Process Water Recirculation during Hydrothermal Carbonization of Waste Biomass: Current Knowledge and Challenges
Abstract
1. Introduction
2. Effect of PW Recirculation on Hydrochar Properties
2.1. Effect on the Mass Yield
| Feedstock | Temperature (°C) | RT 1 | B/W 2 | RS 3 | MY 4 (%) | HHV 5 (MJ/Kg) | pH | TOC 6 (g/L) | References |
|---|---|---|---|---|---|---|---|---|---|
| Cornstalk (CK) | 220 | 2 h | 0.2 | 4 | 55.9–66.2 | 22.7–23.3 | 3.7–3.9 | 11.7–29.1 | [46] |
| BSG 7 | 200 | 2 h | 0.13 | 2 | 59.0–60.4 | 28.7–27.9 | 4.5–4.3 | 17.0–15.1 | [66] |
| BSG | 200 | 4 h | 0.13 | 2 | 54.6–51.3 | 28.5–29.0 | 4.6–4.5 | 21.8–12.4 | [66] |
| BSG | 220 | 2 h | 0.13 | 2 | 56.0–70.2 | 29.4–30.5 | 4.6–4.5 | 15.6–28.5 | [66] |
| BSG | 220 | 4 h | 0.13 | 2 | 54.2–65.9 | 30.5–31.7 | 4.7–4.5 | 14.9–20.4 | [66] |
| Laminaria (LM) | 220 | 2 h | 0.05 | 12 | 13.3–17.8 | 18.4–22.7 | 4.8–5.6 | Na 8 | [67] |
| Loblolly pine (LP) | 200 | 5 min | 0.2 | 9 | 82.1–90.0 | 20.6–20.9 | 3.4–3.3 | 11.7–37.8 | [68] |
| Loblolly pine (LP) | 230 | 5 min | 0.2 | 9 | 75.0–82.9 | 21.5–21.8 | 2.9–3.0 | 18.3–29.4 | [68] |
| Loblolly pine (LP) | 260 | 5 min | 0.2 | 5 | 60.0–69.3 | 25.0–25.3 | 2.8–2.9 | na | [68] |
| SPW 9 | 220 | 1 h | 0.2 | 4 | 60.4–66.3 | 23.0–23.7 | na | na | [69] |
| MGW 10 | 180 | 5 h | 0.15 | 10 | 81.0–85.0 | 25.4–26.6 | 4.6–4.5 | 21.4–30.9 | [70] |
| MGW | 220 | 5 h | 0.15 | 10 | 60.0–70.0 | 28.8–29.8 | 4.3–4.5 | 19.6–22.7 | [70] |
| Miscanthus (MS) | 260 | 5 min | 0.17 | 10 | 47.0–57.0 | 26.1–26.6 | 2.7–2.7 | 28.6–59.0 | [71] |
| Paper (PP) | 200 | 16 h | 0.1 | 4 | 56.4–75.8 | 21.7–20.0 | 2.7–2.8 | na | [72] |
| Chlorella (CH) | 220 | 4 h | 0.1 | 4 | 20.5–26.7 | 30.8–32.1 | na | 27.9–88.7 | [73] |
| Soybean straw (SS) | 220 | 4 h | 0.1 | 4 | 47.7–54.7 | 21.6–22.5 | na | 13.2–32.7 | [73] |
| Poplar wood (PW) | 220 | 4 h | 0.2 | 4 | 60.1–61.2 | 25.0–26.2 | 3.4–3.4 | 17.4–39.2 | [74] |
| Grape pomace (GP) | 225 | 10 min | 0.25 | 3 | 64.3–75.2 | 25.5–25.9 | 3.9–4.1 | 12.1–26.3 | [75] |
| Orange pomace (OP) | 225 | 30 min | 0.25 | 3 | 51.6–57.5 | 24.0–23.7 | 3.8–3.9 | 32.1–57.9 | [75] |
| Poultry litter (PL) | 225 | 15 min | 0.25 | 3 | 53.0–62.4 | 21.2–20.8 | 5.5–5.7 | 27.4–49.7 | [75] |
| Lemon peel (LPW) | 180 220 | 1 h | 0.2 | 2 | 50.1–55.9 | 22.4–22.2 | 3.9–4.0 | 16.0–20.0 | [76] |
| Lemon peel (LPW) | 220 | 1 h | 0.2 | 2 | 49.2–51.2 | 24.4–24.7 | 4.4–4.5 | 13.3–17.8 | [76] |
| Lemon peel (LPW) | 250 | 1 h | 0.2 | 2 | 40.9–42.5 | 26.7–27.2 | 4.7–4.7 | 9.7–18.0 | [76] |
2.2. Effect on HHV and Energy Yield
2.3. Effect on Chemical Composition
3. Effect of PW Recirculation on Liquid Phase Properties
4. Influence of Operating Conditions on PW Recirculation
5. Challenges and Research Opportunities
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Yilmaz, S.; Selim, H. A review on the methods for biomass to energy conversion systems design. Renew. Sustain. Energy Rev. 2013, 25, 420–430. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Lee, M.; Lin, Y.L.; Te Chiueh, P.; Den, W. Environmental and energy assessment of biomass residues to biochar as fuel: A brief review with recommendations for future bioenergy systems. J. Clean. Prod. 2020, 251, 119714. [Google Scholar] [CrossRef]
