Cost-efficient, Effect of Low-Quality PbI2 Purification to Enhance Performances of Perovskite Quantum Dots and Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Materials for Perovskite Precursors and Perovskite Quantum Dots
2.1.2. Materials for Perovskite Solar Cells
2.2. Methods
2.2.1. Methods for Lead Iodide Synthesis and Purification
2.2.2. Synthesis of CsPbI3 Perovskite Quantum Dots
2.2.3. Characterizations of PbI2 Precursor and CsPbI3 Perovskite Quantum Dots
2.2.4. Perovskite Solar Cell Fabrication and Photoconversion Efficiency Measurement
3. Results and Discussion
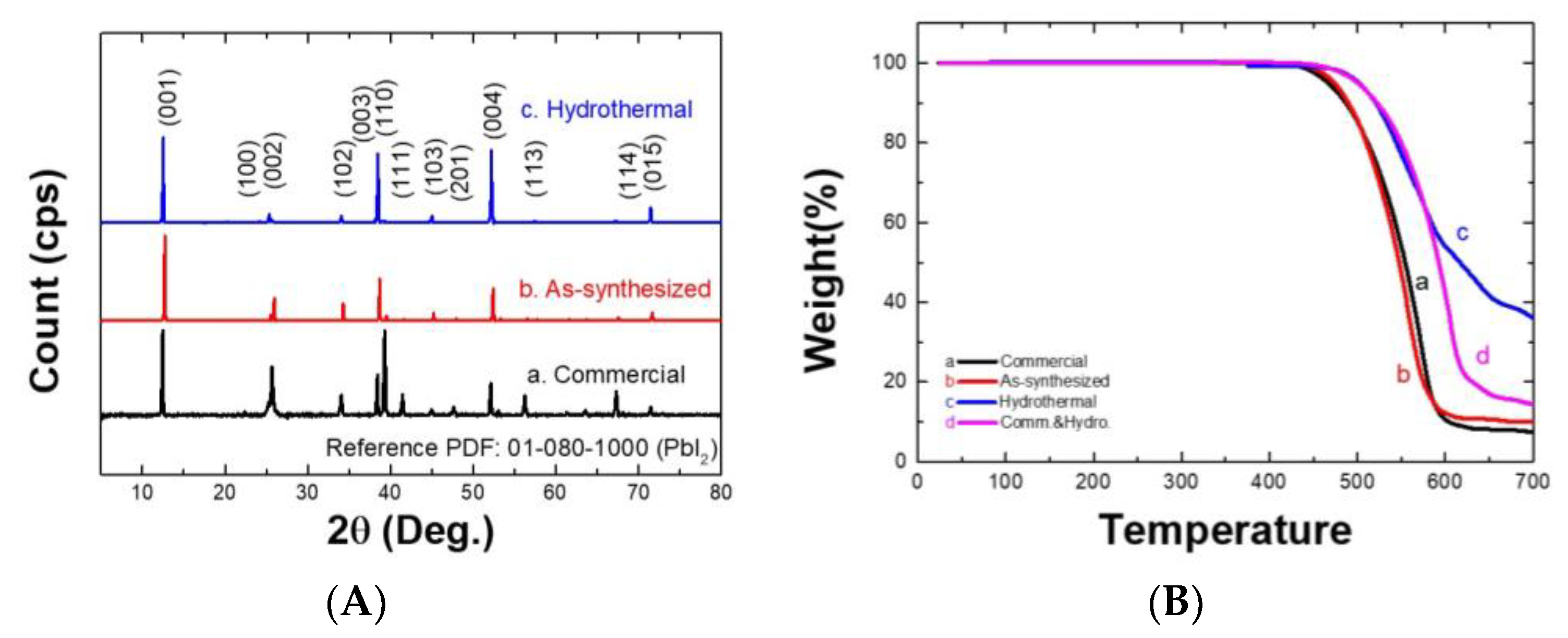
3.1. PbI2 Synthesis and Purification Using Economical Low-Grade Sources
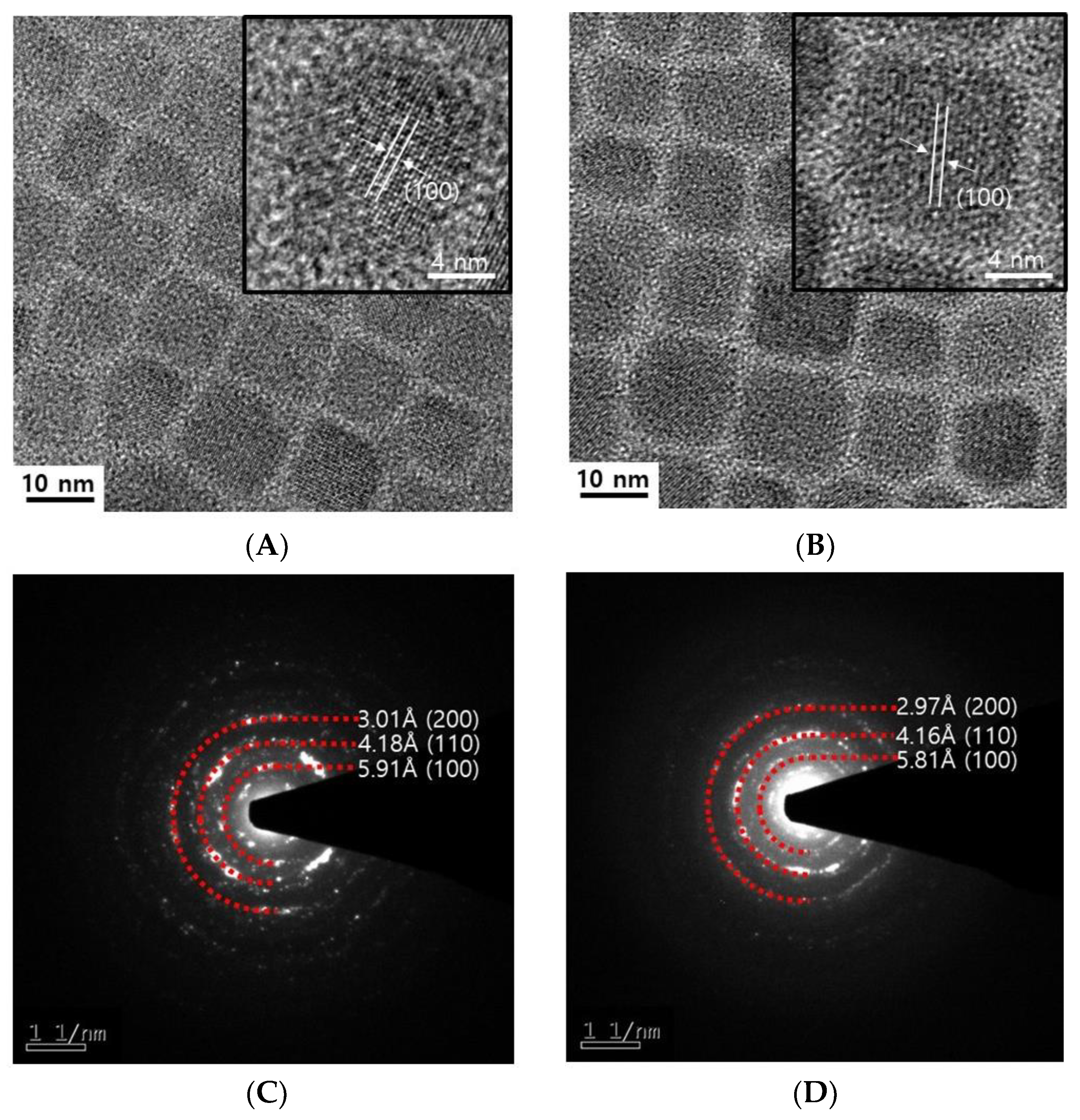
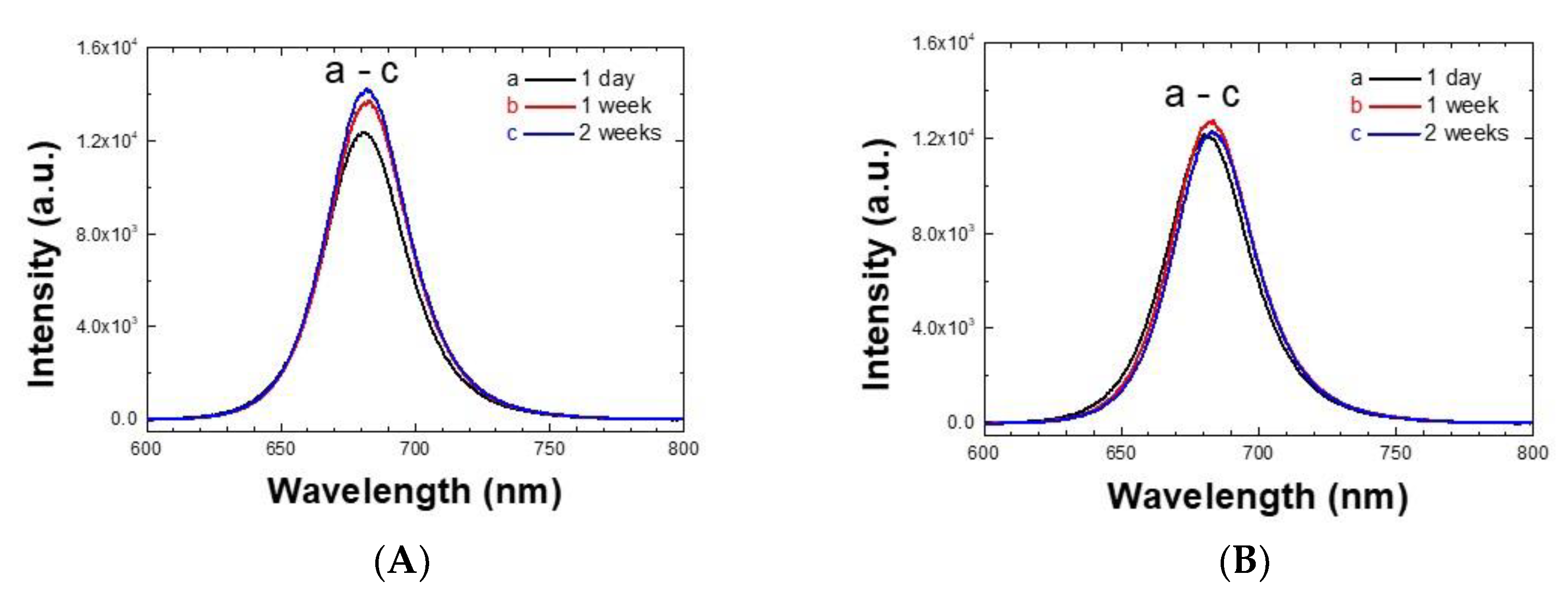
3.2. Similarity of CsPbI3 Perovskite Quantum Dots’ Photophysical Properties and Stabilities Using Either Crystallized PbI2 or Commercial PbI2 with 99.99% Purity
3.3. Application of the Hydrothermal Process to Problematic PbI2 to Make Better Quality of MAPbI3 Perovskite Film for Enhanced Photoconversion Efficiency of PSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, J.J.; Zhu, H.; Li, B.C.; Isikgor, F.H.; Hao, Y.; Xu, Q.H.; Ouyang, J.Y. Boosting the performance of planar heterojunction perovskite solar cell by controlling the precursor purity of perovskite materials. J. Mater. Chem. A 2016, 4, 887–893. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.S.; Jin, Z.W.; Zhang, J.R.; Gao, Z.F.; Li, Y.F.; Liu, S.Z.F. Energy-Down-Shift CsPbCl3:Mn Quantum Dots for Boosting the Efficiency and Stability of Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 1479–1486. [Google Scholar] [CrossRef]
