1. Introduction
Energy is a prerequisite for development in every society of the world, and it plays an important role in ecosystems and human civilization [
1,
2]. However, the conventional energy sources are not renewable, and their high utilization could result in a severe energy crisis [
3]. In addition, excessive utilization of fossil fuels pollutes the environment, causing severe health hazards and accelerating global warming [
4]. Therefore, bioenergy as a powerful renewable substitution to fossil fuels is emerging rapidly worldwide, particularly in developed countries, to meet the energy requirement of the world population and to mitigate global warming [
2,
4]. Furthermore, sustainable bioenergy production can effectively decrease the risks of energy crisis and help the economic growth of countries [
5,
6]. The exploitation of energy crops or agricultural crops are mainly grown for energy production, could reduce the world’s dependence on fossil fuels and can decrease the release of greenhouse gases [
2,
4,
7]. Energy crops are either native species or cultivated, new or traditional, which yield the biomass as the main output that can be utilized for different energy purposes, such as combustion or raw material for pyrolysis and gasification, and fermentation for ethanol production [
4,
6,
8]. Thus, the concept of bioenergy crops is gaining significant consideration in the scientific community because of its renewability and environmental-friendly nature [
3,
4].
Maize (
Zea mays L.), sorghum [
Sorghum bicolor (L.) Moench], and pearl millet (
Pennisetum glaucum L.) are important energy crops [
4,
9,
10] with high biomass production and diverse possible bioenergy uses [
4,
9]. They are C4 crops with exceptional daily dry matter accumulation and high nitrogen use efficiency [
11]. Maize is an important feedstock crop because of its high biomass 32–54 t ha
−1 [
4,
12,
13] and superior starch accumulation [
14]. Given its high percentage of volatile compounds and simple conversion, maize is a suitable crop for bioconversion [
6]. In addition, maize is usually used for the starchy raw material and bioethanol present in grains and biomass, respectively. Biomass can be used for combustion or production of second-generation bioethanol [
3,
6]. Biomass or bioenergy sorghum is potentially an ideal feedstock for the cellulosic ethanol industry because it produces high yields and has suitable chemical properties for fuel conversion [
15]. It is an important crop in warm areas and achieves higher yields with fewer inputs than many other bioenergy crops in arid and semiarid environments [
16,
17,
18]. The aerial biomass yield of sorghum is high, ranging from 35 t ha
−1 to 54 t ha
−1 [
19,
20,
21]. Pearl millet is utilized worldwide as a grain crop, a forage crop, or a high-biomass feedstock [
22,
23]. It has high levels of tolerance to drought, salinity, and temperature adaptation and can successfully be grown on poor and degraded lands [
24,
25]. It requires very low rainfall (less than 300 mm) and less nitrogen amount than most other cereal crops [
26], which qualifies it as an important crop for biomass production. Some genotypes of pearl millet can yield high dry matter, ranging from 15 t ha
−1 to 25 t ha
−1 [
27]. Study on ethanol production from pearl millet has not received sufficient attention up until now, but the presence of high biomass coupled with its ethanol-friendly properties ensures a high possibility of this crop becoming the future bio-fuel crop of the marginal lands in semi-arid areas.
World Water Development Report 2014 showed a strong link between energy and water. Energy and water are interdependent, and any action taken in one sector can significantly influence the other sector positively or negatively [
28]. Energy demand is progressively increasing in the world because of the fast-growing population. The rapidly growing population has also enhanced the rate of wastewater in recent decades. The application of untreated wastewater is however not suggested because it possesses different organic and inorganic chemicals, biodegradable materials, toxic substances, and disease-causing agents [
29]. Wastewater is contaminated with heavy metals and trace elements, such as lead (Pb), arsenic (As), chromium (Cr), cadmium (Cd), zinc (Zn), copper (Cu), boron (B), manganese (Mn), cobalt (Co), and molybdenum (Mo), many of which are toxic to crop plants, animals, and humans [
30]. Prolonged consumption of foods and vegetables grown from unsafe wastewater with high concentrations of heavy metals can cause potential health risks in humans [
30]. Plants cultivated in wastewater-irrigated soils exhibit higher concentrations of heavy metals (Cd, Pb, and Ni) in the edible portions compared with plants grown in the reference soil [
30,
31].
Thus, wastewater should be treated and then reutilized for crop production to solve these problems. Reuse of wastewater at a reasonable rate can enhance plant growth and productivity [
32], thereby increasing the economic benefits for growers due to the reduced need for chemical fertilizers because wastewater contains different plant nutrients [
30,
33]. Irrigation with treated wastewater (TWW) to agriculture has been increasing rapidly worldwide [
34,
35] and is highly encouraged as an alternative source of water and nutrients for plant growth and yield, particularly in aid and semi-arid regions. Arid and semi-arid regions are the areas that have unfavorable environmental circumstances, such as low precipitation rate, shortage of freshwater resources, and high temperatures and evapotranspiration. Accordingly, the management of such environments is challenging particularly with climate change concerns. The application of TWW for irrigation helps conserve good-quality water resources and increases environmental performance [
35,
36,
37]. Accordingly, recycling nutrients through irrigation bioenergy crops with TWW can provide a suitable option to reduce the synthetic fertilizer application into agricultural lands and improve agricultural sustainability because synthetic fertilizers require a high consumption of fossil fuel during their production process [
4].
