Abstract
NOx emissions in compression-ignition engines can be reduced, either through the application of engine-internal methods, i.e., making sure that as little NOx as possible is produced as a result of the mixture combustion processes, or through the use of catalytic converters designed to reduce nitrogen oxides, including NH3-SCR or HC-SCR converters. Converters using ammonia offer high conversion rates, but they tend to be problematic in terms of their operation. For this reason, converters using hydrocarbons for the purpose of NOx reduction have been gaining in popularity. An Ag/Al2O3-SiO2 converter is an example of such a catalytic converter. This paper describes the process of preparing such a converter and characterises the porosity and acidity of its surface. The dispersion of silver was assessed based on oxygen absorption tests on crystallites of sliver and based on TEM images of crystallites of silver. The conversion of NO2, NO and propane was assessed depending on the temperature. Additionally, NO2, NO and propane conversion was assessed at 500 °C, depending on the admixture of a reducer, and propane selectivity in the process of reduction of NO to N2 was calculated. The test results indicate that the developed converter can be considered the basis for further research into the development of this compression-ignition engine exhaust after-treatment technology.
1. Introduction
Based on the analysis of the test results of the compression-ignition engines produced so far and their development prospects, it can be concluded that they relatively easily meet the standards limiting emissions of carbon monoxide and hydrocarbons, while it is much more difficult to meet the requirements limiting the emission of particulates and nitrogen oxides [1]. Emission of nitrogen oxides, due to their toxic properties and large amounts emitted into the atmosphere, is becoming one of the main problems that need to be solved in future designs of these engines. The introduction of the Euro regulations in the LDV and HDV vehicles type-approval tests forced vehicle manufacturers to significantly reduce the emission of harmful substances, including NOx emission. Research is being conducted to create, in the laboratory conditions [2,3], driving cycles corresponding to the actual operation of vehicles, so that it is possible to quickly and accurately assess the actual emission of harmful components to the environment, including nitrogen oxides in particular. Reduction of NOx [1] can be obtained through:
- Engine-internal methods. These methods consist in conducting the processes taking place during the preparation and then the combustion of the mixture [4] in such a manner as to make sure that NOx concentration levels in the engine exhaust gas are as low as possible. The process consists in finding the optimum balance between the power developed by the engine, unit fuel consumption and the emission of harmful substances,
- The use of catalytic converters designed to reduce nitrogen oxides [1], including NH3-SCR and possibly HC-SCR converters.
- The following methods are commonly used in parallel to reduce NOx emissions:
- Exhaust gas recirculation (EGR), which lowers [5] the production of NOx in the engine’s cylinder, and
- The use of catalytic converters [6,7] designed to selectively reduce NOx, with ammonia separated from a water-urea solution.
EGR, applied to an extent that does not cause a significant increase in fuel consumption and PM emissions, results in only a slight reduction of NOX emissions and is not an effective method in terms of ensuring compliance with Euro 6 and VI requirements. Nevertheless, its application contributes to reduced NOX concentrations upstream of SCR converters, and thus facilitates their operation. Therefore, it is necessary to use NOX selective catalytic reduction converters.
Selective chemical reduction is the mechanism that enables the removal of nitrogen oxides in the presence of oxygen. Depending on the type of reducing agent, we can distinguish two types of selective chemical reduction:
- Reduction with ammonia (NH3-SCR)
In the NH3-SCR systems, gaseous ammonia can be released from ammonia sorbents or ammonium salt decomposition, and it can be introduced into exhaust gases [8] at any temperature, especially under low load conditions of the internal combustion engine. For SCR converters active at these temperatures, NOx emission can be lowered at temperatures well below 200 °C.
As ammonia sorbents [9], MgCl2 composites, can be used, with various carbon materials such as graphite or graphene.
A commonly used method of delivering ammonia [10] to the NH3-SCR converter is the injection of a water urea solution (AdBlue fluid), from which gaseous ammonia is obtained in thermolysis processes. The dimensions of the sprayed AdBlue droplets [10] have a significant influence on the effectiveness of selective catalytic reduction of NOx.
The water urea solution subjected to hydrolysis tends to form sediments which are difficult to deal with during operation. Several combinations of urea decomposition reactions and the mechanisms of sediment formation were presented in [11] paper. A significant problem of effective NOx reduction is [12] the appropriate control of AdBlue injection in an open loop or more precisely in a closed loop, taking into account the fact of gaseous ammonia storage.
The most frequently used catalytically active materials [13,14] are V2O5/TiO2. or V2O5/WO3/TiO2 oxides. Also zeolites are used as catalytically active materials [15]. Problems with unburned hydrocarbons stored in zeolites with large pores motivated the search for materials with small pores. The chabazite reactors containing Cu or Fe ions with the designation Cu/SSZ-13 or Fe/SSZ-13 were developed for the application of NH3-SCR catalysis [15]. Despite these disadvantages and because of the high NOX conversion to N2, these converters are widely used in LDV and HDV vehicles.
- Reduction with hydrocarbons (HC-SCR), or their derivatives containing oxygen, light alcohols in most cases. These converters have the characteristic qualities:
- ○
- Achieve [16] relative low NOX conversion rate,
- ○
- NOX conversions are achieved [16] only in a narrow range of high temperatures,
- ○
- they require high doses of hydrocarbons due to their low selectivity, which contributes to higher converter temperatures [16,17]. It is often observed that the higher the dose of injected hydrocarbons, the wider the NOX reduction temperature range, yet a high dose of injected hydrocarbons may cause an increase in the temperature of the converter to levels that are uncommon for oxidising converters used in compression-ignition engines. An injection of 3% of the fuel dose upstream of the HC-SCR converter causes an increase in the temperature of the converter’s bed by 30 °C, while a 6% fuel injection increases the temperature by 50 °C.
