Experimental Validation of a Low-Energy-Consumption Heating Model for Recirculating Aquaponic Systems
Abstract
1. Introduction
2. Numerical Modelling
2.1. Simulation Model
2.2. Grid Generation
2.3. Numerical Solution Method
2.4. Boundary Conditions
3. Experimental Setup
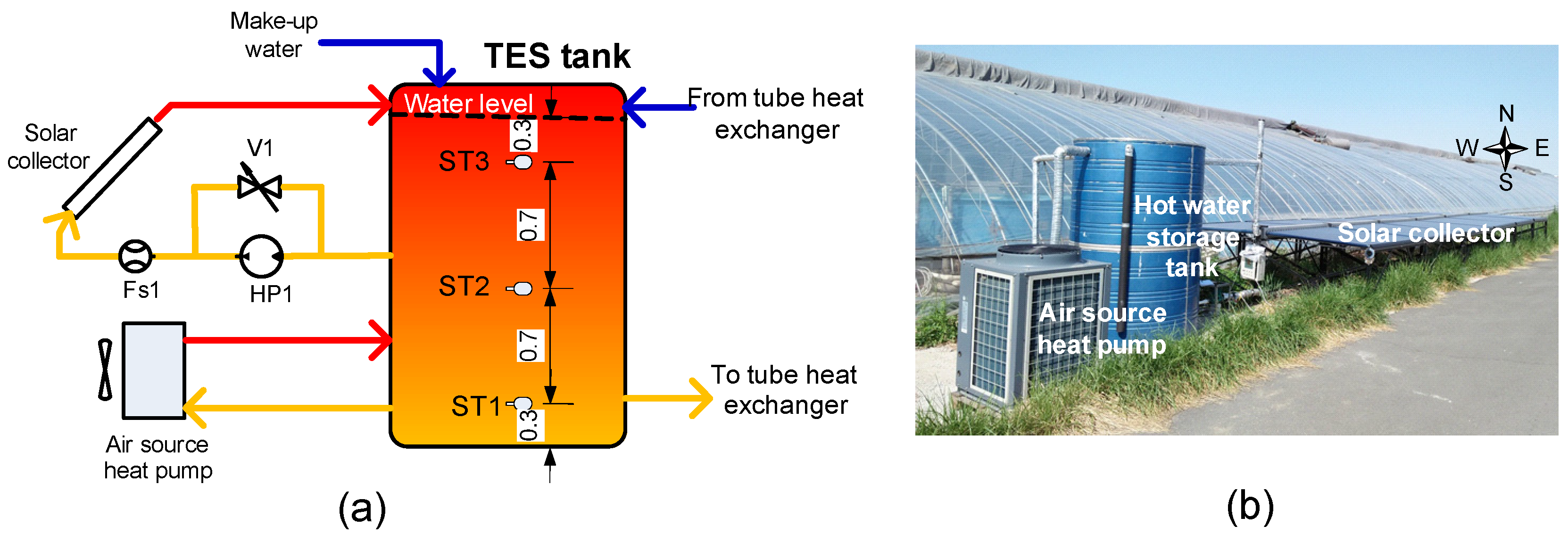
3.1. Thermal Energy Storage Tank
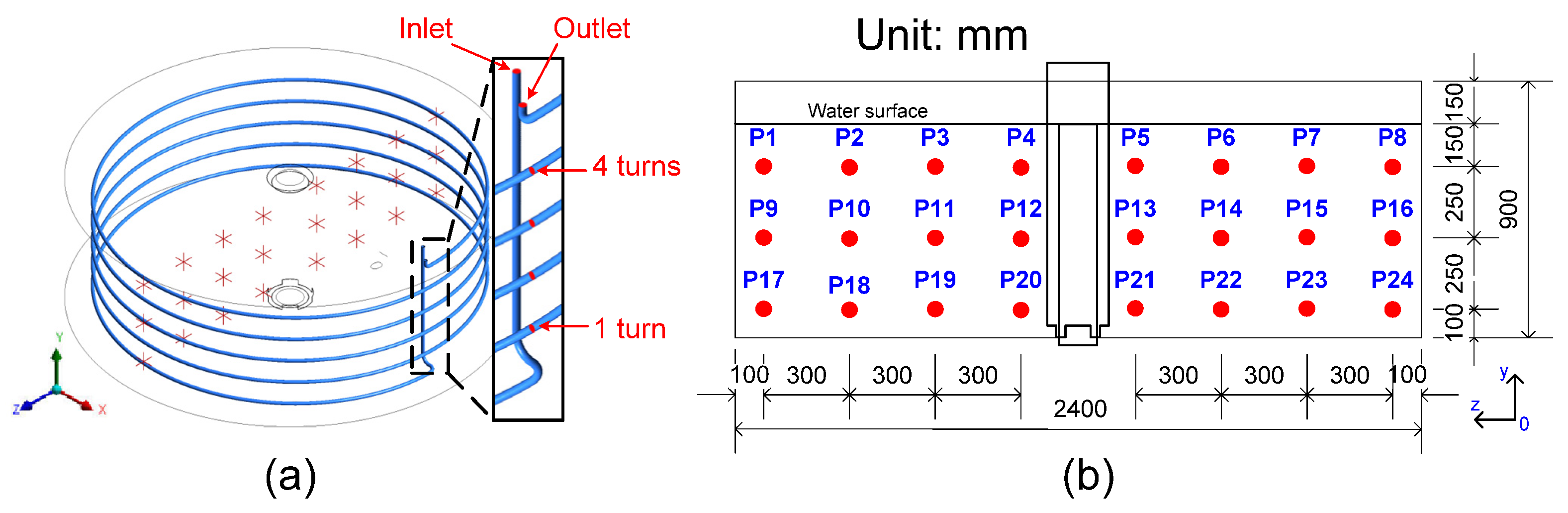
3.2. Helically Coiled Heat Exchanger
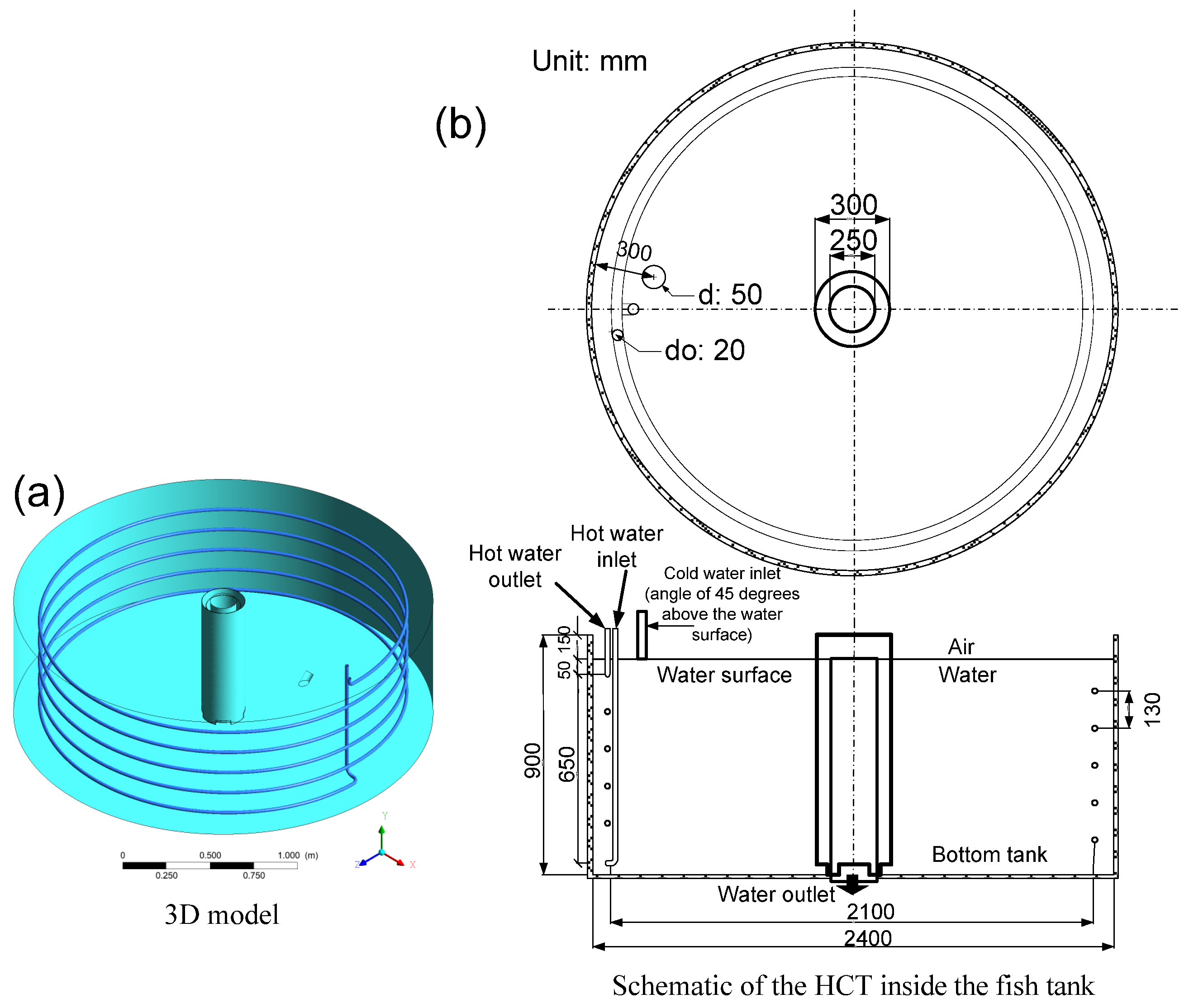
