Abstract
Hydrothermal gasification (HTG) experiments were carried out to extract hydrogen from biomass. Although extensive research has been conducted on hydrogen production with HTG, limited research exists on the use of biochar as a raw material. In this study, woodland residues (wood chip) and biochar from wood-chip pyrolysis were used in HTG treatment to generate hydrogen. This research investigated the effect of temperature (300–425 °C) and biomass/water (0.5–10) ratio on gas composition. A higher temperature promoted hydrogen production because the water–gas shift reaction and steam-reforming reaction were promoted with an increase in temperature. The methane concentration was related positively to temperature because of the methanation and hydrogenation reactions. A lower biomass/water ratio promoted hydrogen production but suppressed carbon-monoxide production. Most reactions that produce hydrogen consume water, but water also affects the water–gas shift reaction balance, which decreases the carbon-monoxide concentration. By focusing on the practical application of HTG, we attempted biochar treatment by pyrolysis (temperature of heating part: 700 °C), and syngas was obtained from hydrothermal treatment above 425 °C.
1. Introduction
Renewable and environmentally-friendly energy sources have attracted attention because of global warming and the gradual depletion of fossil resources. Biomass from plants can be used as a material or energy source [1], which is representative of a renewable energy source, and includes woodland residues, municipal waste, energy crops, aquatic plants, agricultural crops and animal waste. Plants accumulate energy and absorb carbon dioxide, and release energy and carbon when burnt. Therefore, bioenergy is a carbon-neutral energy source [2]. In Japan, eight million tons of forest residue are generated annually, but the utilization rate is only 9% [3]; most forest residue is landfilled in situ. The purpose of this study was to extract biofuel from conifer wood chips. Coniferous wood chips contain 31–49% cellulose, 9–23% hemicellulose and 20–35% lignin [4]. Because lignin hinders cellulose and hemicellulose decomposition, to convert wood chips to bioenergy, lignin needs to be decomposed first. Common methods, such as burning, hydrothermal treatment and pyrolysis have been studied to decompose the lignin [5].
Pyrolysis can be traced back to the ancient Egyptian period and has been used extensively in coke and charcoal production in modern times [5]. Biomass pyrolysis is a process of thermal degradation of biomass raw materials without air/oxygen and is used to produce solid (biochar), liquid (tar and other organic matter) and gas products [6]. In general, biochar is burned and used for power generation. Biochar can be used to generate hydrogen or syngas that contains carbon monoxide and hydrogen by hydrothermal gasification (HTG) [7]. Hydrogen is considered a clean energy carrier. When burned, hydrogen combines with oxygen to produce only water. Therefore, infrastructure that is based on hydrogen energy has been regarded as an ideal solution to environmental problems [8,9,10]. Syngas could convert to C8–C16 by the Fischer–Tropsch process reaction, to form the main content of jet fuel [11,12,13].
Hydrothermal treatment is a chemical reaction produced by high temperature and pressure with water. In this critical situation, water acts as a solvent and has a catalytic effect. For hydrothermal gasification, the effect of temperature and biomass/water (B/W) ratio is important. Temperature affects the decomposition rate of cellulose, hemicellulose, and lignin, and has a significant impact on the conversion of low-molecular-mass compounds. In HTG, almost half of the hydrogen is produced from water, which influences the B/W ratio’s effect on gas yield and composition [7].
Although the use of HTG and pyrolysis technology to treat biomass has been studied extensively, limited research exists that combines pyrolysis and HTG. We have found some documents that use hydrothermal gasification to treat char, but almost no biochar comes from pyrolysis. In fact, the author did not find any literature using hydrothermal gasification to treat char from pyrolysis [7]. Compared with conventional thermal power generation, HTG removes impurities, such as ash, from char, so the amounts of SOx and NOx that are emitted from syngas use are small [14]. The HTG of pyrolysis chars is environmentally friendly and leads to cost reductions because it can operate without desulfurization equipment. This research confirmed the optimal condition to produce syngas by conducting batch HTG experiments. The biochar from pyrolysis is treated by HTG under optimal conditions to produce syngas.
