Thermo-Economic Analysis on Integrated CO2, Organic Rankine Cycles, and NaClO Plant Using Liquefied Natural Gas
Abstract
1. Introduction
Literature Review
- A new multigeneration system is proposed using LNG as a heat source and sink;
- Energy, exergy, and economic investigations of the new configuration system are carried out;
- Various products are generated, i.e., electrical power, heating and cooling loads, PW, hydrogen, and NaCl.
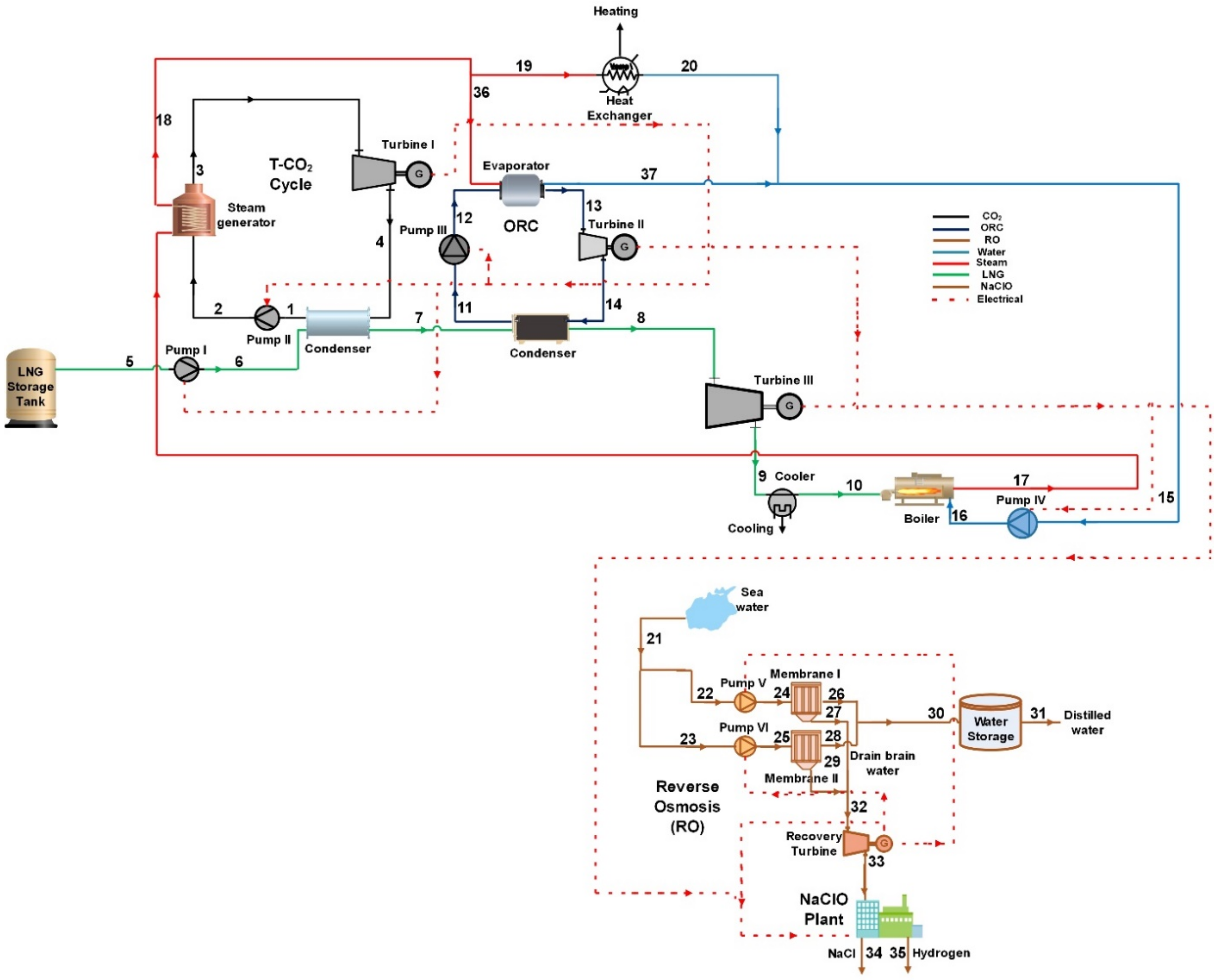
2. Materials and Methods
- (a)
- The system works in steady-state conditions;
- (b)
- The ambient pressure and temperature are 288 K and 1 bar, respectively;
- (c)