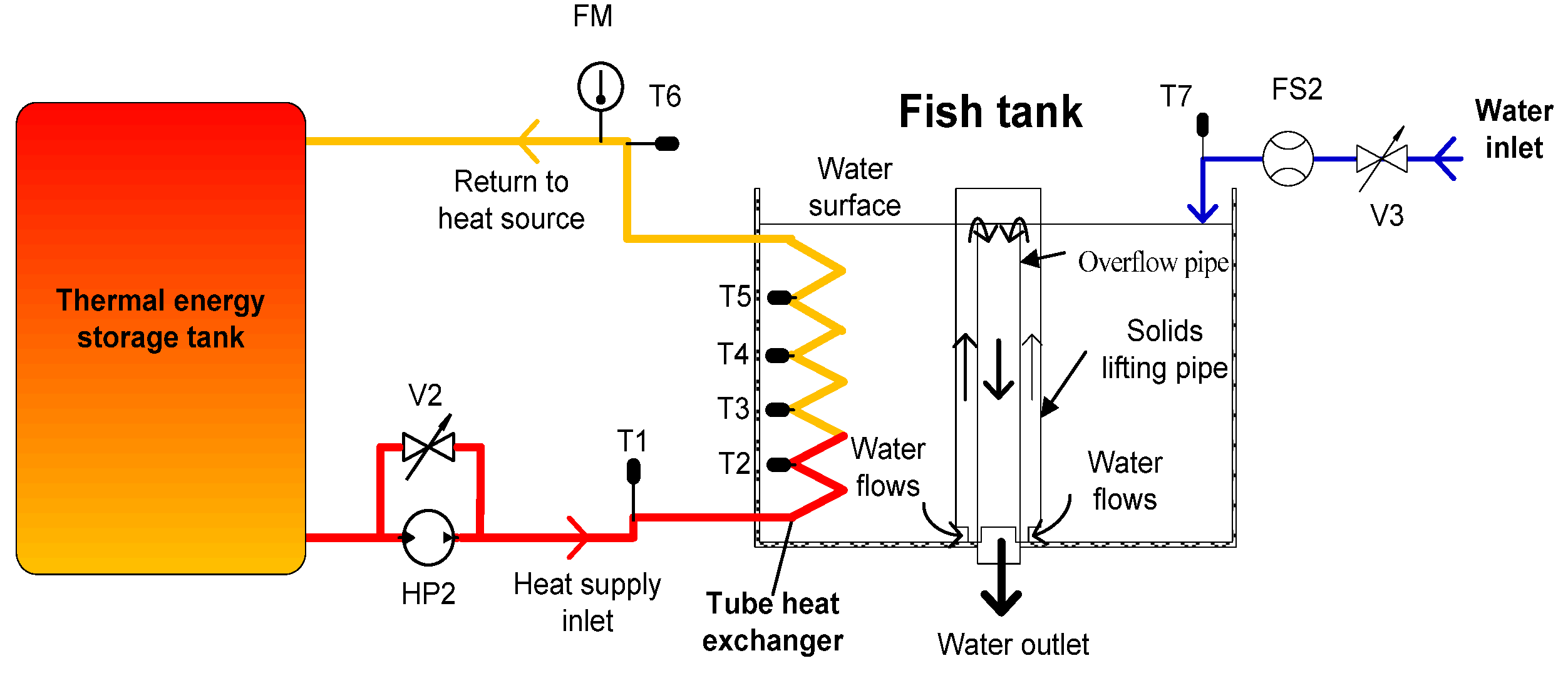
- The geometric dimensions of the HCT and fish tank are similar to those of the simulation model, as shown in Figure 1b and listed in Table 1. The water level in the cylindrical tank was maintained at a depth of 0.75 m by the overflow pipe to maintain a water volume of 3.4 m3, with a recirculating water flow of approximately 0.930 kg/s. The nozzle supplying the water into the fish tank was positioned at a 45° angle above the water surface.
