Viability of Various Sources to Ignite A2L Refrigerants
Abstract
1. Introduction
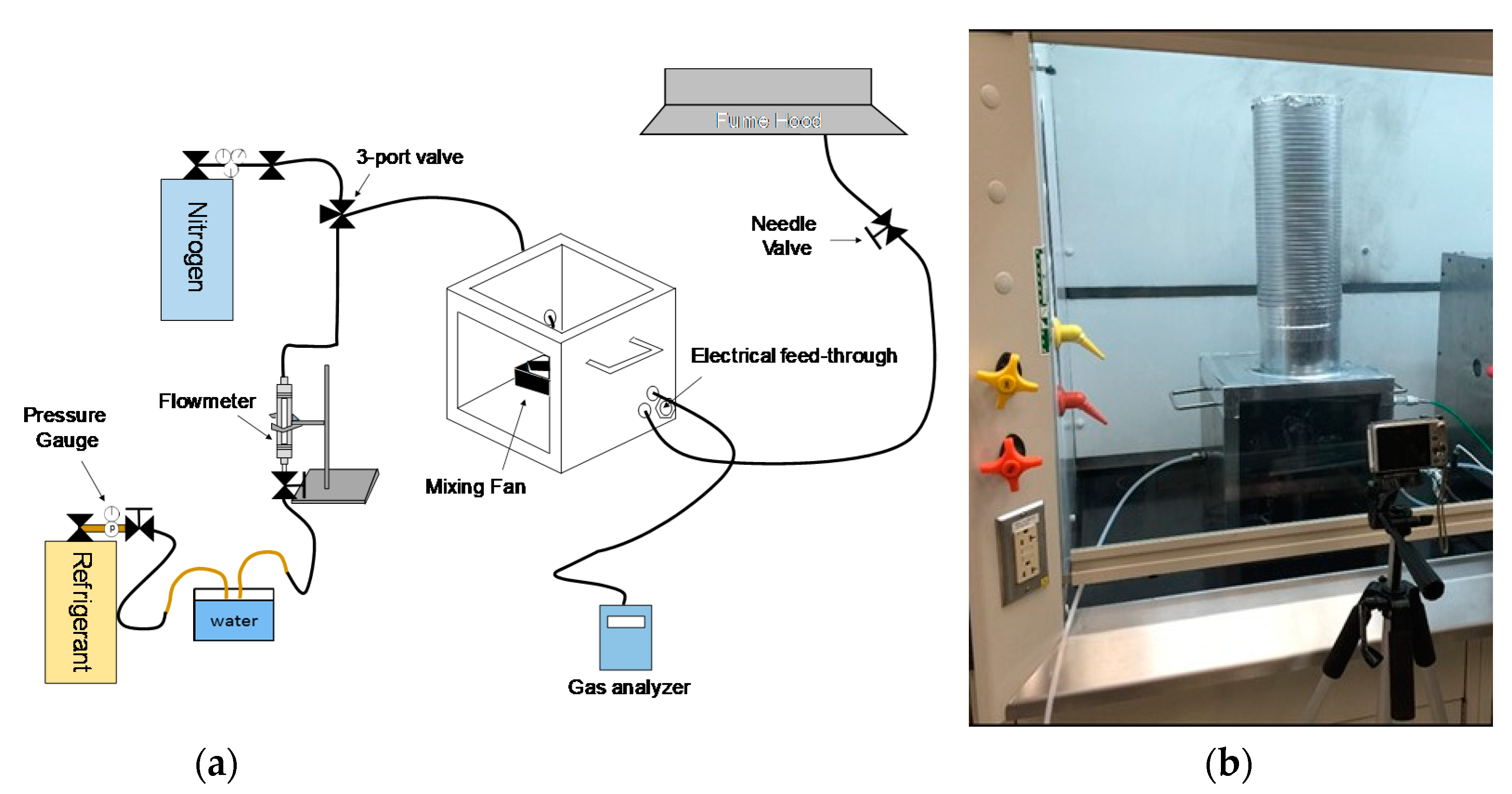
2. Experimental Methods
3. Results
3.1. Premixed Tests with Resistively Heated Wires
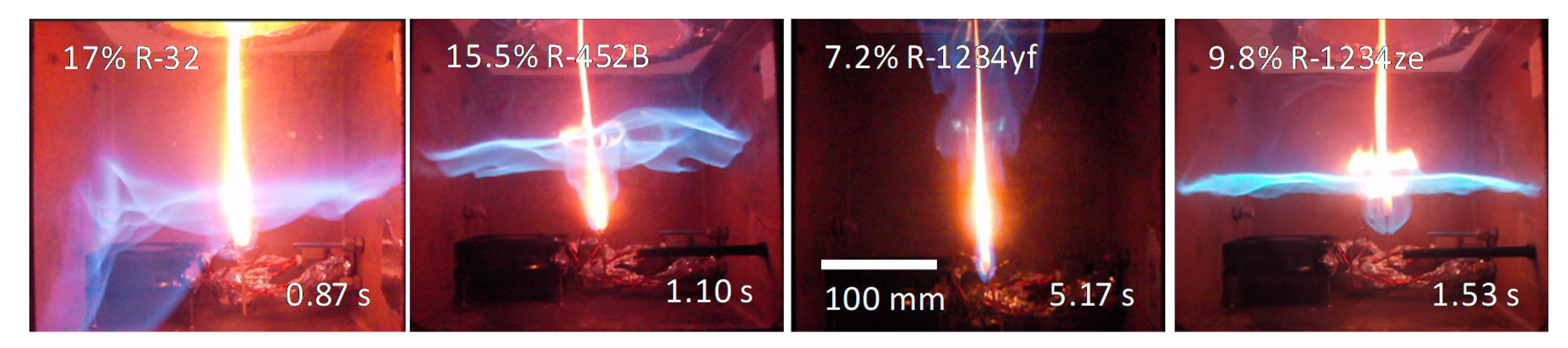
3.2. Premixed Tests with Open Flames
3.3. Jet Tests with Open Flames
3.4. Non-Viable Ignition Sources
3.5. Halocarbons as Fuels or Suppressants
4. Conclusions
- NiCr wires heated resistively to 740 °C and open flames (burning safety matches and butane) are viable ignition sources for quiescent premixtures of refrigerant and air above the LFL. Large deflagrations were observed that propagated up to the ceiling and then down to the floor.
- When the refrigerant concentration was increased slowly, every candle or butane flame extinguished before initiating any large deflagrations.
- Eleven other potential residential ignition sources did not produce any flames in quiescent stoichiometric refrigerant/air mixtures. These included a smoldering cigarette, a pizeoelectric butane lighter, electric arcs, motors, and various residential heating elements at 540 °C and below.
- Depending on the conditions, these A2L halocarbon refrigerants can act as either fuels or suppressants. They act as fuels for a strong ignition source like a resistively heated hot wire or for an open flame suddenly introduced into a flammable mixture. For weaker sources or refrigerant mixtures below the LFL, they act as suppressants.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| AIT | Autoignition temperature |
| D | Mass diffusivity |
| I | Current |
| L | Approximate diffusion layer thickness |
| LFL | Lower flammability limit |
| LHV | Lower heating value enthalpy of combustion |
| LOI | Limiting oxygen index |
| LPM | Liter per minute |
| Lq | Quenching distance |
| MIE | Minimum ignition energy |
| N | Number of trials |
| Q | Refrigerant volumetric flow rate |
| SL | Laminal flame speed |
| T | Temperature |
| t | Time |
| UFL | Upper flammability limit |
| V | Chamber volume; also excitation |
| X | Refrigerant mole fraction |
References