- Pressure loss in the heat exchanger is assumed to be 2%;
- (d)
- The kinetic and potential energies are ignored;
- (e)
- The pressure loss in the cycles is ignored;
- (f)
- The turbine and pump polythrophic efficiencies are assumed to be 80%;
- (g)
- The heat exchanger effectiveness factor is assumed to be 80%;
- (h)
- The salt concentration in the electrolyzer is assumed constant;
- (i)
- The inlet CO2 and LNG of the pump are in a liquid state;
- (j)
- The RO recovery ratio is 0.3.
2.1. Mathematical Modeling Approach
2.1.1. Mass and Energy Balance
2.1.2. Exergy Analysis
2.1.3. Thermo-Economic Analysis
3. Results and Discussion
3.1. Simulation Method Description
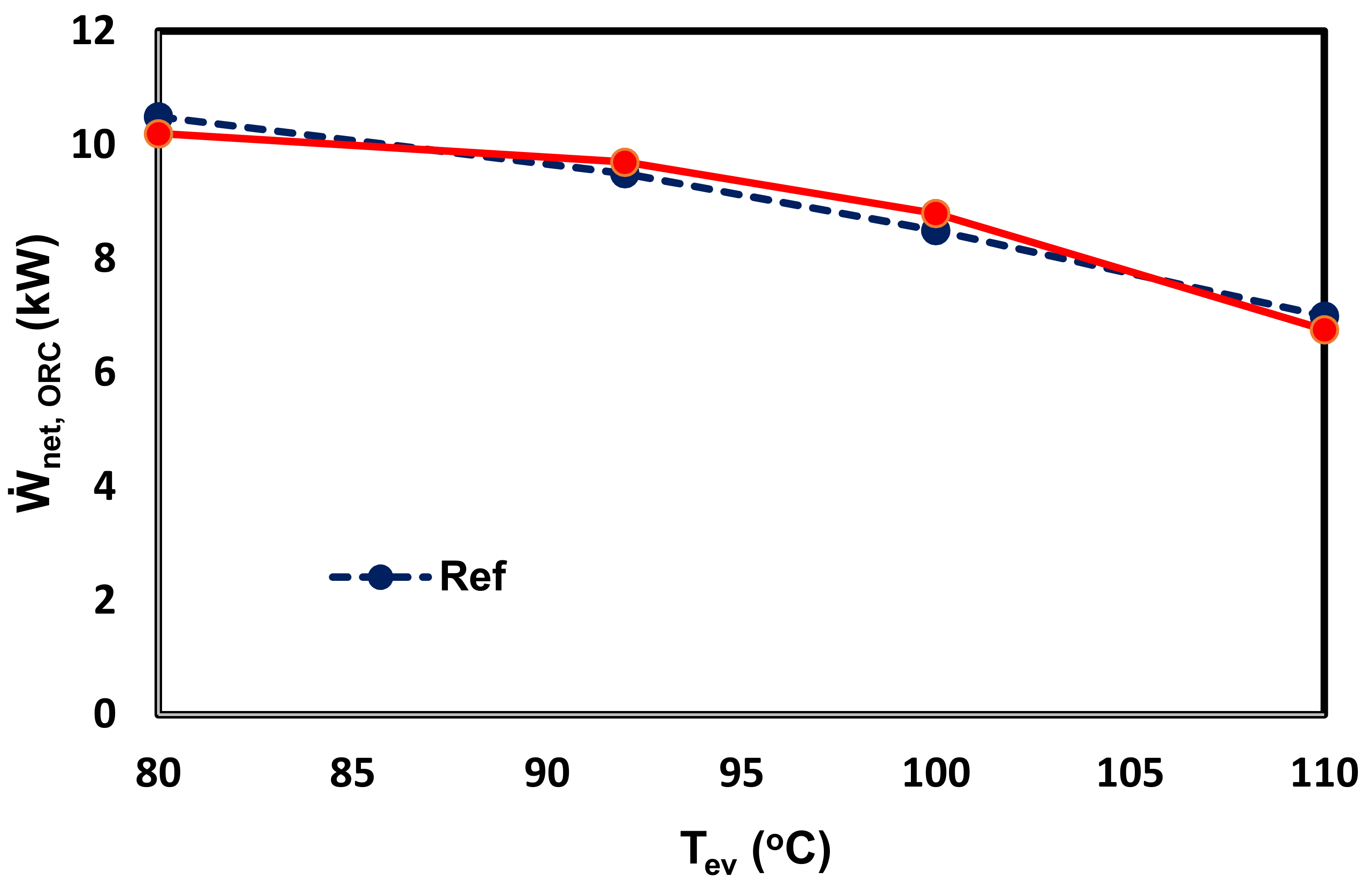
3.2. Model Validation
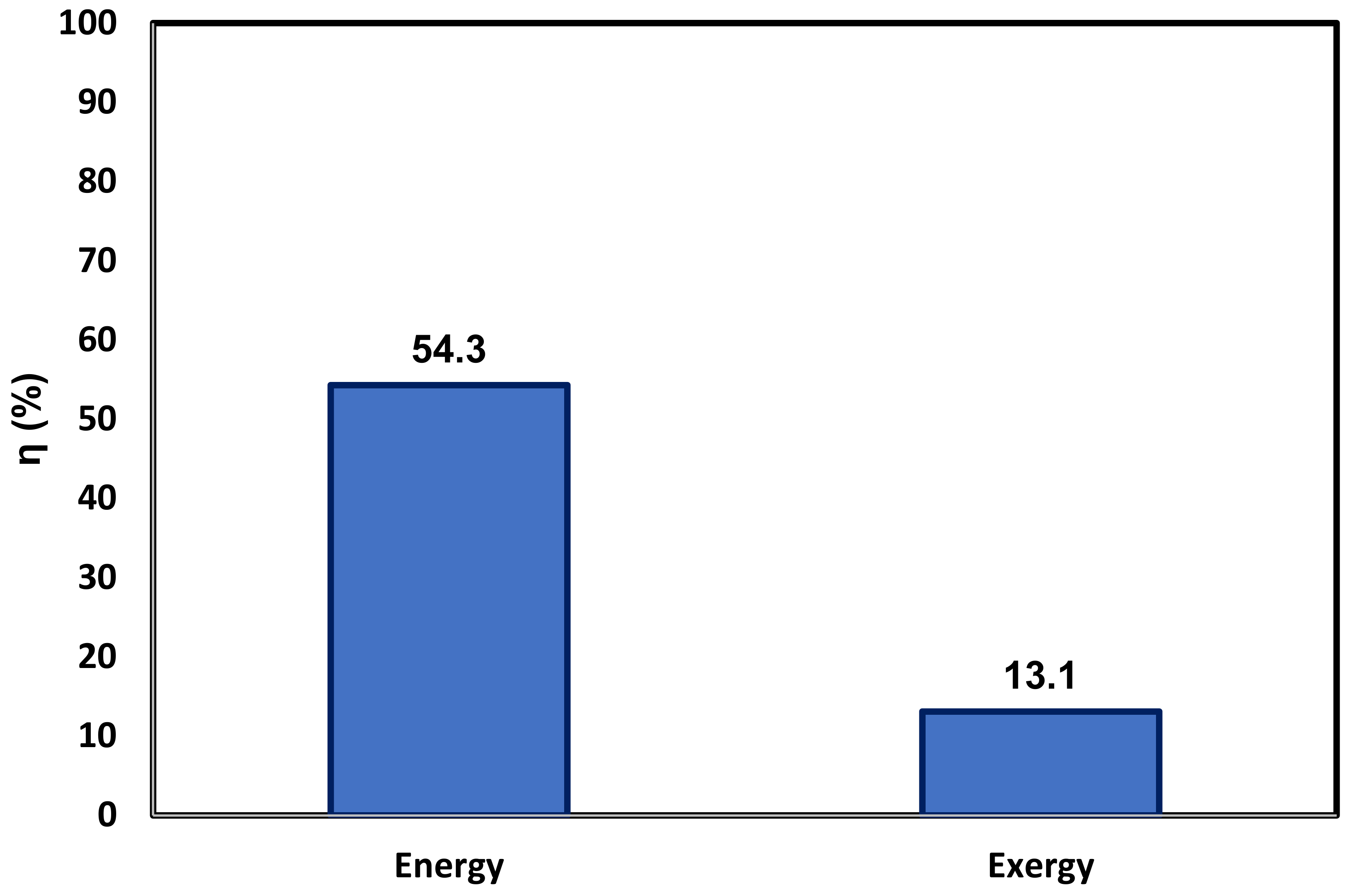
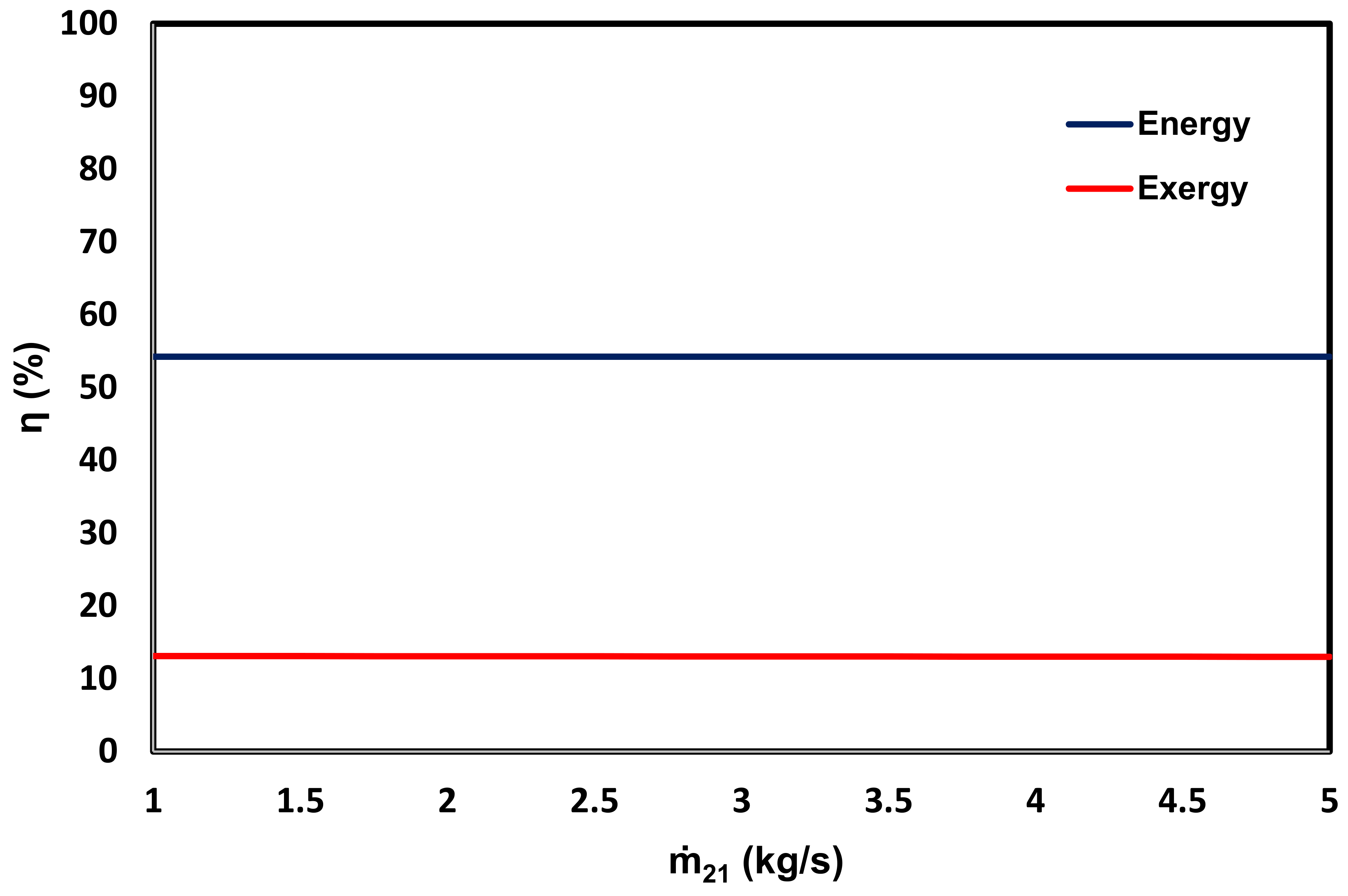
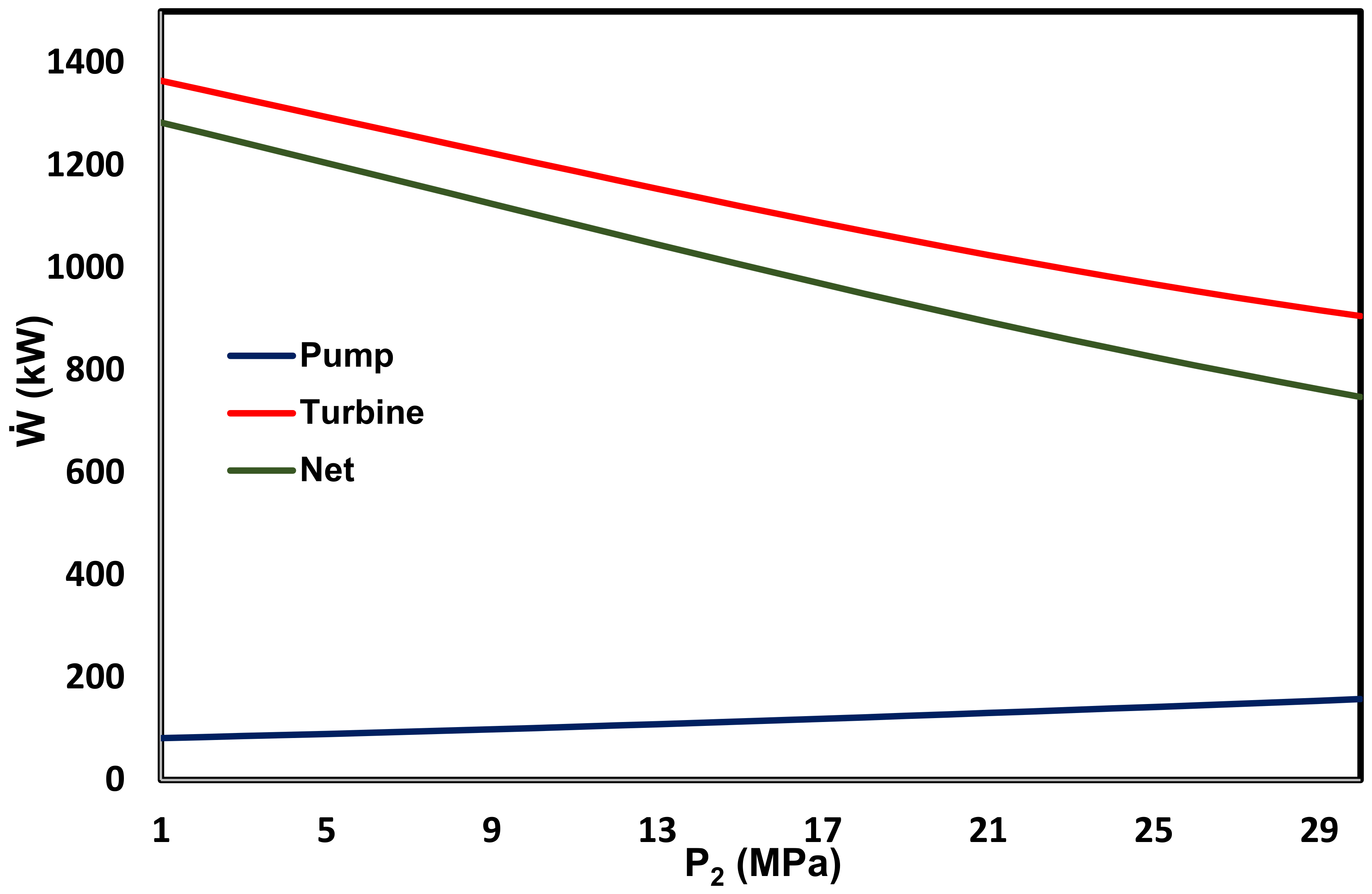
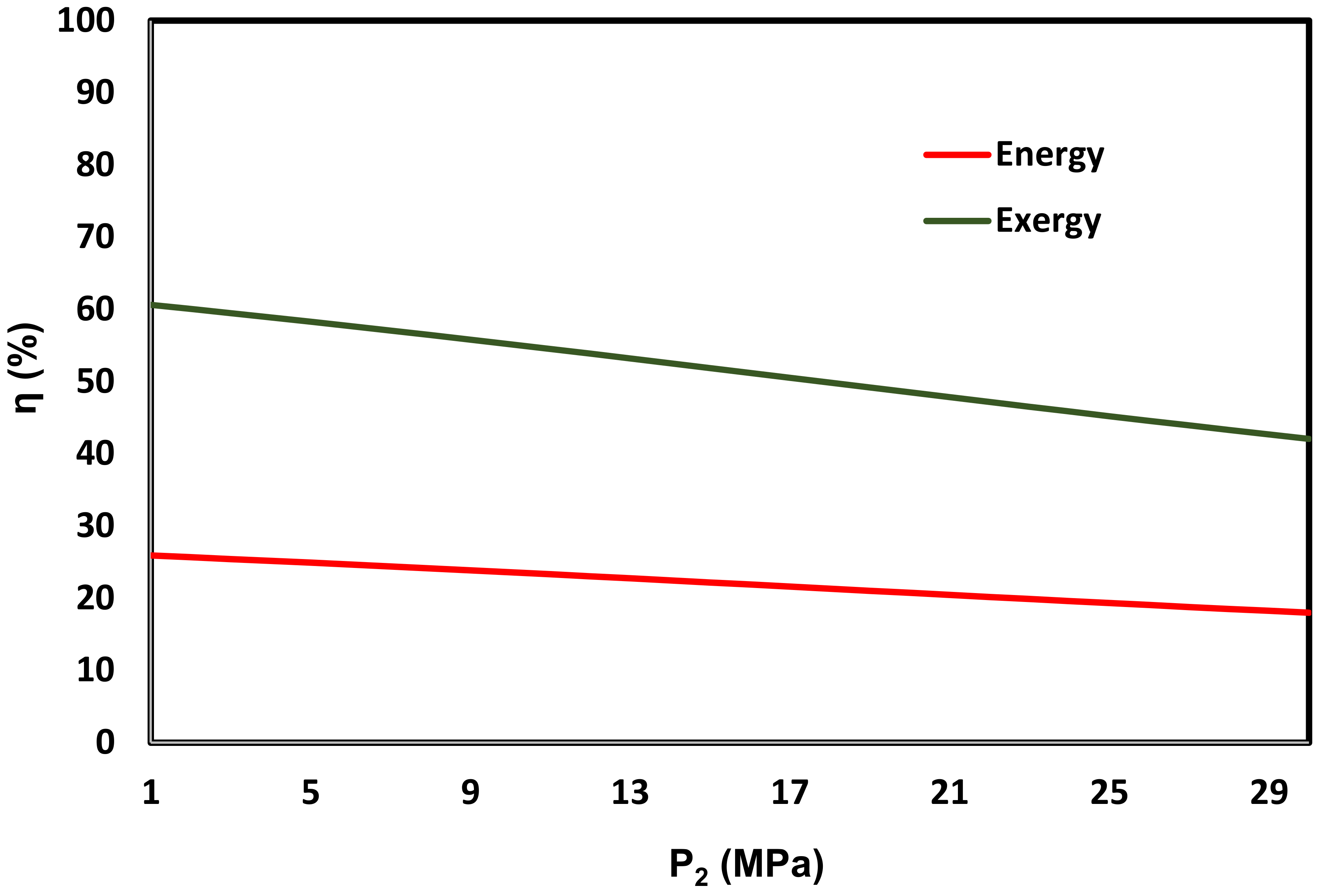
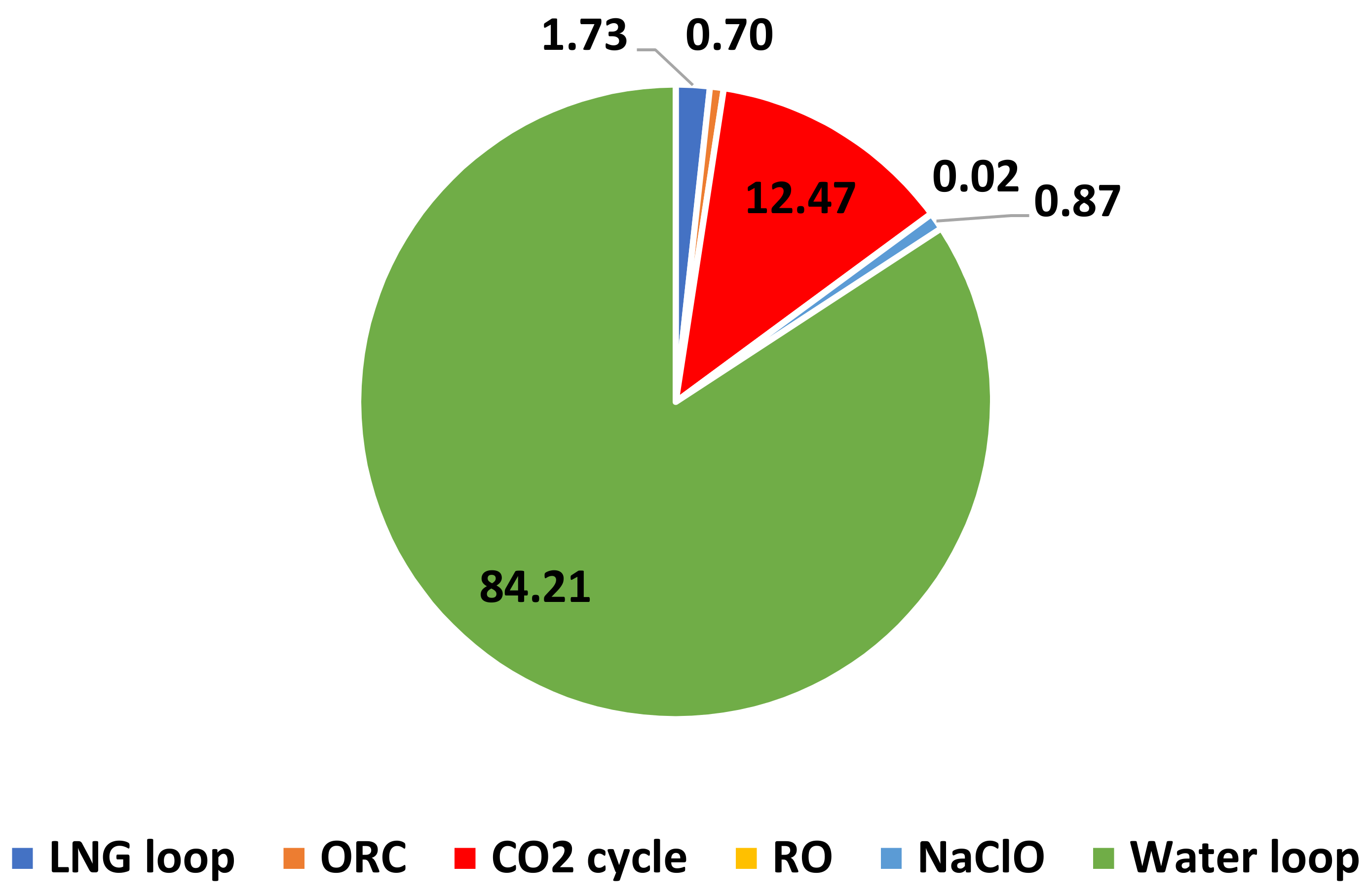
3.3. Energy and Exergy Analyses
3.4. Thermo-Economic Analyses
- (a)
- Increase in the initial cost of the NaClO and RO plants (negative effect);
- (b)
- Increase in the system product costs (NaCl, PW, and hydrogen) due to an increase in these products;
- (c)
- Decrease in the electrical power product cost due to an increase in RO and NaClO plant power consumption.