During wastewater treatments, not all nutrients are separated (particularly nitrogen and phosphorus) from wastewater. The TWW contains nutrients such as nitrate (18.1 mg L
−1), P (5.2 mg L
−1), K (28.5 mg L
−1), Ca (141.8 mg L
−1), Mg (49.32 mg L
−1), and Zn (0.87 mg L
−1) [
36]. In another investigation, the TWW contained NH
4+ (10.02 mg L
−1); NO
3− (61.08 mg L
−1); PO
43− (8.7 mg L
−1), and K
+ (46.8 mg L
−1) [
32]. Maucieri et al. [
38] reported that TWW contained a total of N 9.2 mg L
−1; a total of P 6.8 mg L
−1. Nutrients may be present and available to crops irrigated with that water source, thus decreasing plant fertilizer demand [
32,
38,
39]. Various crops, such as vetch (
Vicia sativa), wheat, barley, and maize irrigated with TWW, show an increase in biological yields [
35,
40,
41]. The grain yields of maize, wheat, millet, beans, and rapeseed irrigated with TWW are much higher than those in dry farming, indicating that the TWW can supply essential nutrients to crop plants [
33,
42]. The crop growth and productivity under wastewater irrigation can be further enhanced if the soil is fertilized with a suitable quantity of chemical fertilizers. Soil nutrient level decreases with the passage of time when crops are continuously grown and harvested. These nutrients are refilled either through natural decomposition or by the addition of fertilizers. Therefore, fertilizers are an essential component of modern agriculture and beneficial to plants in providing deficient nutrients [
43,
44]. However, their excessive use causes serious environmental problems, such as water soil and air pollution, land degradation, and increased greenhouse gas emissions [
43,
45]. In general,, chemical fertilizers are applied to increase crop yield, but only 10–40% of the applied fertilizers can be available to plants [
46]. The remaining fertilizers in the soil are lost through leaching and volatilization, which cause a major threat to terrestrial and aquatic environments and affect biodiversity [
47,
48]. High fertilizer amounts, particularly nitrogen, can easily pollute the water table in the instance of heavy rains or in sandy soils. In addition, the nitrous oxide gas that can be emitted for volatilization during field spreading of nitrogenous fertilizers is a key greenhouse gas. Similarly, phosphorus, an essential mineral for plant growth and development [
4,
49], can cause water pollution [
41,
50,
51]. For example, high rates of phosphorus and nitrogen result in eutrophication by soil erosion due to rain or wind [
51].
Thus, judicious use of chemical fertilizers is necessary. Basing on the above background, we hypothesized that reduced application of NPK fertilizers along with TWW increases the aboveground plant biomass and exerts positive effects on plant growth. Therefore, the main objectives of this study are to (1) evaluate the safety of TWW for irrigation of three energy crops fertilized with 100% or 50% of the total recommended doses of NPK to maintain the environment and reduce pollution, and (2) cover part of increasing demand for freshwater by using TWW for irrigation of energy crops. In this study, we evaluated the impact of long- and short-term irrigation with TWW on the growth, yield, quality, and energy production of different field crops intended for bioenergy production, compared with groundwater use. This impact was investigated on three field crops, namely, maize, pearl millet, and sorghum.
3. Results
Maize plants grown on old-cultivated soil irrigated with treated wastewater (L1 + TWW) were the tallest (202.0 cm), followed by plants (171.75 cm) grown on virgin soil irrigated with treated wastewater (L2 + TWW). Maize plants grown on virgin soil and irrigated with groundwater (L3 + GW) were the lowest plants (162.50 cm) (
Table 4). A similar trend was recorded in sorghum and pearl millet plants. Both crops produced taller plants (sorghum 281.38 cm; pearl millet 163.19 cm) when grown on old-cultivated soil and irrigated with TWW, whereas the shortest plants (sorghum 162.56 cm; pearl millet 151.25 cm) were obtained on virgin soil irrigated with GW. The individual effect of NPK fertilizer on the height of maize, sorghum, and pearl millet plants was not significant (
Table 4).
Total chlorophyll content in terms of SPAD values increased in all three tested crops grown on old-cultivated soil and irrigated with TWW (
Table 4). In maize and sorghum, the highest total chlorophyll content (45.32 and 51.03 SPAD, respectively) was noted when these crops were grown on old-cultivated soil and irrigated with TWW, followed by those in crops grown on virgin soil with TWW, and the lowest was observed in the crops grown on virgin soil and irrigated with GW. In pearl millet, the total chlorophyll content in plants grown on old soil and irrigated with TWW was higher by 15.4% or 13.0% than those obtained from plants grown on virgin soil irrigated with GW or TWW, respectively. The individual effect of NPK fertilizer doses was significant in maize and pearl millet but not in sorghum crop. Application of 100% recommended dose of NPK considerably increased the total chlorophyll content in maize and pearl millet (40.82 and 54.44 SPAD, respectively) compared with 50% recommended dose of NPK (33.14 and 47.37 SPAD, respectively).