- Six temperature sensors, T1–T6, were used to measure temperature for the hot liquid inlet, 1 turn, 2 turns, 3 turns, 4 turns, and the outlet of the HCT. The temperature sensors were inserted into the HCT through the probe. Temperature sensor T7 was used to measure temperature for the water inlet of the fish tank, and an additional sensor was used to measure the environmental temperature, as shown in Figure 6.
- A flow meter (FM) and flow valve (FV2) were used to measure and control the liquid flow into the HCT. Similarly, a flow sensor (FS2) and flow valve (FV3) were used for the fish tank (Figure 3).
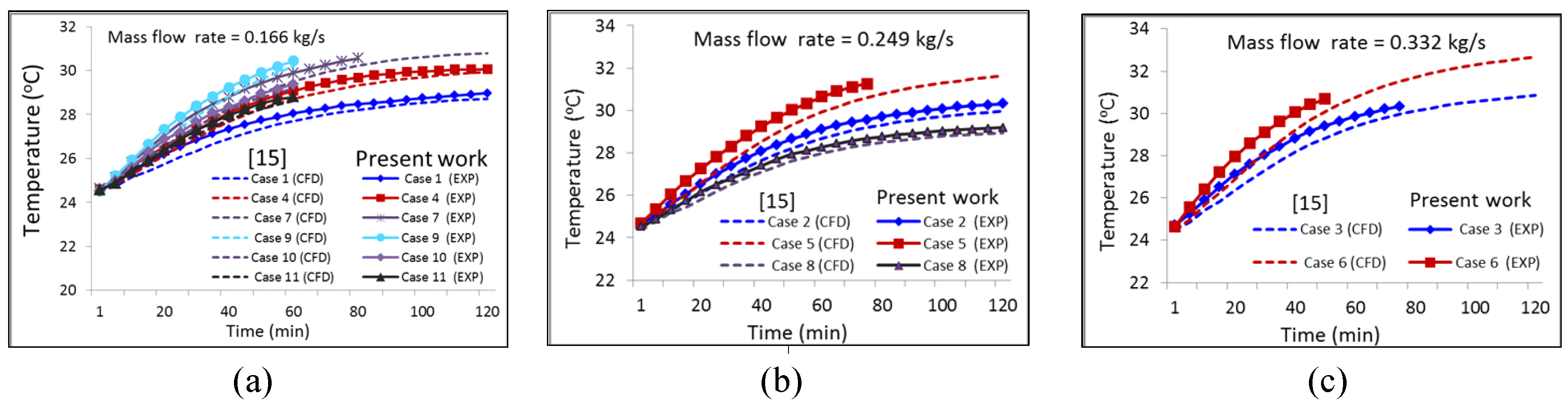
- The operation modes for the experiment are listed in Table 2. Among them, the results of cases 1–8 were analyzed and compared to those of Le et al. [15]. Three additional cases 9–11 were studied further to investigate the transient discharging energy efficiency of the TES tank. The initial temperature for all the liquids of the HCHE was set to 24.5 ± 0.5 °C.
3.3. Instrumentation
- In the HCHE model, the experimental setup used 32 temperature sensors, including seven sensors to test the temperature of the points from T1 to T7 (Figure 2), 24 sensors to measure the temperature of the water in the fish tank (Figure 4), and one sensor to measure the environment temperature. A waterproof digital temperature sensor was used, and it allowed for multi-sensor support over the same 1-Wire bus [26]. The sensor has a resolution of 0.0625 °C (12 bits), operating temperature range of −55 to +125 °C, and ±0.5 °C accuracy from −10 °C to +85 °C.
- A flow meter was used to measure the hot water flow rate of the HCT with a working temperature range of −20 °C to 100 °C, flow rate range of 1–50 L/min, accuracy of 0.5%, and working pressure ≤4.0 MPa. The flow meter was installed according to the manufacturer’s recommendations (horizontal).
- Two water flow sensors (Hall effect sensors) were used to measure the mass flow rate of the water into the fish tank and solar collectors. The flow sensor has a range of 5–150 L/min, accuracy of ±3%, working pressure of ≤1.75 MPa, and working temperature range of −20 °C to 85 °C.
- Two centrifugal pumps were used to pump the circulating liquid of the HCHE and the solar collectors. The pump model was a Wilo PH-101EH-200W from Shandong, China, and had an electric power of 200 W and a maximum flow rate of 130 L/min at a rated head of 8 m.