4. Conclusions
- (a)
- In comparison with a system featuring LNG only as a heat sink [14], which uses solar energy through a flat plate collector as the heat source, the system energy and exergy efficiencies are further improved from 12.4% and 4.45% to 54% and 13.1%, respectively.
- (b)
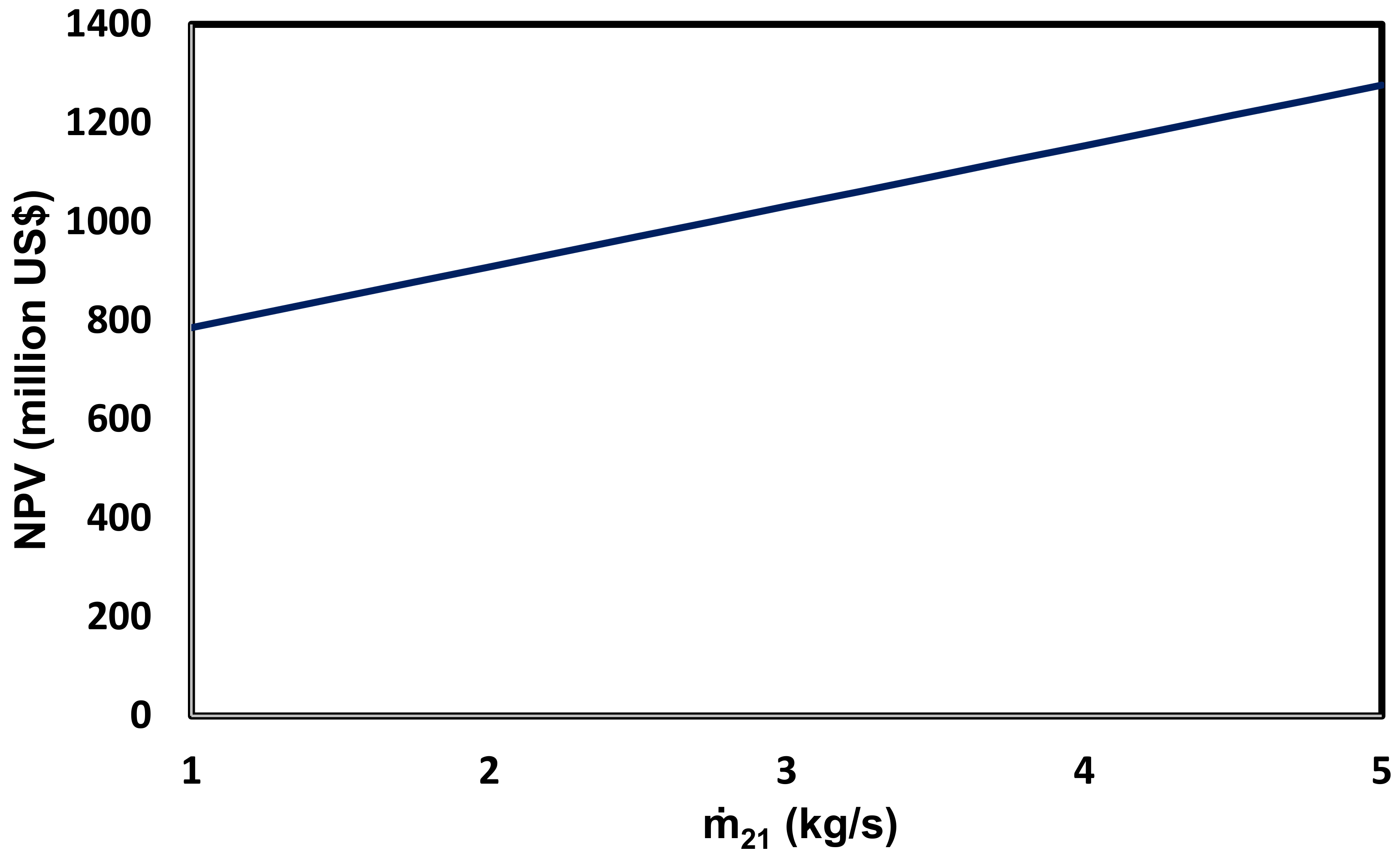
- The NPV of this system is equal to 908.9 million USD.
- (c)
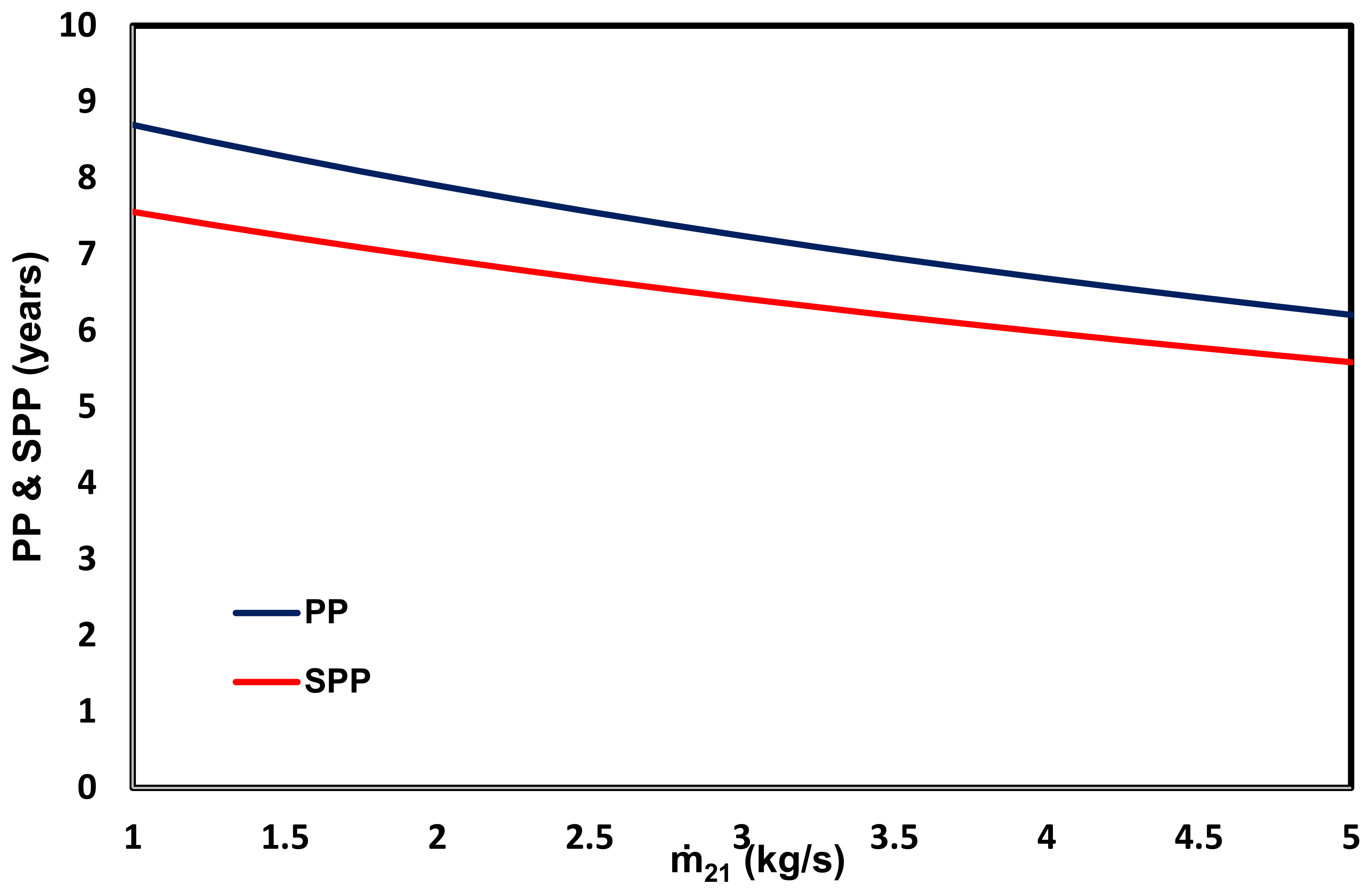
- The PP and SPP of this system are 7.9 and 6.9 years, respectively.
- (d)
- The IRR value of this system is equal to 0.138.
