Increasing Biogas Yield from Fodder by Microbial Stimulation of Propionic Acid Synthesis in Grass Silages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Flask Cultures Experiments
2.3. Grass Silage Production
2.4. Biogas Yield and Biogas Content Determination
2.5. Analytical Methods
2.6. Statistical Analyses
3. Results
3.1. Stimulation of Propionic Acid Synthesis in the Co-Fermentation of L. buchneri KKP 2047p and L. diolivorans KKP 2057p
3.2. Stimulation of Propionic Acid Synthesis in L. buchneri KKP 2047p and P. acidilactici KKP 2065p Co-culture
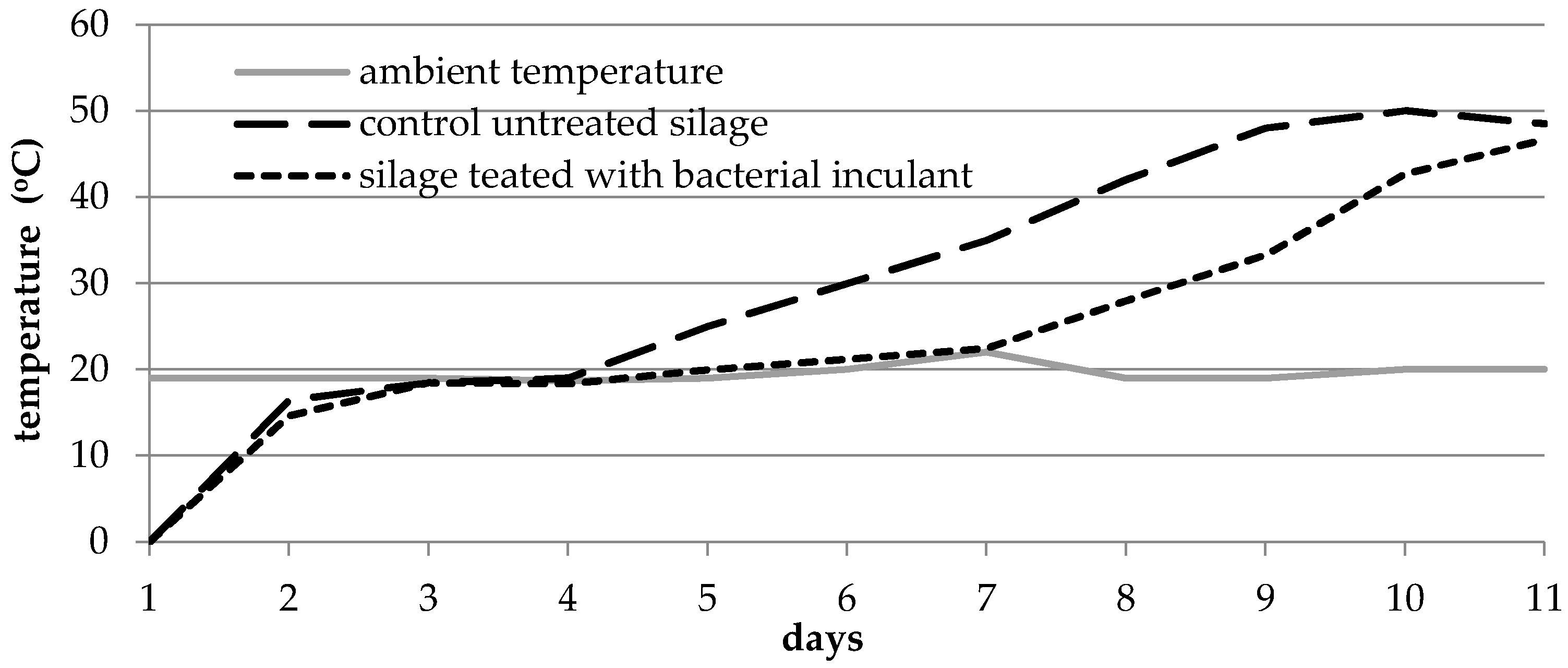
3.3. Improvement of Aerobic Stability of Grass Silage and Biogas Yield under the Influence of the Lactic Acid Bacteria Inoculants
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sun, Z.; Yu, J.; Dan, T.; Zhang, W.; Zhang, H. Phylogenesis and Evolution of Lactic Acid Bacteria. In Lactic Acid Bacteria; Metzler, J.B., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–101. [Google Scholar]
- Nishino, N.; Yoshida, M.; Shiota, H.; Sakaguchi, E. Accumulation of 1,2-propanediol and enhancement of aerobic stability in whole crop maize silage inoculated with Lactobacillus buchneri. J. Appl. Microbiol. 2003, 94, 800–807. [Google Scholar] [CrossRef] [Green Version]
- Jatkauskas, J.; Vrotniakiene, V.; Ohlsson, C.; Lund, B. The effects of three silage inoculants on aerobic stability in grass, clover-grass, lucerne and maize silages. Agric. Food Sci. 2013, 22, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Soucaille, P.; Meynial-Salles, I.; Voelker, F.; Figge, R. Microorganisms and Methods for Production of 1,2-Propanediol and Acetol. U.S. Patent Application 9051588, 9 June 2015. [Google Scholar]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass Valorization 2017, 8, 267–283. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, C.; Idler, C.; Heiermann, M. Improving aerobic stability and biogas production of maize silage using silage additives. Bioresour. Technol. 