2. Materials and Methods
Wood chips from north Hokkaido conifers were used in the HTG experiment, and Larix kaempferi wood chips from Sunagawa were used in the pyrolysis experiments. All standard samples were used as received. Sulfuric acid was filtered with 0.45 µm filter paper.
Conifer wood chips were used in the HTG experiment to determine the optimal conditions for hydrogen production (experimental group 1). An autoclave200 (Model 122841, SUS 316 Tsukuba, Japan) was used for the HTG experiments. A diagram that shows the structure of the reactor is shown in Figure 1. The experimental conditions of Group 1 are outlined in Table 1 and the experimental process is shown in Figure 2. Firstly, we fixed the reactor body by a heater and unscrewed the screw to open the reactor. Wood chips (1 g) and pure water were placed in the reactor (the quality of pure water was determined from the B/W ratio, which is the mass ratio of biomass and pure water). Then, we tightened the screws to close the reactor. Nitrogen purging was conducted three times to remove air from the reactor. In each purge, the internal pressure of the reactor reached 4.0 MPa. To obtain the most accurate gas component measurement, all nitrogen gas was discharged and the initial pressure of group 1 was 0.1 MPa. Heating started after nitrogen purging. The heating rate was adjusted by the proportional–integral–derivative control system, and the holding time after exceeding the target temperature was defined as the reaction time. After each reaction, the reactor was cooled to room temperature overnight. The gas and solid residues were collected, separately, from the reactor. Gas composition analysis was performed by gas chromatography (GC). An electric furnace (FO300) (Yamato Scientific Co., Ltd., Tokyo, Japan) was used to dry the solid phase at 105 °C for 24 h in air, and the mass balance was calculated. The calculation of mass balance will be introduced below. The HTG experiment was carried out from 300 °C to 425 °C, with a B/W ratio of between 0.5 to 10.
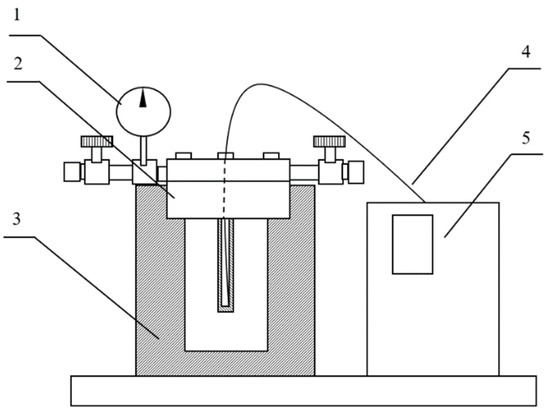
Figure 1.
Schematic diagram of a hydrothermal reactor. 1 = pressure gauge; 2 = reactor body; 3 = heater; 4 = thermocouple; 5 = control unit.

Table 1.
Experimental conditions for HTG (group 1).
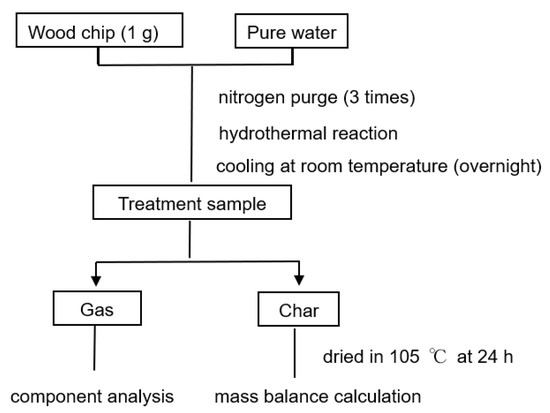
Figure 2.
Experimental flow of HTG.