Likewise, leaf area plant
−1 significantly increased in the maize (1564.81 cm
2), sorghum (1175.92 cm
2), and pearl millet (521.91 cm
2) by 18.33, 12.76, and 8.70% when grown on old-cultivated soil and irrigated with TWW compared to those grown on virgin soil and irrigated with GW, respectively (
Table 4). The leaf area of maize and pearl millet plants grown on virgin soil and irrigated with TWW was higher than those of plants grown on virgin soil and irrigated with GW. However, there were no significant differences between the leaf area obtained from sorghum plants grown on virgin soil and irrigated with TWW or GW (
Table 4). The individual effect of NPK fertilizer was not significant for maize and pearl millet, but it significantly influenced sorghum leaf area (
Table 4). Application of 100% recommended dose of NPK fertilizer enhanced the leaf area plant
−1 (1140.73 cm
2) in sorghum by 12.63% as compared with 50% recommended dose of NPK (996.65 cm
2). The interactive effect of NPK fertilizer with soil locations and irrigation sources was analyzed. With the application of 50% or 100% recommended NPK dose, the largest leaf area was observed in the maize, sorghum, and pearl millet grown on old soil and irrigated with treated wastewater, followed by those in the crops grown virgin soil irrigated with TWW, and the smallest was noted in the crops grown on virgin soil and irrigated with GW (
Table 5).
Plantation of maize on old-cultivated soil and irrigated with TWW resulted in higher biomass by 56.32% and 50.23% than those grown on virgin soil and irrigated with GW and TWW, respectively (
Table 4). Likewise, sorghum and pearl millet produced higher biomass (43.83 and 56.73%, respectively) when grown on old-cultivated soil and irrigated with TWW rather than when grown on virgin soil and irrigated with GW. Use of 100% recommended dose of NPK fertilizer significantly increased the biomass in maize (24.64 t ha
−1) by 7.1% compared with 50% recommended dose of NPK fertilizer (22.89 t ha
−1); however, NPK doses exerted no significant effect on the dry biomass of sorghum and pearl millet (
Table 4). Interaction of NPK fertilizer with soil locations and irrigation sources exhibited that use of 50% or 100% recommended dose of NPK in old-soil irrigated with TWW resulted in higher biomass in crops (maize, sorghum, and pearl millet) followed by virgin soil irrigated with TWW (
Table 5) compared with those grown on virgin soil and irrigated with GW.
Maize, sorghum, and pearl millet grown on old-cultivated soil and irrigated with TWW possessed higher energy content (16.87, 17.54, and 16.11 MJ kg
−1 DM, respectively), followed by virgin soil irrigated with TWW, and lower energy content from these crops were recorded when grown on virgin soil irrigated with GW (16.41, 15.53, and 15.53 MJ kg
−1 DM, respectively), (
Table 6). The individual effect of NPK fertilizer on the energy content of all crops was not significant. In the case of gross energy, maize (620.30 GJ ha
−1), sorghum (605.83 GJ ha
−1), and pearl millet (303.83 GJ ha
−1) showed higher gross energy value when grown on old-cultivated soil and irrigated with TWW, whereas minimum gross energy (263.54, 301.28, and 166.17 GJ ha
−1, respectively) was observed when they were grown on virgin soil irrigated with GW. This means that gross energy obtained from maize, sorghum, and pearl millet grown in old-cultivated soil and irrigated with TWW was increased by 57.51, 50.26, and 45.30% compared with those grown on virgin soil irrigated with GW, respectively. The individual effect of NPK fertilizer on gross energy in maize was significant but not in sorghum and pearl millet crops. Higher gross energy was recorded from maize (409.02 GJ ha
−1) when a 100% recommended dose of NPK fertilizer was applied as compared with a 50% recommended dose of NPK fertilizer (383.86 GJ ha
−1). The highest ash content (maize 12.02%, sorghum 9.92%, and pearl millet 15.61%) was recorded when the crops were cultivated on virgin soil and irrigated with GW, followed by crops cultivated on virgin soil irrigated with TWW, and the lowest ash content for the same crops (9.24%, 7.54%, and 13.49%) was recorded when they were sown on old-cultivated soil and irrigated with TWW. The individual effect of NPK fertilizer dose on the ash content of crops was not significant (
Table 6).
The interactive effect of irrigation water sources and different doses of NPK showed that irrigation with TWW and fertilization with a 50% recommended dose of NPK resulted in higher gross energy values of maize (610.41 GJ ha
−1), sorghum (604.29 GJ ha
−1), and pearl millet (301.13) than irrigation with GW and fertilization with 100% recommended dose of NPK (274.58, 302.62, and 189.40 GJ ha
−1, respectively), (
Table 7). However, the energy content was higher in maize (16.94 MJ kg
−1 DM) and sorghum (17.56 MJ kg
−1 DM) irrigated with TWW and fertilized with 50% recommended dose of NPK fertilizer in old soil than in the crops irrigated with GW and fertilized with 100% recommended dose of NPK fertilizer in virgin soil (16.28 and 15.44 MJ kg
−1 DM, respectively). However, pearl millet produced higher energy content (16.21 MJ kg
−1 DM) when irrigated with TWW and fertilizer with 100% recommended dose of NPK than when irrigated with GW and 100% recommended dose of NPK (15.44 MJ kg
−1 DM).