- Ibarra-Gonzalez, P.; Rong, B.G. A review of the current state of biofuels production from lignocellulosic biomass using thermochemical conversion routes. Chin. J. Chem. Eng. 2019, 27, 1523–1535. [Google Scholar] [CrossRef]
- Volpe, R.; Messineo, S.; Volpe, M.; Messineo, A. Catalytic effect of char for tar cracking in pyrolysis of citrus wastes, design of a novel experimental set up and first results. Chem. Eng. Trans. 2016, 50, 181–186. [Google Scholar]
- Luz, F.C.; Volpe, M.; Fiori, L.; Manni, A.; Cordiner, S.; Mulone, V.; Rocco, V. Spent Coffee Enhanced Biomethane Potential via an Integrated Hydrothermal Carbonization-Anaerobic Digestion Process. Bioresour. Technol. 2018, 256, 102–109. [Google Scholar]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic Digestion of Lignocellulosic Biomass: Challenges and Opportunities; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 178, ISBN 1808956354. [Google Scholar]
- Panwar, N.L.; Kothari, R.; Tyagi, V.V. Thermo chemical conversion of biomass-Eco friendly energy routes. Renew. Sustain. Energy Rev. 2012, 16, 1801–1816. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification and Pyrolysis; Elsevier: Oxford, UK, 2010; ISBN 9780123749888. [Google Scholar]
- Gopu, C.; Gao, L.; Volpe, M.; Fiori, L.; Goldfarb, J.L. Valorizing municipal solid waste: Waste to energy and activated carbons for water treatment via pyrolysis. J. Anal. Appl. Pyrolysis 2018, 133, 48–58. [Google Scholar] [CrossRef]
- Saha, N.; Volpe, M.; Fiori, L.; Volpe, R.; Messineo, A.; Reza, M.T. Cationic dye adsorption on hydrochars of winery and citrus juice industries residues: Performance, mechanism, and thermodynamics. Energies 2020, 13, 4686. [Google Scholar] [CrossRef]
- Merzari, F.; Goldfarb, J.; Andreottola, G.; Mimmo, T.; Volpe, M.; Fiori, L. Hydrothermal carbonization as a strategy for sewage sludge management: Influence of process withdrawal point on hydrochar properties. Energies 2020, 13, 2890. [Google Scholar] [CrossRef]
- Chen, W.T.; Haque, M.A.; Lu, T.; Aierzhati, A.; Reimonn, G. A perspective on hydrothermal processing of sewage sludge. Curr. Opin. Environ. Sci. Health 2020, 14, 63–73. [Google Scholar] [CrossRef]
- Magdeldin, M.; Kohl, T.; De Blasio, C.; Järvinen, M.; Park, S.W.; Giudici, R. The BioSCWG project: Understanding the trade-offs in the process and thermal design of hydrogen and synthetic natural gas production. Energies 2016, 9, 838. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nagappan, S.; Bhosale, R.R.; Lay, C.H.; Duc Nguyen, D.; Pugazhendhi, A.; Chang, S.W.; Kumar, G. Review on sustainable production of biochar through hydrothermal liquefaction: Physico-chemical properties and applications. Bioresour. Technol. 2020, 310, 123414. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- He, C.; Chen, C.L.; Giannis, A.; Yang, Y.; Wang, J.Y. Hydrothermal gasification of sewage sludge and model compounds for renewable hydrogen production: A review. Renew. Sustain. Energy Rev. 2014, 39, 1127–1142. [Google Scholar] [CrossRef]