As catalytically active material [17,18], dispersed silver on Al2O3 is often used, and as reducing agents, hydrocarbons and light alcohols. In order to increase the activity of the silver catalytic converter [19], a doping of the intermediate layer with Al2O3 with MgO and CeO2 oxides was used. Zeolites doped with metal ions [20] are often used as active materials in HC-SCR technology. Thus, in the paper [21] the influence of the use of zeolites substituted with metal ions and other catalytically active materials of different surface acidity, on the NOx reduction activity, was investigated. It has been found that the most active HC-SCR converters should have strong acid places, an active phase such as copper or cobalt, or elements such as silver or a mixture of metals for broader activity. To reduce deactivation, the materials should also contain noble metals such as platinum or rhodium. The effect of hydrogen addition to various hydrocarbon reducing agents on the NOx reduction efficiency was carried out [22] both in the laboratory experiments and on an engine test bench at temperatures below 315 °C on an Ag/Al2O3 converter with 2.5% silver mass content. Hydrogen increased the NOx reduction efficiency at low temperatures (245–315 °C).
The catalytic reduction of NOx on an Ag/Al2O3 catalytic converter was also investigated with the use of such reagents as [23] liquid hydrocarbons (GTL) and butanol. It has been proven that the obtained effects result from the high reactivity, polarity and diffusivity of butanol in the catalytic converter, increasing the NOx conversion.
The phenomenon of cold plasma was used in the research on increasing the NOx reduction activity, especially at low temperatures. Thus, the efficiency of NOx reduction by HC SCR technology with catalytic converters based on Pt and Ag on the Al2O3 support at various temperatures [24] was evaluated using hydrogen and hydrocarbons as reducers supplied directly or generated on an engine stand by plasma reforming. The cold plasma generated in a corona discharge reactor [25] was used to reform diesel fuel for selective catalytic reduction (HC-SCR) of NOx on Ag/Al2O3 catalytic converters.
2. Preparation of Ag/Al2O3-SiO2 Converters
Converters intended to reduce nitrogen oxides are made of the following elements:
- A substrate in the form of a steel foil monolith,
- A foundation layer made of aluminium polyphosphate,
- A catalytic carrier being a mixture of al2o3 and sio2 oxides,
- Catalytically active silver applied onto the carrier.
The undesired process of converting active and large surface forms of γ-Al2O3 to α-Al2O3 can be prevented by introducing into the carrier elements or compounds that thermally stabilise aluminium oxide variants. SiO2, produced through the hydrolysis of Si(OC2H5)4, is an example of such a compound [26]. An additional positive effect of introducing SiO2 into the carrier layer may be an increase in the acidity of the carrier surface. The ceramic foundation layer, i.e., the one made of aluminium polyphosphate, is coated with a layer of silver carrier composed of aluminium hydroxide with an admixture of silicon hydroxide. Al2O3 and SiO2 are produced in the firing process to form the actual layer of the catalytic carrier. This layer is applied using the sol-gel process from the Al(OC4H9)3 and Si(OC2H5)4 solution subjected to hydrolysis. Figure 1 shows scheme of the sol-gel method used to produce aluminium oxide with an admixture of silicon dioxide.
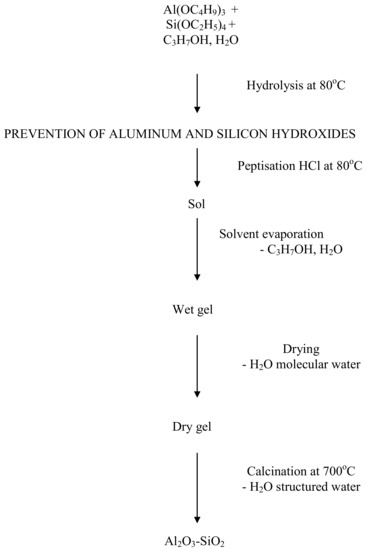
Figure 1.
Schemes of the sol-gel method used in order to produce aluminium oxide with an admixture of silicon dioxide.
The catalytically active element (silver) is applied onto the carrier layers through impregnation. The following converter models were prepared with the following dimensions: Φ30 mm and length of 60 mm and a channels density of 32/cm2 for testing catalytic activity:
- Al2O3-SiO2 converter-carrier—the intermediate layer with Al2O3-SiO2 carrier applied onto the steel foil monolith,
- IAg/Al2O3-SiO2 converter—the carrier was impregnated with silver nitrate solution, which was used to coat the sample with the Al2O3-SiO2 carrier applied onto the steel foil monolith in order to achieve a silver content of 2 g/dm3,
- IIAg/Al2O3-SiO2 converter—also in this case the carrier was impregnated with a silver nitrate solution and the silver content achieved was 4 g/dm3.
The following samples were made for testing surface acidity, porosity and silver dispersion.
- Steel foil plates covered with the Al2O3-SiO2 carrier without aluminium polyphosphate base.
- Steel foil plates covered with the Al2O3-SiO2 carrier without an aluminium polyphosphate base, impregnated with a nitrate solution, obtaining a silver content of 4 g/dm3.