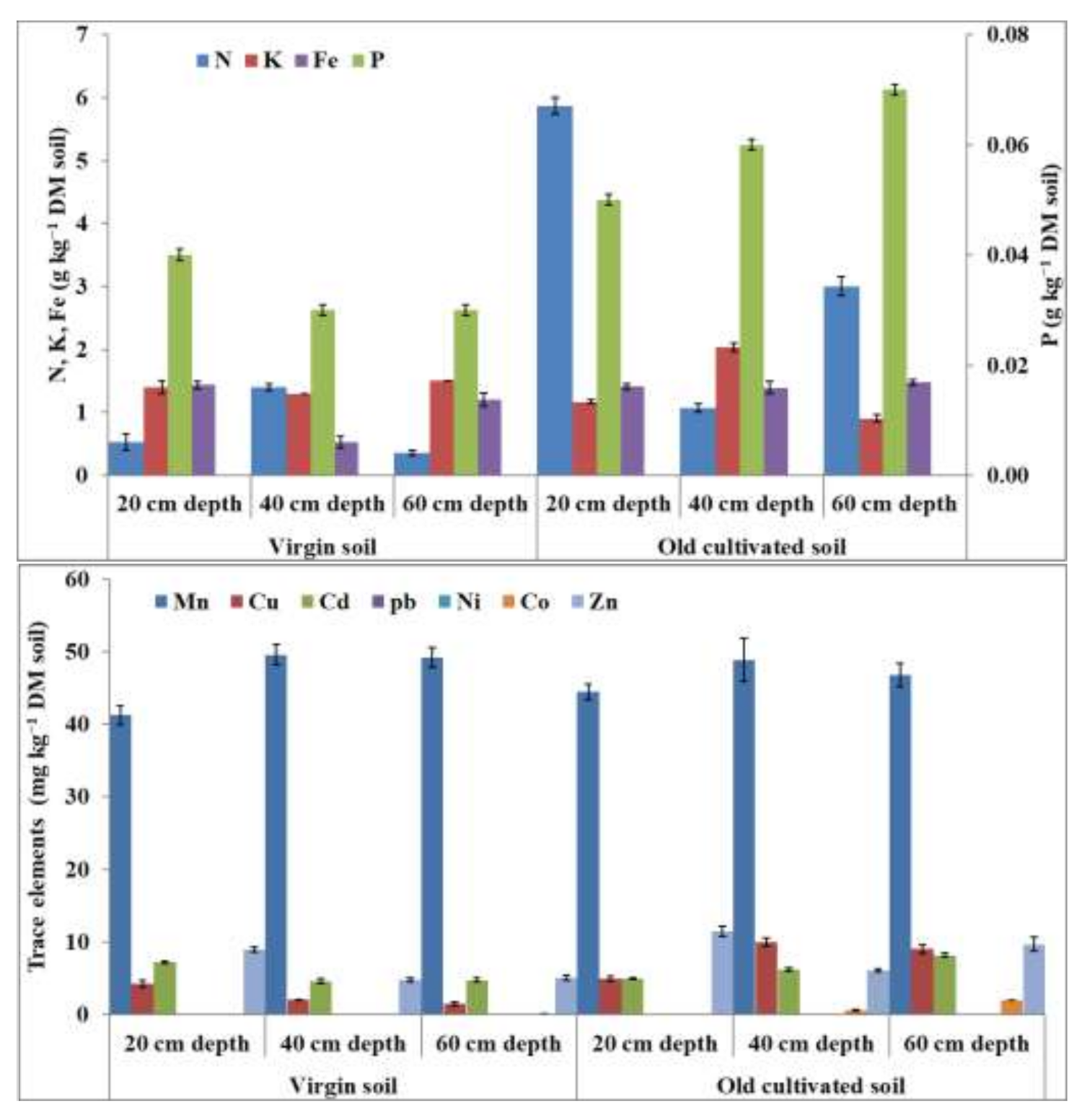
Interaction effects of NPK fertilizer and soil with irrigation sources on macronutrients, such as nitrogen (N), phosphorus (P), and potassium (K), in maize, sorghum, and pearl millet plants were significant (
Table 8). Results showed that the biomass of different crops contained the highest nutrients when grown on old- soil irrigated with TWW, followed by virgin soil irrigated with TWW, and the lowest nutrients were recorded in crops grown on virgin soil irrigated with GW (
Table 8 and
Figure 2). Maize crop cultivated on old-soil irrigated with TWW and fertilized with 100% or 50% recommended dose of NPK showed the highest N (23.0 and 21.0 g kg
−1 DM) and P content (2.1 and 2.0 g kg
−1 DM) compared with virgin soil irrigated with GW and fertilized with 100% or 50% recommended dose of NPK (7.9 and 5.0 g kg
−1 DM for N; 1.5 and 1.2 g kg
−1 DM for P, respectively). A similar trend was recorded in sorghum and pearl millet plants, where N and P contents were higher in biomass cultivated on old soil irrigated with TWW and fertilized with 100% or 50% recommended dose of NPK than in biomass cultivated on virgin soil irrigated with TWW or virgin soil irrigated with GW. Similarly, the K contents in maize (15.18 g kg
−1 DM), sorghum (19.85 g kg
−1 DM), and pearl millet (16.10 g kg
−1 DM) increased when grown on old-cultivated soil with TWW and applied with 100% recommended dose of NPK without significant differences with 50% recommended dose of NPK compared with the virgin soil irrigated with TWW or GW along with the recommended doses of NPK (
Table 8). However, the interaction effect of NPK fertilizer doses (50% and 100% recommended dose) on sorghum or pearl millet irrigated with TWW or GW was not significant for the content of K in biomass.