2015, 197, 393–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Zollitsch, W.; Mayer, K.; Gruber, L. Biogas production from maize and dairy cattle manure—Influence of biomass composition on the methane yield. Agric. Ecosyst. Environ. 2007, 118, 173–182. [Google Scholar] [CrossRef]
- McEniry, J.; Allen, E.; Murphy, J.; O’Kiely, P. Grass for biogas production: The impact of silage fermentation characteristics on methane yield in two contrasting biomethane potential test systems. Renew. Energy 2014, 63, 524–530. [Google Scholar] [CrossRef]
- Muck, R.; Nadeau, E.; McAllister, T.; Contreras-Govea, F.; Santos, M.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Kung, L.; Taylor, C.; Lynch, M.; Neylon, J. The Effect of Treating Alfalfa with Lactobacillus buchneri 40788 on Silage Fermentation, Aerobic Stability, and Nutritive Value for Lactating Dairy Cows. J. Dairy Sci. 2003, 86, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Elferink, S.J.W.H.O.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic Conversion of Lactic Acid to Acetic Acid and 1,2-Propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Krooneman, J.; Faber, F.; Alderkamp, A.C.; Elferink, S.J.H.W.O.; Driehuis, F.; Cleenwerck, I.; Swings, J.; Gottschal, J.C.; Vancanneyt, M. Lactobacillus diolivorans sp. nov., a 1,2-propanediol-degrading bacterium isolated from aerobically stable maize silage. Int. J. Syst. Evol. Microbiol. 2002, 52, 639–646. [Google Scholar] [CrossRef]
- Toraya, T. The structure and the mechanism of action of coenzyme B12-dependent diol dehydratases. J. Mol. Catal. B Enzym. 2000, 10, 87–106. [Google Scholar] [CrossRef]
- Taranto, M.P.; Vera, J.L.; Hugenholtz, J.; De Valdez, G.F.; Sesma, F. Lactobacillus reuteri CRL1098 Produces Cobalamin. J. Bacteriol. 2003, 185, 5643–5647. [Google Scholar] [CrossRef] [Green Version]
