,241 ± 345 | 1590 C | 1670 C | 73.75 ± 1.38 | 40.70 ± 2.61 | 5.20 ± 0.38 | 1.81 ± 0.24 | 0.10 ± <0.01 | 25.94 ± 3.25 | 1.52 ± 0.02 | 0.48 ± 0.03 |
| 170 °C | 38.07 ± 0.57 | 16,716 ± 257 | 1562 C | 1646 C | 70.09 ± 1.43 | 41.44 ± 1.34 | 5.19 ± 0.19 | 1.62 ± 0.11 | 0.12 ± 0.03 | 21.72 ± 1.57 | 1.49 ± 0.01 | 0.39 ± <0.01 |
| 185 °C | 40.79 ± 0.44 | 16,672 ± 325 | 1643 C | 1703 C | 68.96 ± 1.35 | 39.94 ± 1.61 | 4.80 ± 0.23 | 1.47 ± 0.08 | 0.10 ± <0.01 | 22.65 ± 1.48 | 1.43 ± 0.01 | 0.43 ± 0.02 |
| p-values | ||||||||||||
| Raw vs. 150 | - | n.s. | - | - | *** | n.s. | n.s. | n.s. | *** | n.s. | - | - |
| Raw vs. 170 | - | * | - | - | *** | n.s. | n.s. | ** | *** | *** | - | - |
| Raw vs. 185 | - | * | - | - | *** | n.s. | n.s. | ** | *** | *** | - | - |
| 150 vs. 170 | n.s. | ** | - | - | *** | n.s. | n.s. | ** | *** | *** | - | - |
| 150 vs. 185 | n.s. | ** | - | - | *** | n.s. | * | *** | n.s. | ** | - | - |
| 170 vs. 185 | n.s. | n.s. | - | - | n.s. | * | *** | *** | *** | n.s. | - | - |
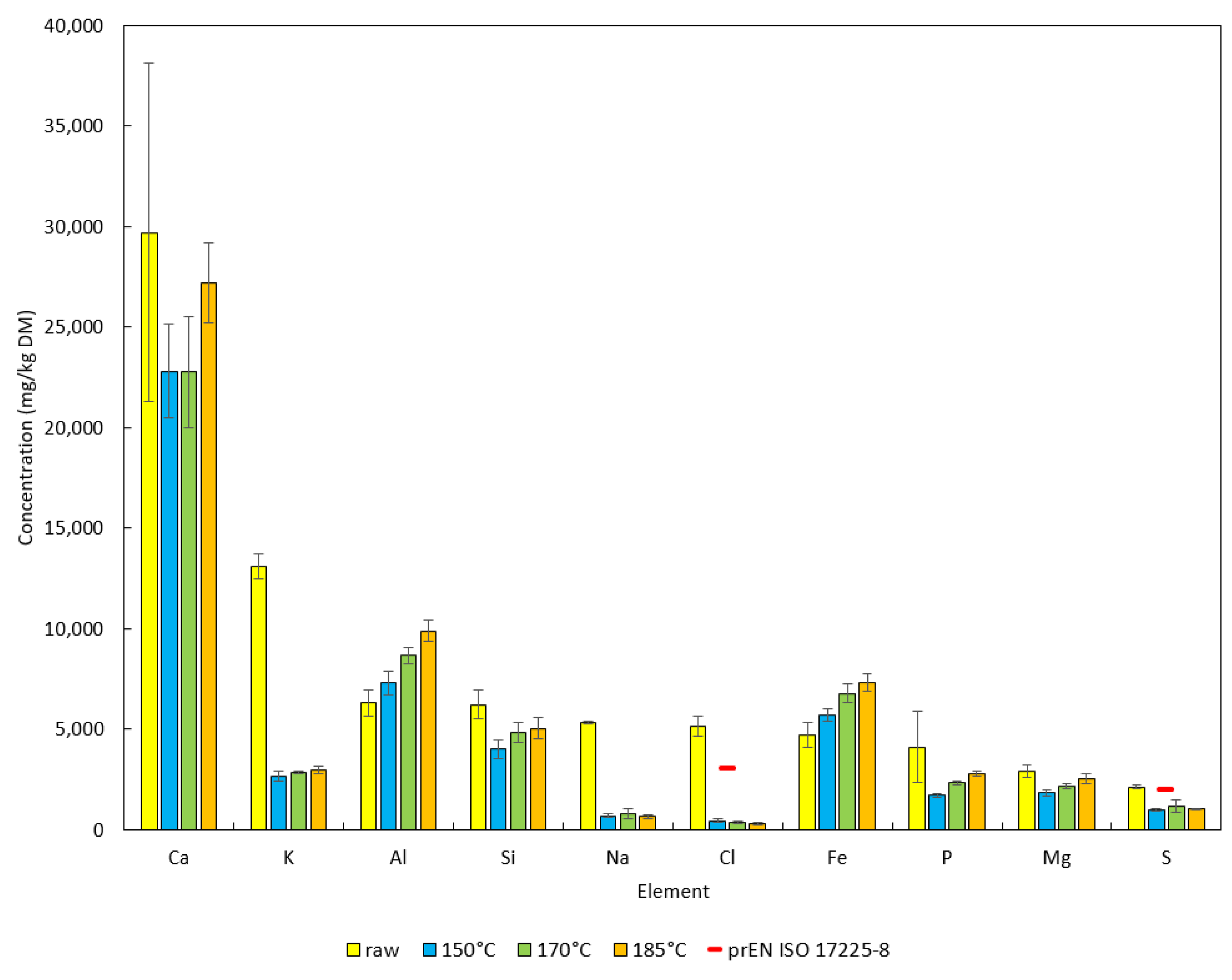
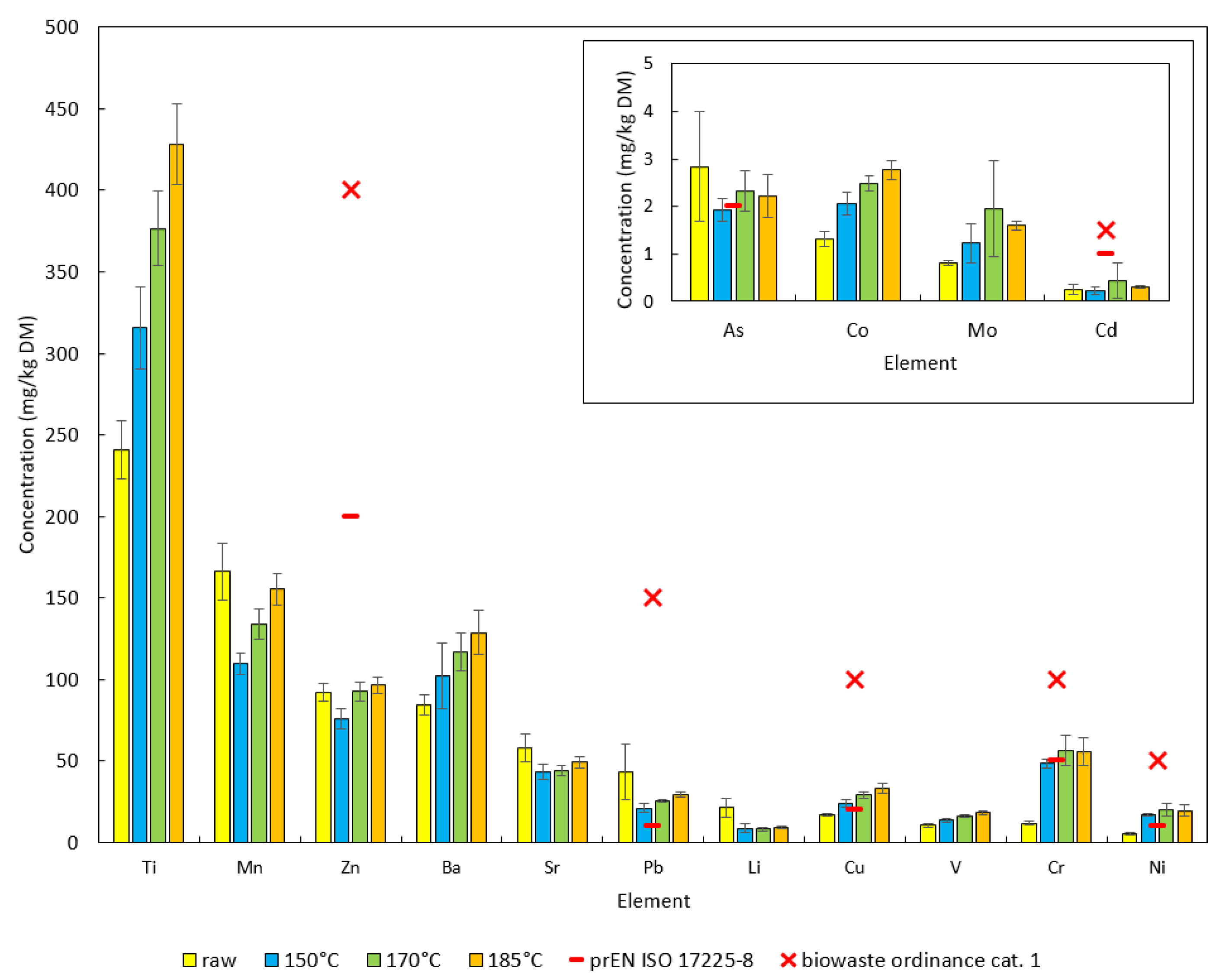
| Samples | Ca | K | Al | Si | Na | Cl | Fe | P | Mg | S | Ti | Mn | Zn |
