Artificial Neural Networks for Predicting Hydrogen Production in Catalytic Dry Reforming: A Systematic Review
Abstract
1. Introduction
2. Search Strategy and the Protocol of the Study
2.1. Search Strategy
2.2. Inclusion Criteria and Data Extraction
2.3. Artificial Neural Network Modeling
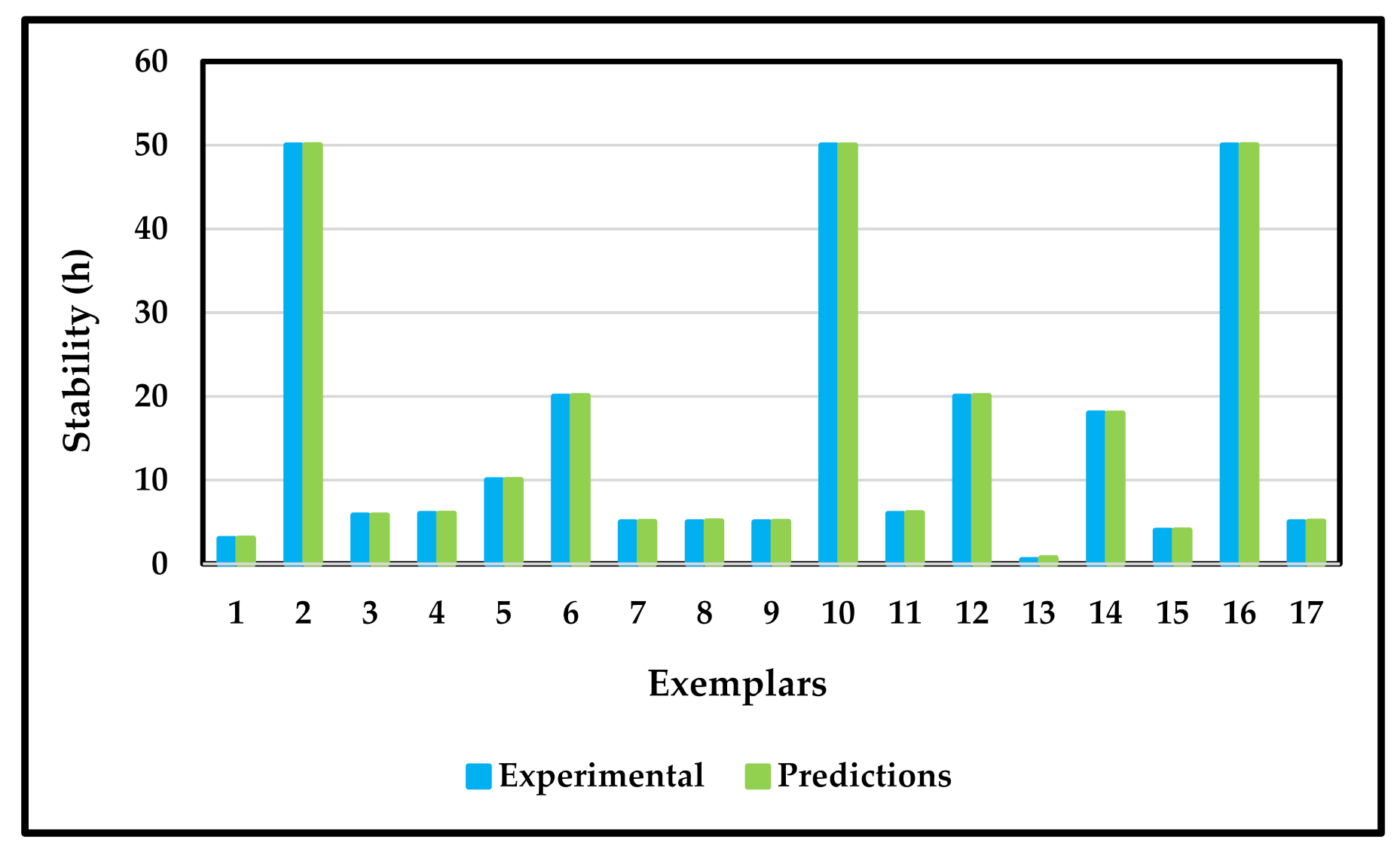
3. Results and Discussion
3.1. Data Accessibility
3.2. Artificial Neural Network Modeling
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tran, N.T.; Pham, T.L.M.; Nguyen, T.D.; Van Cuong, N.; Siang, T.J.; Phuong, P.T.; Jalil, A.; Truong, Q.D.; Abidin, S.Z.; Hagos, F.Y.; et al. Improvements in hydrogen production from methane dry reforming on filament-shaped mesoporous alumina-supported cobalt nanocatalyst. Int. J. Hydrog. Energy 2020. [Google Scholar] [CrossRef]
- Judd, S.; Al Momani, F.; Znad, H.; Al Ketife, A. The cost benefit of algal technology for combined CO2 mitigation and nutrient abatement. Renew. Sustain. Energy Rev. 2017, 71, 379–387. [Google Scholar] [CrossRef]
- Almomani, F.; Al Ketife, A.; Judd, S.; Shurair, M.; Bhosale, R.R.; Znad, H.; Tawalbeh, M. Impact of CO2 concentration and ambient conditions on microalgal growth and nutrient removal from wastewater by a photobioreactor. Sci. Total Environ. 2019, 662, 662–671. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, G.; Xu, Y.; Zhang, R. Dry reforming of methane over Co-Ce-M/AC-N catalyst: Effect of promoters (Ca and Mg) and preparation method on catalytic activity and stability. Int. J. Hydrog. Energy 2019, 44, 22972–22982. [Google Scholar] [CrossRef]
- Abdullah, B.; Ghani, N.A.A.; Vo, D.-V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef]
- Oliveira, A.A.S.; Medeiros, R.L.; Figueredo, G.P.; Macedo, H.P.; Braga, R.M.; Maziviero, F.V.; Melo, M.A.; Melo, D.M.; Vieira, M.M. One-step synthesis of LaNiO3 with chitosan for dry reforming of methane. Int. J. Hydrog. Energy 2018, 43, 9696–9704. [Google Scholar] [CrossRef]
- Almomani, F.; Al-Jaml, K.L.; Bhosale, R.R. Solar photo-catalytic production of hydrogen by irradiation of cobalt co-doped TiO2. Int. J. Hydrog. Energy 2021, 46, 12068–12081. [Google Scholar] [CrossRef]
- Bhosale, R.; Kumar, A.; Almomani, F.; Ghosh, U.; Anis, M.S.; Kakosimos, K.; Shende, R.; Rosen, M.A. Solar Hydrogen Production via a Samarium Oxide-Based Thermochemical Water Splitting Cycle. Energies 2016, 9, 316. [Google Scholar] [CrossRef]
- Lee, B.; Kim, H.; Lee, H.; Byun, M.; Won, W.; Lim, H. Technical and economic feasibility under uncertainty for methane dry reforming of coke oven gas as simultaneous H2 production and CO2 utilization. Renew. Sustain. Energy Rev. 2020, 133, 110056. [Google Scholar] [CrossRef]