- Kruse, A. Hydrothermal biomass gasification. J. Supercrit. Fluids 2009, 47, 391–399. [Google Scholar] [CrossRef]
- Pavlovič, I.; Knez, Ž.; Škerget, M. Hydrothermal reactions of agricultural and food processing wastes in sub- and supercritical water: A review of fundamentals, mechanisms, and state of research. J. Agric. Food Chem. 2013, 61, 8003–8025. [Google Scholar] [CrossRef]
- Volpe, M.; Fiori, L. From olive waste to solid biofuel through hydrothermal carbonisation: The role of temperature and solid load on secondary char formation and hydrochar energy properties. J. Anal. Appl. Pyrolysis 2017, 124, 63–72. [Google Scholar] [CrossRef]
- Merzari, F.; Lucian, M.; Volpe, M.; Andreottola, G.; Fiori, L. Hydrothermal carbonization of biomass: Design of a bench-Scale reactor for evaluating the heat of reaction. Chem. Eng. Trans. 2018, 65, 43–48. [Google Scholar]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldfarb, J.L.; Fiori, L. Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Fiori, L. Hydrothermal carbonization kinetics of lignocellulosic agro-wastes: Experimental data and modeling. Energies 2019, 12, 516. [Google Scholar] [CrossRef]
- Wang, R.; Wang, C.; Zhao, Z.; Jia, J.; Jin, Q. Energy recovery from high-ash municipal sewage sludge by hydrothermal carbonization: Fuel characteristics of biosolid products. Energy 2019, 186, 115848. [Google Scholar] [CrossRef]
- Park, K.Y.; Lee, K.; Kim, D. Characterized hydrochar of algal biomass for producing solid fuel through hydrothermal carbonization. Bioresour. Technol. 2018, 258, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Luis, J.; Moscoso, G.; Kumar, S.; Cao, X.; Mao, J.; Schafran, G. Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J. Environ. Manag. 2012, 109, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Correa, C.; Ngamying, C.; Klank, D.; Kruse, A. Investigation of the textural and adsorption properties of activated carbon from HTC and pyrolysis carbonizates. Biomass Convers. Biorefinery 2018, 8, 317–328. [Google Scholar] [CrossRef]
- Volpe, M.; Fiori, L.; Merzari, F.; Messineo, A.; Andreottola, G. Hydrothermal carbonization as an efficient tool for sewage sludge valorization and phosphorous recovery. Chem. Eng. Trans. 2020, 80, 199–204. [Google Scholar]
- Marin-Batista, J.D.; Mohedano, A.F.; Rodriguez, J.J.; de La Rubia, M.A. Energy and phosphorous recovery through hydrothermal carbonization of digested sewage sludge. Waste Manag. 2020, 105, 566–574. [Google Scholar] [CrossRef]
- Becker, G.C.; Wüst, D.; Köhler, H.; Lautenbach, A.; Kruse, A. Novel approach of phosphate-reclamation as struvite from sewage sludge by utilising hydrothermal carbonization. J. Environ. Manag. 2019, 238, 119–125. [Google Scholar] [CrossRef]
- Shi, Y.; Luo, G.; Rao, Y.; Chen, H.; Zhang, S. Hydrothermal conversion of dewatered sewage sludge: Focusing on the transformation mechanism and recovery of phosphorus. Chemosphere 2019, 228, 619–628. [Google Scholar] [CrossRef]
