1. Introduction
Those living in rural areas of developing nations face many daily problems, including but not limited to, having access to a clean cook fuel with an appropriate energy content. Women and children are often forced to spend multiple hours a day trekking for firewood and other materials to burn for cooking. Inefficient burning of inappropriate material leads to health issues, time spent collecting these materials is time away from education and earning a living. By carbonizing biomass to create a solid fuel that can be used for cooking, it would be possible to help in solving the above problems faced daily for those living in rural areas of developing nations. To aid the integration of concentrated solar technology with the thermochemical techniques used to create a biofuel, an energy demand calculator has been made and applied to Uganda. Uganda was selected as the case study nation due to the strong links between the University of Leeds and the Centre for Research in Energy and Energy Conservation (CREEC) based at the Makerere University, Kampala, Uganda.
The integration of biomass and concentrated solar technology (CST) has been proven successful multiple times shown within literature. With enough solar radiation to power the earth 4200 times, and multiple ways to thermochemically treat biomass into a fuel, integration of the two is only logical. Solar pyrolysis is not a new nor a novel technology and is described many times within literature. It has been present in academia since the 1980s, using solar simulators (furnace images) and elliptic mirrors as a source of radiation [
1]. Solar pyrolysis is defined as an endothermic process of converting biomass into an inert atmosphere in which the required heat for the reaction to occur is provided by concentrated solar energy [
2]. Solar pyrolysis thus allows for solar energy to be stored within a chemical compound. Throughout literature, it has become apparent that solar pyrolysis can occur via two techniques, that being through either direct or indirect radiation. Either biomass is directly heated by concentrated solar radiation through either a borosilicate or quartz glass, or is indirectly heated via convection or a heat transferring fluid as shown by R. Adinberg et al. (2014) [
1,
3]. Indirect reactors have external walls heated by concentrated solar radiation. Conduction through the walls heats the reactants. Most indirect reactors are catalytic tubular reformers, whereby a gas flows across a heated catalyst [
4,
5]. Double cavity reactors have recently been developed for thermochemical purposes. In the double cavity reactor, the reaction chamber is physically separated from the reactor that receives the solar radiation [
4,
6].
There has been work shown in literature assessing various solar pyrolysis techniques, temperatures, hold times, and other variables, allowing a compilation of the key research challenges that lie ahead with the technology. Morales et al. (2014) studied the effect of solar pyrolysis on orange peel [
2]. The study was conducted with the feedstock directly heated within a borosilicate glass tube with helium as the gas carrier as part of a parabolic trough array. The irradiance profile of the parabolic trough was plotted using the SolTrace software provided by the [US] National Renewable Energy Laboratory (NREL). The parabolic trough had an aperture width of 1.3 m and a reflectivity of 0.94. The borosilicate glass receiver had an external diameter of 2 inches. Peak solar irradiance during the experiment was 25,084 W/m
2, with the average being 12,553 W/m
2. During pyrolysis of the orange peel, temperatures averaged 290 °C with a peak temperature of 495 °C. Under these conditions the orange peel lost 79.08% of its mass producing mainly a liquid bio-oil. Of the product, 77.64% was liquid, 1.43% was gas and the remainder was char. The results for the solar pyrolysis of the orange peel compared well to literature results of an electric furnace based pyrolysis of orange peel [
2]. S. Morales went on to after reviewing his work to note that with an increase in aperture width, the maximum temperature and efficiency of his process would increase [
2].