| Raw vs. 150 | n.s. | *** | * | *** | *** | *** | n.s. | *** | *** | *** | *** | *** | *** |
| Raw vs. 170 | n.s. | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ** | n.s. |
| Raw vs. 185 | n.s. | *** | *** | *** | *** | *** | *** | *** | ** | *** | *** | n.s. | n.s. |
| 150 vs. 170 | n.s. | * | *** | *** | n.s. | n.s. | *** | *** | *** | *** | *** | *** | *** |
| 150 vs. 185 | *** | *** | *** | *** | n.s. | * | *** | *** | *** | n.s. | *** | *** | *** |
| 170 vs. 185 | *** | * | *** | n.s. | n.s. | ** | ** | *** | *** | *** | *** | *** | n.s. |
| Samples | Ba | Sr | Pb | Li | Cu | V | Cr | Ni | As | Co | Mo | Cd | |
| Raw vs. 150 | n.s. | *** | *** | *** | * | ** | *** | ** | ** | ** | * | n.s. | |
| Raw vs. 170 | *** | *** | *** | *** | *** | ** | *** | ** | n.s. | ** | ** | n.s. | |
| Raw vs. 185 | *** | ** | *** | *** | ** | ** | *** | *** | n.s. | ** | ** | n.s. | |
| 150 vs. 170 | *** | n.s. | *** | n.s. | *** | *** | * | * | *** | *** | ** | *** | |
| 150 vs. 185 | *** | *** | *** | * | *** | *** | * | * | * | *** | * | *** | |