- Ali, S.; Khader, M.M.; Almarri, M.J.; Abdelmoneim, A.G. Ni-based nano-catalysts for the dry reforming of methane. Catal. Today 2020, 343, 26–37. [Google Scholar] [CrossRef]
- Abd Ghani, N.A.; Azapour, A.; Syed Muhammad, A.F.A.; Abdullah, B. Dry reforming of methane for hydrogen production over NiCo catalysts: Effect of NbZr promoters. Int. J. Hydrog. Energy 2019, 44, 20881–20888. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, J.; Liu, J.; Wang, C.; Huang, Q.; Xu, X.; Peng, H.; Liu, W.; Wang, X.; Zhou, W. Methane dry reforming over Ni/Mg-Al-O: On the significant promotional effects of rare earth Ce and Nd metal oxides. J. CO2 Util. 2018, 25, 242–253. [Google Scholar] [CrossRef]
- Pizzolitto, C.; Pupulin, E.; Menegazzo, F.; Ghedini, E.; Di Michele, A.; Mattarelli, M.; Cruciani, G.; Signoretto, M. Nickel based catalysts for methane dry reforming: Effect of supports on catalytic activity and stability. Int. J. Hydrog. Energy 2019, 44, 28065–28076. [Google Scholar] [CrossRef]
- Mohd Arif, N.N.; Abidin, S.Z.; Osazuwa, O.U.; Vo, D.-V.N.; Azizan, M.T.; Taufiq-Yap, Y.H. Hydrogen production via CO2 dry reforming of glycerol over ReNi/CaO catalysts. Int. J. Hydrog. Energy 2019, 44, 20857–20871. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Di Bartolomeo, E. Co and Ni supported on CeO2 as selective bimetallic catalyst for dry reforming of methane. Int. J. Hydrog. Energy 2012, 37, 15992–15999. [Google Scholar] [CrossRef]
- Andraos, S.; Abbas-Ghaleb, R.; Chlala, D.; Vita, A.; Italiano, C.; Laganà, M.; Pino, L.; Nakhl, M.; Specchia, S. Production of hydrogen by methane dry reforming over ruthenium-nickel based catalysts deposited on Al2O3, MgAl2O4, and YSZ. Int. J. Hydrog. Energy 2019, 44, 25706–25716. [Google Scholar] [CrossRef]
- Shang, Z.; Li, S.; Li, L.; Liu, G.; Liang, X. Highly active and stable alumina supported nickel nanoparticle catalysts for dry reforming of methane. Appl. Catal. B Environ. 2017, 201, 302–309. [Google Scholar] [CrossRef]
- Akiki, E.; Akiki, D.; Italiano, C.; Vita, A.; Abbas-Ghaleb, R.; Chlala, D.; Ferrante, G.D.; Laganà, M.; Pino, L.; Specchia, S. Production of hydrogen by methane dry reforming: A study on the effect of cerium and lanthanum on Ni/MgAl2O4 catalyst performance. Int. J. Hydrog. Energy 2020, 45, 21392–21408. [Google Scholar] [CrossRef]
- Drif, A.; Bion, N.; Brahmi, R.; Ojala, S.; Pirault-Roy, L.; Turpeinen, E.; Seelam, P.K.; Keiski, R.L.; Epron, F. Study of the dry reforming of methane and ethanol using Rh catalysts supported on doped alumina. Appl. Catal. A Gen. 2015, 504, 576–584. [Google Scholar] [CrossRef]