Zeng et al. (2014) investigated the solar pyrolysis of wood in a lab-scale solar reactor, assessing the influence of temperature and gas flow. The pyrolysis temperature ranged from 600 to 2000 °C with a hold time of 12 min, and argon flow rates between 3 Nl/min and 12 Nl/min. The heating rate was constant at 50 °C/s across all experiments conducted [
7]. Zeng et al. reported on how the use of arc image furnaces in literature are pronounced for providing a higher liquid yield compared to conventional furnaces. Liquid yield unlike gas and char yield are not significantly dependent on the heat flux density [
7]. K. Zeng et al. however were studying solar pyrolysis conditions in order to gain a high gas yield from Beechwood samples. The results showed that for the highest possible gas yield, the temperature needs to be as high as possible. Gas yield constantly increased across the experiments, with a final yield of 51%, but the biggest increase occurred between 600 °C–1000 °C (15–37%). Liquid yield and char yield both decreased with increasing temperature. At 600 °C the liquid yield was 71% dropping to 52% at 1000 °C and further to 41% at 2000 °C. The char remained low throughout the experiment dropping from 14%–8% [
7]. Gas flow rate had the opposite effect. At 1200 °C increasing the argon flow rate within the reaction chamber leads to a slight decrease in gas yield and an increase in liquid and char yield. This result would be due to the removal rate of products from the hot zone of the reactor. Zeng et al. has helped show that the main products to be produced form solar pyrolysis are either a liquid or a gas [
7]. Char yield never passed 15% within the experiments and so this would need to be taken into consideration if taking this technology forward.
Li et al. conducted experiments similar to K. Zeng et al. as outlined above. Li et al. attempted to produce a pyrolysis gas from pine sawdust, peach pit, grape stalk and grape marc within a temperature range of 800 °C–2000 °C with the use of a solar dish. As expected, gas yield increased with increasing temperature and temperature rate, with liquid yield and char yield reacting oppositely. Li et al. did note that the feedstocks with a higher lignin content provided a higher char yield [
8]. In a review conducted by Chintala (2018), the process variables of solar biomass were investigated. Chintala confirmed through citing literature that by increasing the reaction temperature the gas yield increased. Chintala also investigated the effects of biomass particle size and claimed that a larger particle size will increase char yield [
9].
Two examples of a high solid yield produced by solar pyrolysis, which are perhaps of a higher relevancy to this study, include work conducted by Ramos et al. and Hans et al. Ramos was able to produce 70 g of biochar from 180 g of wood implying a char yield of 38%. His parabolic solar concentrator had a surface area of 1.37 m
2 and its receiver hit temperatures of above 270 °C. For the conversion to efficiently take place, the process occurred over 5 h during peak day time hours [
10]. Hans et al. also managed to produce a solid fuel. Hans et al. took agricultural wastes such as wheat straw and pyrolyzed them in a solar driven reactor for 90 min at 500 °C. The solid fuel produced gained energy density, increasing it from 16.9 MJ/kg to 24–28 MJ/kg. Details of his design are based in the reference Hans et al. [
11].
There are limited reports of modelling solar pyrolysis in the literature, however Sanchez et al. (2018) has published a useful system for modelling and evaluation the thermochemical technique [
12]. Sanchez et al. breaks the model down into two scenarios (i) heating of the biomass from an ambient to an operating temperature and (ii) the pyrolysis reactions at the operating temperature, details of which can be found in the reference Sanchez et al. [
12]. The model aims to predict the length needed for the pyrolysis reactor for a set feed rate. By varying the operating temperature and hold times, the optimum reactor size can be predicted. The equations for the model are outlined in the reference and the simulation software used was MatLAB. Sanchez et al. ran the model based off of data from Seville, Spain. During optimum conditions the model predicted a maximum char yield from a woody biomass feedstock to be 40.8 wt%. However the system would not constantly be able to run at optimum conditions, leaving the average annual yield to be a meek 10.1 wt% [
12]. The model and the predictions made by Sanchez et al. are observed to be accurate and could be applied to the future work of this thesis. The design of the system that Sanchez is basing the model off from however does not seem optimum. The use of better materials and a parabolic dish or trough instead of linear Fresnel would hopefully improve the efficiency during non-optimum conditions. Improving the efficiency for optimum conditions would also increase the overall operating temperature and therefore decrease the char yield—which for the production of a solid fuel would be detrimental. Work conducted by Zeng et al., Li et al. and Soria et al. provided a useful solution for this. By creating effectively a shutter system from a carbon composite, they were able to have a larger control of the operating temperature [
7].