- Sriramulu, D.D.; Liang, M.; Hernandez-Romero, D.; Raux-Deery, E.; Lünsdorf, H.; Parsons, J.B.; Warren, M.J.; Prentice, M.B. Lactobacillus reuteri DSM 20016 Produces Cobalamin-Dependent Diol Dehydratase in Metabolosomes and Metabolizes 1,2-Propanediol by Disproportionation. J. Bacteriol. 2008, 190, 4559–4567. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, K.; Fabiszewska, A.; Świątek, M.; Szymanowska-Powałowska, D. Evaluation of the ability to metabolize 1,2-propanediol by heterofermentative bacteria of the genus Lactobacillus. Electron. J. Biotechnol. 2017, 26, 60–63. [Google Scholar] [CrossRef]
- Zielińska, K.J.; Fabiszewska, A.U. Improvement of the quality of maize grain silage by a synergistic action of selected lactobacilli strains. World J. Microbiol. Biotechnol. 2018, 34, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrighetto, C.; Zampese, L.; Lombardi, A. RAPD-PCR characterization of lactobacilli isolated from artisanal meat plants and traditional fermented sausages of Veneto region (Italy). Lett. Appl. Microbiol. 2001, 33, 26–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.G.; Kubelik, A.R.; Livak, K.J.; Rafalski, J.A.; Tingey, S.V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990, 18, 6531–6535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oneca, M.; Irigoyen, A.; Ortigosa, M.; Torre, P. PCR and RAPD identification ofL. plantarumstrains isolated from ovine milk and cheese. Geographical distribution of strains. FEMS Microbiol. Lett. 2003, 227, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Zielinska, K.; Fabiszewska, A.; Stefanska, I. Different aspects of Lactobacillus inoculants on the improvement of quality and safety of alfalfa silage. Chil. J. Agric. Res. 2015, 75, 298–306. [Google Scholar] [CrossRef] [Green Version]