3.4. Experimental Procedure
4. Mathematical Formalism
5. Results and Discussion
5.1. Temperature Field for Water in the HCT
5.2. Temperature Fields of Water in the Fish Tank
5.3. Heat Transfer Rate
5.4. Energy Efficiency
6. Conclusions
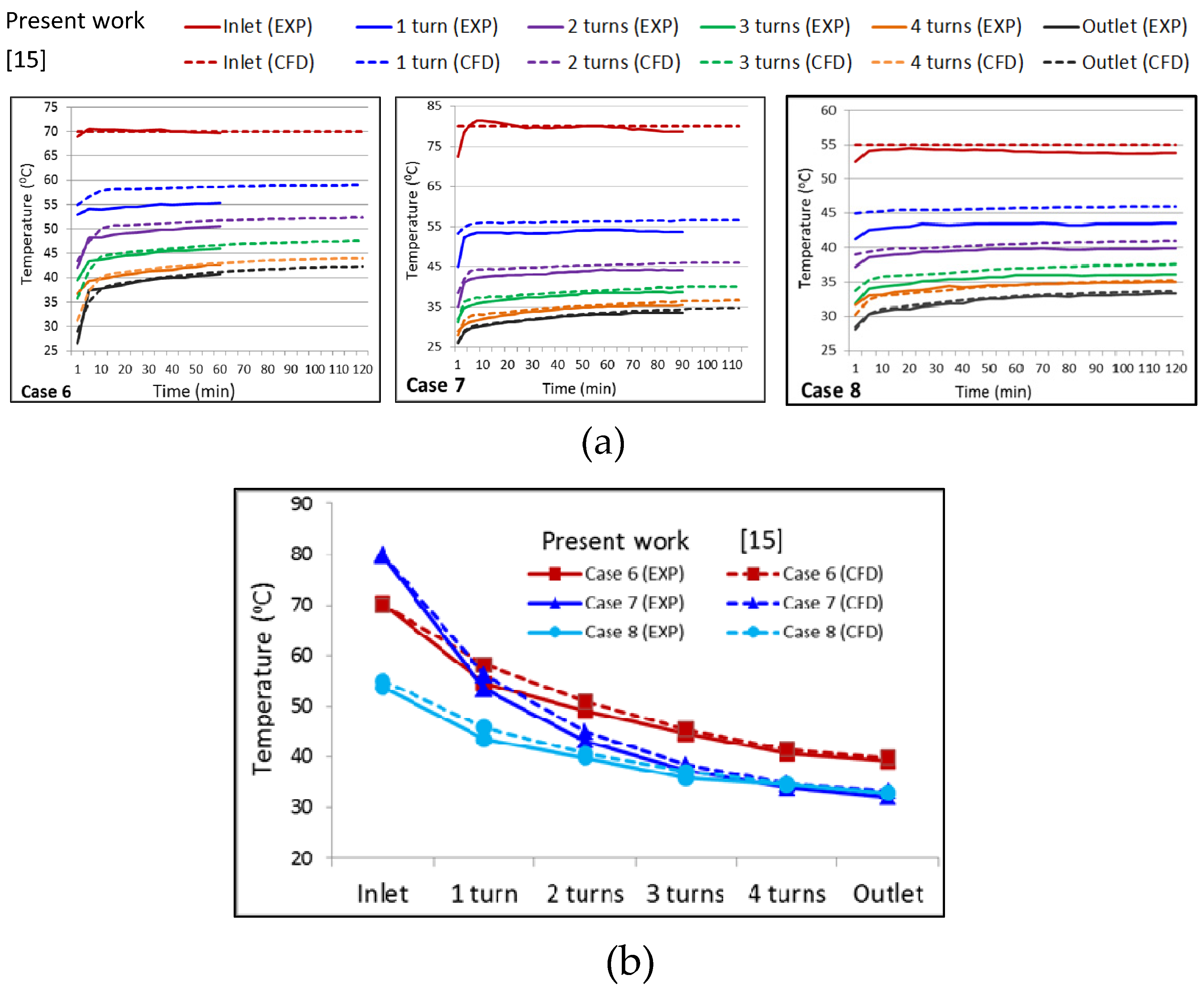
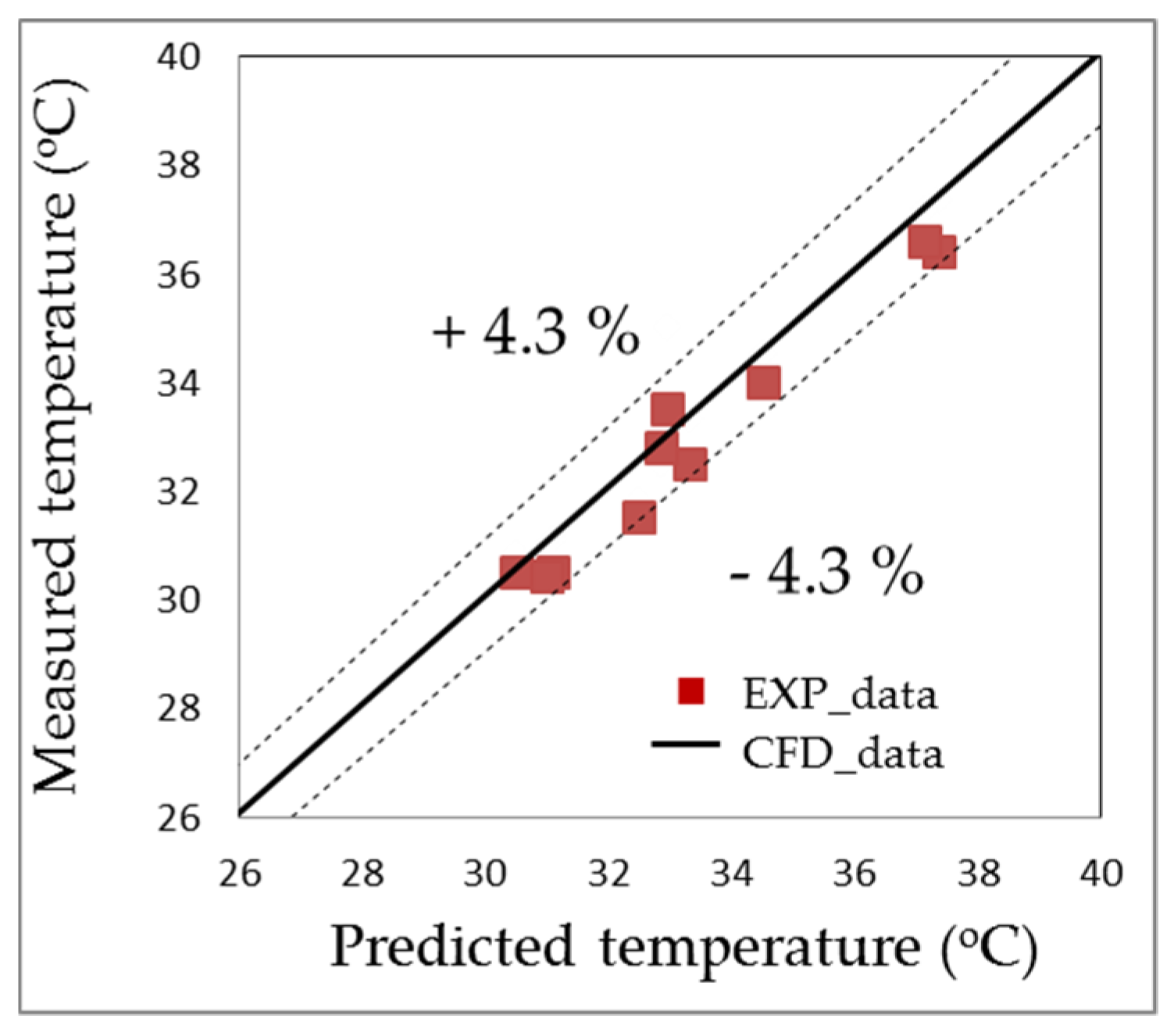