- (e)
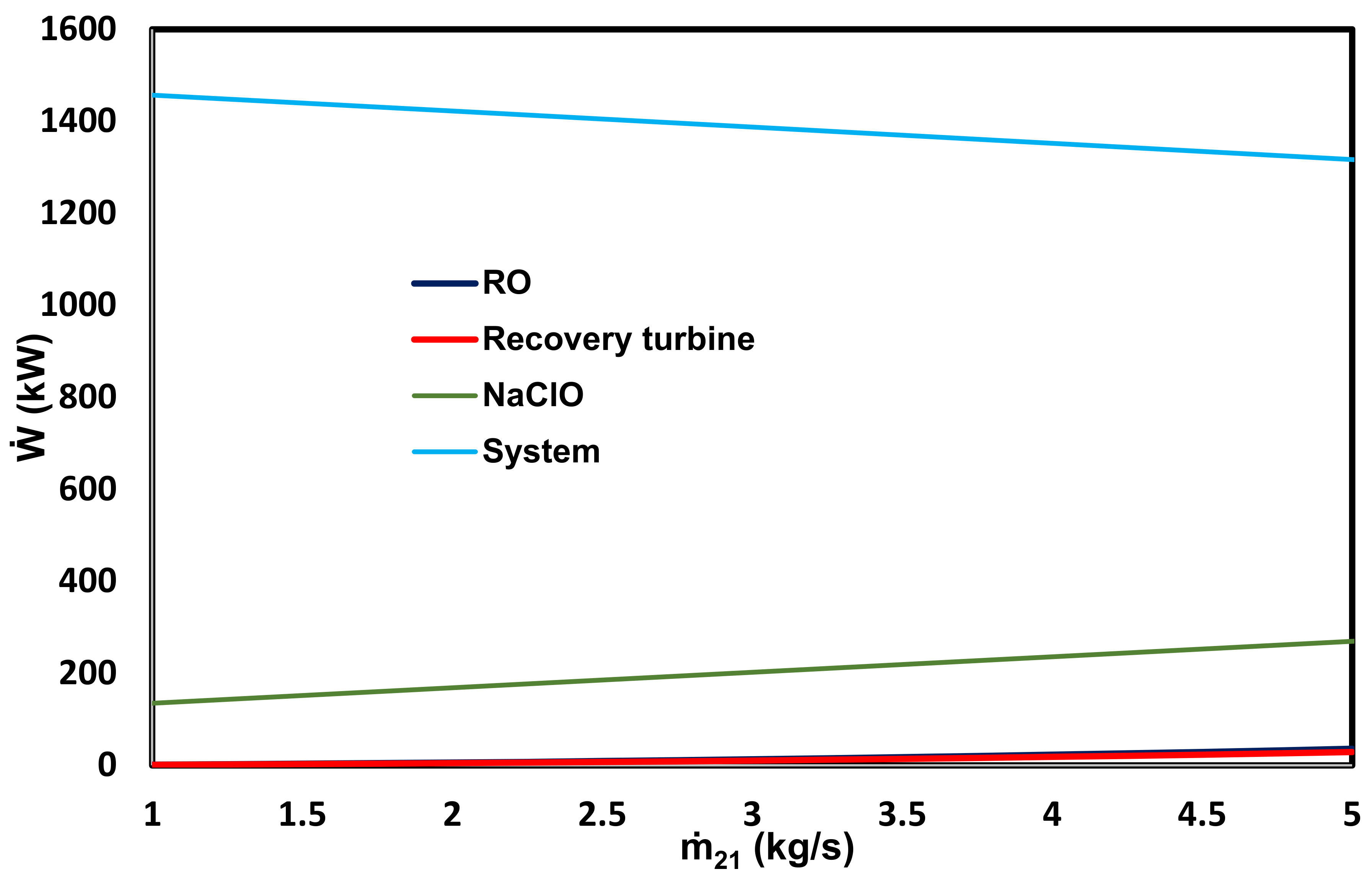
- Greater desalination of seawater to produce PW, salt, and hydrogen is beneficial according to the economic evaluation.
- (f)
- Greater seawater desalination does not have a considerable effect on the system energy and exergy efficiencies.
- (g)
- The highest and lowest contributions to the exergy destruction rates were presented by the water loop and RO system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbols (units) | Description |
| A (m2) | Area |
| (USD) | System investment cost |
| (USD) | System investment cost in a specific year |
| CF (USD) | Multigeneration annual income |
| e (kJ/kg) | Specific exergy |
| Exergy rate | |
| Ft (-) | Correction factor |
| g (m/s2) | Gravitational acceleration |
| h (kJ/kg) | Specific enthalpy |
| c (USD/kWh) | Product-specific cost |
| C (USD) | Investment cost |
| RW (1/K) | Water permeability coefficient |
| (kg/s) | Mass flow rate |
| N (years) | Project lifetime |
| P (kPa) | Pressure |
| (kW) | Heat transfer rate |
| r (-) | Discount factor |
| R (kJ/kmoleK) | Global gas constant |
| s (kJ/kgK) | Specific entropy |
| T | Temperature |
| U (W/m2K) | Overall heat transfer coefficient |
| V (m3) | Volume |
| (kW) | Power |
| x (-) | Mass fraction, concentration of salt |
| y (-) | Mole fraction |
| Y (USD/kWh, USD/kg) | Annual capacity of system production |
| Greek Symbols | Description |
| η (-) | Polythrophic efficiency |
| (Pa) | Net pressure membrane |
| Abbreviations | Description (Units) |
| IRR | Internal rate of return (-) |
| NPV | Net present value (USD) |
| PP | Payback period (-) |
| RR | Recovery ratio (years) |
| SPP | Simple payback period (kW) |
References
- Omoregie, U. After the Crash: Oil Price Recovery and LNG Project Viability. Nat. Resour. 2019, 10, 179–186. [Google Scholar] [CrossRef]
- Ehyaei, M.; Ahmadi, A.; Assad, M.E.H.; Rosen, M.A. Investigation of an integrated system combining an Organic Rankine Cycle and absorption chiller driven by geothermal energy: Energy, exergy, and economic analyses and optimization. J. Clean. Prod. 2020, 258, 120780. [Google Scholar] [CrossRef]
- Kumar, S.; Kwon, H.-T.; Choi, K.-H.; Lim, W.; Cho, J.H.; Tak, K.; Moon, I. LNG: An eco-friendly cryogenic fuel for sustainable development. Appl. Energy 2011, 88, 4264–4273. [Google Scholar] [CrossRef]
- Chen, Y.; He, L.; Guan, Y.; Lu, H.; Li, J. Life cycle assessment of greenhouse gas emissions and water-energy optimization for shale gas supply chain planning based on multi-level approach: Case study in Barnett, Marcellus, Fayetteville, and Haynesville shales. Energy Convers. Manag. 2017, 134, 382–398. [Google Scholar] [CrossRef]
- Lv, Q.; Liu, H.; Wang, J.; Liu, H.; Shang, Y. Multiscale analysis on spatiotemporal dynamics of energy consumption CO2 emissions in China: Utilizing the integrated of DMSP-OLS and NPP-VIIRS nighttime light datasets. Sci. Total. Environ. 2020, 703, 134394. [Google Scholar] [CrossRef]
- Uwitonze, H.; Han, S.; Jangryeok, C.; Hwang, K.S. Design process of LNG heavy hydrocarbons fractionation: Low LNG temperature recovery. Chem. Eng. Process. Process. Intensif. 2014, 85, 187–195. [Google Scholar] [CrossRef]
- Nakaiwa, M.; Akiya, T.; Owa, M.; Tanaka, Y. Evaluation of an energy supply system with air separation. Energy Convers. Manag. 1996, 37, 295–301. [Google Scholar] [CrossRef]
- Messineo, A.; Panno, G. LNG cold energy use in agro-food industry: A case study in Sicily. J. Nat. Gas Sci. Eng. 2011, 3, 356–363. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.-S. A conceptual demonstration of freeze desalination–membrane distillation (FD–MD) hybrid desalination process utilizing liquefied natural gas (LNG) cold energy. Water Res. 2012, 46, 4037–4052. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Woudstra, N. Understanding the exergy of cold: Theory and practical examples. Int. J. Exergy 2010, 7, 693–713. [Google Scholar] [CrossRef]
- Liu, E.; Lv, L.; Yi, Y.; Xie, P. Research on the Steady Operation Optimization Model of Natural Gas Pipeline Considering the Combined Operation of Air Coolers and Compressors. IEEE Access 2019, 7, 83251–83265. [Google Scholar] [CrossRef]
- Otsuka, T. Evolution of an LNG terminal: Senboku Terminal of Osaka gas. In Proceedings of the 23rd World Gas Conference, Amsterdam, The Netherlands, 5–9 June 2006; pp. 2617–2630. [Google Scholar]
- Li, Z.-G.; Cheng, H.; Gu, T.-Y. Research on dynamic relationship between natural gas consumption and economic growth in China. Struct. Chang. Econ. Dyn. 2019, 49, 334–339. [Google Scholar] [CrossRef]
- Naseri, A.; Bidi, M.; Ahmadi, M.H. Thermodynamic and exergy analysis of a hydrogen and permeate water production process by a solar-driven transcritical CO2 power cycle with liquefied natural gas heat sink. Renew. Energy 2017, 113, 1215–1228. [Google Scholar] [CrossRef]
- Qi, M.; Park, J.; Kim, J.; Lee, I.; Moon, I. Advanced integration of LNG regasification power plant with liquid air energy storage: Enhancements in flexibility, safety, and power generation. Appl. Energy 2020, 269, 115049. [Google Scholar] [CrossRef]
- Franco, A.; Casarosa, C. Thermodynamic analysis of direct expansion configurations for electricity production by LNG cold energy recovery. Appl. Therm. Eng. 2015, 78, 649–657. [Google Scholar] [CrossRef]
- Yu, H.; Kim, D.; Gundersen, T. A study of working fluids for Organic Rankine Cycles (ORCs) operating across and below ambient temperature to utilize Liquefied Natural Gas (LNG) cold energy. Energy 2019, 167, 730–739. [Google Scholar] [CrossRef]
- Ahmadi, A.; El Haj Assad, M.; Jamali, D.; Kumar, R.; Li, Z.; Salameh, T.; Al-Shabi, M.; Ehyaei, M. Applications of geothermal organic Rankine Cycle for electricity production. J. Clean. Prod. 2020, 274, 122950. [Google Scholar] [CrossRef]
- Ghaebi, H.; Namin, A.S.; Rostamzadeh, H. Exergoeconomic optimization of a novel cascade Kalina/Kalina cycle using geothermal heat source and LNG cold energy recovery. J. Clean. Prod. 2018, 189, 279–296. [Google Scholar] [CrossRef]
- Angelino, G.; Invernizzi, C.M. The role of real gas Brayton cycles for the use of liquid natural gas physical exergy. Appl. Therm. Eng. 2011, 31, 827–833. [Google Scholar] [CrossRef]
- Ahmadi, A.; Jamali, D.; Ehyaei, M.; Assad, M.E.H. Energy, exergy, economic and exergoenvironmental analyses of gas and air bottoming cycles for production of electricity and hydrogen with gas reformer. J. Clean. Prod. 2020, 259, 120915. [Google Scholar] [CrossRef]
- Pattanayak, L.; Padhi, B.N. Thermodynamic analysis of combined cycle power plant using regasification cold energy from LNG terminal. Energy 2018, 164, 1–9. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Mehrpooya, M.; Pourfayaz, F. Exergoeconomic analysis and multi objective optimization of performance of a Carbon dioxide power cycle driven by geothermal energy with liquefied natural gas as its heat sink. Energy Convers. Manag. 2016, 119, 422–434. [Google Scholar] [CrossRef]