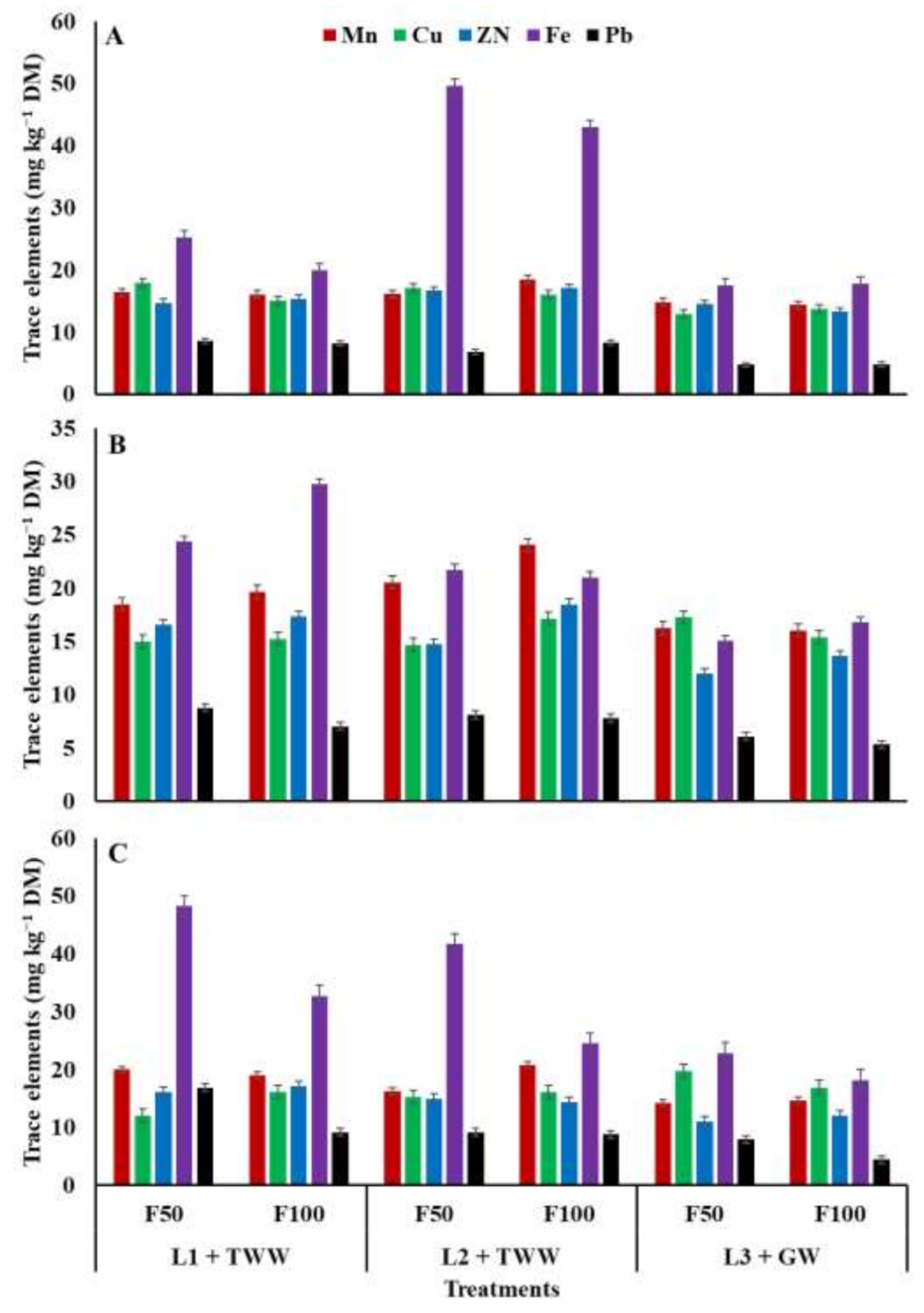
The application of NPK fertilizer and soil locations with irrigation sources affected the trace elements (i.e., Mn, Cu, Zn, Fe, Pb, Ni, Co, and Cd) in plant biomasses (maize, sorghum, and pearl millet) (
Figure 2 and
Figure 3). Mn content increased in maize (16.40 mg kg
−1 DM), sorghum (18.50 mg kg
−1 DM), and pearl millet (20.0 mg kg
−1 DM) plants grown on old-soil and virgin irrigated with TWW and fertilized with 50% recommended dose of NPK than virgin soil irrigated with GW (14.95, 16.25, and 14.23 mg kg
−1 DM, respectively). However, the highest Mn content in maize, sorghum, and pearl millet was recorded when these crops were grown on virgin soil irrigated with TWW and fertilized with 100% recommended dose of NPK fertilizer (18.50, 24.05, and 20.78 mg kg
−1 DM, respectively). Aside from these results, fertilizer doses exerted no significant effect on Mn content in plants grown on old soil irrigated with TWW and virgin soil irrigated with GW.