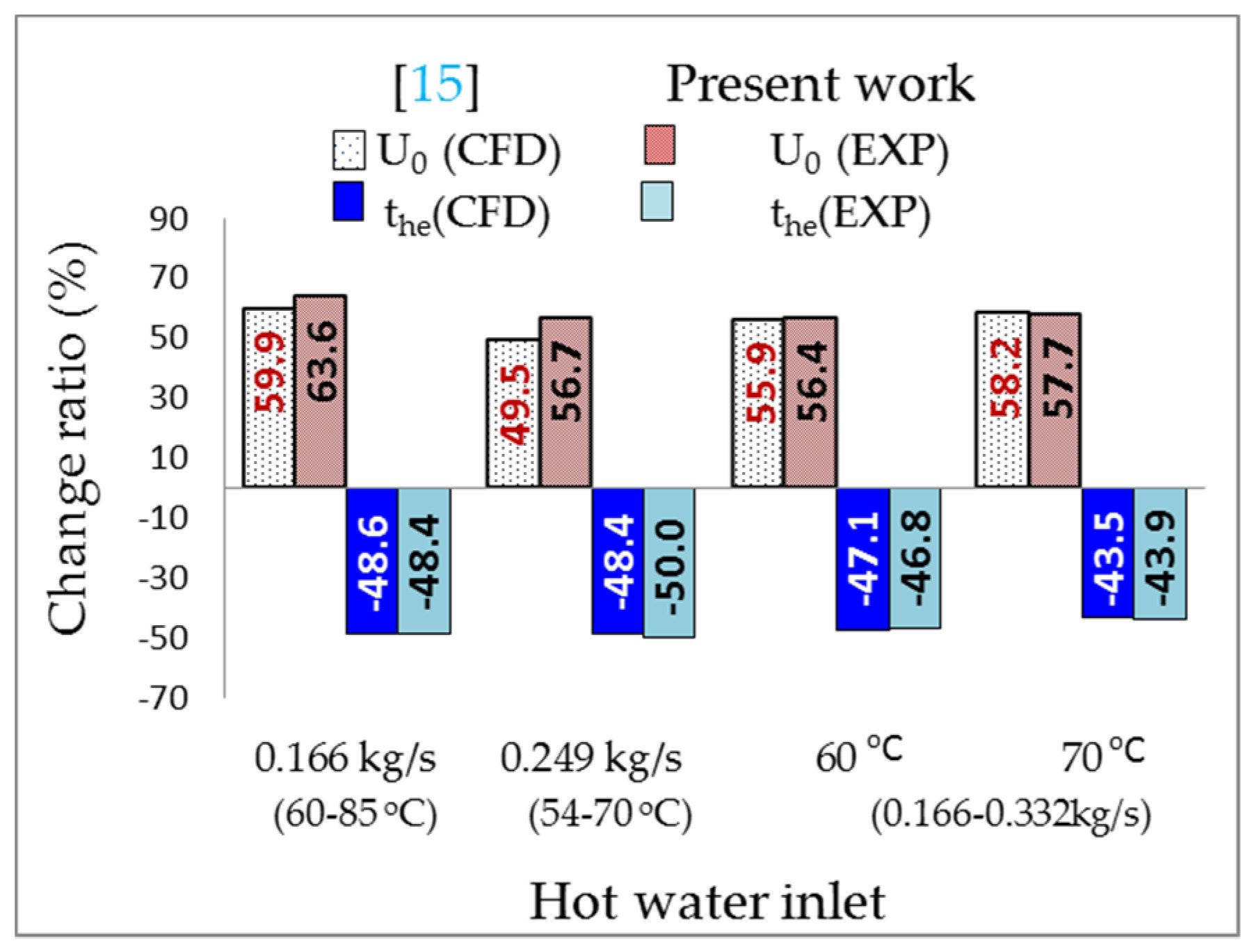
- The data obtained from the experiment agreed with the numerical simulation results. The time difference to heat the water in the fish tank () from 24.5 °C to 28 °C between the two results was from 3 min to 8 min for all cases. The average temperature error of the water in the fish tank was within ±3.6%.