To study the practicality of HTG, a combined pyrolysis/HTG experiment was carried out (experiment group 2). In this experiment, biochar from pyrolysis with HTG was treated to produce hydrogen under the optimal condition, as determined from group 1. The specific condition is shown in Table 2. Figure 3 shows the structure of the pyrolysis reactor. The experimental conditions of group 2 are outlined in Table 3, and the experimental flow is shown in Figure 4. After improving the equipment in the reference, the continuous pyrolysis experiment was undertaken at Mitsui Chemical Factory (Hokkaido, Japan). The wood chip was fed into the bottom of the pyrolysis reactor and risen by rotation of the screw. The heating part released heat and raised the temperature of the heat conduction part to reach the reaction temperature. At a high temperature, wood chip undergoes a pyrolysis reaction in the reactor; the oil is removed and collected through various outlets, and the char is discharged from the top of the reactor. The wood chips were heated for 8 h at 700 °C in air to recover the char and oil. The oil phase was filtered using 0.45 µm filter paper (mixed cellulose esters membrane). For biochar, elemental analysis was performed to calculate the high heating value (HHV) using the DuLong formula. Char (1 g) and pure water (2 mL) were used in each HTG experiment, with a flow that was the same as group 1. Gas from the HTG was measured by GC.

Table 2.
Experimental conditions for HTG (group 2).
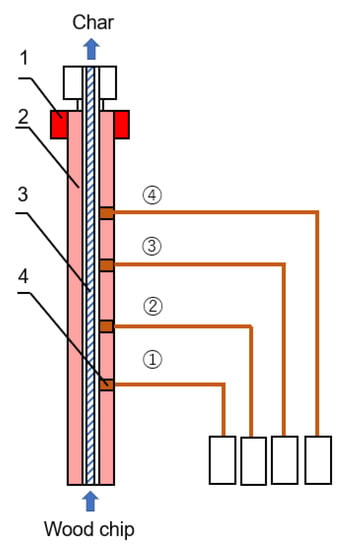
Figure 3.
Continuous pyrolysis reactor. 1: heating part; 2: heat conduction part; 3: screw; 4: outlet.

Table 3.
Experimental conditions for pyrolysis (group 2).
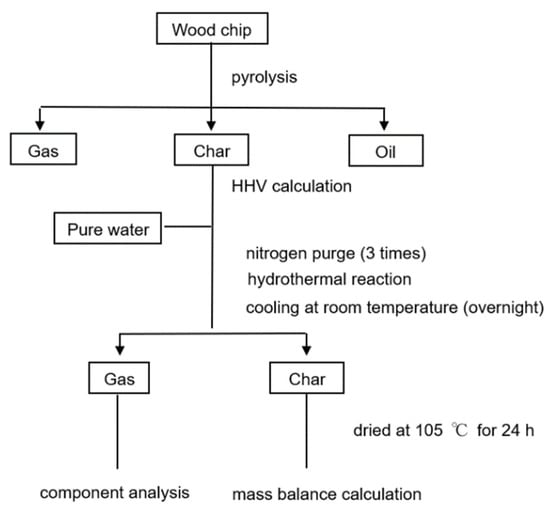
Figure 4.
Experimental flow of combined experiment.
The mass balance calculation is expressed by Equations (1)–(3) [15]. After filtering and drying the char, the dry char mass was calculated as the total mass after drying minus the dry filter paper mass. The total liquid mass (on the filter paper and char) was calculated as the char and filter mass before drying minus their masses after drying. The total liquid mass was determined by adding the mass of the separated liquid portion. According to the principle of conservation of mass, the mass of generated gas was obtained by subtracting the char and liquid mass from the sum of the total mass of pure water and biomass before treatment.
Msolid = solid mass after drying
Mliquid = measured liquid mass + solid mass before drying − Msolid
Mgas = total material mass input − Msolid − Mliquid
The concentrations of C, H and N were measured at the Institute of Creation Research (Hokkaido University, Sapporo, Japan). The oxygen concentration was calculated using Equation (4). The elemental analysis and the DuLong formula (Equation (5)) [16] were used to calculate the HHV. The HHV is an important property to define the energy content of the fuel [7].