- Son, I.H.; Lee, S.J.; Roh, H.-S. Hydrogen production from carbon dioxide reforming of methane over highly active and stable MgO promoted Co–Ni/γ-Al2O3 catalyst. Int. J. Hydrog. Energy 2014, 39, 3762–3770. [Google Scholar] [CrossRef]
- Harun, N.; Abidin, S.Z.; Osazuwa, O.U.; Taufiq-Yap, Y.H.; Azizan, M.T. Hydrogen production from glycerol dry reforming over Ag-promoted Ni/Al2O3. Int. J. Hydrog. Energy 2019, 44, 213–225. [Google Scholar] [CrossRef]
- Abdullah, N.; Ainirazali, N.; Ellapan, H. Structural effect of Ni/SBA-15 by Zr promoter for H2 production via methane dry reforming. Int. J. Hydrog. Energy 2020. [Google Scholar] [CrossRef]
- Pan, C.; Guo, Z.; Dai, H.; Ren, R.; Chu, W. Anti-sintering mesoporous Ni–Pd bimetallic catalysts for hydrogen production via dry reforming of methane. Int. J. Hydrog. Energy 2020, 45, 16133–16143. [Google Scholar] [CrossRef]
- Le, V.T.; Almomani, F.; Vasseghian, Y.; Vilas–Boas, J.A.; Dragoi, E.-N. Graphene-based nanomaterial for desalination of water: A systematic review and meta-analysis. Food Chem. Toxicol. 2021, 148, 111964. [Google Scholar] [CrossRef] [PubMed]
- Vasseghian, Y.; Rad, S.S.; Vilas–Boas, J.A.; Khataee, A. A global systematic review, meta-analysis, and risk assessment of the concentration of vanadium in drinking water resources. Chemosphere 2021, 267, 128904. [Google Scholar] [CrossRef]
- Dragoi, E.-N.; Vasseghian, Y. Modeling of mass transfer in vacuum membrane distillation process for radioactive wastewater treatment using artificial neural networks. Toxin Rev. 2020, 1–10. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Farhadi, A.; Nematollahi, A.; Vasseghian, Y.; Fakhri, Y. A systematic review and meta-analysis to investigate the concentration and prevalence of trichothecenes in the cereal-based food. Trends Food Sci. Technol. 2020, 102, 193–202. [Google Scholar] [CrossRef]
- Storn, R.; Price, K. Differential Evolution—A Simple and Efficient Heuristic for global Optimization over Continuous Spaces. J. Glob. Optim. 1997, 11, 341–359. [Google Scholar] [CrossRef]
- Fernández, J.C.; Hervás, C.; Martínez-Estudillo, F.; Gutiérrez, P.A. Memetic Pareto Evolutionary Artificial Neural Networks to determine growth/no-growth in predictive microbiology. Appl. Soft Comput. 2011, 11, 534–550. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Berkani, M.; Almomani, F.; Dragoi, E.-N. Data mining for pesticide decontamination using heterogeneous photocatalytic processes. Chemosphere 2021, 270, 129449. [Google Scholar] [CrossRef]
- Abbass, H.A. A Memetic Pareto Evolutionary Approach to Artificial Neural Networks. In AI 2001: Advances in Artificial Intelligence; Stumptner, M., Corbett, D., Brooks, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 2256, pp. 113–152. [Google Scholar]