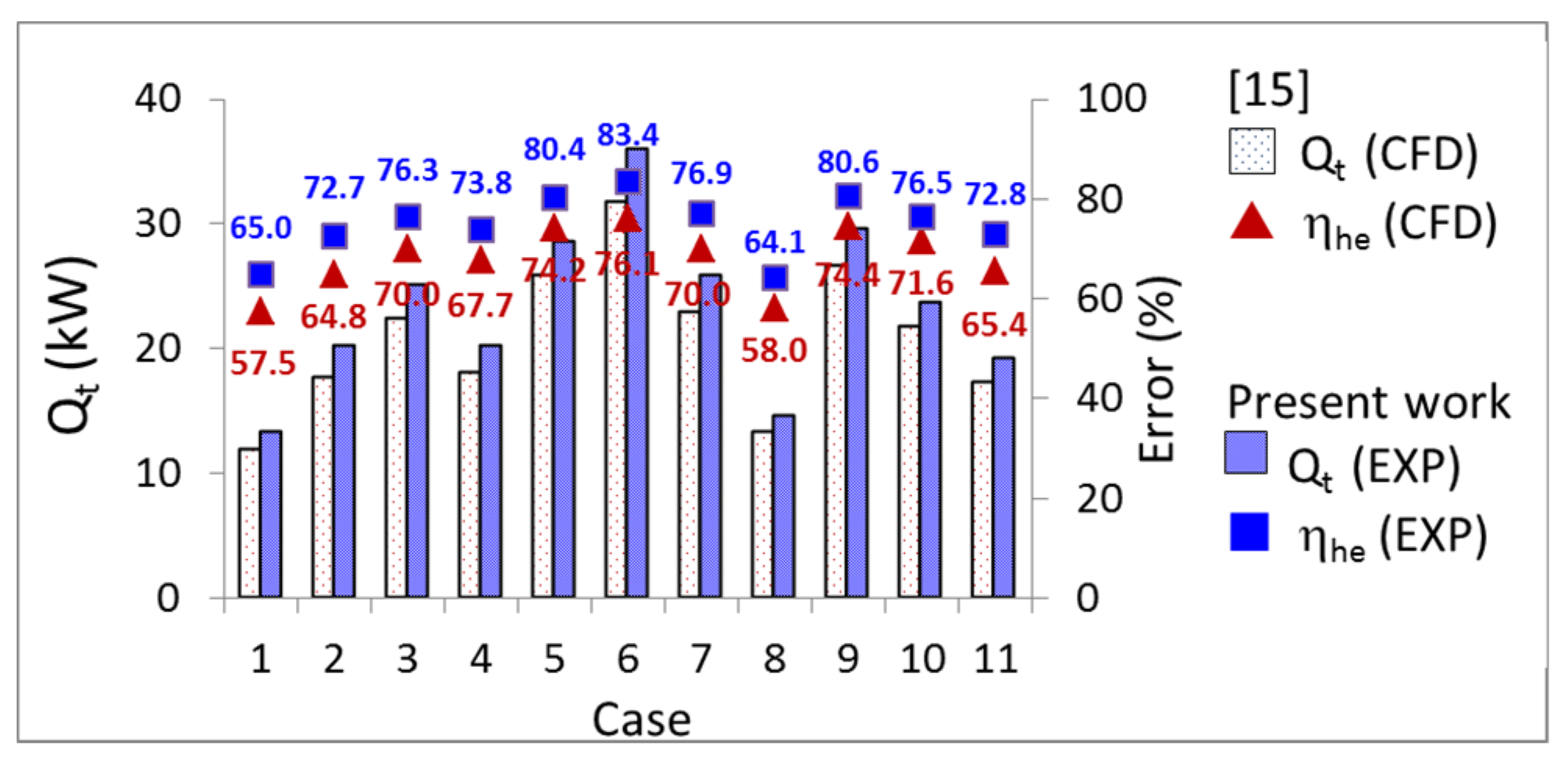
- The error values of the heat transfer rate () and the heat transfer rate per unit length () between the two results was within ±4.2%, and ranged from 0.5% to 7.2%, respectively. The thermal energy () was between 65.9% and 83.4% with an error range of less than 8.0%.
- The values ranged from 59.51 MJ (case 6) to 76.39 MJ (case 1), and the error values ranged from 6.4% to 11.5%. To heat 3.4 m3 of fish tank water from 24.5 °C to 28 °C, case 9 used 5.6% of the thermal energy of the TES tank, case 10 used 6.4%, and case 11 used 7.2%.