The gas concentration was measured by a GC4000 (GL Sciences Inc., Tokyo, Japan). The concentrations of CO, CO2 and CH4 were measured by an FID detector, using H2 as a carrier gas. H2 was measured by a thermal conductivity detector. All experiments were repeated once, and the measurement and calculation results were averaged.
3. Results and Discussion
3.1. Group 1
3.1.1. Mass Balance
Group 1 was used to determine the optimal condition for hydrogen production. The mass balance results are provided in Figure 5. As the treatment temperature increased from 300 °C to 380 °C, the yield of liquid decreased significantly and the gas yield increased. A high temperature is required to promote gas production because extensive biomass depolymerization occurs when the temperature is sufficient to destroy the bonds and the concentration of free radicals increases [2]. As the free radical reaction was promoted, the gas yield increased and the oil yield decreased [17,18,19]. The char showed a more stable yield with a change in temperature. As the temperature increased, the solid-phase yield decreased. The solid phase included ash, unreacted biomass and biochar that was generated by the hydrothermal reaction [7,20]. As the temperature increased, biomass decomposition was promoted, which converted biomass to gas, oil and biochar, and resulted in a decrease in total solid yield.
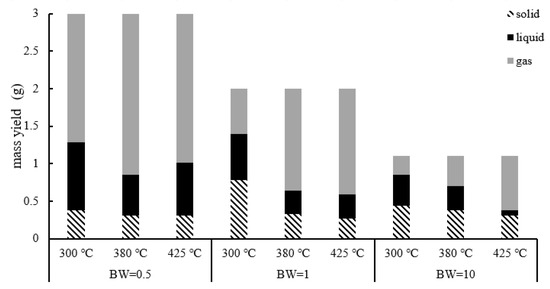
Figure 5.
Mass balance of group 1.
In addition, for the experiment with a B/W ratio of 10, an interesting result could be observed. The yield of the liquid first decreased as the temperature rose, and after a reaction temperature over 380 °C, it increased again. As shown in Figure 6, most of the liquid products came from ionic reactions. In the experiment with a B/W ratio of 0.1, the ion product in the water increased due to the increasing temperature, which promoted the ionic reaction and increased the generation rate of the liquid phase. When exceeding 380 °C, the generation rate of the liquid phase exceeded the consumption rate, resulting in an increase in the yield of the liquid phase. In the experiments of B/W ratios of 1 and 0.1, the specific gravity of water was relatively low, which led the rate increase of the ionic reaction to be insignificant, meaning the liquid yield did not increase even after the temperature rose above 380 °C.
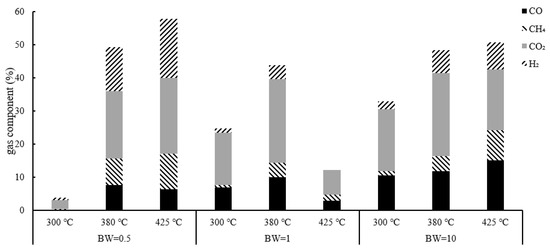
Figure 6.
Gas component analysis result of group 1.
3.1.2. Gas Component Analysis
The gas components were analyzed by gas chromatography. Figure 6 shows that a higher temperature could yield a higher gas concentration. Except for hydrogen, methane, carbon monoxide and carbon dioxide, the remaining gases were almost all nitrogen. The principle of HTG for lignocellulosic biomass is outlined in Figure 7 [21,22,23,24]. Temperature is the most important parameter in the hydrothermal reaction. Temperature affects the rate of radical reaction, and indirectly, it affects the rate of ionic reaction by affecting the ionic product and permittivity of the solvent [25,26,27,28]. The ion product is greatest at 300 °C [29], which means that the ionic reaction is promoted; cellulose, hemicellulose and lignin are decomposed to produce char and tar. When the HTG temperature exceeds 300 °C, the ion product decreases with an increase in temperature, which means that the free-radical reaction becomes dominant. The biomass decomposes into low-molecular-mass materials, such as aldehydes and ketones, and produces gas. Because this research was carried out above 300 °C, the ion product decreased with an increase in temperature and the free-radical reaction was promoted, which resulted in increased gas production.