The converters and plates, mentioned above, were dried at 100 °C for three hours. Ag/Al2O3-SiO2 converters were obtained through a thermal disintegration of silver nitrate at 500 °C for five hours.
3. Research Part
The aim of the article was to build HC-SCR converters using catalytically active silver and to characterize the properties of the method of removing nitrogen oxides through their selective catalytic reduction using hydrocarbons on the developed Ag/Al2O3-SiO2 converter, in particular:
- Evaluation of the physicochemical properties, significant for the catalytic activity, of the Al2O3-SiO2 carrier surfaces and the Ag/Al2O3-SiO2 silver coated carrier, by examining the structure, porosity and acidity of the carrier surface and examining the degree of silver dispersion;
- Proving the catalytic activity of the catalytic support Al2O3-SiO2;
- Evaluation of the catalytic activity of a converter with various silver loads;
- Determination of the reduction ratio of NO2 and NO on the reducing catalytic converter depending on the conversion temperature and the addition of reducing agent in the form of propane.
The work was carried out in the following stages:
- Stage 1
- NOx conversion tests on the Al2O3-SiO2 carrier-reactor and the IAg/Al2O3-SiO2 and IIAg/Al2O3-SiO2 reactors depending on the temperature of the catalytic process with a constant dose of the reducer placed in an electric tubular furnace enabling setting the temperature of reactor models up to 700 °C with actual diesel engine exhaust gases flow.
- Stage 2
- Testing the acidity of the surface of the carrier in the form of a powder isolated from a steel foil coated with an Al2O3-SiO2 carrier without an aluminum polyphosphate base, using ammonia desorption method.
- Stage 3
- Measurements of the specific surface area and porosity of a catalytic support in the form of a powder isolated from a steel foil coated with an Al2O3-SiO2 support without an aluminum polyphosphate base: specific BET surface,
- volume and surface of mesopores distribution by the BJH method,
- volume and surface of micropore distribution by the DFT method.
- Stage 4
- Tests of silver dispersion on IIAg/Al2O3-SiO2 powder isolated from a coated steel foil without an aluminum polyphosphate base, by the method:
- oxygen adsorption on silver crystallites,
- analysis of the images from the transmission electron microscope.
- Stage 5
- Tests of NO, NO2 and C3H8 conversions and propane selectivity in the reduction of NO to N2 of the IIAg/Al2O3-SiO2 converter model placed in an electric tubular furnace depending on the NOx conversion temperature at a constant dose of the reducer (C3H8) and depending on the dose of the reducer at a constant NOx conversion temperature.
3.1. Comparative Studies of NOx Conversion in a Carrier-Converter and Silver-Impregnated Converters
The tests were carried out using models of Al2O3-SiO2, IAg/Al2O3-SiO2 and IIAg/Al2O3-SiO2 converters. The models of the converters were placed in a converter in the form of a quartz glass tube placed in an electric tube kiln. A portion of the exhaust gas from a Perkins 854E-E34TA compression-ignition engine fuelled with low sulphur (8 ppm) diesel, operating at set parameters (engine speed and load) was transported at a constant relative volume velocity, through a heated gas route, to the converter with temperature set using a PID controller. Concentrations of exhaust gases components measured with the AVL AMA i60 analyzer before and after the catalytic converter were: NOX = 240 ppm, CO2 = 4.4%, CO = 0.04%, HC = 840 ppm, O2 = 14.5%, H2O = 4.2%. The relative volumetric flow velocity of the exhausts was SV = 14,000 h−1.
The gas temperature upstream and downstream of the converters was measured with thermocouples. Propane was added to the exhaust gas produced by the engine, with its flow being regulated by a rotameter. After leaving the converter, the gases were directed, via gas route, to the analyzers measuring CO and CO2 concentrations using the NDIR, O2 by the PMD method, NO and NO2 using the CLD method and THC using the FID method.
For comparison purposes, Figure 2 presents the calculation results for the conversion of nitrogen oxides for each of the tested converter models. The highest nitrogen oxides reduction rate in the widest range of exhaust gas temperature was achieved for the IAg/Al2O3-SiO2 and IIAg/Al2O3-SiO2 converters. At approx. 400 °C, the nitrogen oxides reduction rate was 50%, while at temperatures above 500 °C the rate was approx. 80–90%. Between 500 and 600 °C, a high reduction rate of 85% was maintained for the IAg/Al2O3-SiO2 converter, and of 90% for the IIAg/Al2O3-SiO2 converter.
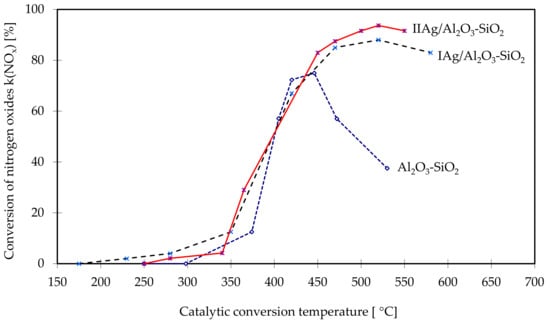
Figure 2.
Conversion of nitrogen oxides in the tested converters as a function of the temperature of the catalytic process.
The effect of silver coating of the Al2O3-SiO2 carrier on the NOx conversion is clearly seen at temperatures above 400 °C when the conversion on the carrier sharply decreases from about 70% to 38% at 530 °C.