- German National Standard. German Standard Methods for the Examination of Water, Waste Water and Sludge; Sludge and Sediments (Group S); Determi-Nation of the Amenability to Anaerobic Digestion (S 8); DIN 38414-8; Deutsches Institut Fur Normung E.V.: Berlinm Germany, 1985. [Google Scholar]
- Honig, H. Determination of Aerobic Deterioration; System Volkenrode–Institut fur Grunland und Futterpflanzenforschung der Bundesforsungszns talt fur Landwirtschaft Braunschweig-Volkenrode (FAL): Berlin, Germany, 1985. [Google Scholar]
- Nelson, W. Applied Life Data Analysis; Wiley: Hoboken, NJ, USA, 1982. [Google Scholar]
- Danner, H.; Holzer, M.; Mayrhuber, E.; Braun, R. Acetic Acid Increases Stability of Silage under Aerobic Conditions. Appl. Environ. Microbiol. 2003, 69, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Li, L.; Wang, X.-F.; Zeng, Z.-H.; Hu, Y.-G.; Cui, Z.-J. Effects of Lactobacillus buchneri and Lactobacillus plantarum on fermentation, aerobic stability, bacteria diversity and ruminal degradability of alfalfa silage. World J. Microbiol. Biotechnol. 2009, 25, 965–971. [Google Scholar] [CrossRef]
- Lindsey, J.R.; Kung, L. Effects of combining Lactobacillus buchneri 40788 with various lactic acid bacteria on the fermentation and aerobic stability of corn silage. Anim. Feed Sci. Tech. 2010, 159, 105–109. [Google Scholar] [CrossRef]
- Zhang, C.; Brandt, M.J.; Schwab, C.; Gänzle, M.G. Propionic acid production by cofermentation of Lactobacillus buchneri and Lactobacillus diolivorans in sourdough. Food Microbiol. 2010, 27, 390–395. [Google Scholar] [CrossRef]
- Selwet, M. Influence of inoculation with Lactobacillus on fermentation, production of 1,2-propanediol and 1-propanol as well as Maize silage aerobic stability. Open Life Sci. 2020, 15, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Bannink, A.; Hindrichsen, I.; Kinley, R.; Pellikaan, W.; Milora, N.; Dijkstra, J. The effect of lactic acid bacteria included as a probiotic or silage inoculant on in vitro rumen digestibility, total gas and methane production. Anim. Feed. Sci. Technol. 2016, 211, 61–74. [Google Scholar] [CrossRef]