- Volna, E. Neuroevolutionary optimization. Int. J. Comput. Sci. Issues 2010, 7, 31–37. [Google Scholar]
- Vasseghian, Y.; Moradi, M.; Pirsaheb, M.; Khataee, A.; Rahimi, S.; Badi, M.Y.; Mousavi Khaneghah, A. Pesticide decontamination using UV/ferrous-activated persulfate with the aid neuro-fuzzy modeling: A case study of Malathion. Food Res. Int. 2020, 137, 109557. [Google Scholar] [CrossRef] [PubMed]
- Moghri, M.; Dragoi, E.N.; Salehabadi, A.; Shukla, D.K.; Vasseghian, Y. Effect of various formulation ingredients on thermal characteristics of PVC/clay nanocomposite foams: Experimental and modeling. e-Polymers 2016, 17, 119–128. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Bahadori, A.; Khataee, A.; Dragoi, E.-N.; Moradi, M. Modeling the Interfacial Tension of Water-Based Binary and Ternary Systems at High Pressures Using a Neuro-Evolutive Technique. ACS Omega 2019, 5, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Vasseghian, Y.; Ahmadi, M.; Joshaghani, M. Ultrasound Assisted Ash and Sulphur Removal from Bitumen Using Column Flotation Technique: Experimental, RSM and ANN Methods in Modelling and Optimization of Process. Iran. J. Sci. Technol. Trans. A Sci. 2016, 41, 1149–1163. [Google Scholar] [CrossRef]
- Dragoi, E.-N.; Curteanu, S.; Leon, F.; Galaction, A.-I.; Cascaval, D. Modeling of oxygen mass transfer in the presence of oxygen-vectors using neural networks developed by differential evolution algorithm. Eng. Appl. Artif. Intell. 2011, 24, 1214–1226. [Google Scholar] [CrossRef]
- Drăgoi, E.-N.; Curteanu, S.; Lisa, C. A neuro-evolutive technique applied for predicting the liquid crystalline property of some organic compounds. Eng. Optim. 2012, 44, 1261–1277. [Google Scholar] [CrossRef]
- Esmaeili, A.; Hejazi, E.; Vasseghian, Y. Comparison study of biosorption and coagulation/air flotation methods for chromium removal from wastewater: Experiments and neural network modeling. RSC Adv. 2015, 5, 91776–91784. [Google Scholar] [CrossRef]
- Feoktistov, V. Differential Evolution: In Search of Solutions; Springer: Berlin, Germany, 2006. [Google Scholar]
- Vasseghian, Y.; Dragoi, E.-N. Modeling and Optimization of Acid Blue 193 Removal by UV and Peroxydisulfate Process. J. Environ. Eng. 2018, 144, 06018003. [Google Scholar] [CrossRef]
- Bian, Z.; Kawi, S. Highly carbon-resistant Ni–Co/SiO 2 catalysts derived from phyllosilicates for dry reforming of methane. J. CO2 Util. 2017, 18, 345–352. [Google Scholar] [CrossRef]
- Amin, N.A.S.; Yaw, T.C. Thermodynamic equilibrium analysis of combined carbon dioxide reforming with partial oxidation of methane to syngas. Int. J. Hydrog. Energy 2007, 32, 1789–1798. [Google Scholar] [CrossRef]