- Xue, X.; Guo, C.; Du, X.; Yang, L.; Yang, Y. Thermodynamic analysis and optimization of a two-stage organic Rankine cycle for liquefied natural gas cryogenic exergy recovery. Energy 2015, 83, 778–787. [Google Scholar] [CrossRef]
- Xu, J.; Lin, W. A CO2 cryogenic capture system for flue gas of an LNG-fired power plant. Int. J. Hydrog. Energy 2017, 42, 18674–18680. [Google Scholar] [CrossRef]
- Stradioto, D.A.; Seelig, M.F.; Schneider, P.S. Reprint of: Performance analysis of a CCGT power plant integrated to a LNG regasification process. J. Nat. Gas Sci. Eng. 2015, 27, 18–22. [Google Scholar] [CrossRef]
- Shi, X.; Agnew, B.; Che, D.; Gao, J. Performance enhancement of conventional combined cycle power plant by inlet air cooling, inter-cooling and LNG cold energy utilization. Appl. Therm. Eng. 2010, 30, 2003–2010. [Google Scholar] [CrossRef]
- Atienza-Márquez, A.; Bruno, J.C.; Akisawa, A.; Coronas, A. Performance analysis of a combined cold and power (CCP) system with exergy recovery from LNG-regasification. Energy 2019, 183, 448–461. [Google Scholar] [CrossRef]
- Ayou, D.S.; Eveloy, V. Energy, exergy and exergoeconomic analysis of an ultra low-grade heat-driven ammonia-water combined absorption power-cooling cycle for district space cooling, sub-zero refrigeration, power and LNG regasification. Energy Convers. Manag. 2020, 213, 112790. [Google Scholar] [CrossRef]
- Taheri, M.; Mosaffa, A.; Farshi, L.G. Energy, exergy and economic assessments of a novel integrated biomass based multigeneration energy system with hydrogen production and LNG regasification cycle. Energy 2017, 125, 162–177. [Google Scholar] [CrossRef]
- Liu, Y.; Han, J.; You, H. Performance analysis of a CCHP system based on SOFC/GT/CO2 cycle and ORC with LNG cold energy utilization. Int. J. Hydrog. Energy 2019, 44, 29700–29710. [Google Scholar] [CrossRef]
- Naseri, A.; Bidi, M.; Ahmadi, M.H.; Saidur, R. Exergy analysis of a hydrogen and water production process by a solar-driven transcritical CO 2 power cycle with Stirling engine. J. Clean. Prod. 2017, 158, 165–181. [Google Scholar] [CrossRef]
- Ferrero García, R.; Carbia Carril, J.; Romero Gomez, J.; Romero Gomez, M. Power plant based on three series Rankine cycles combined with a direct expander using LNG cold as heat sink. Energy Convers. Manag. 2015, 101, 285–294. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, L.; Zhang, Z.; Shang, L.; Chen, S. Thermodynamic analysis of KCS/ORC integrated power generation system with LNG cold energy exploitation and CO2 capture. J. Nat. Gas Sci. Eng. 2017, 46, 188–198. [Google Scholar] [CrossRef]
- Xia, W.; Huo, Y.; Song, Y.; Han, J.; Dai, Y. Off-design analysis of a CO2 Rankine cycle for the recovery of LNG cold energy with ambient air as heat source. Energy Convers. Manag. 2019, 183, 116–125. [Google Scholar] [CrossRef]
- Behzadi, A.; Gholamian, E.; Ahmadi, P.; Habibollahzade, A.; Ashjaee, M. Energy, exergy and exergoeconomic (3E) analyses and multi-objective optimization of a solar and geothermal based integrated energy system. Appl. Therm. Eng. 2018, 143, 1011–1022. [Google Scholar] [CrossRef]
- Ghasemian, E.; Ehyaei, M.A. Evaluation and optimization of organic Rankine cycle (ORC) with algorithms NSGA-II, MOPSO, and MOEA for eight coolant fluids. Int. J. Energy Environ. Eng. 2017, 9, 39–57. [Google Scholar] [CrossRef]
- Bejan, A. Advanced Engineering Thermodynamics; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Li, Z.; Ehyaei, M.; Kamran Kasmaei, H.; Ahmadi, A.; Costa, V. Thermodynamic modeling of a novel solar powered quad generation system to meet electrical and thermal loads of residential building and syngas production. Energy Convers. Manag. 2019, 199, 111982. [Google Scholar] [CrossRef]
- Talebizadehsardari, P.; Ehyaei, M.; Ahmadi, A.; Jamali, D.; Shirmohammadi, R.; Eyvazian, A.; Ghasemi, A.; Rosen, M.A. Energy, exergy, economic, exergoeconomic, and exergoenvironmental (5E) analyses of a triple cycle with carbon capture. J. CO2 Util. 2020, 41, 101258. [Google Scholar] [CrossRef]
- Yargholi, R.; Kariman, H.; Hoseinzadeh, S.; Bidi, M.; Naseri, A. Modeling and advanced exergy analysis of integrated reverse osmosis desalination with geothermal energy. Water Sci. Technol. Water Supply 2020, 20, 984–996. [Google Scholar] [CrossRef]
- El-Dessouky, H.T.; Ettouney, H.M. Fundamentals of Salt Water Desalination; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Lazzaretto, A.; Tsatsaronis, G. SPECO: A systematic and general methodology for calculating efficiencies and costs in thermal systems. Energy 2006, 31, 1257–1289. [Google Scholar] [CrossRef]
- Bejan, A.; Tsatsaronis, G.; Moran, M. Thermal Design and Optimization; John Wiley and Sons, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Ehyaei, M.A.; Ahmadi, A.; Rosen, M.A.; Davarpanah, A. Thermodynamic Optimization of a Geothermal Power Plant with a Genetic Algorithm in Two Stages. Processes 2020, 8, 1277. [Google Scholar] [CrossRef]
- Zeinodini, M.; Aliehyaei, M. Energy, exergy, and economic analysis of a new triple-cycle power generation configuration and selection of the optimal working fluid. Mech. Ind. 2019, 20, 501. [Google Scholar] [CrossRef]
- Bellos, E.; Pavlovic, S.; Stefanovic, V.; Tzivanidis, C.; Nakomcic-Smaradgakis, B.B. Parametric analysis and yearly performance of a trigeneration system driven by solar-dish collectors. Int. J. Energy Res. 2019, 43, 1534–1546. [Google Scholar] [CrossRef]
- Tzivanidis, C.; Bellos, E.; Antonopoulos, K.A. Energetic and financial investigation of a stand-alone solar-thermal Organic Rankine Cycle power plant. Energy Convers. Manag. 2016, 126, 421–433. [Google Scholar] [CrossRef]
- Nami, H.; Ertesvåg, I.S.; Agromayor, R.; Riboldi, L.; Nord, L.O. Gas turbine exhaust gas heat recovery by organic Rankine cycles (ORC) for offshore combined heat and power applications—Energy and exergy analysis. Energy 2018, 165, 1060–1071. [Google Scholar] [CrossRef]
- Ehyaei, M.; Ahmadi, A.; Rosen, M.A. Energy, exergy, economic and advanced and extended exergy analyses of a wind turbine. Energy Convers. Manag. 2019, 183, 369–381. [Google Scholar] [CrossRef]
- Alizadeh, S.M.; Ghazanfari, A.; Ehyaei, M.A.; Ahmadi, A.; Jamali, D.H.; Nedaei, N.; Davarpanah, A. Investigation the Integration of Heliostat Solar Receiver to Gas and Combined Cycles by Energy, Exergy, and Economic Point of Views. Appl. Sci. 2020, 10, 5307. [Google Scholar] [CrossRef]
- Flinnsci. Sodium Chloride Laboratory Grade 500 g. 2020. Available online: https://www.flinnsci.com/sodium-chloride-laboratory-grade-500-g/s0063/ (accessed on 3 June 2020).