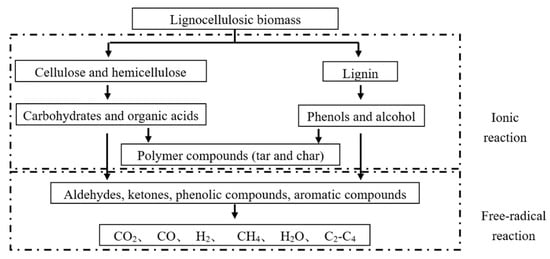
Figure 7.
HTG mechanism of lignocellulosic biomass.
The hydrogen concentration increased with an increase in temperature because of the promoting effect on the water–gas shift reaction and the steam-reforming reaction (Equations (6) and (7)) [8]. An increase in temperature also promoted methane production. The reactions that produced methane were mainly methanation (Equations (8) and (9)) and hydrogenation (Equation (10)) reactions. Although the reaction was exothermic, methane was produced even above 425 °C [21].
A larger water input resulted in a higher hydrogen concentration. Approximately half of the hydrogen was produced by the water–gas shift reaction [28,29]. Most water-producing reactions consume water. Therefore, the increase in water input promoted hydrogen production. The concentration of carbon monoxide increased as the B/W ratio increased. The water–gas shift reaction had a large effect on HTG. A larger water input resulted in more carbon monoxide conversion to hydrogen. Among the given conditions, the most suitable condition for producing hydrogen was 425 °C and a B/W ratio of 0.5.
3.2. Group 2
① Pyrolysis
3.2.1. Mass Balance
The continuous pyrolysis test treated 488 g wood chips in 700 °C over 8 h. The mass balance is shown in Figure 8. Approximately half of the wood chips were converted to biochar.
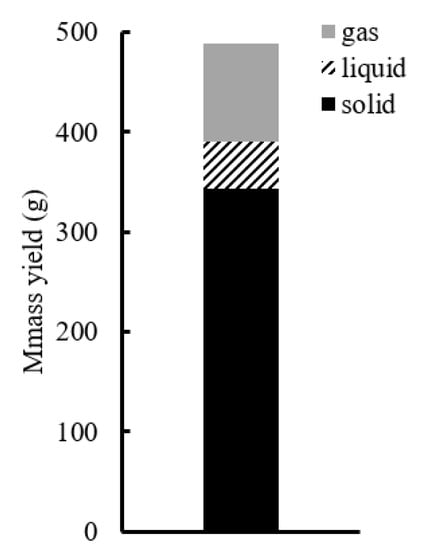
Figure 8.
Mass balance of pyrolysis.
3.2.2. Elemental Analysis and HHV Calculation
Table 4 shows the elemental analysis of the biochar from pyrolysis. The HHV was 26.26 MJ/kg, which is higher than lignite coal (15–20 MJ/kg) [30].

Table 4.
Elemental analysis of biochar.
② HTG
3.2.3. Mass Balance
The temperature and pressure change curves of HTG using biochar as a raw material are shown in Figure 9. From 15 min, the pure water temperature exceeded the boiling point, steam was generated and the pressure began to increase. Considering the HTG mass balance, as shown in Figure 10, temperature appeared to have little effect on the mass balance. A comparison of the HTG mass balances of groups 1 and 2 (Figure 5) shows that the liquid yield of the biochar-based experiment increased, and the gas yield decreased. Biochar contains char, unreacted biomass and ash. Because char is difficult to decompose, the generation speed of low-molecular-mass compounds, which is required for gas production, is low. Low-molecular-mass substances react with water to generate gas. Therefore, a lower concentration of low-molecular-mass substances leads to reduced water consumption. Compared with the experiment using wood, the HTG of group 2 yielded less gas (300 °C: –27%, 380 °C: –27%, 425 °C: –24%) and more liquid (300 °C: +45%, 380 °C: +89%, 425 °C: +89%).