Unfortunately, the catalytic properties occur at relatively high temperatures T > 400 °C, although within quite a wide range of them. At above 500 °C, a drop in the rate of reduction of nitrogen oxides is observed, which is insignificant due to the temperature of exhaust gases produced by compression-ignition engines used on LDVs in the European NEDC and WLTP emission test cycles and the US FTP75 cycle, do not reach these temperatures.
3.2. Tests of Acidity of the Surface of the Al2O3-SiO2 Carrier
Tests found in the available literature suggest that there is a direct correlation between the catalytic activity in terms of the reduction of nitrogen oxides and the acidic properties of the active surface of the converter carrier [27]. As the test results for the Al2O3-SiO2 carrier clearly demonstrate its activity in the process of reducing nitrogen oxides, tests were conducted in order to identify the acidity of the surface of the Al2O3-SiO2 carrier used for the construction of the Ag/Al2O3-SiO2 converter.
The acidity of the surface of the catalyst carrier was determined using a Shimadzu GCMS-QP2020 gas chromatograph featuring a TCD. Argon was used as the carrier gas. Ammonia vapour was dosed until the ammonia peak occurred, then the sample was heat treated at approx. 100 °C until the signal from the ammonia disappeared, at which point the ammonia desorption was initiated:
- 3 min at approx. 100 °C (372 K),
- sample heating at the rate of 12 degrees/minute up to approx. 420 °C (692 K),
- heat treatment of the sample for 30 min until the signal from ammonia disappears.
The area between the curve of the peaks resulting from desorption and the zero line was calculated and compared with the area in the test, where 4 cm3 of ammonia was completely adsorbed on the strong centres of the catalyst.
Table 1 presents the amount of adsorbed ammonia, as well as the area of the converter coated with ammonia, calculated based on the BET model (a single particle can be adsorbed on a single acid centre of the converter), which can serve as the measure of the acidity of its surface. The calculations were performed for two temperature ranges; 372–692 K and 600–692 K.

Table 1.
Results of determination of acidity of the surface of the converter’s carrier.
The results presented in Table 1 indicate that the prepared carrier offers high surface acidity. The concentration levels of acidic centres for aluminosilicates amount to approx. 1012–1014 centres per 1 cm2, which corresponds to 0.2–20% occupation of the surface of the catalytic converter. The support developed has a surface area of about 8% greater than the acid surface present in aluminosilicates in the temperature range essential for NOx conversion.
3.3. Studies of the Specific Surface Area and Porosity of the Catalytic Support
Measurements of the BET specific surface area of the Al2O3-SiO2 catalytic support were carried out based on the nitrogen adsorption isotherm equation using an ASAP 2420 device (Micromeritics Inc., Norcross, GA, USA). The measured area value was 195.4 m2/g.
Porosity tests were carried out separately for mesopores with the size range 1.7 to 23 nm, using the Barrett-Joyner-Halenda (BJH) method [28], and for micropores with the size range 0.8 to 2.2 nm, using the Density Functional Theory (DFT) method [29].
3.3.1. Tests of Distribution of Volume and Surface of Mesopores of the Catalytic Carrier
The relationship between vapour pressure over a curved surface characterised by rk curvature radius, and vapour pressure over the po surface has been described by the Kelvin equation:
In order to quantitatively describe mesoporous solids, the phenomenon of capillary condensation is used. This phenomenon occurs in mesopores where the diameter is, according to the IUPAC classification, within the range from 2–50 nm.
Figure 3 presents the results of measurements, carried out using the BJH method, of the total surface of mesopores, as well as the increase in their surface during adsorption and desorption of nitrogen, whereas Figure 4 presents the total volume and increase in the volume of mesopores.
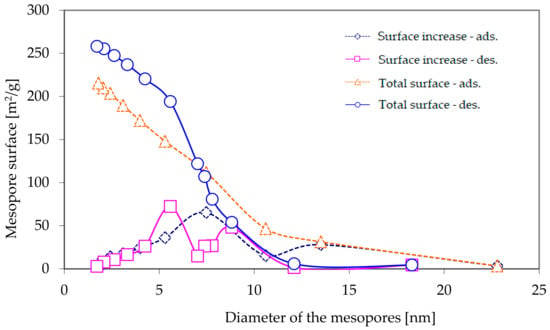
Figure 3.
The total surface of mesopores and the increase in the surface of the mesopores as a function of their diameter, as calculated using the BJH method, based on nitrogen adsorption and desorption isotherms.
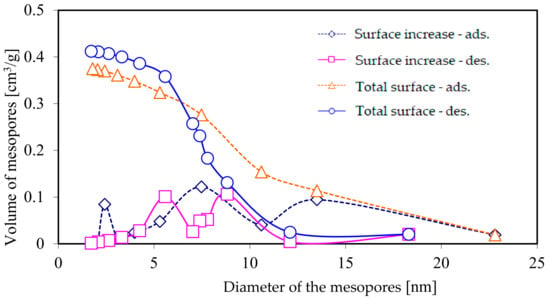
Figure 4.
The total volume of mesopores and the increase in the volume of the mesopores as a function of their diameter, as calculated using the BJH method, based on nitrogen adsorption and desorption isotherms.
3.3.2. Tests on the Distribution of Volume and Surface of Micropores of the Catalytic Carrier
The tests were carried out with the method using isotherms calculated based on the density functional theory, determining the distribution of pore size with the set cavity geometry based on experimental isotherms.
Figure 5 presents the relationship between the surface/volume of the micropores and their width, as calculated using the DFT method based on the nitrogen adsorption isotherms.