Developing solar pyrolysis may not the technology of choice for this work, but some of its principles can be carried on. For a high char yield it would seem that a slow pyrolysis under cooler conditions would be optimal. This would be beneficial as it would help with the simplicity of the design. Building a solar collector to reach temperatures between 200–600 °C would be easier and more economically viable compared to one that needed to reach 2000 °C. Little research has been conducted in the field of integrating solar with lower temperature hydrothermal treatments, but will be investigated in this work.
As can be seen through the review of literature the design of the pyrolysis system is key to what operating conditions can be achieved, and thus what pyrolysis products can be produced. Chintala summarized the key research challenges in their review. These challenges are as follow [
9]:
Uniform distribution of the heat flux
Heat losses from the surface of the reactor/high wind speeds
High capital costs
Reactor design for effective thermochemical conversion, including reactor material
Variation in solar flux (time of day/season)
However, by modeling a variety of options before setting on a design choice the challenges may be overcome. By selecting the correct solar reactor type, either direct or indirect with respect to the product required and building the system from the correct materials, the system should be as efficient as possible. An efficient system will reduce the overall capital costs (though they may still be high) as there will be no wasted materials [
13]. For a small scale system, the solar reactor may only be required to run during peak hours and so the variation in solar flux may be a non-issue. In a large scale reactor, molten salt technology and solar thermal storage may play a role to account for the varying solar conditions.
This report provides a method for calculating the energy demand required for a thermochemical process, and relating that to the area of a solar collector required to produce the needed energy input. The energy demand assessment will report on the energy requirements for the thermochemical technologies that are possible for integration with a solar resource. A theoretical thermodynamic approach following the principals of the first law of thermodynamics forms the basis of the calculator. The energy demand calculator is then applied to Uganda. A quantitative and qualitative review of the waste biomass in Uganda is also reported. It is essential that the feedstock selected must be a waste stream and not disrupt current practice. The qualitative biomass review of Uganda will form the basis on to which the quantitative review of the biomass will be formed. The quantitative review will normalize the biomass by reporting the higher heating values (HHVs), showing which biomass sources will make for a feasible feedstock within Uganda.
3. Overview of the Qualitative Assessment of Biomass in Uganda
The biomass assessment of Uganda reports on what source and use of energy is most needed by those in rural areas, what biomass is readily available, how it is used, and what goes to waste. The aim of the assessment was to discover if there was a readily available biomass waste stream that could be used to produce a useful fuel.
In order to achieve an overall and fair assessment of the country, the maximum distance was covered within the time available. Homesteads, schools, farms, plantations and factories were all visited, as well as general observations made. From interviews and observations with the local people, especially those in schools, a cook fuel/solid fuel for heat would ultimately be the best source of fuel to provide Uganda and other developing countries. Currently the main source of fuel used is untreated forest wood which has a number of disadvantages; deforestation, poor efficiency and high emissions. The potential feedstocks that were identified from the assessment of Uganda include: agricultural and forestry residue (including water hyacinth), sewage sludge and municipal solid waste (MSW). These feedstocks were shown to be largely going to waste, suggesting that if incorporated into the system they would not currently disrupt the lifestyles of the local people.
Literature assessment of the thermochemical technology available and comparing that with concentrated solar technology showed that the possible and practical integration options included a hydrothermal/steam treatment or pyrolysis with either a linear Fresnel or parabolic trough.
5. Calculator Utilisation
The energy demand calculator is a tool which theoretically determines the amount of energy required or produced from the solar/biomass processes. It does not take into account the practicality of solutions. For example, wanting to produce a solar pyrolysis system requiring high temperatures of 500 °C in a rural location of a developing country would require huge amounts of mirrors. Solar energy is dilute, whilst chemical energy is concentrated, thus you need a vast amount of solar energy to make a significant amount of chemical energy. This being the primary reason as to why high temperature thermochemical treatments such as pyrolysis (or gasification) not being a practical solution. A large central receiver (power tower) solar system would be required to generate the heat needed for the process. This would increase the cost and the complexity of the system drastically. With that stated a low temperature steam treatment (with a low biomass to water ratio) or low temperature HTC (ideally a low water to biomass ratio) are ideal thermochemical treatments. With temperatures between 100–250 °C a simple linear Fresnel concentrating method will be able to meet the temperatures required by a low temperature technique.