- Nami, H.; Akrami, E. Analysis of a gas turbine based hybrid system by utilizing energy, exergy and exergoeconomic methodologies for steam, power and hydrogen production. Energy Convers. Manag. 2017, 143, 326–337. [Google Scholar] [CrossRef]
- Lee, H.-J.; Yoo, S.-H.; Huh, S.-Y. Economic benefits of introducing LNG-fuelled ships for imported flour in South Korea. Transp. Res. Part D Transp. Environ. 2020, 78, 102220. [Google Scholar] [CrossRef]
- Lecompte, S.; Huisseune, H.; Broek, M.V.D.; De Schampheleire, S.; De Paepe, M. Part load based thermo-economic optimization of the Organic Rankine Cycle (ORC) applied to a combined heat and power (CHP) system. Appl. Energy 2013, 111, 871–881. [Google Scholar] [CrossRef]
- Alshammari, F.; Karvountzis-Kontakiotis, A.; Pesyridis, A.; Usman, M. Expander Technologies for Automotive Engine Organic Rankine Cycle Applications. Energies 2018, 11, 1905. [Google Scholar] [CrossRef]
- Bejan, A.; Tsatsaronis, G.; Moran, M.J. Thermal Design and Optimization; John Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- Couper, J.R.; Penney, W.R.; Fair, J.R.; Walas, S.M. (Eds.) Chapter 21—Costs of Individual Equipment. In Chemical Process Equipment, 2nd ed.; Gulf Professional Publishing: Burlington, VT, USA, 2005; pp. 719–728. [Google Scholar]
- Roosen, P.; Uhlenbruck, S.; Lucas, K. Pareto optimization of a combined cycle power system as a decision support tool for trading off investment vs. operating costs. Int. J. Therm. Sci. 2003, 42, 553–560. [Google Scholar] [CrossRef]
- Yuanda Henan China. 1000 kg/H Steam Boiler Natural Gas/LPG/CNG/LNG Fired Steam Boiler with High Quality—China Steam Boiler, Gas Fired Steam Boiler. Available online: https://yuandaboiler.en.made-in-china.com/product/oXqEAynPZOcI/China-1000kg-H-Steam-Boiler-Natural-Gas-LPG-CNG-LNG-Fired-Steam-Boiler-with-High-Quality.html (accessed on 15 June 2020).
- Du, Y.; Xie, L.; Liu, J.; Wang, Y.; Xu, Y.; Wang, S. Multi-objective optimization of reverse osmosis networks by lexicographic optimization and augmented epsilon constraint method. Desalination 2014, 333, 66–81. [Google Scholar] [CrossRef]
- Ahmadi, A.; Esmaeilion, F.; Esmaeilion, A.; Ehyaei, M.A.; Silveira, J.L. Benefits and Limitations of Waste-to-Energy Conversion in Iran. Renew. Energy Res. Appl. 2020, 1, 27–45. [Google Scholar] [CrossRef]
- Marques, R.C. Comparing private and public performance of Portuguese water services. Hydrol. Res. 2007, 10, 25–42. [Google Scholar] [CrossRef]
- Pierobon, L.; Nguyen, T.-V.; Larsen, U.; Haglind, F.; Elmegaard, B. Multi-objective optimization of organic Rankine cycles for waste heat recovery: Application in an offshore platform. Energy 2013, 58, 538–549. [Google Scholar] [CrossRef]
- Berhane, H.G.; Gonzalo, G.G.; Laureano, J.; Dieter, B. Design of environmentally conscious absorption cooling systems via multi-objective optimization and life cycle assessment. Appl. Energy 2009, 86, 1712–1722. [Google Scholar] [CrossRef]
- Shafer, T. Calculating Inflation Factors for Cost Estimates. In City of Lincoln Transportation and Utilities Project Delivery; Public Works: Lincoln, NE, USA, 2021. [Google Scholar]
- Alaloul, W.S.; Musarat, M.A.; Liew, M.; Qureshi, A.H.; Maqsoom, A. Investigating the impact of inflation on labour wages in Construction Industry of Malaysia. Ain Shams Eng. J. 2021. [Google Scholar] [CrossRef]
- Edalati, S.; Ameri, M.; Iranmanesh, M.; Tarmahi, H.; Gholampour, M. Technical and economic assessments of grid-connected photovoltaic power plants: Iran case study. Energy 2016, 114, 923–934. [Google Scholar] [CrossRef]
- Emara, H.I. Nutrient salts, inorganic and organic carbon contents in the waters of the Persian Gulf and the Gulf of Oman. J. Persian Gulf 2010, 1, 33–44. [Google Scholar]
- FILMTEC™ Membranes. How to Evaluate the Active Membrane Area of Seawater Reverse Osmosis Elements. 2015. Available online: dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/45-D01504-en.pdf (accessed on 3 June 2020).


| No. | Energy Resource | LNG | Components | Products | Results | Ref | |
|---|---|---|---|---|---|---|---|
| Heat Source | Heat Sink | ||||||
| 1 | Solar | ● | CO2 cycle, FPC, RO, NaClO plant, Stirling engine | Electrical, NaCl, hydrogen, PW | The exergy destruction rate was decreased from 16.7% to 8.8% upon replacement of the condenser by a Stirling engine | [32] | |
| 2 | Solar | ● | Transcritical CO2 cycle, FPC, RO, NaClO plant | Electrical, NaCl, hydrogen, PW | The system net output power was increased by increasing the inlet temperature of the boiler and turbine | ||
| 3 | Geothermal | ● | CO2 cycle | Electrical | The system exergy efficiency was equal to 20.5% The product cost rate was equal to 263,592.15 USD/year | [14] | |
| 4 | Exhaust hot gas of combined cycle | ● | Two-stage ORC | Electrical | The energy and exergy efficiencies were 25.64% and 31.02% CPP was equal to 6.3 USD/W | ||
| 5 | LNG | ● | RC, ASU, LAES | Electrical, clean air | The maximum amount of net output power ranged from 85.7–94.8 kJ/kg LNG | [23] | |
| 6 | LNG | ● | ASU, GC, CO2 capture | Electrical, CO2 | 90% CO2 recovery | ||
| 7 | NG | ● | RC, direct expander | Electrical | The maximum exergy efficiency was obtained by 302.8 kJ/kg LNG | [24] | |
| 8 | LNG | ● | ● | CCGT, LNG regasification process | Electrical | The electrical efficiency was increased from 6.32% to 9.09% | |
| 9 | LNG | ● | ● | CCPP, IAC, intercooling | Electrical | The electrical efficiency was improved by 2.8% | [15] |
| 10 | LNG | ● | ● | CCPP | Electrical | This system produced 3.36 MW and 21 MW of electrical power and cooling | |
| 11 | Geothermal | ● | Absorption power/cooling cycle | Electrical | The system energy and exergy efficiencies were equal to 39% and 36%, respectively | [25] | |
| 12 | Biomass | ● | ARS, GC, RC, ORC, PEM Elec | Electrical, cooling, and hydrogen | Upon increasing the fuel mass flow rate from 4 to 10 kg/s, the system energy efficiency decreased by 8.5% and the cost rate increased by 122.8% | ||
| 13 | LNG | ● | ● | SOFC, GC, CO2 cycle, ORC, CO2 capture | Electrical, cooling, heating, and CO2 | The system exergy efficiency was equal to 62.3% | [33] |
| 14 | Exhaust gas | ● | KC, ORC, CO2 capture | Electrical, CO2 | The system energy efficiency was equal to 36% The system exergy efficiency was equal to 41.4% The net electrical power output was equal to 394,658 kW | ||
| 15 | Ambient air | ● | CO2 Rankine cycle | Electrical | The system thermal efficiency was equal to 6.75% The net output power was 108.7 kW | [26] | |
| No. | Components | Mass Balance | Energy Equation |
|---|---|---|---|
| CO2 cycle | |||