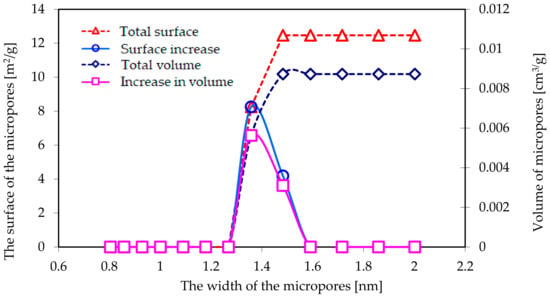
Figure 5.
The relationship between the surface/volume of the micropores and their width as calculated with DFT method based on the nitrogen adsorption isotherms.
3.4. Tests of Silver Dispersion on IIAg/Al2O3-SiO2 Powder Isolated from a Steel Foil Coated without an Aluminium Polyphosphate Base
Oxygen Absorption on Silver Crystallites Method
The selective chemisorption of gases is the most common method for determining the dispersion of metal and medium-sized metal particles when examining catalytic converters. The given gas is subjected to chemisorption in such conditions that enable the formation of a monolayer of this gas on the surface of the metal, with no significant presence of chemisorption on the carrier. When examining the degree of dispersion of silver, molecular oxygen is the most suitable gas, for which the process of adsorption is as follows:
Prior to taking the primary measurements, a 300 mg sample of the catalyst in the form of IIAg/Al2O3-SiO2 powder was reduced in a stream of hydrogen at 400 °C for one hour in order to remove the impurities remaining after the sample preparation process.
Oxygen adsorption was measured through the following steps:
- Oxidation of the sample with oxygen doses at 500 °C,
- The elimination of oxygen from the sample in a stream of helium,
- Supplying several 109 mm3 doses of oxygen in a stream of helium with a flow rate of 30 cm3/min every 90 s at 170 °C.
Figure 6 presents the process of adsorption of a dose of oxygen on the tested sample. Assuming that stoichiometry for the adsorption of atomic oxygen on silver O/Ag = 1, it was determined that the tested sample contains 28 µmol of surface atoms of silver. The average size of crystallites [30] can be determined based on the following relationship:
L20 = 1.44 C
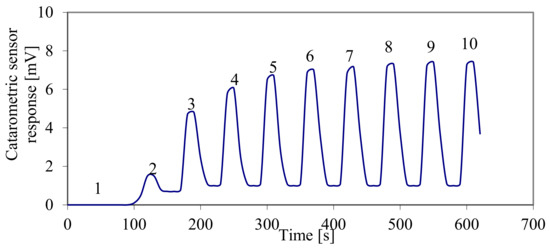
Figure 6.
The process of adsorption of 10 doses of oxygen with the volume of 109 mm3 in a stream of helium on the tested IIAg/Al2O3-SiO2 catalytic converter sample. Oxygen concentrations downstream of the sample were proportional to the voltage signal of the thermal conductivity detector.
The average size of crystallites determined using this method corresponds to the crystallite size in a homogenous substitute set of crystallites, and to an identical total surface of all crystallites in the actual set. In order to determine the silver content, the sample was subjected to an elementary analysis. According to the analysis results, the silver content in the examined sample was 8.0% of the sample mass, which means that the average size of silver crystallites in the examined sample was approx. 12 nm.
3.5. Method of Analyzing Images from the Transmission Electron Microscope
In order to determine the size of silver crystallites, the converter sample, in the form of Ag/Al2O3-SiO2 powder, was examined using a JEOL 2000EX transmission electron microscope. Figure 7 presents an image of the preparation, magnified 100,000 times, as seen through the microscope. There are precipitations with the size of a few nanometres, and those with the size of a dozen or so nanometres visible in the amorphous substrate.
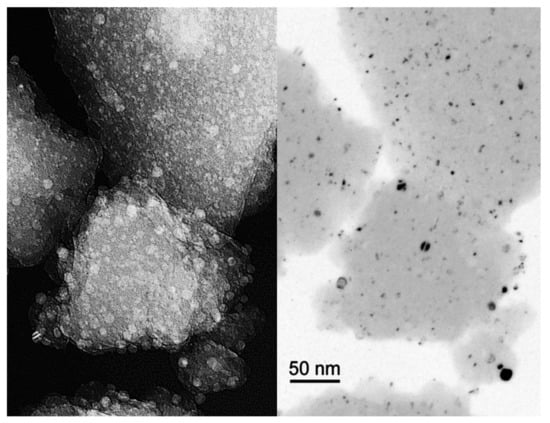
Figure 7.
Example TEM images of the preparation, magnified 100,000 times (on the left-hand side) and the image obtained through the use of the dark field method, which enables the crystallites to be counted (on the right-hand side).
Three microscopic images were analysed in order to better characterise and describe the microstructure of the catalyst in terms of the distribution of nanocrystallites of silver. The dimensions of approx. 1000 crystallites were measured. The obtained set of results was sorted into classes, with the width of each class equal to 1 nm. Figure 8 presents a graphic representation of the results of the measurements in the form of a relative count and a relative cumulative number.
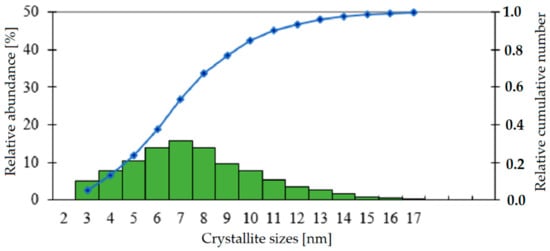
Figure 8.