Uganda has been selected as the case study for this report, the model can be made applicable to all biomass types. Shown below are five practical examples based on common waste biomass streams from Uganda. Each example aims to hydrothermally carbonise 10/15/20 kg of biomass sample at 200 °C in a location with 750 W/m
2 of direct irradiance.
Table 9 shows the constant input variables and
Table 10 shows the specific heat capacity of the different biomasses used for comparison. However, it should be noted that heat capacities do vary within biomasses and will be dependent on how much moisture is in the biomass. Reported heat capacities are approximations for an example and lab tested heat capacities should be used for live projects.
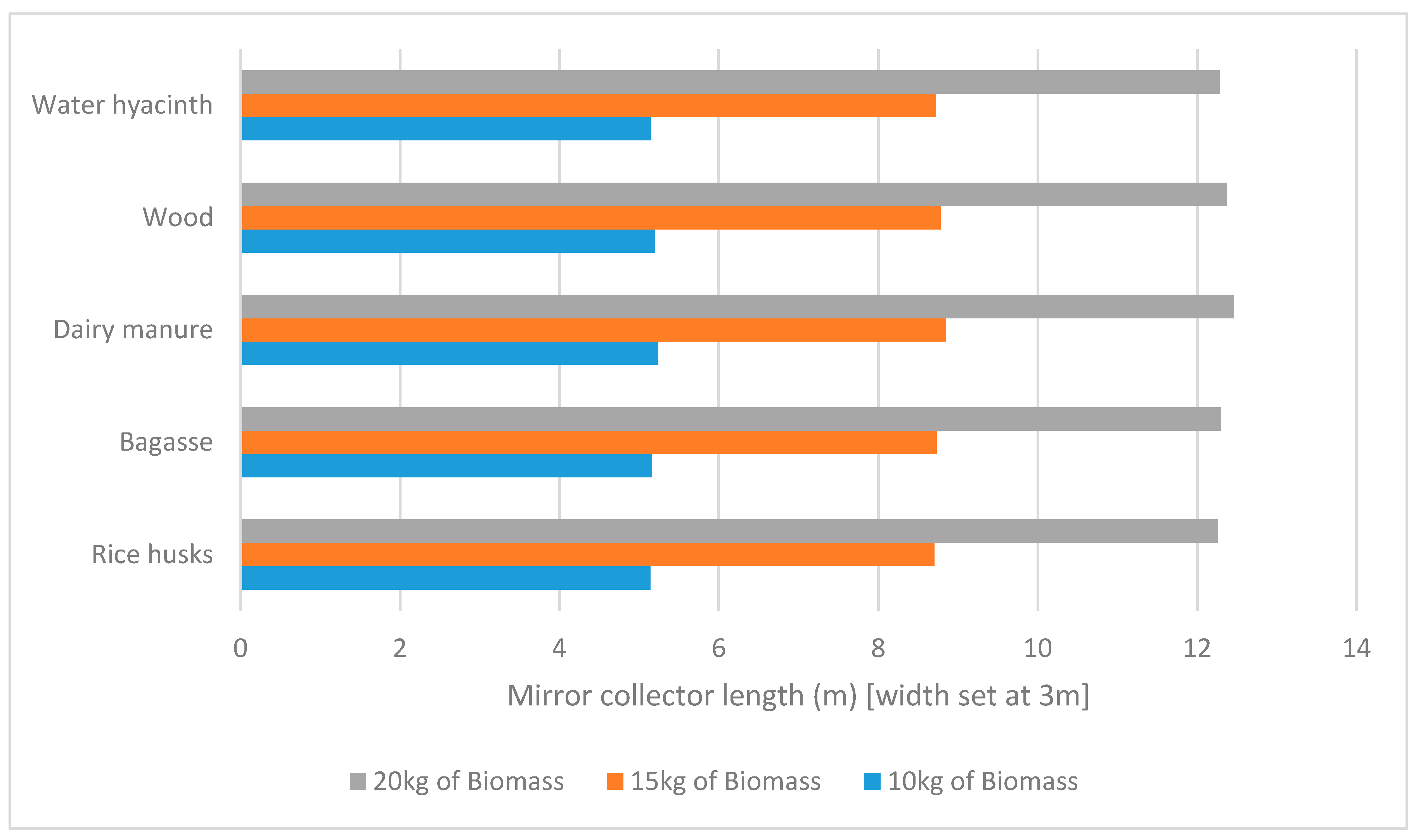
Figure 4 shows the length of mirror required for the process. As HTC requires a large body of water in the system, a ratio of 1:10, the specific heat capacity and therefore