- Khota, W.; Pholsen, S.; Higgs, D.; Cai, Y. Fermentation quality and in vitro methane production of sorghum silage prepared with cellulase and lactic acid bacteria. Asian Australas. J. Anim. Sci. 2017, 30, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Szlachta, J.; Prask, H.; Fugol, M.; Luberański, A. Effect of Mechanical Pre-Treatment of the Agricultural Substrates on Yield of Biogas and Kinetics of Anaerobic Digestion. Sustainability 2018, 10, 3669. [Google Scholar] [CrossRef] [Green Version]
- Saad, M.M.; Eida, A.A.; Hirt, H. Tailoring plant-associated microbial inoculants in agriculture: A roadmap for successful application. J. Exp. Bot. 2020, 71, 3878–3901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
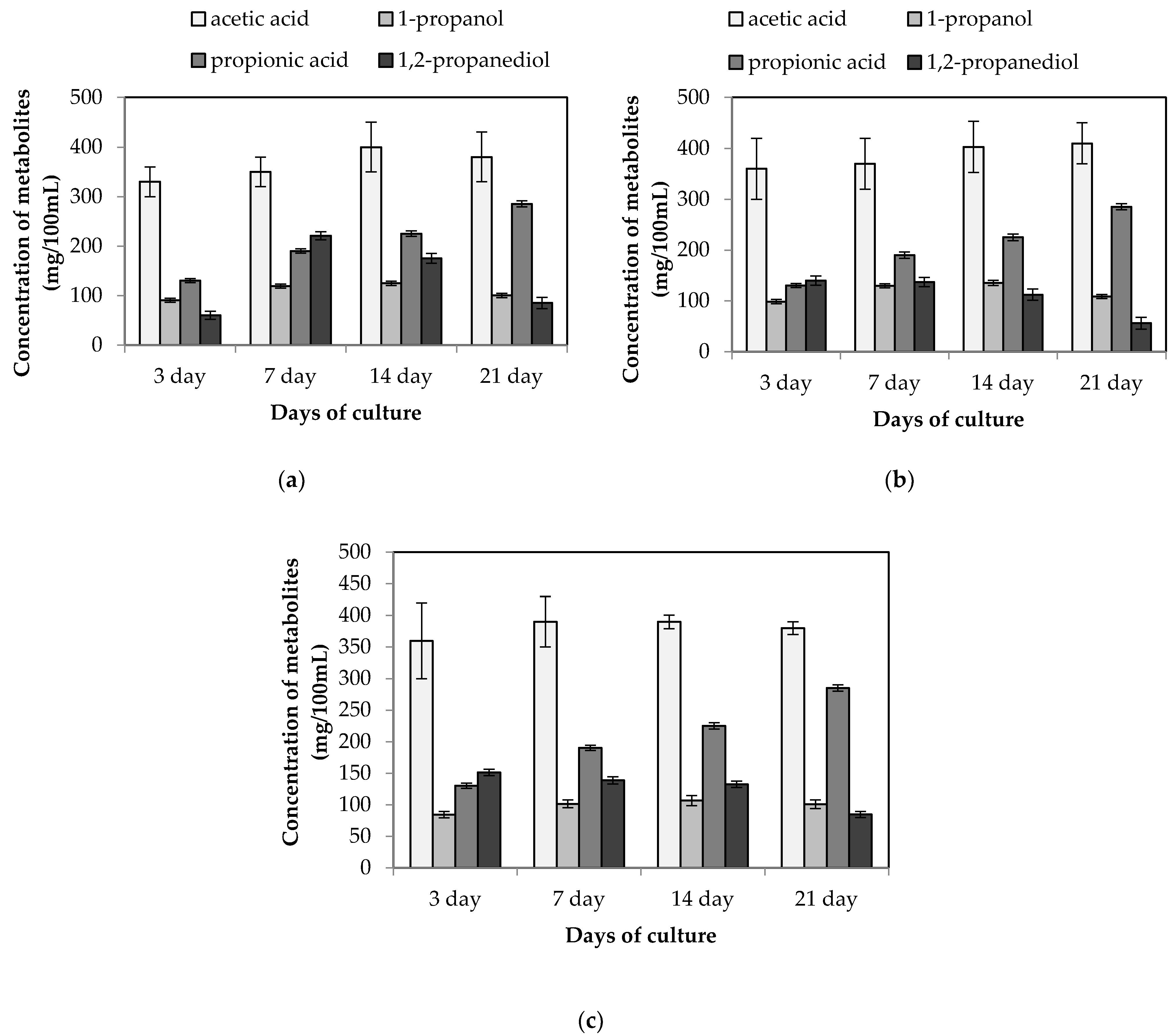
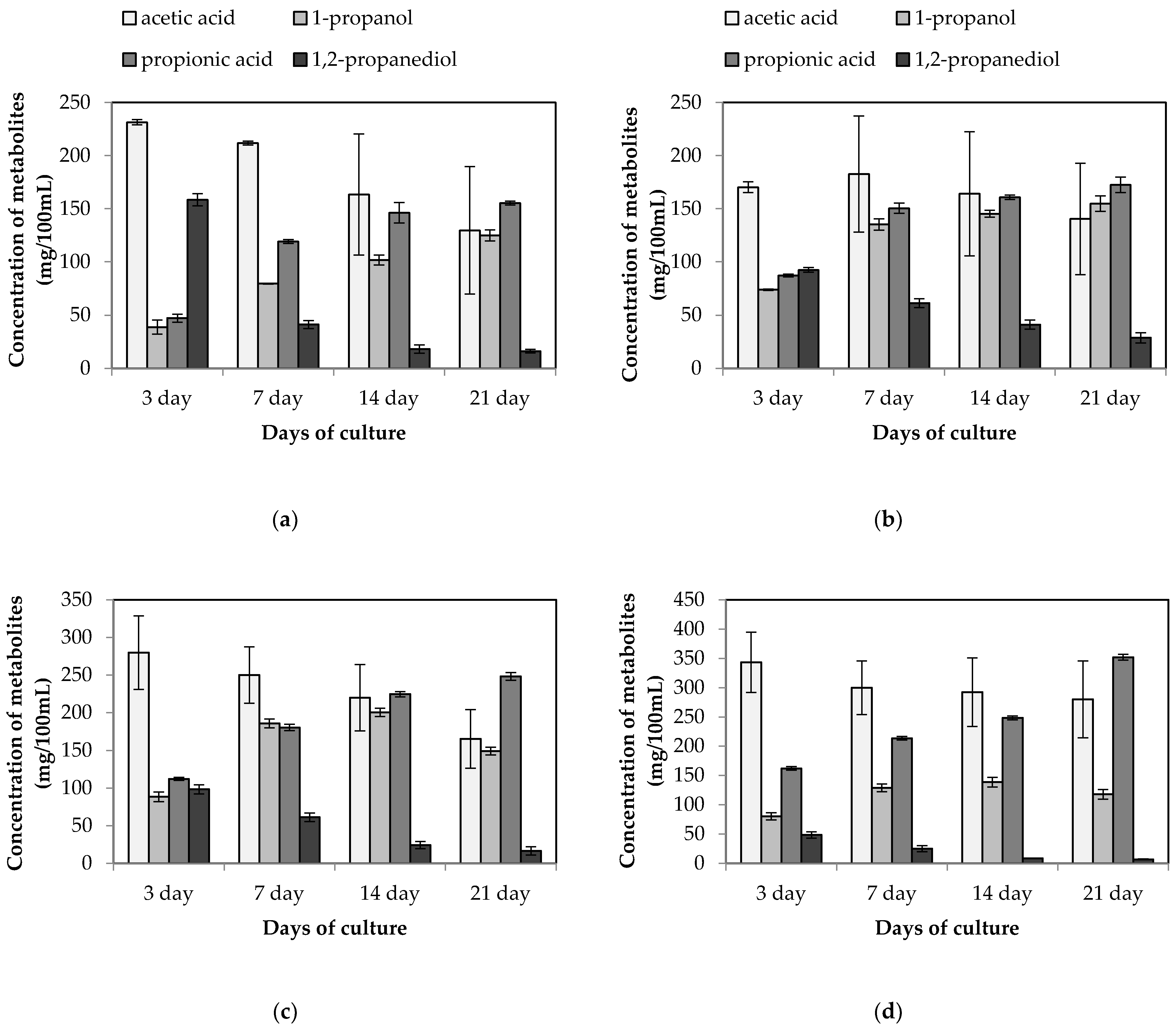
| Lactobacillus Strain | Source of Species/Strain Isolation | Specific Features | Reference | |
|---|---|---|---|---|
| 1,2-propanediol * Content, Synthesized from Glucose, mg/100 mL | Propionic Acid Content, mg/100 mL | |||
| L. buchneri KKP 2047p | maize silage | 400.00 * | 174.9 1 | 17,18 |
| L. buchneri KKP 907p | meadow sward | 120.00 * | no detected | 24,37 |
| L. diolivorans LMG 19667 | maize silage | no | 125.8 2 | 13 |
| L. diolivorans KKP 2057p | maize silage | no | 125.4 1 | 18 |
| P. acidilactici KKP 2065p | buckwheat grain | no | 248.20 3 | --- |
| Silage | pH | Volatile Carboxylic Acids Content, g/100 g of Fresh Mass Silage | Metabolite Content, mg/100 g of Fresh Mass Silage | ||||
|---|---|---|---|---|---|---|---|
| Lactic | Acetic | 3-Hydroxy Butyric | 1,2-Propanediol | 1-Propanol | Propionic Acid | ||
| Untreated (control A) | 5.10 ± 0.1 | 1.18 a ± 0.2 | 0.22 a ± 0.1 | 0.09 a ± 0.15 | n.d. a | n.d. a | 10.8 a ± 3.4 |