Relative count and cumulative number for the sizes of crystallites of silver, as determined based on TEM images.
The data presented in Figure 8 indicates that, in the tested sample, containing approx. 1000 crystallites, the largest share (approx. 15%) is represented by crystallites with a size of approx. 7 nm, and that their dimensions range from approx. 2 nm to approx. 18 nm.
The dispersion of noble metals typical of three-way catalysts used in the automotive industry, constructed with similar methods based on platinum, palladium and rhodium, is similar [31]. The average size of crystallites ranges from 3 to 5 nm. According to the work [32], the size of platinum crystallites in a new TWC is approx. 9 nm.
3.6. Tests of Catalytic Activity of a Silver-Impregnated Carrier
Tests of the IIAg/Al2O3-SiO2 silver-based converter model were conducted in two series. In the first series, an exhaust gas sample from the Perkins 854E-E34TA engine was passed through the converter at a constant relative volume velocity and at various temperatures of the catalytic conversion process, adjusted with the temperature of the electric furnace. Nitrogen oxides were reduced through the use of a constant amount of propane admixture upstream of the catalytic converter. In the course of the second series, the exhaust gas sample was passed through the converter at a constant relative volume velocity and at a constant process temperature of 500 °C, yet with various amounts of propane admixture regulated using the rotameter valve. The concentrations of exhaust gas components measured with the AVL AMA i60 analyser are described in Section 3.1.
The first stage in the reduction of NOx is the reduction of NO2 to NO at the temperature range from 250 to 400 °C. This is an almost 100% reduction stage with T50 temperature equal to 300 °C. At 350 °C, the reduction of NO to N2 and N2O begins, reaching its peak value of ~90% at above 500 °C, with T50 at the level of 420 °C.
The conversion of propane at a temperature corresponding to the maximum reduction of NOX reaches 40%. The selectivity of propane in the process of reducing NO to N2 (in reaction conditions with an excess of propane and without considering the formation of N2O) reaches its maximum value of approx. 8%. Figure 9 shows (described above) conversion of nitrogen oxide and propane as well as selectivity of propane as a function of the temperature of the catalytic proces.
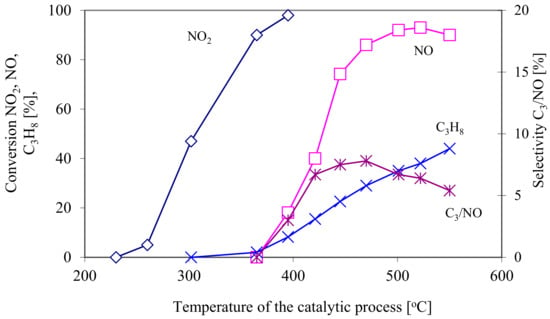
Figure 9.
Conversion of nitrogen oxide and propane as well as selectivity of propane as a function of the temperature of the catalytic process.
The tests for the conversion of nitrogen oxides and propane selectivity, depending on the propane dose, were conducted at a constant temperature of the catalytic conversion process equal to 500 °C (Figure 10).
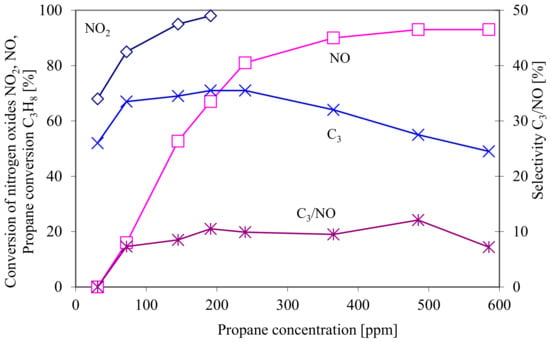
Figure 10.
The conversion of nitrogen oxides and of propane, as well as the selectivity of propane as a function of propane concentration upstream of the catalytic converter at the constant temperature of the catalytic process T = 500 °C.
An almost 100% conversion of NO2 requires the concentration of propane at 200 ppm, whereas the maximum reduction of NO at the level of approx. 90% requires the addition of more than 400 ppm of propane.
The selectivity of propane in the process of reducing NO to N2 was determined assuming that reduction occurs in the presence of oxygen, but without its participation, as illustrated by the following reaction:
10 NO + C3H8 → 3CO2 + 4H2O + 5N2
Selectivity was defined as follows:
In those conditions, nitrous oxide was produced in an amount corresponding to approx. 10% of the initial NOX amount. The selectivity of propane in the process of reducing NO to N2 (without considering the formation of N2O) reaches its maximum value of approx. 10%.
4. Discussion
The results can be summarised as follows:
- (1)
- The catalytic Al2O3-SiO2 support developed is a good basis for depositing dispersed silver, because it is characterized by a large specific surface (BET surface equal to about 195 g/dm3) and an extensive network of meso and micropores, as well as surface acidity exceeding by 8% the values of aluminosilicates. This support is active in reducing nitrogen oxides, achieving a maximum conversion of 70% at the temperature of 450 °C.
- (2)
- Silver crystallites applied by impregnation method have dimensions in the order of 7–12 nm and they are comparable with the dimensions of platinum crystallites in the TWC converters.