- Huang, W.X.; Yoon, S.J.; Sapkota, P. Effect of Light Illumination on Mixed Halide Lead Perovskites: Reversible or Irreversible Transformation. ACS Appl. Energy Mater. 2018, 1, 2859–2865. [Google Scholar] [CrossRef]
- Kim, G.; Min, H.; Lee, K.S.; Lee, D.Y.; Yoon, S.M.; Seok, S.I. Impact of strain relaxation on performance of α-formamidinium lead iodide perovskite solar cells. Science 2020, 370, 108–112. [Google Scholar] [CrossRef]
- Asif, A.A.; Singh, R.; Alapatt, G.F. Technical and economic assessment of perovskite solar cells for large scale manufacturing. J. Renew. Sustain. Energy 2015, 7, 043120. [Google Scholar] [CrossRef]
- Mitzi, D.B.; Feild, C.; Schlesinger, Z.; Laibowitz, R. Transport, optical, and magnetic properties of the conducting halide perovskite CH3NH3SnI3. J. Solid State Chem. 1995, 114, 159–163. [Google Scholar] [CrossRef]
- Kagan, C.; Mitzi, D.; Dimitrakopoulos, C. Organic-inorganic hybrid materials as semiconducting channels in thin-film field-effect transistors. Science 1999, 286, 945–947. [Google Scholar] [CrossRef]
- Mitzi, D.B.; Chondroudis, K.; Kagan, C.R. Design, structure, and optical properties of organic− inorganic perovskites containing an oligothiophene chromophore. Inorg. Chem. 1999, 38, 6246–6256. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Senanayak, S.P.; Yang, B.; Thomas, T.H.; Giesbrecht, N.; Huang, W.; Gann, E.; Nair, B.; Goedel, K.; Guha, S.; Moya, X. Understanding charge transport in lead iodide perovskite thin-film field-effect transistors. Sci. Adv. 2017, 3, e1601935. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths >175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Iocozzia, J.; Zhang, M.; Ye, M.; Yan, S.; Jin, H.; Wang, S.; Zou, Z.; Lin, Z. The charge carrier dynamics, efficiency and stability of two-dimensional material-based perovskite solar cells. Chem. Soc. Rev. 2019, 48, 4854–4891. [Google Scholar] [CrossRef] [PubMed]
- Saba, M.; Cadelano, M.; Marongiu, D.; Chen, F.; Sarritzu, V.; Sestu, N.; Figus, C.; Aresti, M.; Piras, R.; Lehmann, A.G. Correlated electron–hole plasma in organometal perovskites. Nat. Commun. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, S.G.; Lee, D.K.; Park, N.G. CH3NH3PbI3 and HC(NH2)(2)PbI3 Powders Synthesized from Low-Grade PbI2: Single Precursor for High-Efficiency Perovskite Solar Cells. ChemSusChem 2018, 11, 1813–1823. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Abdelhady, A.L.; Murali, B.; Alarousu, E.; Burlakov, V.M.; Peng, W.; Dursun, I.; Wang, L.F.; He, Y.; Maculan, G.; et al. High-quality bulk hybrid perovskite single crystals within minutes by inverse temperature crystallization. Nat. Commun. 2015, 6, 7586. [Google Scholar] [CrossRef]
- Gu, Z.K.; Huang, Z.D.; Li, C.; Li, M.Z.; Song, Y.L. A general printing approach for scalable growth of perovskite single-crystal films. Sci. Adv. 2018, 4, eaat2390. [Google Scholar] [CrossRef]
- Bush, K.A.; Frohna, K.; Prasanna, R.; Beal, R.E.; Leijtens, T.; Swifter, S.A.; McGehee, M.D. Compositional Engineering for Efficient Wide Band Gap Perovskites with Improved Stability to Photoinduced Phase Segregation. ACS Energy Lett. 2018, 3, 428–435. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical Management for Colorful, Efficient, and Stable Inorganic-Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Wakamiya, A.; Endo, M.; Sasamori, T.; Tokitoh, N.; Ogomi, Y.; Hayase, S.; Murata, Y. Reproducible Fabrication of Efficient Perovskite-based Solar Cells: X-ray Crystallographic Studies on the Formation of CH3NH3PbI3 Layers. Chem. Lett. 2014, 43, 711–713. [Google Scholar] [CrossRef]
- Sadhanala, A.; Ahmad, S.; Zhao, B.; Giesbrecht, N.; Pearce, P.M.; Deschler, F.; Hoye, R.L.; Gödel, K.C.; Bein, T.; Docampo, P. Blue-green color tunable solution processable organolead chloride–bromide mixed halide perovskites for optoelectronic applications. Nano Lett. 2015, 15, 6095–6101. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Kerner, R.A.; Grede, A.J.; Brigeman, A.N.; Rand, B.P.; Giebink, N.C. Diode-pumped organo-lead halide perovskite lasing in a metal-clad distributed feedback resonator. Nano Lett. 2016, 16, 4624–4629. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Pradhan, B.; Hofkens, J.; Roeffaers, M.B.; Steele, J.A. Solar-Driven Metal Halide Perovskite Photocatalysis: Design, Stability, and Performance. ACS Energy Lett. 2020, 5, 1107–1123. [Google Scholar] [CrossRef]
- Kim, I.S.; Pellin, M.J.; Martinson, A.B. Acid-compatible halide perovskite photocathodes utilizing atomic layer deposited TiO2 for solar-driven hydrogen evolution. ACS Energy Lett. 2019, 4, 293–298. [Google Scholar] [CrossRef]
- Suarez, I.; Valles-Pelarda, M.; Gualdron-Reyes, A.F.; Mora-Sero, I.; Ferrando, A.; Michinel, H.; Salgueiro, J.R.; Pastor, J.P.M. Outstanding nonlinear optical properties of methylammonium- and Cs-PbX3 (X = Br, I, and Br-I) perovskites: Polycrystalline thin films and nanoparticles. APL Mater. 2019, 7, 041106. [Google Scholar] [CrossRef]