| Treated with bacterial inoculant Biogas A | 4.48 ± 0.1 | 1.48 a ± 0.3 | 0.45 ab ± 0.15 | n.d. b | 97.8 b ± 11.3 | 90.6 b ± 11.4 | 116.4 b ± 6.8 |
| Untreated (control B) | 4.55 ± 0.1 | 1.25 a ± 0.2 | 0.27 a ± 0.1 | 0.18 a ± 0.15 | n.d. a | n.d. a | 8.2 a ± 3.4 |
| Treated with bacterial inoculant Biogas B | 4.53 ± 0.1 | 1.40 a ± 0.3 | 0.67 b ± 0.15 | n.d. b | 100.8 b ± 9.3 | 90.4 b ± 9.4 | 138.32 c ± 6.5 |
| Silage | Dry Matter, % | Loading the Fermentation Mixture with Organic Dry Matter odm *, % | Biogas Yield NI/kg odm * | Methane in Biogas, % |
|---|---|---|---|---|
| Untreated (control A) | 45.0 ± 0.9 | 70.4 ± 2.0 | 367.3 ± 18.7 | 58.4 ± 1.4 |
| Treated with bacterial inoculant Biogas A | 44.1 ± 0.9 | 69.8 ± 2.1 | 527.4 ± 25.5 | 66.9 ± 2.1 |
| Untreated (control B) | 50.8 ± 3.6 | 66.4 ± 2.1 | 461.9 ± 23.1 | 61.9 ± 2.2 |
| Treated with bacterial inoculant Biogas B | 41.5 ± 0.9 | 66.6 ± 2.1 | 507.3 ± 23.5 | 68.6 ± 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, K.; Fabiszewska, A.; Piasecka-Jóźwiak, K.; Choińska, R. Increasing Biogas Yield from Fodder by Microbial Stimulation of Propionic Acid Synthesis in Grass Silages. Energies 2021, 14, 2843. https://doi.org/10.3390/en14102843
Zielińska K, Fabiszewska A, Piasecka-Jóźwiak K, Choińska R. Increasing Biogas Yield from Fodder by Microbial Stimulation of Propionic Acid Synthesis in Grass Silages. Energies. 2021; 14(10):2843. https://doi.org/10.3390/en14102843
Chicago/Turabian StyleZielińska, Krystyna, Agata Fabiszewska, Katarzyna Piasecka-Jóźwiak, and Renata Choińska. 2021. "Increasing Biogas Yield from Fodder by Microbial Stimulation of Propionic Acid Synthesis in Grass Silages" Energies 14, no. 10: 2843. https://doi.org/10.3390/en14102843
APA StyleZielińska, K., Fabiszewska, A., Piasecka-Jóźwiak, K., & Choińska, R. (2021). Increasing Biogas Yield from Fodder by Microbial Stimulation of Propionic Acid Synthesis in Grass Silages. Energies, 14(10), 2843. https://doi.org/10.3390/en14102843