- (3)
- In the course of the experiment, an approx. 90% reduction of nitrogen oxides was achieved for the silver-based catalytic converter. Unfortunately, the catalytic properties occur at relatively high temperatures (T > 400 °C), but within a rather wide range of temperatures. Above 500 °C, a drop in the rate of reduction of nitrogen oxides is observed, which is insignificant in the case of exhaust gases produced by compression-ignition engines. Unfortunately, the selectivity of propane in the process of NOx reduction is low and does not exceed 20%, which makes it necessary to supply the reducer in high concentrations.
- (4)
- The reduction of nitrogen oxides is a two-stage process. In the first stage, NO2 is reduced at a temperature of approx. 200 °C; in the second stage, NO is reduced to N2 at a temperature ranging from 200 to 550 °C.
- (5)
- The activity of the IIAg/Al2O3-SiO2 catalytic converter, measured using the conversion rate of NOX, was examined at relatively low values of the relative volume velocity of the exhaust gas flow of 14,000 h−1. NOx conversion at higher SV values is still to be investigated; the same applies to the construction of a complete catalytic converter with a relatively large volume, which could be fitted into an actual petrol engine.
- (6)
- Replacement of the PGM group metals with silver may contribute to a significant reduction in production costs of this type of reactors. Thus, for 1 dm3 of DOC volume containing usually 1g/dm3 of platinum, the cost of precious metal is about $34, while the corresponding cost of silver in the amount of 4g/dm3 is about $3.2, which means that it is about 10 times smaller.
Based on the results produced, it can be concluded that there is a real possibility for the development of an HC-SCR catalytic converter based on a IIAg/Al2O3-SiO2 converter for compression-ignition engines. The development of a system for the catalytic oxidisation of excessive hydrocarbons used for NOX reduction will be an important problem to be solved in the process of designing a utilitarian converter model.
Author Contributions
S.W.K. and P.O. conceived and designed the experiments; S.W.K., P.O. and M.Ś. performed the experiments and processed the data; S.W.K., P.O. and M.Ś. analysed the data; S.W.K. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Symbols
| φ | the angle of wetting for the meniscus of the liquid in relation to the wall of the capillary [º] |
| δ | surface tension of the liquid [N/m] |
| C | silver content of the sample [% of the mass] |
| c(C3)k | propane concentration downstream of the catalytic converter |
| c(C3)p | propane concentration upstream of the catalytic converter |
| c(NO)k | NO concentration downstream of the catalytic converter |
| c(NO)p | NO concentration upstream of the catalytic converter |
| L20 | average diameter of crystallites of silver [nm] |
| p | vapour pressure over a flat surface [Pa] |
| po | vapour pressure above a curved (cylindrical) surface [Pa] |
| R | universal gas constant [J/(mol · K)] |
| s(C3/NO) | selectivity of propane in the nitrogen reduction process |
| T | temperature [K] |
| Vm | liquid volume [mol] |
References
- Kruczyński, S.W.; Merkisz, J.; Ślęzak, M. Zanieczyszczenia Powietrza Spalinami Przez Transport Samochodowy; Wydawnictwo WKiŁ: Warszawa, Poland, 2019. (In Polish) [Google Scholar]
- Puchalski, A.; Komorska, I.; Ślęzak, M.; Niewczas, A. Synthesis of naturalistic vehicle driving cycles using the Markov Chain Monte Carlo method. Eksploat. Niezawodn. 2020, 22. [Google Scholar] [CrossRef]
- Wiśniowski, P.; Ślęzak, M.; Niewczas, A.; Szczepański, T. Method for synthesizing the laboratory exhaust emission test from car engines based on road tests. IOP Conf. Ser. Mater. Sci. Eng. 2018, 421, 042080. [Google Scholar] [CrossRef]
- Zamboni, G.; Moggia, S.; Capobianco, M. Effects of a dual-loop exhaust gas recirculation system and variable nozzle turbine control on the operating parameters of an automotive diesel engine. Energies 2017, 10, 47. [Google Scholar] [CrossRef]
- Johnson, T.V. Diesel emissions in review. SAE Int. J. Engines 2011, 4, 143–157. [Google Scholar] [CrossRef]
- Li, L.; Lin, W.; Zhang, Y. A new dynamic injection system of urea-water solution for a vehicular select catalyst reduction system. Energies 2017, 10, 12. [Google Scholar] [CrossRef]
- Latha, H.S.; Prakash, K.V.; Veerangouda, M.; Maski, D.; Ramappa, K.T. A Review on SCR System for NOx Reduction in Diesel Engine. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1553–1559. [Google Scholar] [CrossRef]
- Karkamkar, A.; Deshmane, C. Next Generation SCR-Dosing System Investigation 2016, Annual Merit Review (Report). Available online: https://www.energy.gov/sites/prod/files/2016/06/f32/ace027_karkamkar_2016_o_web.pdf (accessed on 9 June 2016).
- Grimaldos Osorio, N. Novel Ammonia Storage Materials for SCR Systems: Carbon Materials–Salt Composites. 2019. Available online: https://www.diva-portal.org/smash/get/diva2:1333451/FULLTEXT01.pdf (accessed on 15 September 2020).