- Sokolov, S.; Kondratenko, E.V.; Pohl, M.-M.; Barkschat, A.; Rodemerck, U. Stable low-temperature dry reforming of methane over mesoporous La2O3-ZrO2 supported Ni catalyst. Appl. Catal. B Environ. 2012, 113-114, 19–30. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Z.; Jiao, Y. Dry reforming of methane towards CO-rich hydrogen production over robust supported Ni catalyst on hierarchically structured monoclinic zirconia nanosheets. Int. J. Hydrog. Energy 2016, 41, 17907–17921. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; Chen, X.; Rui, N.; Betancourt, L.E.; Lin, L.; Xu, W.; Sun, C.-J.; Abeykoon, A.M.; Rodriguez, J.A.; et al. Effects of Zr Doping into Ceria for the Dry Reforming of Methane over Ni/CeZrO2 Catalysts: In Situ Studies with XRD, XAFS, and AP-XPS. ACS Catal. 2020, 10, 3274–3284. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Zhang, X.; Wang, Z.; Wang, X.; Peng, H. Design of Ni-ZrO2@SiO2 catalyst with ultra-high sintering and coking resistance for dry reforming of methane to prepare syngas. J. CO2 Util. 2018, 27, 297–307. [Google Scholar] [CrossRef]
- Sajjadi, S.M.; Haghighi, M.; Eslami, A.A.; Rahmani, F. Hydrogen production via CO2-reforming of methane over Cu and Co doped Ni/Al2O3 nanocatalyst: Impregnation versus sol–gel method and effect of process conditions and promoter. J. Sol. Gel Sci. Technol. 2013, 67, 601–617. [Google Scholar] [CrossRef]
- Schwengber, C.A.; Da Silva, F.A.; Schaffner, R.A.; Fernandes-Machado, N.R.C.; Ferracin, R.J.; Bach, V.R.; Alves, H.J. Methane dry reforming using Ni/Al2O3 catalysts: Evaluation of the effects of temperature, space velocity and reaction time. J. Environ. Chem. Eng. 2016, 4, 3688–3695. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Ribeiro, M.C.; Ma, W.; Shafer, W.D.; Jacobs, G.; Graham, U.M.; Davis, B.H. Fischer-Tropsch synthesis: Metal-support interfacial contact governs oxygenates selectivity over CeO2 supported Pt-Co catalysts. Appl. Catal. A Gen. 2011, 393, 17–23. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Tsubaki, N.; Tan, Y.; Han, Y. Carbon dioxide reforming of methane over Ni nanoparticles incorporated into mesoporous amorphous ZrO 2 matrix. Fuel 2015, 147, 243–252. [Google Scholar] [CrossRef]
- Adamu, S.; Bawah, A.R.; Muraza, O.; Malaibari, Z.; Hossain, M.M. Effects of metal support interaction on dry reforming of methane over Ni/Ce-Al2O3 catalysts. Can. J. Chem. Eng. 2020, 98, 2425–2434. [Google Scholar] [CrossRef]
- Newnham, J.; Mantri, K.; Amin, M.H.; Tardio, J.; Bhargava, S.K. Highly stable and active Ni-mesoporous alumina catalysts for dry reforming of methane. Int. J. Hydrog. Energy 2012, 37, 1454–1464. [Google Scholar] [CrossRef]