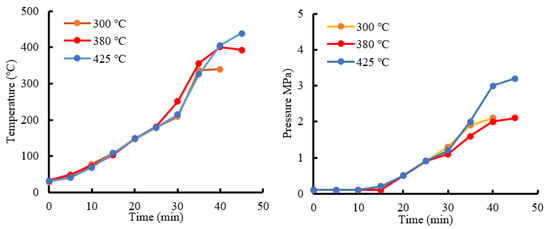
Figure 9.
Temperature–pressure changes of group 2 HTG.
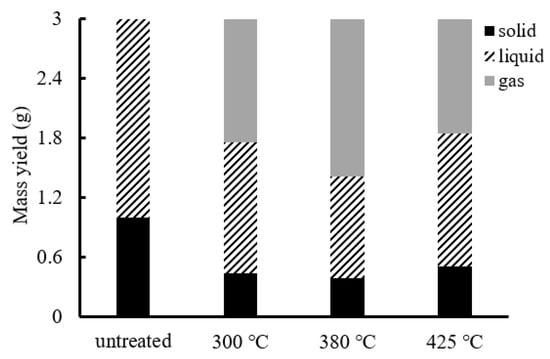
Figure 10.
Mass balance of HTG (group 2).
3.2.4. Gas Component Analysis
The gas components are shown in Figure 11. Carbon dioxide was detected in gases from HTG at 300 and 380 °C. An analysis of gas from 425 °C HTG also indicated the presence of carbon dioxide, carbon monoxide, methane and hydrogen. The same as Figure 6, except for hydrogen, methane, carbon monoxide and carbon dioxide, the remaining gases were almost all nitrogen. Because char is difficult to decompose, the rate of production of low-molecular-mass compounds required for gas production was slow. Therefore, HTG at 300 and 380 °C produced almost no gas. Above 425 °C, the char decomposed to produce low-molecular-mass compounds, such as acetic acid and HMF, which were converted to gas. The reactions that produce methane are mainly methanation (Equations (8) and (9)) and hydrogenation (Equation (10)) reactions. Hydrogen is produced mainly by the water–gas shift reaction and the steam-reforming reaction (Equations (6) and (7)). The reaction that produces carbon monoxide is the water–gas shift reaction (Equation (6)). Although it is possible to generate hydrogen from pyrolysis char by HTG, a temperature above 425 °C is required.
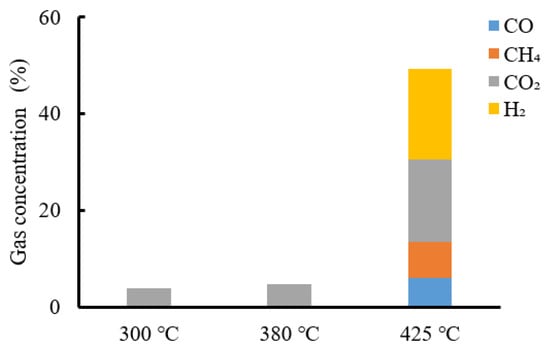
Figure 11.
Gas component analysis result of group 2.
The char obtained by pyrolysis has a higher HHV, which leads to a higher yield of hydrogen, which can be obtained by treating these chars by hydrothermal gasification. Because pyrolysis requires a lot of energy, we do not recommend only adding pyrolysis as a pre-treatment for producing hydrogen; also, phenolic components and tar become causes of trouble and blockage in the single continuous pyrolysis system. However, since the purpose of pyrolysis is mostly bio-oil production, the treatment of the remaining char will be very important. We believe hydrothermal gasification is a premium method of treating char from the pyrolysis of biomass.
4. Conclusions
This study compared the use of wood chip or pyrolytic char as the feedstock to examine optimal HTG conditions for hydrogen generation. The temperature of HTG affects the concentration of each gas. A higher temperature promotes hydrogen and methane production as it favors the water–gas shift, methanation and hydrogenation reactions. Because of the effect of the B/W ratio, a larger water input yielded a higher hydrogen concentration. Of the given conditions, the most appropriate conditions to produce hydrogen are 425 °C and a B/W value of 0.5. It is possible to treat biochar with HTG to produce carbon monoxide and hydrogen. A temperature greater than 425 °C is found to be required; biochar is a stable product. The present research proposes that hydrogen is generated from biochar by combining continuous pyrolysis and HTG.