- Davis, S.G.; Pagliaro, J.L.; DeBold, T.F.; van Wingerden, M.; van Wingerden, K. Flammability and explosion characteristics of mildly flammable refrigerants. J. Loss Prev. Proc. Ind. 2017, 49, 662–674. [Google Scholar] [CrossRef]
- Kujak, S. Flammability and new refrigerant options. ASHRAE J. 2017, 59, 16–24. [Google Scholar]
- Kim, D.K.; Klieger, A.E.; Lomax, P.Q.; McCoy, C.G.; Reymann, J.Y.; Sunderland, P.B. An improved test method for refrigerant flammability limits in a 12 L vessel. Sci. Tech. Built Environ. 2018, 24, 861–866. [Google Scholar] [CrossRef]
- Burrell, R.R.; Pagliaro, J.L.; Linteris, G.T. Effects of stretch and thermal radiation on difluoromethane/air burning velocity measurements in constant volume spherically expanding flames. Proc. Combust. Inst. 2019, 37, 4231–4238. [Google Scholar] [CrossRef] [PubMed]
- Pierantozzi, M.; Tomassetti, S.; Nicola, G. Climate change and refrigerants: Thermodynamic properties of low-GWP fluids for domestic applications and binary systems for low-temperature options. Appl. Sci. 2020, 10, 2014. [Google Scholar] [CrossRef]
- Goetzler, W.; Bendixen, L.; Bartholomew, P. Risk Assessment of HFC-32 and HFC-32/134a (30/70 wt.%) in Split System Residential Heat Pumps; Final Report No. DOE/CE/23810-92; ARTI MCLR Project Number 665-52402; Arthur D. Little, Inc.: Cambridge, MA, USA, 1998; 88p. [Google Scholar]
- Clodic, D.; Riachi, Y. A method for determining practical flammability risk when using refrigerant blends. HVAC&R Res. 2009, 15, 819–834. [Google Scholar]
- Goetzler, W.; Burgos, J. Study of input parameters for risk assessment of 2L flammable refrigerants in residential air conditioning and commercial refrigeration applications. ASHRAE Trans. 2014, 120, 437–448. [Google Scholar]
- Lewandowski, T.A. Risk Assessment of Residential Heat Pump Systems Using 2L Flammable Refrigerants; AHRI Project 8004 Final Report; Air-Conditioning, Heating, and Refrigeration Institute: Arlington, VA, USA, 2012. [Google Scholar]
- Boussouf, A.; Lecoustre, V.R.; Li, H.; By, R.; Sunderland, P.B. Ignition of R-32 and R-410A Refrigerant Mixtures with Lubricating Oil. In Proceedings of the Purdue Conference on Refrigeration and Air Conditioning, West Lafayette, IN, USA, 14–17 July 2014. [Google Scholar]