The concentrations of Cu and Zn showed no significant differences in maize, sorghum, and pearl millet plants grown on old-cultivated soil irrigated with TWW or on virgin soil irrigated with TWW (
Figure 2). However, maize plants grown on old and virgin soils irrigated with TWW had higher Cu (17.95 and 17.15 mg kg
−1 DM, respectively) content than the plants grown on virgin soil irrigated with GW (12.98 mg kg
−1 DM). Meanwhile, higher Cu content was noted when sorghum and pearl millet plants were grown on virgin soil irrigated with GW and used 50% recommended dose of NPK. Similarly, Fe content significantly increased in sorghum (29.75 mg kg
−1 DM) and pearl millet (17.10 mg kg
−1 DM) grown on old soil and virgin soil irrigated TWW and used 100% recommended dose of NPK than in the plants grown on virgin soil irrigated with GW. However, the highest Fe content (49.65 mg kg
−1 DM) was noted in maize plants sown on virgin soil irrigated with TWW, followed by maize plants sown on old soil irrigated with treated wastewater, and the lowest was found in maize plants grown on virgin soil and irrigated with GW (
Figure 2). Effect of NPK fertilizer doses with soil location and irrigation sources showed that application of 50% recommended dose of NPK effectively increased Fe content in plants, except in maize where 100% recommended dose of NPK fertilizer enhanced Fe uptake.
Similarly, Pb content was higher in the crops grown on old soil and virgin soil irrigated with TWW and fertilized with 50% or 100% recommended dose of NPK fertilizer than in the crops grown on virgin soil irrigated with GW (
Figure 2). The individual effect of NPK fertilizer dose showed not much variation for Pb content in plant biomass. The content of Ni significantly increased in the plant biomass of all the crops (maize, sorghum, and pearl millet) grown on old soil irrigated with TWW and fertilized with 50% or 100% recommended dose of NPK fertilizer followed by virgin soil irrigated with TWW. However, only a small quantity of Ni was noted in maize, sorghum, and pearl millet plant biomass planted on virgin soil irrigated with GW (
Figure 3). The individual effect of NPK fertilizer indicated that application of 50% recommended dose of NPK fertilizer enhanced the Ni content significantly in maize and pearl millet, but it did not exert a significant effect on sorghum plants.
Similarly, Cd content was higher in the plant biomass of maize, sorghum, and pearl millet sown on whether old or virgin soil irrigated TWW along with 50% and 100% recommended dose of NPK fertilizer, whereas a minimum quantity of Cd was measured in plants cultivated on virgin soil irrigated with GW. Overall, the application of a 50% recommended dose of NPK fertilizer increased the Cd content in plant biomass compared with the 100% recommended dose of NPK. The plant analysis for Co showed that Co content was higher in plant biomass (maize, sorghum, and pearl millet) grown on virgin soil irrigated with TWW and fertilized with 50% or 100% recommended dose of NPK fertilizer than in plant biomass grown on virgin soil irrigated with GW and old-soil irrigated with TWW.