- Volpe, M.; Goldfarb, J.L.; Fiori, L. Hydrothermal carbonization of Opuntia ficus-indica cladodes: Role of process parameters on hydrochar properties. Bioresour. Technol. 2018, 247, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Fiori, L.; Volpe, R.; Messineo, A. Upgrading of Olive Tree Trimmings Residue as Biofuel by Hydrothermal Carbonization and Torrefaction: A Comparative Study. Chem. Eng. Trans. 2016, 50, 13–18. [Google Scholar]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Rahim, M.H.A.; Park, J.W.; Kim, H.J. Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: A review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Pauline, A.L.; Joseph, K. Hydrothermal carbonization of organic wastes to carbonaceous solid fuel–A review of mechanisms and process parameters. Fuel 2020, 279, 118472. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Hitzl, M.; Corma, A.; Pomares, F.; Renz, M. The hydrothermal carbonization (HTC) plant as a decentral biorefinery for wet biomass. Catal. Today 2015, 257, 154–159. [Google Scholar] [CrossRef]
- Leng, L.; Zhou, W. Chemical compositions and wastewater properties of aqueous phase (wastewater) produced from the hydrothermal treatment of wet biomass: A review. Energy Sour. Part A Recovery Utilization Environ. Effects 2018, 40, 2648–2659. [Google Scholar] [CrossRef]
- Becker, R.; Dorgerloh, U.; Paulke, E.; Mumme, J.; Nehls, I. Hydrothermal carbonization of biomass: Major organic components of the aqueous phase. Chem. Eng. Technol. 2014, 37, 511–518. [Google Scholar] [CrossRef]
- Langone, M.; Basso, D. Process waters from hydrothermal carbonization of sludge: Characteristics and possible valorization pathways. Int. J. Environ. Res. Publ. Health 2020, 17, 6618. [Google Scholar] [CrossRef]
- Berge, N.D.; Ro, K.S.; Mao, J.; Flora, J.R.V.; Chappell, M.A.; Bae, S. Hydrothermal Carbonization of Municipal Waste Streams. Environ. Sci. Technol. 2011, 45, 5696–5703. [Google Scholar] [CrossRef]
- Leng, S.; Leng, L.; Chen, L.; Chen, J.; Chen, J.; Zhou, W. The effect of aqueous phase recirculation on hydrothermal liquefaction/carbonization of biomass: A review. Bioresour. Technol. 2020, 318, 124081. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Zhan, H.; Song, Y.; He, C.; Huang, Y.; Yin, X.; Wu, C. Insights into the evolution of chemical structures in lignocellulose and non-lignocellulose biowastes during hydrothermal carbonization (HTC). Fuel 2019, 236, 960–974. [Google Scholar] [CrossRef]
- Shen, Y. A review on hydrothermal carbonization of biomass and plastic wastes to energy products. Biomass Bioenergy 2020, 134, 105479. [Google Scholar] [CrossRef]
- Wang, R.; Jin, Q.; Ye, X.; Lei, H.; Jia, J.; Zhao, Z. Effect of process wastewater recycling on the chemical evolution and formation mechanism of hydrochar from herbaceous biomass during hydrothermal carbonization. J. Clean. Prod. 2020, 277, 123281. [Google Scholar] [CrossRef]
- Antero, R.V.P.; Alves, A.C.F.; de Oliveira, S.B.; Ojala, S.A.; Brum, S.S. Challenges and alternatives for the adequacy of hydrothermal carbonization of lignocellulosic biomass in cleaner production systems: A review. J. Clean. Prod. 2020, 252, 119899. [Google Scholar] [CrossRef]
- Reza, M.T.; Freitas, A.; Yang, X.; Coronella, C.J. Wet Air Oxidation of Hydrothermal Carbonization (HTC) Process Liquid. ACS Sustain. Chem. Eng. 2016, 4, 3250–3254. [Google Scholar] [CrossRef]