- Yangi, L.K.; Li, Y.W.; Pei, Y.X.; Wang, J.Q.; Lin, H.; Li, X. A novel 2D perovskite as surface “patches ” for efficient flexible perovskite solar cells. J. Mater. Chem. A 2020, 8, 7808–7818. [Google Scholar] [CrossRef]
- Zhou, N.J.; Bekenstein, Y.; Eisler, C.N.; Zhang, D.D.; Schwartzberg, A.M.; Yang, P.D.; Alivisatos, A.P.; Lewis, J.A. Perovskite nanowire-block copolymer composites with digitally programmable polarization anisotropy. Sci. Adv. 2019, 5, eaav8141. [Google Scholar] [CrossRef]
- Chen, L.-C.T.; Tien, C.-H.; Tseng, Z.-L.; Ruan, J.-H. Enhanced Efficiency of MAPbI3 Perovskite Solar Cells with FAPbX3 Perovskite Quantum Dots. Nanomaterials 2019, 9, 121. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.H.; Ding, C.; Kobayashi, S.; Izuishi, T.; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T.; et al. Highly Luminescent Phase-Stable CsPbl(3) Perovskite Quantum Dots Achieving Near 100% Absolute Photoluminescence Quantum Yield. ACS Nano 2017, 11, 10373–10383. [Google Scholar] [CrossRef]
- Zhu, X.H.; Wangyang, P.H.; Sun, H.; Yang, D.Y.; Gao, X.Y.; Tian, H.B. Facile growth and characterization of freestanding single crystal PbI2 film. Mater. Lett. 2016, 180, 59–62. [Google Scholar] [CrossRef]
- Koziol, J. Studies on Flavins in Organic Solvents-i*. Spectral Characteristics of Riboflavin, Riboflavin Tetrabutyrate and Lumichrome. Photochem. Photobiol. 1966, 5, 41–54. [Google Scholar] [CrossRef]
- Golden Rain. Available online: https://edu.rsc.org/exhibition-chemistry/golden-rain/2000048.article (accessed on 20 December 2020).