biomass type has a very small effect on the length of mirror required. The mass has a much greater effect on the amount of energy required as shown in
Figure 4 Keeping the constant variables, the same, but increasing the mass of biomass to 15 kg, it is clear that mass has a much larger affect than biomass type. As with every extra 1 kg of biomass added to the system, 10 kg of water is required under current HTC practices.
The results shown in
Figure 4 show that for a solar driven HTC process, the mass of biomass being processed has a significantly greater effect on the area of mirror collector required, than the type of biomass being processed.
6. Conclusions
A calculator has been produced to predict the area of the solar collector required to process a given mass of biomass in a given time, the amount of biomass that can be processed in a set time with set solar collector area or the time required to process a given mass of biomass with a set collector area. The basis of the model is formed from Equations (1)–(3) shown in
Section 2.1. A thermodynamic approach with an efficiency factor was deemed as the most practical method for the model. Comparing steam treatment, HTC and pyrolysis in two examples, pyrolysis was shown to be the least energy demanding process. The main factor contributing to this was the amount of water that required heating during steam treatment and HTC. Though pyrolysis is the least energy demanding process, the feedstock selected and practicality should be considered before which thermochemical process is selected for an integrated system.
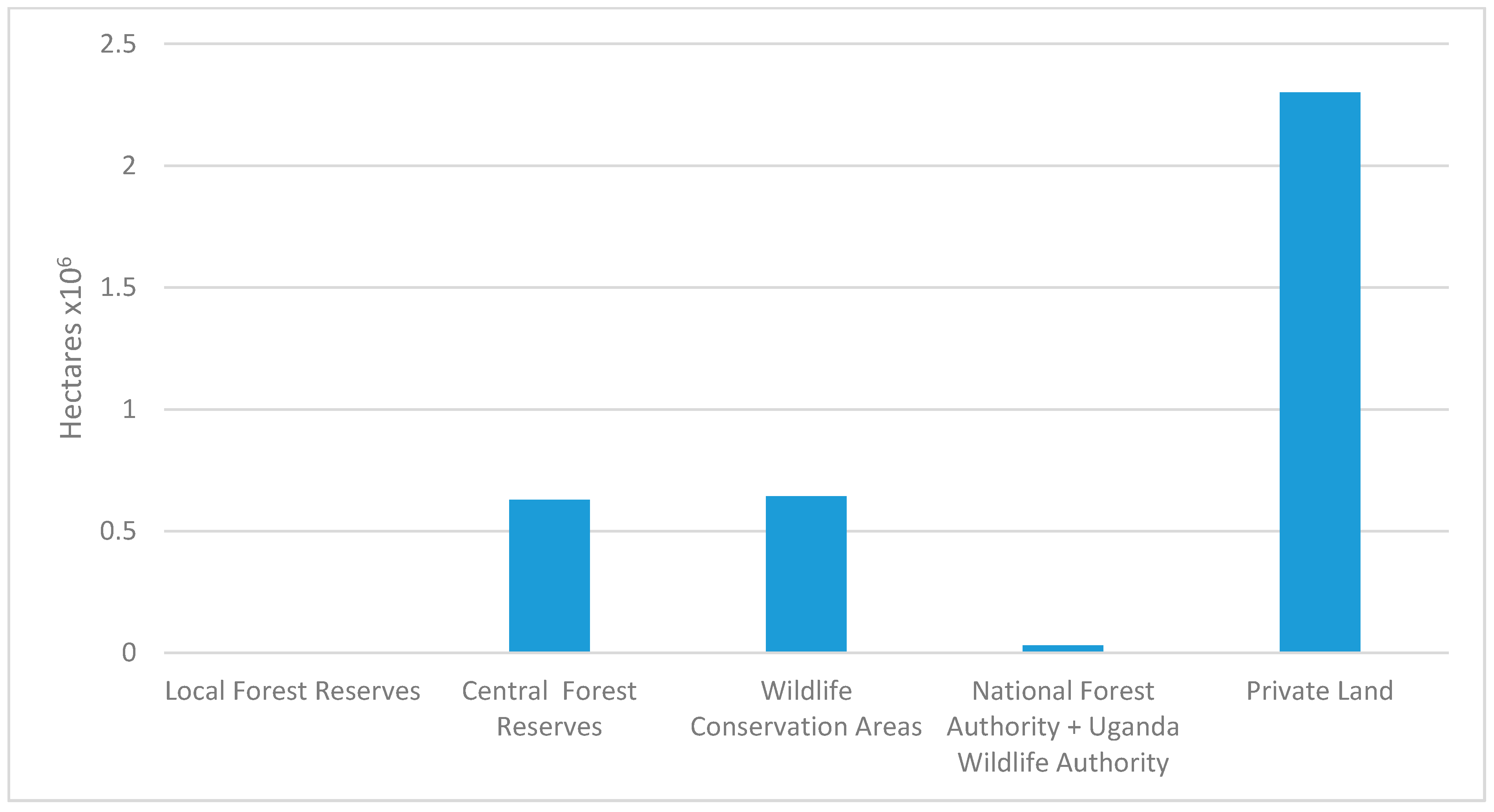
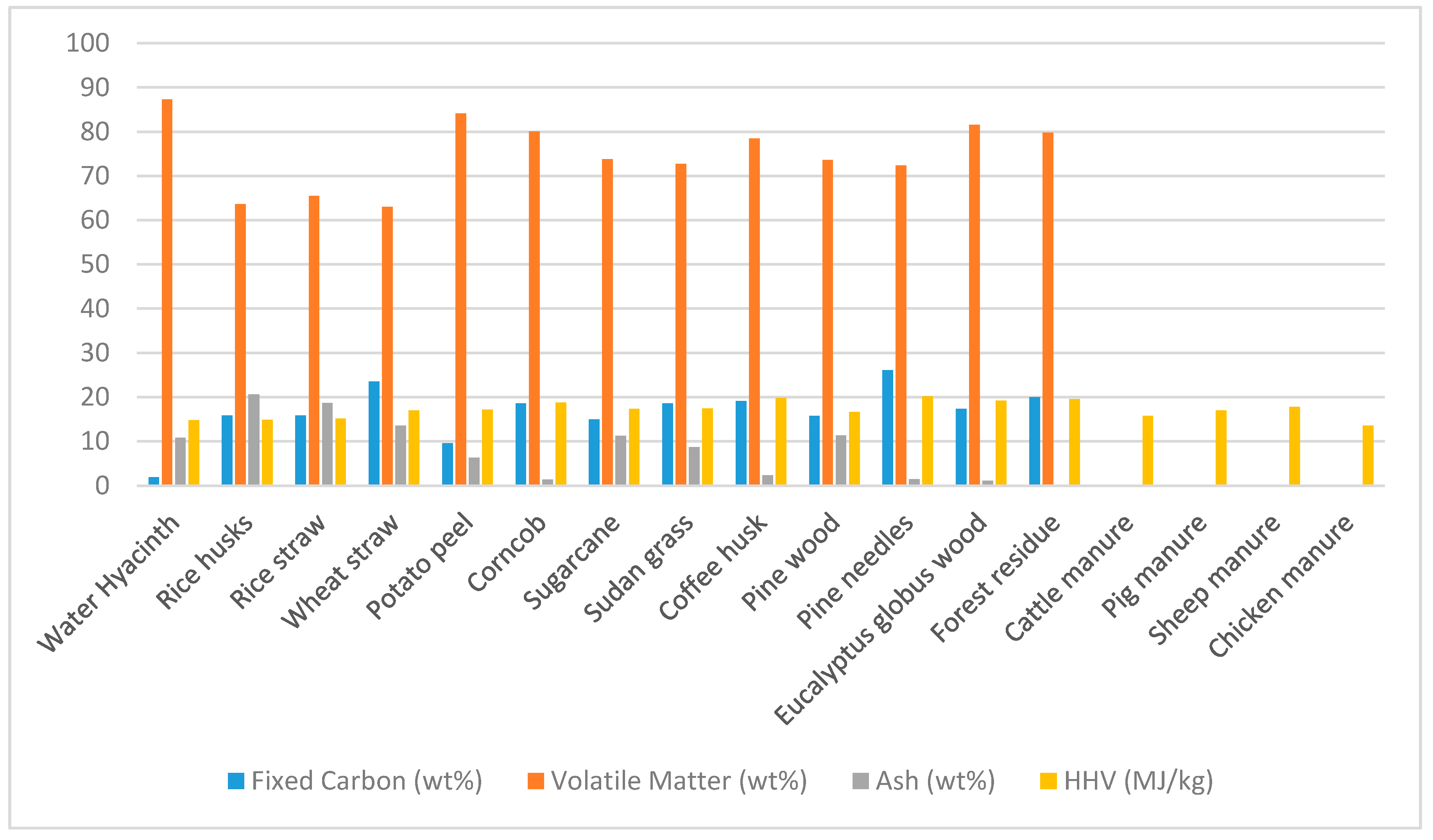
Uganda is a country rich in biomass waste. From the quantitative review accessing the amount of biomass waste available, it is shown that from the top five most produced crops in Uganda, there is approximately 3.75 × 10