- Stobernack, N.; Mayer, F.; Malek, C.; Bhandari, R. Evaluation of the energetic and environmental potential of the hydrothermal carbonization of biowaste: Modeling of the entire process chain. Bioresour. Technol. 2020, 318, 124038. [Google Scholar] [CrossRef]
- Wang, F.; Yi, W.; Zhang, D.; Liu, Y.; Shen, X.; Li, Y. Anaerobic co-digestion of corn stover and wastewater from hydrothermal carbonation. Bioresour. Technol. 2020, 315, 123788. [Google Scholar] [CrossRef]
- Fernandez, S.; Srinivas, K.; Schmidt, A.J.; Swita, M.S.; Ahring, B.K. Anaerobic digestion of organic fraction from hydrothermal liquefied algae wastewater byproduct. Bioresour. Technol. 2017, 247, 250–258. [Google Scholar] [CrossRef]
- Brown, A.E.; Finnerty, G.L.; Camargo-valero, M.A.; Ross, A.B. Bioresource Technology Valorisation of macroalgae via the integration of hydrothermal carbonisation and anaerobic digestion. Bioresour. Technol. 2020, 312, 123539. [Google Scholar] [CrossRef]
- Kruse, A.; Funke, A.; Titirici, M.-M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef]
- Reza, M.T.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J. Hydrothermal carbonization of loblolly pine: Reaction chemistry and water balance. Biomass Convers. Biorefinery 2014, 4, 311–321. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, d-xylose, and wood meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Volpe, M.; Messineo, A.; Mäkelä, M.; Barr, M.R.; Volpe, R.; Corrado, C.; Fiori, L. Reactivity of cellulose during hydrothermal carbonization of lignocellulosic biomass. Fuel Proc. Technol. 2020, 206, 106456. [Google Scholar] [CrossRef]
- Fang, Z.; Sato, T.; Smith, R.L.; Inomata, H.; Arai, K.; Kozinski, J.A. Reaction chemistry and phase behavior of lignin in high-temperature and supercritical water. Bioresour. Technol. 2008, 99, 3424–3430. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Upgrading the characteristics of biochar from cellulose, lignin, and xylan for solid biofuel production from biomass by hydrothermal carbonization. J. Ind. Eng. Chem. 2016, 42, 95–100. [Google Scholar] [CrossRef]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef]
- Lynam, J.G.; Coronella, C.J.; Yan, W.; Reza, M.T.; Vasquez, V.R. Acetic acid and lithium chloride effects on hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 6192–6199. [Google Scholar] [CrossRef]
- Arauzo, P.J.; Olszewski, M.P.; Wang, X.; Pfersich, J.; Sebastian, V.; Manyà, J.; Hedin, N.; Kruse, A. Assessment of the effects of process water recirculation on the surface chemistry and morphology of hydrochar. Renew. Energy 2020, 155, 1173–1180. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Gu, C.; Han, Y.; Zan, S.; Wu, S. Effects of process water recirculation on solid and liquid products from hydrothermal carbonization of Laminaria. Bioresour. Technol. 2019, 292, 121996. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, P.; Narayanan, S.K. Effect of hydrothermal carbonization reaction parameters on. Environ. Prog. Sustain. Energy 2014, 33, 676–680. [Google Scholar]