From hydrothermal gasification at B/W ratio 0.5 and 425 °C, the gas obtained has almost the same composition as that from biomass (H2 17.85%, CO2 22.96%, CH4 10.61%, CO 6.42%) and biochar (H2 18.73%, CO2 17.12%, CH4 7.53%, CO 5.97%). Rather than using biochar, we recommend using biomass as the raw material for HTG to produce hydrogen. From the perspective of energy production, although the same concentration of hydrogen can be extracted from the unit weight of biochar and biomass, the gas yield of HTG using biomass as a raw material (2 g) is higher than that of biochar (1.2 g). In addition, in the pyrolysis experiment of this study, only about 3.5 g of biochar could be extracted from 5 g of biomass. This means that in order to produce the same amount of hydrogen, pyrolysis-HTG treatment would consume more biomass than the HTG treatment. Even though pyrolysis also produces some by-products with higher HHV, we believe that the pyrolysis-HTG reaction is disadvantageous compared with the HTG reaction.
However, the research provides a new idea for the treatment of biochar in thermal decomposition, which is the utilization of HTG to treat biochar to produce hydrogen. This method can not only obtain a higher concentration of hydrogen but also reduce the emission of harmful gases such as SO2, which is environmentally friendly.
Author Contributions
Conceived and performed experiments, analyzed data, prepared the manuscript draft, writing, B.Z.; Conceptualization, methodology, review, editing, supervision, N.S. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data on the compounds are available from the authors.
Acknowledgments
The continuous pyrolysis reactor was provided by Mitsui Chemical, Hokkaido. We thank Laura Kuhar, from Edanz (https://www.edanz.com/ac; accessed date 4 June 2021), for editing a draft of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yokoyama, S.; Imou, K. Biomass Energy; Morikita Publishing Co., Ltd.: Tokyo, Japan, 2009. [Google Scholar]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Sugisaki, H. Survey on Trends in Utilization of Woody Biomass Energy. Wood Inform. 2017, 311, 17–20. [Google Scholar]
- Kambara, S.; Moritomi, H. Behavior of tar in pyrolysis and gasification reactions of biomass (Special feature: New development of biomass technology). Kagaku Kogyo 2009, 54, 231–235. [Google Scholar]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomass pyrolysis—A technological review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Optimization and modeling of process parameters during HTG of biomass model compounds to generate hydrogen-rich gas products. Int. J. Hydrogen Energy 2020, 45, 18275–18288. [Google Scholar] [CrossRef]
- Navarro, R.M.; Pena, M.A.; Fierro, J.L.G. Hydrogen production reactions from carbon feedstocks: Fossil fuels and biomass. Chem. Rev. 2007, 107, 3952–3991. [Google Scholar] [CrossRef] [PubMed]
- JO’M, B. The origin of ideas on a hydrogen economy and its solution to the decay of the environment. Int. J. Hydrogen Energy 2002, 27, 731–740. [Google Scholar] [CrossRef]
- Ogden, J.M. Testimony to the Committee on Science; US House of Representatives: Washington, DC, USA, 2003.