| 1 | Pump II (P) | ||
| 2 | Steam generator | ||
| 3 | Turbine I (T) | ||
| 4 | Condenser | ||
| ORC | |||
| 5 | Pump III (P) | ||
| 6 | Steam generator | ||
| 7 | Turbine II (T) | ||
| 8 | Condenser | ||
| LNG loop | |||
| 9 | Pump I (P) | ||
| 10 | Turbine III (T) | ||
| 11 | Cooler | ||
| Boiler | |||
| 12 | Boiler | ||
| Water loop | |||
| 13 | Pump IV (P) | ||
| 14 | Heater | ||
| No. | Components | Mass Balance | Energy Equation | x |
|---|---|---|---|---|
| 1 | Pump V | |||
| 2 | Pump VI | |||
| 3 | Membrane I | |||
| 4 | Membrane II | |||
| 5 | Recovery turbine |
| Mass Balance | |
| Concentration Balance | |
| Energy Balance |
| No. | Components | Exergy Efficiency | Exergy Destruction Rate (kW) |
|---|---|---|---|
| CO2 cycle | |||
| 1 | Pump II (P) | ||
| 2 | Steam generator | ||
| 3 | Turbine I (T) | ||
| 4 | Condenser | ||
| ORC | |||
| 5 | Pump III (P) | ||
| 6 | Evaporator | ||
| 7 | Turbine II (T) | ||
| 8 | Condenser | ||
| LNG loop | |||
| 9 | Pump I (P) | ||
| 10 | Turbine III (T) | ||
| 11 | Cooler | ||
| Boiler | |||
| 12 | Boiler | ||
| Water loop | |||
| 13 | Pump IV (P) | ||
| 14 | Heater | ||
| RO | |||
| 15 | Pump V | ||
| 16 | Pump VI | ||
| 17 | Membrane I | ||
| 18 | Membrane II | ||
| 19 | Recovery turbine | ||
| NaClO | |||
| 20 | NaClO plant | ||
| Specific Cost | Value | Ref |
|---|---|---|
| cpower | 0.21 USD/kWh | [46] |
| cPW | 0.0004 USD/kg | [47] |
| ccooling/cheating | 0.07 USD/kWh | [48] |
| cNaCl | 10.47 USD/kg | [49] |
| cH2 | 13.96 USD/kg | [50] |
| cLNG | 0.025 USD/kWh | [48] |
| No. | Components | Cost Function | Ref |
|---|---|---|---|
| CO2 cycle | |||
| 1 | Pump | [23] | |
| 2 | Steam generator | [23] | |
| 3 | Turbine | [23] | |
| 4 | Condenser | [23] | |
| ORC | |||
| 5 | Pump | [55] | |
| 6 | Evaporator | [55] | |
| 7 | Turbine | [56] | |
| 8 | Condenser | [55] | |
| LNG loop | |||
| 9 | LNG turbine | [57] | |
| 10 | LNG pump | [58] | |
| 11 | LNG cooler | [58] | |
| Water loop | |||
| 12 | Pump | [59] | |
| 13 | Boiler | 33,600,000 | [60] |
| 14 | Heater | ln(10.76A)) ln(10.76A) ln (10.76A) | [58] |
| RO | |||
| 15 | Pump | [61] | |
| 16 | Membrane | 50 | [62] |
| 17 | Storage Tank | [63] | |
| 18 | Recovery turbine | [61] | |
| NaClO | |||
| 19 | NaClO (HD:6000) | 45,000 | [14] |
| No. | Components | |
|---|---|---|
| 2 | Boiler | 500 |
| 3 | Heat exchanger | 700 |
| 4 | Condenser | 800 |
| Parameter | Unit | Value | Ref |
|---|---|---|---|
| kg/s | 6.73 | [41] | |
| T1 | K | 220 | [41] |
| T2 | K | 224.9 | [41] |
| T3 | K | 493.1 | [41] |
| P1 | kPa | 600 | [41] |
| P2 | kPa | 12,490 | [41] |
| x21 | mg/L | 40,200 | [69] |
| x30 | mg/L | 150 | [69] |
| Am | m2 | 35.3 | [70] |
| RR | - | 0.3 | [14] |
| ṁ21 | kg/s | 2 | - |
| No. | Parameter | Unit | Present Work | Ref [32] | Error (%) |
|---|---|---|---|---|---|
| 1 | kW | 14.2 | 14.66 | 3.1 | |
| 2 | kW | 4.98 | 4.778 | 4.2 | |
| 3 | kW | 7.19 | 7.464 | 3.6 | |
| 4 | kW | 3.81 | 3.693 | 3.1 |
| No. | Parameter | Model | Ref [14] | Error (%) |
|---|---|---|---|---|
| 10 | 1.092 | 1.104 | 0.7 | |
| 2 | 0.468 | 0.456 | 2.6 | |
| 3 | 3.45 | 3.711 | 7 | |
| 4 | 8.42 | 8.96 | 6 |
| No | T (K) | P (kPa) | ṁ (kg/s) | X (-) | h (kJ/kg) | e (kJ/kg) |
|---|---|---|---|---|---|---|
| 1 | 220.0 | 600 | 6.73 | - | −420 | 210.7 |
| 2 | 224.9 | 12,490 | 6.73 | - | −407.2 | 220.1 |
| 3 | 493.2 | 12,490 | 6.73 | - | 141 | 288.3 |
| 4 | 220.5 | 610 | 6.73 | - | −74.91 | 104.7 |
| 5 | 111.5 | 101.4 | 9.547 | - | −911.7 | 1015 |
| 6 | 115.3 | 6580 | 9.547 | - | −889 | 1019 |
| 7 | 210.5 | 6440 | 9.547 | - | −372.5 | 655.8 |
| 8 | 283.2 | 6310 | 9.547 | - | −104.5 | 599.4 |
| 9 | 255.3 | 4000 | 9.547 | - | −148.7 | 542.9 |
| 10 | 288.2 | 4000 | 9.547 | - | −4672 | 548.9 |
| 11 | 271.9 | 280 | 10.26 | - | 50.18 | 35.22 |
| 12 | 295.3 | 709.1 | 10.26 | - | 89.45 | 35.27 |
| 13 | 403.2 | 709.1 | 10.26 | - | 369.2 | 60.63 |
| 14 | 272.9 | 280 | 10.26 | - | 249.7 | 23.31 |
| 15 | 303.2 | 101.3 | 115.5 | - | 125.8 | 1.579 |
| 16 | 303.2 | 150 | 115.5 | - | 125.8 | 1.627 |
| 17 | 513.2 | 150 | 115.5 | - | 2952 | 705 |
| 18 | 493.2 | 150 | 115.5 | - | 2912 | 687.9 |
| 19 | 493.2 | 150 | 114.3 | - | 2912 | 687.9 |
| 20 | 303.2 | 101.3 | 114.3 | - | 125.8 | 1.579 |
| 21 | 288.2 | 101.3 | 2 | 40,020 | 59.45 | 13.46 |
| 22 | 288.2 | 101.3 | 1 | 40,020 | 59.45 | 13.46 |
| 23 | 288.2 | 101.3 | 1 | 40,020 | 59.45 | 13.46 |
| 24 | 288.2 | 4767 | 1 | 40,020 | 63.69 | 17.98 |
| 25 | 288.2 | 4767 | 1 | 40,020 | 63.69 | 17.98 |
| 26 | 288.2 | 4767 | 0.3 | 150 | 67.49 | 4.659 |
| 27 | 288.2 | 4767 | 0.7 | 57,107 | 61.83 | 6.596 |
| 28 | 288.2 | 4767 | 0.3 | 150 | 67.49 | 4.659 |
| 29 | 288.2 | 4767 | 0.7 | 57,107 | 61.83 | 6.596 |
| 30 | 288.2 | 4767 | 0.6 | 150 | 67.49 | 4.659 |
| 31 | 288.2 | 101.3 | 0.6 | 150 | 63.05 | 0.000242 |
| 32 | 288.2 | 4767 | 1.4 | 57,107 | 61.83 | 6.596 |
| 33 | 288.2 | 303.9 | 1.4 | 57,107 | 57.84 | 2.323 |
| 34 | 507.2 | 101.3 | 0.01333 | - | 205.9 | 156.5 |
| 35 | 298.2 | 101.3 | 0.02568 | - | 7885 | 1226 |
| 36 | 493.2 | 150 | 1.288 | - | 2912 | 687.9 |
| 37 | 303.2 | 101.3 | 1.288 | - | 125.8 | 1.579 |
| 38 | 288.2 | 101.3 | 14 | - | 57.84 | 2.323 |
| Product | Unit | Value |
|---|---|---|
| Wnet,system | GWh/year | 12.75 |
| Qcooling | GWh/year | 276.4 |
| Qheating | GWh/year | 1783 |
| VPW | m3/year | 17,280 |
| mNaCl | Ton/year | 383.76 |
| mH2 | Ton/year | 739.56 |
| No. | Parameter | Unit | Value |
|---|---|---|---|
| 1 | NPV | million USD | 908.9 |
| 2 | PP | years | 7.9 |
| 3 | SPP | years | 6.9 |
| 4 | IRR | - | 0.138 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tjahjono, T.; Ehyaei, M.A.; Ahmadi, A.; Hoseinzadeh, S.; Memon, S. Thermo-Economic Analysis on Integrated CO2, Organic Rankine Cycles, and NaClO Plant Using Liquefied Natural Gas. Energies 2021, 14, 2849. https://doi.org/10.3390/en14102849
Tjahjono T, Ehyaei MA, Ahmadi A, Hoseinzadeh S, Memon S. Thermo-Economic Analysis on Integrated CO2, Organic Rankine Cycles, and NaClO Plant Using Liquefied Natural Gas. Energies. 2021; 14(10):2849. https://doi.org/10.3390/en14102849
Chicago/Turabian StyleTjahjono, Tri, Mehdi Ali Ehyaei, Abolfazl Ahmadi, Siamak Hoseinzadeh, and Saim Memon. 2021. "Thermo-Economic Analysis on Integrated CO2, Organic Rankine Cycles, and NaClO Plant Using Liquefied Natural Gas" Energies 14, no. 10: 2849. https://doi.org/10.3390/en14102849
APA StyleTjahjono, T., Ehyaei, M. A., Ahmadi, A., Hoseinzadeh, S., & Memon, S. (2021). Thermo-Economic Analysis on Integrated CO2, Organic Rankine Cycles, and NaClO Plant Using Liquefied Natural Gas. Energies, 14(10), 2849. https://doi.org/10.3390/en14102849