- Malevu, T.D.; Ocaya, R.O.; Tshabalala, K.G. Phase transformations of high-purity PbI2 nanoparticles synthesized from lead-acid accumulator anodes. Phys. B Condens. Matter 2016, 496, 69–73. [Google Scholar] [CrossRef]
- Dualeh, A.; Gao, P.; Seok, S.I.; Nazeeruddin, M.K.; Graetzel, M. Thermal Behavior of Methylammonium Lead-Trihalide Perovskite Photovoltaic Light Harvesters. Chem. Mater. 2014, 26, 6160–6164. [Google Scholar] [CrossRef]
- Ten Brinck, S.; Zaccaria, F.; Infante, I. Defects in Lead Halide Perovskite Nanocrystals: Analogies and (Many) Differences with the Bulk. ACS Energy Lett. 2019, 4, 2739–2747. [Google Scholar] [CrossRef]
- Kim, J.; Park, B.W.; Baek, J.; Yun, J.S.; Kwon, H.W.; Seidel, J.; Min, H.; Coelho, S.; Lim, S.; Huang, S.J. Unveiling the Relationship between the Perovskite Precursor Solution and the Resulting Device Performance. J. Am. Chem. Soc. 2020, 142, 6251–6260. [Google Scholar] [CrossRef]


| Purity, Chemical (Brand) | Retail Price, US$ (Quantity (g)) | US$ g−1 |
|---|---|---|
| 99.5% Pb(CH3COO)2, (Fisher Chemical) | 34.38 (250) | 0.138 |
| 99.5%, KI (Samchun Chemicals) | 56.40 (500) | 0.113 |
| 99.99%, trace metals basis, PbI2, (TCI) | 160.43 (25) | 6.42 |
| 99.9985%, metals basis PbI2, (Alfa Aesar) | 108.33 (25) | 4.33 |
| 98.5% PbI2, (Alfa Aesar) | 73.41 (50) | 1.47 |
| PbI2 Sources | Pb (wt%) | I (wt%) | Molar Ratio (I/Pb) |
|---|---|---|---|
| Commercially available | 43.0 | 57.0 | 2.164 |
| As-synthesized (without purification) | 43.2 | 56.8 | 2.147 |
| Hydrothermal (from as-synthesized) | 42.7 | 57.3 | 2.191 |
| Hydrothermal (from commercial) | 42.8 | 57.2 | 2.182 |
| PbI2 Sources | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) Best (Average ± stan. dev.) |
|---|---|---|---|---|
| Commercial | 1.029 | 22.03 | 72.31 | 16.39 (14.77 ± 1.82) |
| Commercial with hydrothermal | 1.031 | 22.14 | 75.88 | 17.31 (15.18 ± 1.92) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Shin, Y.; Jeon, G.G.; Kang, D.; Jung, J.; Jeon, B.; Park, J.; Kim, J.; Yoon, S.J. Cost-efficient, Effect of Low-Quality PbI2 Purification to Enhance Performances of Perovskite Quantum Dots and Perovskite Solar Cells. Energies 2021, 14, 201. https://doi.org/10.3390/en14010201
Lee C, Shin Y, Jeon GG, Kang D, Jung J, Jeon B, Park J, Kim J, Yoon SJ. Cost-efficient, Effect of Low-Quality PbI2 Purification to Enhance Performances of Perovskite Quantum Dots and Perovskite Solar Cells. Energies. 2021; 14(1):201. https://doi.org/10.3390/en14010201
Chicago/Turabian StyleLee, ChaeHyun, YeJi Shin, Gyeong G. Jeon, Dongwoo Kang, Jiwon Jung, Byeongmin Jeon, Jongin Park, Jincheol Kim, and Seog Joon Yoon. 2021. "Cost-efficient, Effect of Low-Quality PbI2 Purification to Enhance Performances of Perovskite Quantum Dots and Perovskite Solar Cells" Energies 14, no. 1: 201. https://doi.org/10.3390/en14010201
APA StyleLee, C., Shin, Y., Jeon, G. G., Kang, D., Jung, J., Jeon, B., Park, J., Kim, J., & Yoon, S. J. (2021). Cost-efficient, Effect of Low-Quality PbI2 Purification to Enhance Performances of Perovskite Quantum Dots and Perovskite Solar Cells. Energies, 14(1), 201. https://doi.org/10.3390/en14010201