- The proposed low-energy-consumption heating method can replace electric heaters, which will reduce electricity consumption when heating aquaculture water in RAS systems.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| specific heat (J/kg K) | |
| D | coils diameter (m) |
| tube inner diameter (mm) | |
| tube outer diameter (mm) | |
| E | electric power |
| mass flow rate (kg/s) | |
| N | number of turns (dimensionless) |
| P | tube pitch (m) |
| total thermal energy (J) | |
| electrical energy consumption (J) | |
| heat transfer rate (W) | |
| usable heat transfer rate (W) | |
| thermal energy stored (J) | |
| energy accumulated | |
| T | temperature (°C) |
| heat transfer duration (s) | |
| V | volume (m3) |
| heat transfer rate per unit length (W/m) | |
| Greek | |
| ΔT | temperature difference (°C) |
| δ | curvature ratio (dimensionless, di/D) |
| ρ | water density (kg/m3 ) |
| thermal efficiency, dimensionless | |
| transient discharging energy efficiency, dimensionless | |
| usable energy efficiency, dimensionless | |
| Subscripts | |
| CFD | computational fluid dynamics |
| FV | flow valve |
| FS | flow sensor |
| FM | flow meter |
| H | height (m) |
| HCHE | helically coiled heat exchanger |
| HCT | helically coiled tubes |
| HP | pump |
| h | hot liquid |
| he | heat exchanger |
| hi | hot in |
| ho | hot out |
| in | initial |
| L | length (m) |
| PPRC | polypropylene random copolymers pipe |
| RAS | recirculating aquaponic system |
| RES | renewable energy source |
| st | stored |
| TES | thermal energy storage |
| t | tank |
| W | wide (m) |
References
- Quagrainie, K.K.; Flores, R.M.V.; Kim, H.-J.; McClain, V. Economic analysis of aquaponics and hydroponics production in the US Midwest. J. Appl. Aquac. 2018, 30, 1–14. [Google Scholar] [CrossRef]
- Maucieri, C.; Forchino, A.A.; Nicoletto, C.; Junge, R.; Pastres, R.; Sambo, P.; Borin, M. Life cycle assessment of a micro aquaponic system for educational purposes built using recovered material. J. Clean. Prod. 2018, 172, 3119–3127. [Google Scholar] [CrossRef]
- Forchino, A.A.; Lourguioui, H.; Brigolin, D.; Pastres, R. Aquaponics and sustainability: The comparison of two different aquaponic techniques using the Life Cycle Assessment (LCA). Aquac. Eng. 2017, 77, 80–88. [Google Scholar] [CrossRef]
- Fang, Y.; Hu, Z.; Zou, Y.; Fan, J.; Wang, Q.; Zhu, Z. Increasing economic and environmental benefits of media-based aquaponics through optimizing aeration pattern. J. Clean. Prod. 2017, 162, 1111–1117. [Google Scholar] [CrossRef]
- Al-Hafedh, Y.S.; Alam, A.; Beltagi, M.S. Food production and water conservation in a recirculating aquaponic system in Saudi Arabia at different ratios of fish feed to plants. J. World Aquacult. Soc. 2008, 39, 510–520. [Google Scholar] [CrossRef]
- Love, D.C.; Fry, J.P.; Li, X.; Hill, E.S.; Genello, L.; Semmens, K.; Thompson, R.E. Commercial aquaponics production and profitability: Findings from an international survey. Aquaculture 2015, 435, 67–74. [Google Scholar] [CrossRef]
- Rakocy, J.E. Ten Guidelines for Aquaponic Systems. Aquaculture 2007, 46, 14–17. [Google Scholar]
- Badiola, M.; Basurko, O.C.; Piedrahita, R.; Hundley, P.; Mendiola, D. Energy use in Recirculating Aquaculture Systems (RAS): A review. Aquac. Eng. 2018, 81, 57–70. [Google Scholar] [CrossRef]
- Delaide, B.; Delhaye, G.; Dermience, M.; Gott, J.; Soyeurt, H.; Jijakli, M.H. Plant and fish production performance, nutrient mass balances, energy and water use of the PAFF Box, a small-scale aquaponic system. Aquac. Eng. 2017, 78, 130–139. [Google Scholar] [CrossRef]
- Love, D.C.; Uhl, M.S.; Genello, L. Energy and water use of a small-scale raft aquaponics system in Baltimore, Maryland, United States. Aquac. Eng. 2015, 68, 19–27. [Google Scholar] [CrossRef]
- Omer, A.M. Energy, environment and sustainable development. Renew. Sust. Energ. Rev. 2008, 12, 2265–2300. [Google Scholar] [CrossRef]
- Shao, S.; Yang, L.; Gan, C.; Cao, J.; Geng, Y.; Guan, D. Using an extended LMDI model to explore techno-economic drivers of energy-related industrial CO2 emission changes: A case study for Shanghai (China). Renew. Sustain. Energy Rev. 2016, 55, 516–536. [Google Scholar] [CrossRef]
- Hassanien, R.H.E.; Li, M.; Dong Lin, W. Advanced applications of solar energy in agricultural greenhouses. Renew. Sustain. Energy Rev. 2016, 54, 989–1001. [Google Scholar] [CrossRef]
- d’Orbcastel, E.R.; Blancheton, J.-P.; Aubin, J. Towards environmentally sustainable aquaculture: Comparison between two trout farming systems using Life Cycle Assessment. Aquac. Eng. 2009, 40, 113–119. [Google Scholar] [CrossRef]
- Le, A.T.; Wang, Y.; Wang, L.; Ta, V.C.; Li, D. Numerical investigation on a low energy-consumption heating method for recirculating aquaponic systems. Comput. Electron. Agric. 2020, 169, 105210. [Google Scholar] [CrossRef]
- Navarro, L.; de Gracia, A.; Colclough, S.; Browne, M.; McCormack, S.J.; Griffiths, P.; Cabeza, L.F. Thermal energy storage in building integrated thermal systems: A review. Part 1. active storage systems. Renew. Energy 2016, 88, 526–547. [Google Scholar] [CrossRef]
- Lin, W.-M.; Chang, K.-C.; Liu, Y.-M.; Chung, K.-M. Field Surveys of Non-Residential Solar Water Heating Systems in Taiwan. Energies 2012, 5, 258–269. [Google Scholar] [CrossRef]
- Thygesen, R. An Analysis of Different Solar-Assisted Heating Systems and Their Effect on the Energy Performance of Multifamily Buildings-A Swedish Case. Energies 2017, 10, 88. [Google Scholar] [CrossRef]
- Hugo, A.; Zmeureanu, R. Residential Solar-Based Seasonal Thermal Storage Systems in Cold Climates: Building Envelope and Thermal Storage. Energies 2012, 5, 3972–3985. [Google Scholar] [CrossRef]
- Bernardo, L.R.; Davidsson, H.; Karlsson, B. Retrofitting Domestic Hot Water Heaters for Solar Water Heating Systems in Single-Family Houses in a Cold Climate: A Theoretical Analysis. Energies 2012, 5, 4110–4131. [Google Scholar] [CrossRef]
- Jonas, D.; Frey, G.; Theis, D. Simulation and performance analysis of combined parallel solar thermal and ground or air source heat pump systems. Sol. Energy 2017, 150, 500–511. [Google Scholar] [CrossRef]
- Parameshwaran, R.; Kalaiselvam, S.; Harikrishnan, S.; Elayaperumal, A. Sustainable thermal energy storage technologies for buildings: A review. Renew. Sustain. Energy Rev. 2012, 16, 2394–2433. [Google Scholar] [CrossRef]
- Najafian, A.; Haghighat, F.; Moreau, A. Integration of PCM in domestic hot water tanks: Optimization for shifting peak demand. Energy Build. 2015, 106, 59–64. [Google Scholar] [CrossRef]
- Yang, W.; Sun, L.; Chen, Y. Experimental investigations of the performance of a solar-ground source heat pump system operated in heating modes. Energy Build. 2015, 89, 97–111. [Google Scholar] [CrossRef]
- Jayakumar, J.S.; Mahajani, S.M.; Mandal, J.C.; Vijayan, P.K.; Bhoi, R. Experimental and CFD estimation of heat transfer in helically coiled heat exchangers. Chem. Eng. Res. Des. 2008, 86, 221–232. [Google Scholar] [CrossRef]
- Programmable Resolution1-Wire Digital Thermometer. Available online: www.datasheets.maximintegrated.com/en/ds/DS18B20.pdf (accessed on 10 April 2020).