- Liao, Y.; Eggenschwiler, P.D.; Spiteri, A.; Nocivelli, L.; Montenegro, G.; Boulouchos, K. Fluid dynamic comparison of AdBlue injectors for SCR applications. SAE Int. J. Engines 2015, 8, 2303–2311. [Google Scholar] [CrossRef]
- Bernhard, A.M.; Peitz, D.; Elsener, M.; Schildhauer, T.; Kröcher, O. Catalytic urea hydrolysis in the selective catalytic reduction of NOx: Catalyst screening and kinetics on anatase TiO2 and ZrO2. Catal. Sci. Technol. 2013, 3, 942–951. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, H.; Gao, Y. Diesel engine SCR control: Current development and future challenges. Emiss. Control Sci. Technol. 2015, 1, 121–133. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Z.; Wang, Y.; Fan, R.; Zhang, C.; Wang, Y.; Zhang, S. Ce and Zr Modified WO3-TiO2 Catalysts for Selective Catalytic Reduction of NOx by NH3. Catalysts 2018, 8, 375. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, S.; Pantaleo, G.; Liotta, L.F. WO3–V2O5 Active Oxides for NOx SCR by NH3: Preparation Methods, Catalysts’ Composition, and Deactivation Mechanism—A Review. Catalysts 2019, 9, 527. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Kollar, M.; Wang, Y.; Szanyi, J.; Peden, C.H. Understanding ammonia selective catalytic reduction kinetics over Cu/SSZ-13 from motion of the Cu ions. J. Catal. 2014, 319, 1–14. [Google Scholar] [CrossRef]
- Kruczyński, S.W.; Kwiatkowski, M.; Zieliński, T. Badania redukcji NOx węglowodorami na przykładzie katalizatorów wysoko- i niskotemperaturowych w spalinach silnika o zapłonie samoczynnym. Czas. Tech. Mech. 2004, 6-M, T-2, 395–401. (In Polish) [Google Scholar]
- He, H.; Yu, Y. Selective catalytic reduction of NOx over Ag/Al2O3 catalyst: From reaction mechanism to diesel engine test. Catal. Today 2005, 100, 37–47. [Google Scholar] [CrossRef]
- Thomas, J.F.; Lewis, S.A.; Bunting, B.G.; Storey, J.M.; Graves, R.L.; Park, P.W. Hydrocarbon selective catalytic reduction using a silver-alumina catalyst with light alcohols and other reductants (No. 2005-01-1082). SAE Tech. Pap. 2005. [Google Scholar] [CrossRef]
- Valanidou, L.; Theologides, C.; Zorpas, A.A.; Savva, P.G.; Costa, C.N. A novel highly selective and stable Ag/MgO-CeO2-Al2O3 catalyst for the low-temperature ethanol-SCR of NO. Appl. Catal. B Environ. 2011, 107, 164–176. [Google Scholar] [CrossRef]
- Lee, K.; Ogita, Y.; Sato, S.; Kosaka, H. NOx reduction with the HC-SCR System over Zeolite Based Catalyst in Diesel Applications. Catalyst 2015, 200, 8. [Google Scholar]
- Mrad, R.; Aissat, A.; Cousin, R.; Courcot, D.; Siffert, S. Catalysts for NOx selective catalytic reduction by hydrocarbons (HC-SCR). Appl. Catal. A Gen. 2015, 504, 542–548. [Google Scholar] [CrossRef]
- Gu, H.; Chun, K.M.; Song, S. The effects of hydrogen on the efficiency of NOx reduction via hydrocarbon-selective catalytic reduction (HC-SCR) at low temperature using various reductants. Int. J. Hydrogen Energy 2015, 40, 9602–9610. [Google Scholar] [CrossRef]
- Herreros, J.M.; George, P.; Umar, M.; Tsolakis, A. Enhancing selective catalytic reduction of NOx with alternative reactants/promoters. Chem. Eng. J. 2014, 252, 47–54. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.O.; Kim, K.T.; Song, Y.H.; Kim, E.; Han, H.S. Hydrogen in plasma-assisted hydrocarbon selective catalytic reduction. Int. J. Hydrogen Energy 2012, 37, 3225–3233. [Google Scholar] [CrossRef]
- Bao, X.; Malik, M.A.; Norton, D.G.; Neculaes, V.B.; Schoenbach, K.H.; Heller, R.; Conway, K.R. Shielded sliding discharge-assisted hydrocarbon selective catalytic reduction of NO x over Ag/Al 2 O 3 catalysts using diesel as a reductant. Plasma Chem. Plasma Process. 2014, 34, 825–836. [Google Scholar] [CrossRef]
- Darkowski, A.; Przyłuski, J.; Maron, E.; Kruczyński, S.W. Preparatyka i właściwości reaktora katalitycznego do redukcji NO ze spalin silników o zapłonie samoczynnym. 23th International Conference on Combustion Engines. J. KONES 1997, 4, 121–139. (In Polish) [Google Scholar]
- Pârvulescu, V.I.; Grange, P.; Delmon, B. Catalytic removal of NO. Catal. Today 1998, 46, 233–316. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Olivier, J.P.; Conklin, W.B.V.; Szombathely, M.V. Determination of pore size distribution from density functional theory: A comparison of nitrogen and argon results. Stud. Surf. Sci. Catal. 1994, 87, 81–89. [Google Scholar]
- Kruczyński, S.W. Wybrane Metody Ograniczania Emisji Tlenków Azotu w Silnikach o Zapłonie Samoczynnym; Wydawnictwo Instytutu Technologii Eksploatacji: Radom, Poland, 2002. [Google Scholar]
- Tabib Zadeh Adibi, P. In situ Studies of Platinum Catalyst Sintering; Chalmers University of Technology: Gothenburg, Sweden, 2016. [Google Scholar]
- Kruczyński, S.W. Maintenance of three way catalytic converter—Thermal deactivation. Eksploat. I Niezawodn. Maint. Reliab. 2012, 14, 239–242. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).