- Chen, X.; Ma, X.; Peng, X.; Lin, Y.; Wang, J.; Zheng, C. Effects of aqueous phase recirculation in hydrothermal carbonization of sweet potato waste. Bioresour. Technol. 2018, 267, 167–174. [Google Scholar] [CrossRef]
- Köchermann, J.; Görsch, K.; Wirth, B.; Mühlenberg, J.; Klemm, M. Hydrothermal carbonization: Temperature influence on hydrochar and aqueous phase composition during process water recirculation. J. Environ. Chem. Eng. 2018, 6, 5481–5487. [Google Scholar] [CrossRef]
- Kambo, H.S.; Minaret, J.; Dutta, A. Process water from the hydrothermal carbonization of biomass: A waste or a valuable product? Waste Biomass Valoriz. 2018, 9, 1181–1189. [Google Scholar] [CrossRef]
- Becker, R.; Dorgerloh, U.; Helmis, M.; Mumme, J.; Diakité, M.; Nehls, I. Hydrothermally carbonized plant materials: Patterns of volatile organic compounds detected by gas chromatography. Bioresour. Technol. 2013, 130, 621–628. [Google Scholar] [CrossRef]
- Leng, S.; Li, W.; Han, C.; Chen, L.; Chen, J.; Fan, L.; Lu, Q.; Li, J.; Leng, L.; Zhou, W. Aqueous phase recirculation during hydrothermal carbonization of microalgae and soybean straw: A comparison study. Bioresour. Technol. 2020, 298, 122502. [Google Scholar] [CrossRef] [PubMed]
- Stemann, J.; Putschew, A.; Ziegler, F. Hydrothermal carbonization: Process water characterization and effects of water recirculation. Bioresour. Technol. 2013, 143, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kabadayi Catalkopru, A.; Kantarli, I.C.; Yanik, J. Effects of spent liquor recirculation in hydrothermal carbonization. Bioresour. Technol. 2017, 226, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Picone, A.; Volpe, M.; Giustra, M.G.; Di Bella, G.; Messineo, A. Hydrothermal carbonization of lemon peel waste: Preliminary results on the effects of temperature during process water recirculation. Appl. Syst. Innov. 2021, 4, 19. [Google Scholar] [CrossRef]
- Axelsson, L.; Franzén, M.; Ostwald, M.; Berndes, G.; Lakshmi, G.; Ravindranath, N.H. Perspective: Jatropha cultivation in southern India: Assessing farmers’ experiences. Biofuels Bioprod. Biorefining 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem. A Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Benavente, V.; Calabuig, E.; Fullana, A. Upgrading of moist agro-industrial wastes by hydrothermal carbonization. J. Anal. Appl. Pyrolysis 2015, 113, 89–98. [Google Scholar] [CrossRef]
- Arauzo, P.J.; Olszewski, M.P.; Kruse, A. Hydrothermal carbonization brewer’s spent grains with the focus on improving the degradation of the feedstock. Energies 2018, 11, 3226. [Google Scholar] [CrossRef]
- Gao, L.; Volpe, M.; Lucian, M.; Fiori, L.; Goldfarb, J.L. Does Hydrothermal Carbonization as a Biomass Pretreatment Reduce Fuel Segregation of Coal-Biomass Blends During Oxidation? Energy Convers. Manag. 2019, 181, 93–104. [Google Scholar] [CrossRef]