- Gandhi, P.; Hunter, G.; Haseman, R.; Rodgers, B. Benchmarking Risk by Whole Room Scale Leaks and Ignitions Testing of A2L Refrigerants; AHRTI Project 9007-01 Final Report; Air-Conditioning, Heating and Refrigeration Institute: Arlington, VA, USA, 2017. [Google Scholar]
- Ballanco, J.; McCrudden, C.; Johnson, P. A2L Refrigerants: Safely Addressing Flammability Concerns. Engineered Systems Magazine, 27 November 2019. [Google Scholar]
- ASHRAE, Inc. ANSI/ASHRAE Standard 34-2016, Designation and Safety Classification of Refrigerants; ASHRAE, Inc.: Atlanta, GA, USA, 2016. [Google Scholar]
- Takizawa, K.; Tokuhashi, K.; Kondo, S. Flammability assessment of CH2=CFCF3: Comparison with fluoroalkenes and fluoroalkanes. Hazard. Mater. 2009, 172, 1329–1338. [Google Scholar] [CrossRef]
- Takizawa, K.; Igarashi, N.; Takagi, S.; Tokuhashi, K.; Kondo, S. Quenching distance measurement of highly to mildly flammable compounds. Fire. Saf. J. 2015, 71, 58–68. [Google Scholar] [CrossRef]
- Kim, D.K.; Sunderland, P.B. Risk Investigation of Energy Produced by Potential Ignition Sources in Residential Application; AHRI Project 8017 Final Report; Air-Conditioning, Heating and Refrigeration Institute: Arlington, VA, USA, 2017. [Google Scholar]
- Gradient Corp. Risk Assessment of Refrigeration Systems Using A2L Flammable Refrigerants; AHRI Project 8009 Final Report; Gradient Corp.: Arlington, VA, USA, 2015. [Google Scholar]
- Minor, B.H.; Herrmann, D.; Gravell, R. Flammability characteristics of HFO-1234yf. Process Saf. Progr. 2010, 29, 150–154. [Google Scholar] [CrossRef]
- Babushok, V.I.; Burgess, D.R., Jr.; Hegetschweiler, M.J.; Linteris, G.T. Flame propagation in the mixtures of O2/N2 oxidizer with fluorinated propene refrigerants (CH2CFCF3, CHFCHCF3, CH2CHCF3). Combust. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Spatz, M.; Minor, B. HFO-1234yf A Low GWP Refrigerant For MAC, Honeywell/DuPont Collaboration. In Proceedings of the International Refrigeration and Air Conditioning Conference, Saalfelden, Austria, 13–14 February 2008. [Google Scholar]
- Honeywell. Solstice Propellant Flammability Assessment. 2016. Available online: http://www.crcind.com/crc/HFO-1234ze.pdf (accessed on 26 December 2020).