4. Discussion
Plant height, leaf area, and dry biomass of maize, sorghum, and pearl millet plants were significantly higher when planted on old and/or virgin soil irrigated with treated wastewater (TWW) than when planted on virgin soil irrigated with groundwater (GW). Furthermore, 50% recommended dose of NPK with TWW showed equivalent results regarding most of growth and biomass traits to 100% recommended dose of NPK fertilizer in all crops. This result suggests that TWW can supply enough necessary nutrients that enable plant growth to achieve plant height. The constant availability of essential nutrients, such as N, P, K, Ca, Mg, and other micronutrients, through TWW [
55] plays an important role in improving the height, leaf area, and dry biomass of maize, sorghum, and pearl millet. For example, phosphorus is the main constituent of DNA and RNA structures and helps in root growth and development, flower initiation, and seed development. Similarly, potassium activates many enzymes involved in the metabolism of plants and controls the opening and closing of leaf stomata. Thus, potassium is an important nutrient for plant growth. Similarly, calcium plays a key role in the synthesis of the cell wall and its plasticity, and indirectly assists in enhancing plant yields and biomass by decreasing soil acidity. Magnesium is an important content of chlorophyll molecules and therefore is actively involved in plant photosynthesis. TWW irrigation increases the uptake of essential nutrients, such as K
+, PO
43−, Cu
2+, and Zn
2+, in plants and improves the growth of fenugreek (
Trigonella foenum L.) and corn mint (
Mentha arvensis L.) [
56]. Such nutrients can play a vital role in various physiological mechanisms of plants, including growth and development, membrane stability, osmotic adjustment, and cellular turgor [
57,
58]. Mousavi et al. [
59] reported that TWW increases the nutrients in soil solution, thereby increasing the leaf area index, dry matter, protein percentage, and Zn and P contents of maize plants. Hence, the increase in plant height, leaf area, and dry biomass of crop plants was due to better growth of plants because of the appropriate quantity of nutrients presented in TWW (i.e., total N 23.5 mg L
−1, NH
4+ 2.9 mg L
−1, NO
3−5.8 mg L
−1, PO
43− 4.1 mg L
−1, K
+ 15.7 mg L
−1). The water required for growing maize or sorghum or millet in the region of the current study is ranged from 8000–9000 m
3 ha
−1. Based on the water requirements and nutrients contents in TWW used in our study, TWW can provide a total N 188.0–211.5 kg ha
−1; NH
4+ 23.2–26.1 kg ha
−1; NO
3− 46.4–52.2 kg ha
−1; PO
43− 32.8–39.7 kg ha
−1; and K
+ 125.6–141.3 kg ha
−1. On the other hand, improving biomass of different crops in the current study when irrigated with TWW, clearly indicates that TWW did not cause stress through the heavy metals which can cause a reduction in the growth and biomass of crops. Similarly, El-Nahhal et al. [
60] reported that the plant height and fresh biomass of Chinese cabbage and maize are significantly higher when irrigated with TWW than with fresh water. Zema et al. [
61] showed that the application of TWW increases plant height by 25.6%, leaf area index by 86.7%, and biomass yield by 63% compared with the conventional water. Moreover, irrigation with TWW increases plant height and growth in tomato (
Solanum lycopersicum Mill.) [
62] and pepper (
Capiscum annuum L.) [
63].
The dry matter of plants increases with increasing leaf area and plant height, providing a large surface for the interception of solar radiation and thus high photosynthetic rate, which increases dry matter accumulation. Plant growth largely depends on nutrients present in the soil, and their deficiency can limit growth, biomass, and productivity [
64]. The quantity of nutrients, such as N, P, K, Cu, Zn, and Fe, was greater in plants irrigated with TWW than in those irrigated with GW (
Table 8 and
Figure 2), which indicates that TWW had higher nutrients. This can be due to the proper availability of nutrients into crops as a result of TWW and synthetic fertilizer. In addition, TWW in the current study contained higher nitrate than ammonium which can be rapidly uptake by crops. This phenomenon can enhance plants to achieve better growth and development. Therefore, using TWW can be a type of fertigation, which subsequently provides nutrients and enhances the photosynthesis, growth, leaf area, and dry matter of plants. Bedbabis et al. [
32] reported an increase in P content as a result of the fertilization impact of TWW applied for irrigation. Application rates of TWW increased the nutrient inputs, higher uptake, and accumulation of nutrients (macro- and micro-nutrients), which consequently increased the dry matter and leaf area index of maize [
60]. In addition, TWW may contain different types of bacteria that degrade or decompose organic matter in the soil, maintain soil fertility, and improve the water-holding capacity of the soil, porosity, and other physicochemical characteristics of the soil. Thus, TWW can be utilized to improve crop production and soil physical properties [
65,
66]. Sorghum and sunflower growth and productivity are improved by irrigating with TWW [
67,
68]. According to Aman et al. [
66], the highest shoot fresh weight is observed in
Cercis siliquastrum,
Caesalpinia gilliesii, and
Robinia pseudoacacia seedlings when irrigated with TWW. Similarly, several other studies showed positive effects of TWW on the growth of different plants [
41,
54,
62,
69,
70,
71,
72,
73,
74,
75] because of optimal nutrient availability and uptake [
76].
The total chlorophyll content of plants is a vital parameter because it shows the physiological status and photosynthetic activity of plants. Results indicated that maize, sorghum, and pearl millet plants exhibited higher total chlorophyll content when irrigated with TWW than with GW. The increase in chlorophyll content of crops may be linked to improvement in the mineral status of plants (
Table 8 and
Figure 2) and/or the increase of leaf area of plants (
Table 4 and
Table 5) that increased the light interception and optimized the CO
2 assimilation and therefore the photosynthetic capacity of plants. In the current study, a high amount of the total N (total N 188.0–211.5 kg ha
−1) was added through TWW. Usually, N is not often immobilized when rich organic material with N is applied into agricultural soil, this could be due to the total enough N for demanding of microorganism. This means that the inorganic N will increase as a result of organic matter mineralization [
33,
39,
41]. The nutrients status in crops can be improved also by the mycorrhizal root colonization as was reported by Seleiman et al. [
39], since the mycorrhizal fungi can enhance the uptake of mineralized N for their host crops. In this context, Faizan et al. [
71] reported higher chlorophyll a, chlorophyll b, and total chlorophyll content in okra (
Abelmoschus esculentus L. Moench) irrigated with TWW compared with GW. In addition, young olive plants irrigated with TWW showed an improvement in growth, soluble sugars and photosynthesis rate, and chlorophyll a, b, and total chlorophyll contents [
77]. Similarly, Helaly et al. [
78] found that TWW irrigation positively affects the total chlorophyll content of Mango (
Mangifera indica L.) trees. Maize plants irrigated with TWW along with municipal wastewater showed an improvement in chlorophyll content and growth [
79].