6 metric tons of waste available. The majority of this waste is left to rot or to go back into the soil if the waste is produced at the farm. Agricultural waste will average an approximate 15–20 MJ/kg as shown in
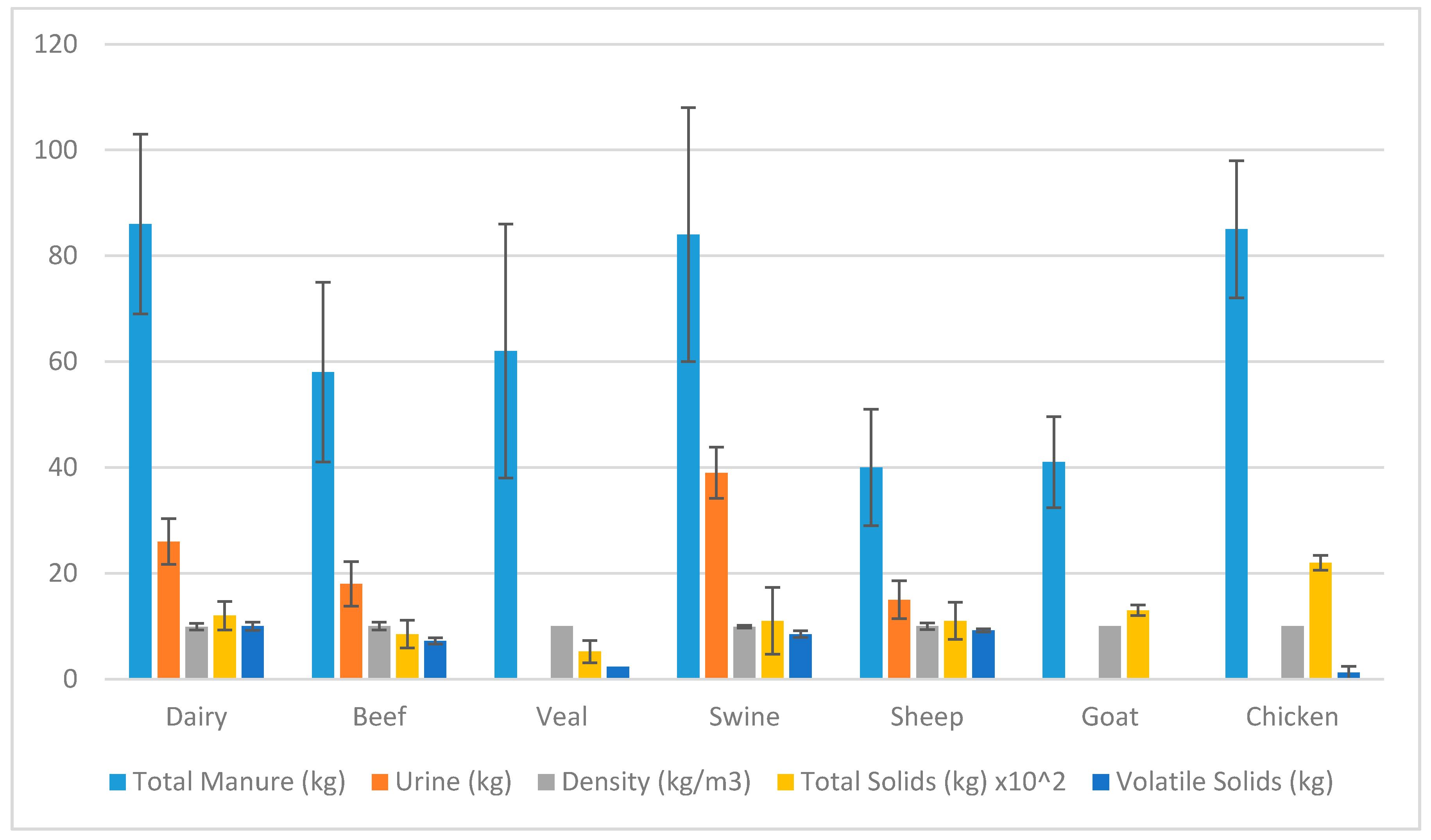
Figure 2. There are 2.3 million hectares of private forest land in Uganda. Forestry residue has been reported to have a HHV of approximately 20 MJ/kg. Within the private forest available, there will be a significant amount of forestry residue available that may be utilized for biofuel. From the qualitative review of biomass, it was stated that the majority of small communities owned their own livestock (individuals, collection of families, schools). Cattle, swine and chicken manure are shown in
Table 4 to have the greatest potential as a feedstock. Though this will vary greatly depending on the conditions the animals are kept in. Conditions of the animals will alter the HHV of their waste as literature reports did vary between 13–20 MJ/kg. Water hyacinth has the potential to be a source of feedstock that will not affect the current supply chain. The quantity in Uganda is unknown, but it has the potential to be farmed and to be used as a feedstock for biofuel for those living near areas of contained water. The biomasses reported all have the potential for upgrading into a solid fuel for use within Uganda and other developing countries to replace firewood or other damaging fuels.
A low temperature steam treatment or low temperature HTC are ideal thermochemical treatments for combining with concentrated solar. With temperatures between 100–250 °C a simple linear Fresnel concentrating method will be able to meet the temperatures required by a low temperature biomass upgrading technique. As seen in
Figure 4, the amount of biomass needed be processed has a much larger effect on the size of the system than the type of feedstock used.