- Poerschmann, J.; Baskyr, I.; Weiner, B.; Koehler, R.; Wedwitschka, H.; Kopinke, F.-D. Hydrothermal carbonization of olive mill wastewater. Bioresour. Technol. 2013, 133, 581–588. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An overview of effect of process parameters on hydrothermal carbonization of biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Nakason, K.; Panyapinyopol, B.; Kanokkantapong, V.; Viriya-empikul, N.; Kraithong, W.; Pavasant, P. Characteristics of hydrochar and liquid fraction from hydrothermal carbonization of cassava rhizome. J. Energy Inst. 2018, 91, 184–193. [Google Scholar] [CrossRef]
- Akhtar, J.; Saidina Amin, N. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Lu, X.; Pellechia, P.J.; Flora, J.R.V.; Berge, N.D. Influence of reaction time and temperature on product formation and characteristics associated with the hydrothermal carbonization of cellulose. Bioresour. Technol. 2013, 138, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Mau, V.; Quance, J.; Posmanik, R.; Gross, A. Phases’ characteristics of poultry litter hydrothermal carbonization under a range of process parameters. Bioresour. Technol. 2016, 219, 632–642. [Google Scholar] [CrossRef]
- Sabio, E.; Álvarez-Murillo, A.; Román, S.; Ledesma, B. Conversion of tomato-peel waste into solid fuel by hydrothermal carbonization: Influence of the processing variables. Waste Manag. 2016, 47, 122–132. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal carbonization as a valuable tool for energy and environmental applications: A review. Energies 2020, 13, 4098. [Google Scholar] [CrossRef]
- Kalderis, D.; Kotti, M.S.; Méndez, A.; Gascó, G. Characterization of hydrochars produced by hydrothermal carbonization of rice husk. Solid Earth 2014, 5, 477–483. [Google Scholar] [CrossRef]
- Knežević, D.; Van Swaaij, W.; Kersten, S. Hydrothermal conversion of biomass. II. Conversion of wood, pyrolysis oil, and glucose in hot compressed water. Ind. Eng. Chem. Res. 2010, 49, 104–112. [Google Scholar] [CrossRef]
- Knežević, D.; van Swaaij, W.P.M.; Kersten, S.R.A. Hydrothermal Conversion of Biomass: I, Glucose Conversion in hot compressed water. Ind. Eng. Chem. Res. 2009, 48, 4731–4743. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Liu, S. Hydrothermal synthesis, characterization, and KOH activation of carbon spheres from glucose. Carbohydr. Res. 2011, 346, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Favas, G.; Jackson, W.R. Hydrothermal dewatering of lower rank coals. 1: Effects of process conditions on the properties of dried product. Fuel 2003, 82, 53–57. [Google Scholar] [CrossRef]
- Sermyagina, E.; Saari, J.; Kaikko, J.; Vakkilainen, E. Hydrothermal carbonization of coniferous biomass: Effect of process parameters on mass and energy yields. J. Anal. Appl. Pyrolysis 2015, 113, 551–556. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Balasubramanian, R. Preparation and characterization of fuel pellets from woody biomass, agro-residues and their corresponding hydrochars. Appl. Energy 2014, 113, 1315–1322. [Google Scholar] [CrossRef]
- Jarvis, J.M.; Billing, J.M.; Corilo, Y.E.; Schmidt, A.J.; Hallen, R.T.; Schaub, T.M. FT-ICR MS analysis of blended pine-microalgae feedstock HTL biocrudes. Fuel 2018, 216, 341–348. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Teh, S.J.; Lim, Y.S. Catalytic hydrothermal upgrading of α-cellulose using iron salts as a lewis acid. BioResources 2015, 10, 5974–5986. [Google Scholar] [CrossRef]