- Honeywell. Honeywell Solstice yf Refrigerants Bulletin. 2012. Available online: https://www.honeywell-refrigerants.com/europe/wp-content/uploads/2013/03/honeywell-solstice-yf-technical-bulletin.pdf (accessed on 26 December 2020).
- Imamura, T.; Sugawa, O. Physical Hazard Evaluation of A2L-Class Refrigerants using Several Types of Conceivable Accident Scenarios. JSRAE 2013, 35–42. Available online: https://www.jsrae.or.jp/info/2012progress_report_e.pdf (accessed on 26 December 2020).
- Imamura, T.; Sugawa, O. Physical Hazard Evaluation of A2L Refrigerants Based on Several Conceivable Accident Scenarios. JSRAE 2014, 48–57. Available online: https://www.jsrae.or.jp/committee/binensei/2014PR_e_ab.pdf (accessed on 26 December 2020).
- Monforte, R.; Caretto, L. Safety Issues in the Application of a Flammable Refrigerant Gas in MAC Systems: The OEM Perspective; SAE International Paper 2009-07-0541; SAE International: Warrendale, PA, USA, 2009. [Google Scholar]
- Imamura, T.; Kamiya, K.; Sugawa, O. Ignition hazard evaluation on A2L refrigerants in situations of service and maintenance. J. Loss Prev. Proc. Ind. 2015, 36, 553–561. [Google Scholar] [CrossRef]
- Richard, R.G.; Spatz, M.W.; Motta, S.F.Y. Hot Surface Ignition with 2L Refrigerants; Honeywell Report; Honeywell Buffalo Research Laboratory: Buffalo, NY, USA, 2012. [Google Scholar]
- Koban, M.; Minor, B.; Coughlan, P.; Gray, N. Hot Surface Ignition Testing of LOW GWP 2L Refrigerants. In Proceedings of the Purdue Conference on Refrigeration and Air Conditioning, West Lafayette, IN, USA, 11–14 July 2016. [Google Scholar]
- Clodic, D.; Jabbour, T. Method of test for burning velocity measurement of flammable gases and results. HVAC&R Res. 2011, 17, 51–75. [Google Scholar]
- Pagliaro, J.L.; Linteris, G.; Sunderland, P.B.; Baker, P.T. Combustion inhibition and enhancement of premixed methane-air flames by halon replacements. Combust. Flame 2015, 162, 41–49. [Google Scholar] [CrossRef]
- Linteris, G.; Pagliaro, J.L.; Sunderland, P.B. Test Results Prepared for Honeywell: Igniter Material Effects in the Japanese High Pressure Gas Law Test; Technical Note (NIST TN)—1902; NIST: Gaithersburg, MD, USA, 2016. [Google Scholar]
- JSRAE. Risk Assessment of Mildly Flammable Refrigerants: Final Report 2016; The Japan Society of Refrigerating and Air Conditioning Engineers: Tokyo, Japan, 2017; pp. 22–34. [Google Scholar]
- Kataoka, O.; Yoshizawa, M.; Ohnishi, H.; Ishida, S. Flammability Evaluation of HFC-32 and HFC-32/134a Under Practical Operating Conditions. In Proceedings of the Purdue Conference on Refrigeration and Air Conditioning, West Lafayette, IN, USA, 23–26 July 1996. [Google Scholar]
- Takahashi, A.; Urano, Y.; Tokuhashi, K.; Kondo, S. Effect of vessel size and shape on experimental flammability limits of gases. J. Hazard. Mater. 2003, 105, 27–37. [Google Scholar] [CrossRef]
- Kul, I.; Gnann, D.L.; Beyerlein, A.L.; DesMarteau, D.D. Lower flammability limit of difluoromethane and percolation theory. Int. J. Thermophys. 2004, 25, 1085–1095. [Google Scholar] [CrossRef]