- Li, J.; Yang, G.; Yoneyama, Y.; Vitidsant, T.; Tsubaki, N. Jet fuel synthesis via Fischer—Tropsch synthesis with varied 1-olefins as additives using Co/ZrO2–SiO2 bimodal catalyst. Fuel 2016, 171, 159–166. [Google Scholar] [CrossRef]
- Ishihara, D.; Kin, G.; Tsubaki, N. Development of Bimodal Catalysts for Jet-fuel Synthesis via Fischer-Tropsch Synthesis. J. Jpn. Inst. Energy 2014, 93, 113–118. [Google Scholar] [CrossRef][Green Version]
- Li, J.; He, Y.; Tan, L.; Zhang, P.; Peng, X.; Oruganti, A.; Yang, G.; Abe, H.; Wang, Y.; Tsubaki, N. Integrated tuneable synthesis of liquid fuels via Fischer—Tropsch technology. Nat. Catalysis 2018, 1, 787–793. [Google Scholar] [CrossRef]
- Ishibashi, Y. The latest situation and issues of integrated coal gasification combined cycle (IGCC). J. At. Energy Soc. Jpn. 2015, 57, 530–534. [Google Scholar] [CrossRef][Green Version]
- Shimizu, N.; Zeng, B.; Kushima, K. Hydrothermal liquefaction of wood chips under supercritical and subcritical water reaction conditions. SN Appl. Sci. 2021, 3, 577. [Google Scholar] [CrossRef]
- Mohammed, I.S.; Na, R.; Kushima, K.; Shimizu, N. Investigating the effect of processing parameters on the products of hydrothermal carbonization of corn stover. Sustainability 2020, 12, 5100. [Google Scholar] [CrossRef]
- Kruse, A.; Gawlik, A. Biomass conversion in water at 330–410 C and 30–50 MPa. Identification of key compounds for indicating different chemical reaction pathways. Ind. Eng. Chem. Res. 2003, 42, 267–279. [Google Scholar] [CrossRef]
- Promdej, C.; Matsumura, Y. Temperature effect on hydrothermal decomposition of glucose in sub-and supercritical water. Ind. Eng. Chem. Res. 2011, 50, 8492–8497. [Google Scholar] [CrossRef]
- Angın, D. Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Li, L.I.N.; Zhang, H. Production and characterization of pyrolysis oil from herbaceous biomass (Achnatherum splendens). Energy Sources 2005, 27, 319–326. [Google Scholar] [CrossRef]
- Álvarez-Murillo, A.; Ledesma, B.; Román, S.; Sabio, E.; Gañán, J. Biomass pyrolysis toward hydrocarbonization. Influence on subsequent steam gasification processes. J. Anal. Appl. Pyrolysis 2015, 113, 380–389. [Google Scholar] [CrossRef]
- Sivasangar, S.; Zainal, Z.; Salmiaton, A.; Taufiq, Y.H. Supercritical water gasification of empty fruit bunches from oil palm for hydrogen production. Fuel 2015, 143, 563–569. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. Biochar as an exceptional bioresource for energy, agronomy, carbon sequestration, activated carbon and specialty materials. Waste Biomass Valorization 2016, 7, 201–235. [Google Scholar] [CrossRef]
- De Caprariis, B.; Filippis, A.; Scarsella, M. Hydrothermal liquefaction of biomass: Influence of temperature and biomass composition on the bio-oil production. Fuel 2017, 208, 618–625. [Google Scholar] [CrossRef]
- Möller, M.; Nilges, P.; Harnisch, F.; Schröder, U. Subcritical water as reaction environment: Fundamentals of hydrothermal biomass transformation. ChemSusChem 2011, 4, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.B.; Chahine, R. Challenges for renewable hydrogen production from biomass. Int. J. Hydrogen Energy 2010, 35, 4962–4969. [Google Scholar] [CrossRef]
- Gong, M.; Nanda, S.; Hunter, H.N.; Zhu, W.; Dalai, A.K.; Koninski, J.A. Lewis acid catalyzed gasification of humic acid in supercritical water. Catal. Today 2017, 291, 13–23. [Google Scholar] [CrossRef]
- Azadi, P.; Afif, E.; Azadi, F.; Farnood, R. Screening of nickel catalysts for selective hydrogen production using supercritical water gasification of glucose. Green Chem. 2012, 14, 1766–1777. [Google Scholar] [CrossRef]
- Yanik, J.; Ebale, S.; Kruse, A.; Sagalam, M.; Yüksel, M. Biomass gasification in supercritical water: Part 1. Effect of the nature of biomass. Fuel 2007, 86, 2410–2415. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Hydrothermal carbonization of anaerobically digested sludge for solid fuel production and energy recovery. Fuel 2014, 130, 120–125. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).