- Heidari, M.; Dutta, A.; Acharya, B.; Mahmud, S. A review of the current knowledge and challenges of hydrothermal carbonization for biomass conversion. J. Energy Inst. 2019, 92, 1779–1799. [Google Scholar] [CrossRef]
- Pagés-Díaz, J.; Huiliñir, C. Valorization of the liquid fraction of co-hydrothermal carbonization of mixed biomass by anaerobic digestion: Effect of the substrate to inoculum ratio and hydrochar addition. Bioresour. Technol. 2020, 317, 123989. [Google Scholar] [CrossRef]
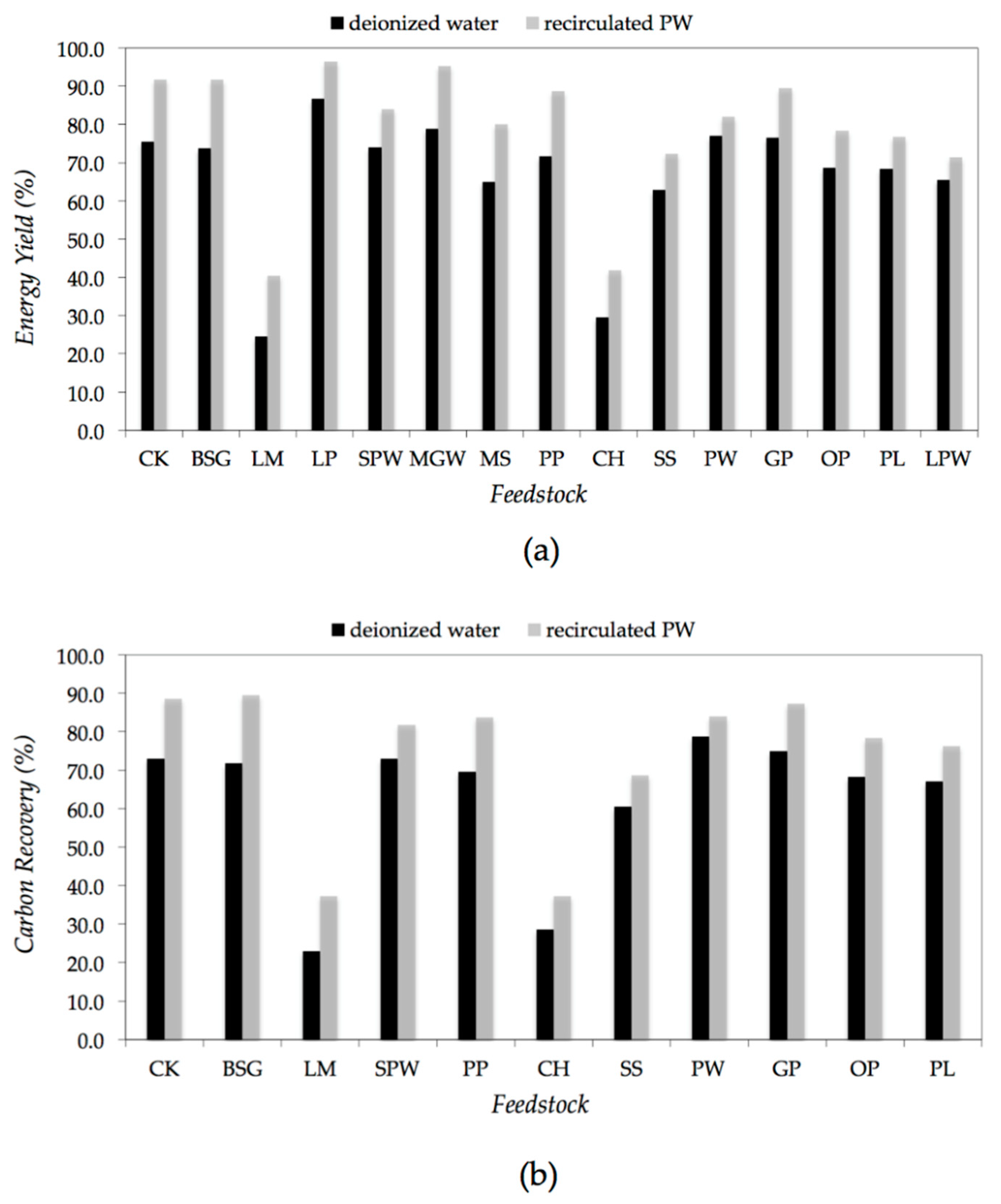

| Feedstock | Proximate Composition (wt% d.b.) | Elemental Composition (wt% d.b.) | References | |||||
|---|---|---|---|---|---|---|---|---|
| VM | Ash | FC | C | H | N | O | ||
| CK | 64.6–61.4 | 2.8–5.2 | 31.2–32.2 | 56.2–57.6 | 5.8–5.7 | 1.8–1.9 | 33.4–29.5 | [46] |
| BSG | 70.7–69.3 | 4.5–3.8 | 24.8–26.91 | 61.9–60.8 | 8.0–7.7 | 3.8–4.0 | 21.3–22.8 | [66] |
| BSG | 69.5–67.7 | 4.7–4.9 | 25.8–27.5 | 62.5–62.7 | 7.6–7.9 | 3.9–4.0 | 20.8–20.1 | [66] |
| BSG | 66.3–65.8 | 4.1–4.6 | 29.6–29.6 | 64.1–65.2 | 7.7–8.1 | 4.1–4.2 | 19.4–17.3 | [66] |
| BSG | 64.0–64.9 | 4.6–4.5 | 31.4–30.6 | 65.5–67.7 | 8.1–8.3 | 4.1–4.3 | 17.2–14.8 | [66] |
| LM | Na 1 | na | na | 45.9–55.7 | 6.3–6.7 | 3.6–4.2 | 34.9–27.5 | [67] |
| SPW | 65.4–64.5 | 6.6–6.8 | 28.0–28.7 | 58.0–59.2 | 5.4–5.5 | 0.2–0.2 | 29.6–28.2 | [69] |
| PP | na | na | na | 54.7–49.9 | 5.7–6.1 | na | 39.4–44.1 | [72] |
| CH | na | na | na | 66.5–68.5 | 7.6–8.0 | 6.5–6.5 | 12.4–11.5 | [73] |
| SS | na | na | na | 52.1–53.5 | 5.3–5.5 | 3.0–3.3 | 24.7–22.9 | [73] |
| PW | na | 0.7–0.4 | na | 62.2–65.2 | 5.6–5.5 | 0.2–0.1 | na | [74] |
| GP | 41.9–43.3 | 2.9–2.37 | na | 61.6–61.9 | 5.9–6.1 | 1.8–2.1 | 27.7–27.4 | [75] |
| OP | 38.0–41.0 | 5.5–7.6 | na | 56.6–58.3 | 5.4–5.1 | 2.4–2.8 | 29.3–26.0 | [75] |
| PL | 39.2–38.1 | 11.3–11.7 | na | 51.8–51.0 | 5.4–5.3 | 3.9–4.2 | 27.6–27.9 | [75] |
| LPW | 65.3–62.6 | 2.3–3.0 | 32.4–34.4 | na | na | na | na | [76] |
| LPW | 58.1–58.1 | 3.1–3.2 | 38.8–38.7 | na | na | na | na | [76] |
| LPW | 50.5–51.7 | 3.2–3.3 | 46.3–45.1 | na | na | na | na | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picone, A.; Volpe, M.; Messineo, A. Process Water Recirculation during Hydrothermal Carbonization of Waste Biomass: Current Knowledge and Challenges. Energies 2021, 14, 2962. https://doi.org/10.3390/en14102962
Picone A, Volpe M, Messineo A. Process Water Recirculation during Hydrothermal Carbonization of Waste Biomass: Current Knowledge and Challenges. Energies. 2021; 14(10):2962. https://doi.org/10.3390/en14102962
Chicago/Turabian StylePicone, Antonio, Maurizio Volpe, and Antonio Messineo. 2021. "Process Water Recirculation during Hydrothermal Carbonization of Waste Biomass: Current Knowledge and Challenges" Energies 14, no. 10: 2962. https://doi.org/10.3390/en14102962
APA StylePicone, A., Volpe, M., & Messineo, A. (2021). Process Water Recirculation during Hydrothermal Carbonization of Waste Biomass: Current Knowledge and Challenges. Energies, 14(10), 2962. https://doi.org/10.3390/en14102962