- Kul, I.; Blaszkowski, C. Flammability studies of isomeric structures of ethane derivatives and percolation theory. Int. J. Thermophys. 2007, 28, 906–917. [Google Scholar] [CrossRef]
- Feng, B.; Yang, Z.; Zhai, R. Climate change and refrigerants: Experimental study on the influence of the flame retardants on the flammability of R1234yf. Energy 2018, 143, 212–218. [Google Scholar] [CrossRef]
- Gu, W.; Cheng, P.; Tang, M. Compilation and evaluation of gas phase diffusion coefficients of halogenated organic compounds. R. Soc. Open Sci. 2018, 5, 171936. [Google Scholar] [CrossRef] [PubMed]
- Senecal, J.A. Flame extinguishing in the cup-burner by inert gases. Fire Saf. J. 2005, 40, 579–591. [Google Scholar] [CrossRef]



| Refrig-erant | Form. | Mole Fraction, % | LHV [14], kJ/g | SL, (cm/s) | Lq [15], mm | AIT, °C | MIE, mJ | ||
|---|---|---|---|---|---|---|---|---|---|
| LFL-UFL | Dry Stoic. [16] | Moist Stoic. [16] | |||||||
| R-32 | CH2F2 | 14.4–29.3 [17] | 17.4 | - | 10.3 | 6.7 [14] | 7.55 | 764 [10] | 65 [18] |
| R-452B | - | 11.9–24 [11] | 14.7 | 14.3 | - | - | - | - | - |
| R-1234yf | C3H2F4 | 6.2–12.3 [17] | 7.8 | 7.2 | 9.3 | 2.7 [19] | 24.8 | 405 [20] | 7500 [18] |
| R-1234ze | C3H2F4 | 7–9.5 [17] | 7.8 | 7.2 | - | 2.1 [19] | - | 368 [21] | 63,000 [17] |
| R-290 | C3H8 | 2.2–10 [17] | 4 | - | 46.4 | 38.7 [14] | 1.75 | 504 [6] | 0.25 [17] |
| Test | Refrigerant | X, % | Mixture | V, VAC | Wire T, °C |
|---|---|---|---|---|---|
| 1 | R-32 | 13 | Lean | 8 | 788 |
| 2 | 17 | Lean | 7.5 | 744 | |
| 3 | 17.4 | Stoichiometric | 8.9 | 863 | |
| 4 | R-452B | 10 | Lean | 8 | 788 |
| 5 | 14.7 | Stoichiometric | 9.4 | 911 | |
| 6 | R-1234yf | 7.8 | Stoichiometric | 9.5 | 921 |
| 7 | R-1234ze | 6.5 | Lean | 8 | 788 |
| 8 | 7.8 | Stoichiometric | 9.5 | 921 |
| Source | V, VAC | I, Amps | T, °C | N | t, s |
|---|---|---|---|---|---|
| Smoldering cigarette | - | - | 490 | 4 | - |
| Butane lighter | - | - | - | 10 | - |
| Friction sparks | - | - | 3000 a | - | 60 |
| Plug and receptacle | 120 | 15 | - | 10 | - |
| Light switch | 120 | 15 | - | 20 | - |
| Hand mixer | 120 | 1.7 | - | - | 120 |
| Cordless drill | 18 | 21 | - | - | 120 |
| Bread toaster | 120 | 6 | 500 | - | 120 |
| Hair dryer | 120 | 13 | 200 | - | 60 |
| Hot plate | 120 | 6 | 540 | 1 | - |
| Space heater | 120 | 12 | 100 | 1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.K.; Sunderland, P.B. Viability of Various Sources to Ignite A2L Refrigerants. Energies 2021, 14, 121. https://doi.org/10.3390/en14010121
Kim DK, Sunderland PB. Viability of Various Sources to Ignite A2L Refrigerants. Energies. 2021; 14(1):121. https://doi.org/10.3390/en14010121
Chicago/Turabian StyleKim, Dennis K., and Peter B. Sunderland. 2021. "Viability of Various Sources to Ignite A2L Refrigerants" Energies 14, no. 1: 121. https://doi.org/10.3390/en14010121
APA StyleKim, D. K., & Sunderland, P. B. (2021). Viability of Various Sources to Ignite A2L Refrigerants. Energies, 14(1), 121. https://doi.org/10.3390/en14010121