- Yang, X.; Xiong, T.; Dong, J.L.; Li, W.X.; Wang, Y. Investigation of the Dynamic Melting Process in a Thermal Energy Storage Unit Using a Helical Coil Heat Exchanger. Energies 2017, 10, 1129. [Google Scholar] [CrossRef]
- Kalogirou, S. Thermal performance, economic and environmental life cycle analysis of thermosiphon solar water heaters. Sol. Energy 2009, 83, 39–48. [Google Scholar] [CrossRef]
- Atia, D.M.; Fahmy, F.H.; Ahmed, N.M.; Dorrah, H.T. Optimal sizing of a solar water heating system based on a genetic algorithm for an aquaculture system. Math. Comput. Model. 2012, 55, 1436–1449. [Google Scholar] [CrossRef]
- Allouhi, A.; Jamil, A.; Kousksou, T.; El Rhafiki, T.; Mourad, Y.; Zeraouli, Y. Solar domestic heating water systems in Morocco: An energy analysis. Energy Convers. Manag. 2015, 92, 105–113. [Google Scholar] [CrossRef]
- Gautam, A.; Chamoli, S.; Kumar, A.; Singh, S. A review on technical improvements, economic feasibility and world scenario of solar water heating system. Renew. Sustain. Energy Rev. 2017, 68, 541–562. [Google Scholar] [CrossRef]
- Saïf ed-Dîn, F.; Bouhal, T.; Gargab, F.; Jamil, A.; Kousksou, T.; Benbassou, A. Design and thermal performance optimization of a forced collective solar hot water production system in Morocco for energy saving in residential buildings. Sol. Energy 2018, 160, 260–274. [Google Scholar] [CrossRef]
- Valdiserri, P. Evaluation and control of thermal losses and solar fraction in a hot water solar system. Int. J. Low-Carbon Technol. 2018, 13, 260–265. [Google Scholar] [CrossRef]








| Parameter | Value |
|---|---|
| Coils diameter, D (m) | 2.1 |
| Tube inner diameter, do (mm) | 20 |
| Tube outer diameter, di (mm) | 19.4 |
| Tube pitch, P (mm) | 130 |
| Curvature ratio, δ (do/D) | 0.0095 |
| Number of turns, N | 5 |
| Tube length, L (m) | 34 |
| Case | Mass Flow Rate (Kg/s) | Temperature (°C) | HP1 Mode | |
|---|---|---|---|---|
| Simulation | Experiment (±2%) | |||
| 1 | 0.166 | 60 | 60 | Turn on |
| 2 | 0.249 | |||
| 3 | 0.332 | |||
| 4 | 0.166 | 70 | 70 | |
| 5 | 0.249 | |||
| 6 | 0.332 | |||
| 7 | 0.166 | 80 | 80 | |
| 8 | 0.249 | 55 | 54 | |
| 9 | 0.166 | 81–87 | 81–87 | Turn off |
| 10 | 71–81 | 71–81 | ||
| 11 | 65–75 | 65–75 | ||
| Case | Maximum Error of Temperature (%) | Heat Transfer Time (min) | Time Difference (min) | |
|---|---|---|---|---|
| Simulation [15] | Experiment (Present Work) | |||
| 1 | 2.3 | 70 | 62 | 8 |
| 2 | 2.5 | 47 | 41 | 6 |
| 3 | 3.2 | 37 | 33 | 4 |
| 4 | 3.1 | 46 | 41 | 5 |
| 5 | 3.6 | 32 | 29 | 3 |
| 6 | 3.4 | 26 | 23 | 3 |
| 7 | 3.0 | 36 | 32 | 4 |
| 8 | 2.4 | 62 | 57 | 5 |
| 9 | 2.0 | 31 | 28 | 3 |
| 10 | 3.0 | 38 | 35 | 3 |
| 11 | 1.3 | 48 | 43 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, A.T.; Wang, L.; Wang, Y.; Vu, N.T.; Li, D. Experimental Validation of a Low-Energy-Consumption Heating Model for Recirculating Aquaponic Systems. Energies 2020, 13, 1958. https://doi.org/10.3390/en13081958
Le AT, Wang L, Wang Y, Vu NT, Li D. Experimental Validation of a Low-Energy-Consumption Heating Model for Recirculating Aquaponic Systems. Energies. 2020; 13(8):1958. https://doi.org/10.3390/en13081958
Chicago/Turabian StyleLe, Anh Tuan, Liang Wang, Yang Wang, Ngoc Tuan Vu, and Daoliang Li. 2020. "Experimental Validation of a Low-Energy-Consumption Heating Model for Recirculating Aquaponic Systems" Energies 13, no. 8: 1958. https://doi.org/10.3390/en13081958
APA StyleLe, A. T., Wang, L., Wang, Y., Vu, N. T., & Li, D. (2020). Experimental Validation of a Low-Energy-Consumption Heating Model for Recirculating Aquaponic Systems. Energies, 13(8), 1958. https://doi.org/10.3390/en13081958