The profitability of bioenergy crops grown for energy is determined by their dry matter yield and energy output [
33]. Dry matter yield depends on the genetic potential of plants, climate and soil conditions, and agricultural practices [
80,
81]. The current study showed that the maize, sorghum, and pearl millet irrigated with TWW using 50% recommended dose of NPK showed higher energy content (heating value), gross energy value, and lower ash content than the crops irrigated with GW along with the same amount of NPK fertilizer (
Table 6 and
Table 7). The increase in gross energy value of the crops irrigated with TWW was due to the increase in the biomass of crops (
Table 4,
Table 5,
Table 6 and
Table 7), considering that the biomass yield is the main factor determining the gross energy yield [
33]. TWW did not cause toxic or heavy metal stress to plants and provided a sufficient amount of nutrients to plants, which increased the biomass and gross energy. Seleiman et al. [
33] reported that sewage sludge, the solid by-product of TWW in wastewater treatment plants, slightly enhances the gross energy yield of hemp and maize compared with other treatments.
The plants (maize, sorghum, and pearl millet) irrigated with TWW showed higher nutrient elements and heavy metals, such as N, P, K, Cu, Zn, Fe, Pb, Ni, Co, and Cd, compared with the plants irrigated with GW (
Table 8 and
Figure 2). However, the concentrations of heavy metals in the plant biomass obtained from the current study were below the permissible limits. The increase in nutrient elements observed in maize, sorghum, and pearl millet plants irrigated with TWW could be due to the sufficient quantities of these elements in TWW and plant root zones that are bioavailable to plants, which consequently lead to their high concentrations in plant biomass. Tzortzakis et al. [
62] revealed that irrigating plants with TWW can enhance the availability of N and P in the root zone; consequently, plants can uptake high N and P contents. Furthermore, Faizan et al. [
71] reported an increase for N, P, and K in okra leaves when plants are irrigated with TWW rather than GW. Thus, the use of TWW for irrigation can enhance the nutrient contents of soil by acting as a fertilizer. It can also enhance the mineralization rate and facilitate nutrient uptake [
82]. Bedbabis et al. [
32] found an increase in P content in plant dry biomass irrigated with TWW. Similarly, El-Nahhal et al. [
60] reported that irrigation with TWW enhances nutrient inputs, high uptake, and accumulation of nutrient elements, resulting in a high dry matter of maize. The use of TWW as a source of irrigation for crops can improve the phosphorus content in the soil and plants and enhance plant growth by preventing phosphorus deficiency in plants [
76]. An increase in heavy metal contents but within permissible limits in plant seeds was observed when plants were irrigated with TWW [
71]. Several other studies have reported an increase in soil nutrient status when irrigated with TWW for crop production [
83,
84]. Therefore, the application of TWW for crop irrigation can supply additional nutrient elements to the soil and can increase their absorption to the plants. Thus, TWW can reduce the quantity of mineral fertilizers applied as found in our investigation.
In the current study, the high performance of the potential bioenergy crops fertilized with a 50% recommended dose of NPK indicates that a 100% recommended dose of NPK applied with TWW might cause salt stress. As a result, the 100% NPK dose did not cause a significant superiority on some growth, productivity, and energy traits in the current study compared with the application of 50% NPK of the recommended dose. Mojid et al. [
84] reported that the high application of fertilizers can cause a restriction of yield increase in the wastewater-irrigated crops. Conversely, Abou El Hassan et al. [
85], Aziz [
70] and El-Aziz [
86] reported that a lower dose of NPK fertilizer performed better than a higher dose of NPK under traditional irrigation water. Similarly, Duarah et al. [
87] observed that 50% NPK along with solubilizing bacteria showed better growth of plants compared with 100% NPK. In addition, wastewater can be used as an alternative source for crop irrigation in arid and semi-arid environments [
32,
88]. Thus, 100% NPK dose along with TWW could be in an excess amount of nutrients and may not be a beneficial application for the growth and productivity of crops used for bioenergy production. Excess dose of fertilizer can alter the soil properties by developing high salt concentration, and this phenomenon can upset beneficial microorganisms in the soil. TWW can increase the fertilizer use efficiency of NPK by increasing the uptake of nutrients added through fertilizer application. Thus, fertilizer rates could be reduced with the use of TWW because TWW can serve not only as the source of water but also of nutrients, particularly N, P, and K, and other micronutrients, such as Zn and B. Moreover, it could help reduce the environmental pollution caused by the over-application of fertilizers.