| 170 vs. 185 | * | *** | *** | ** | *** | *** | n.s. | n.s. | n.s. | *** | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sailer, G.; Knappe, V.; Poetsch, J.; Paczkowski, S.; Pelz, S.; Oechsner, H.; Bosilj, M.; Ouardi, S.; Müller, J. Upgrading the Organic Fraction of Municipal Solid Waste by Low Temperature Hydrothermal Processes. Energies 2021, 14, 3041. https://doi.org/10.3390/en14113041
Sailer G, Knappe V, Poetsch J, Paczkowski S, Pelz S, Oechsner H, Bosilj M, Ouardi S, Müller J. Upgrading the Organic Fraction of Municipal Solid Waste by Low Temperature Hydrothermal Processes. Energies. 2021; 14(11):3041. https://doi.org/10.3390/en14113041
Chicago/Turabian StyleSailer, Gregor, Victoria Knappe, Jens Poetsch, Sebastian Paczkowski, Stefan Pelz, Hans Oechsner, Monika Bosilj, Siham Ouardi, and Joachim Müller. 2021. "Upgrading the Organic Fraction of Municipal Solid Waste by Low Temperature Hydrothermal Processes" Energies 14, no. 11: 3041. https://doi.org/10.3390/en14113041
APA StyleSailer, G., Knappe, V., Poetsch, J., Paczkowski, S., Pelz, S., Oechsner, H., Bosilj, M., Ouardi, S., & Müller, J. (2021). Upgrading the Organic Fraction of Municipal Solid Waste by Low Temperature Hydrothermal Processes. Energies, 14(11), 3041. https://doi.org/10.3390/en14113041