- Li, K.; Chang, X.; Pei, C.; Li, X.; Chen, S.; Zhang, X.; Assabumrungrat, S.; Zhao, Z.-J.; Zeng, L.; Gong, J. Ordered mesoporous Ni/La2O3 catalysts with interfacial synergism towards CO2 activation in dry reforming of methane. Appl. Catal. B Environ. 2019, 259, 118092. [Google Scholar] [CrossRef]
- Huang, F.; Wang, R.; Yang, C.; Driss, H.; Chu, W.; Zhang, H. Catalytic performances of Ni/mesoporous SiO2 catalysts for dry reforming of methane to hydrogen. J. Energy Chem. 2016, 25, 709–719. [Google Scholar] [CrossRef]
- Wang, N.; Yu, X.; Wang, Y.; Chu, W.; Liu, M. A comparison study on methane dry reforming with carbon dioxide over LaNiO3 perovskite catalysts supported on mesoporous SBA-15, MCM-41 and silica carrier. Catal. Today 2013, 212, 98–107. [Google Scholar] [CrossRef]
- Zou, J.; Yang, H.; Zeng, Z.; Wu, C.; Williams, P.T.; Chen, H. Hydrogen production from pyrolysis catalytic reforming of cellulose in the presence of K alkali metal. Int. J. Hydrog. Energy 2016, 41, 10598–10607. [Google Scholar] [CrossRef]





| Target Factor | Training | Validation | Topology | |||
|---|---|---|---|---|---|---|
| MSE | ARE | MSE | ARE | |||
| Conversion | Best | 0.011 | 0.084 | 0.05 | 0.169 | 13:22:10:01 |
| Worst | 0.407 | 0.529 | 0.736 | 0.634 | 13:7:14:6:01 | |
| Average | 0.132 | 0.269 | 0.223 | 0.319 | - | |
| Stability | Best | 0.001 | 0.027 | 0.002 | 0.044 | 13:23:01:01 |
| Worst | 0.383 | 0.514 | 0.775 | 0.867 | 13:24:01 | |
| Average | 0.080 | 0.142 | 0.164 | 0.237 | - | |
| Yield | Best | 0.0001 | 0.015 | 0.001 | 0.02 | 13:18:01 |
| Worst | 0.017 | 0.111 | 0.07 | 0.234 | 13:06:01 | |
| Average | 0.009 | 0.068 | 0.023 | 0.104 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, V.T.; Dragoi, E.-N.; Almomani, F.; Vasseghian, Y. Artificial Neural Networks for Predicting Hydrogen Production in Catalytic Dry Reforming: A Systematic Review. Energies 2021, 14, 2894. https://doi.org/10.3390/en14102894
Le VT, Dragoi E-N, Almomani F, Vasseghian Y. Artificial Neural Networks for Predicting Hydrogen Production in Catalytic Dry Reforming: A Systematic Review. Energies. 2021; 14(10):2894. https://doi.org/10.3390/en14102894
Chicago/Turabian StyleLe, Van Thuan, Elena-Niculina Dragoi, Fares Almomani, and Yasser Vasseghian. 2021. "Artificial Neural Networks for Predicting Hydrogen Production in Catalytic Dry Reforming: A Systematic Review" Energies 14, no. 10: 2894. https://doi.org/10.3390/en14102894
APA StyleLe, V. T., Dragoi, E.-N., Almomani, F., & Vasseghian, Y. (2021). Artificial Neural Networks for Predicting Hydrogen Production in Catalytic Dry Reforming: A Systematic Review. Energies, 14(10), 2894. https://doi.org/10.3390/en14102894







