Studies on the Gasification Performance of Sludge Cake Pre-Treated by Hydrothermal Carbonization
Abstract
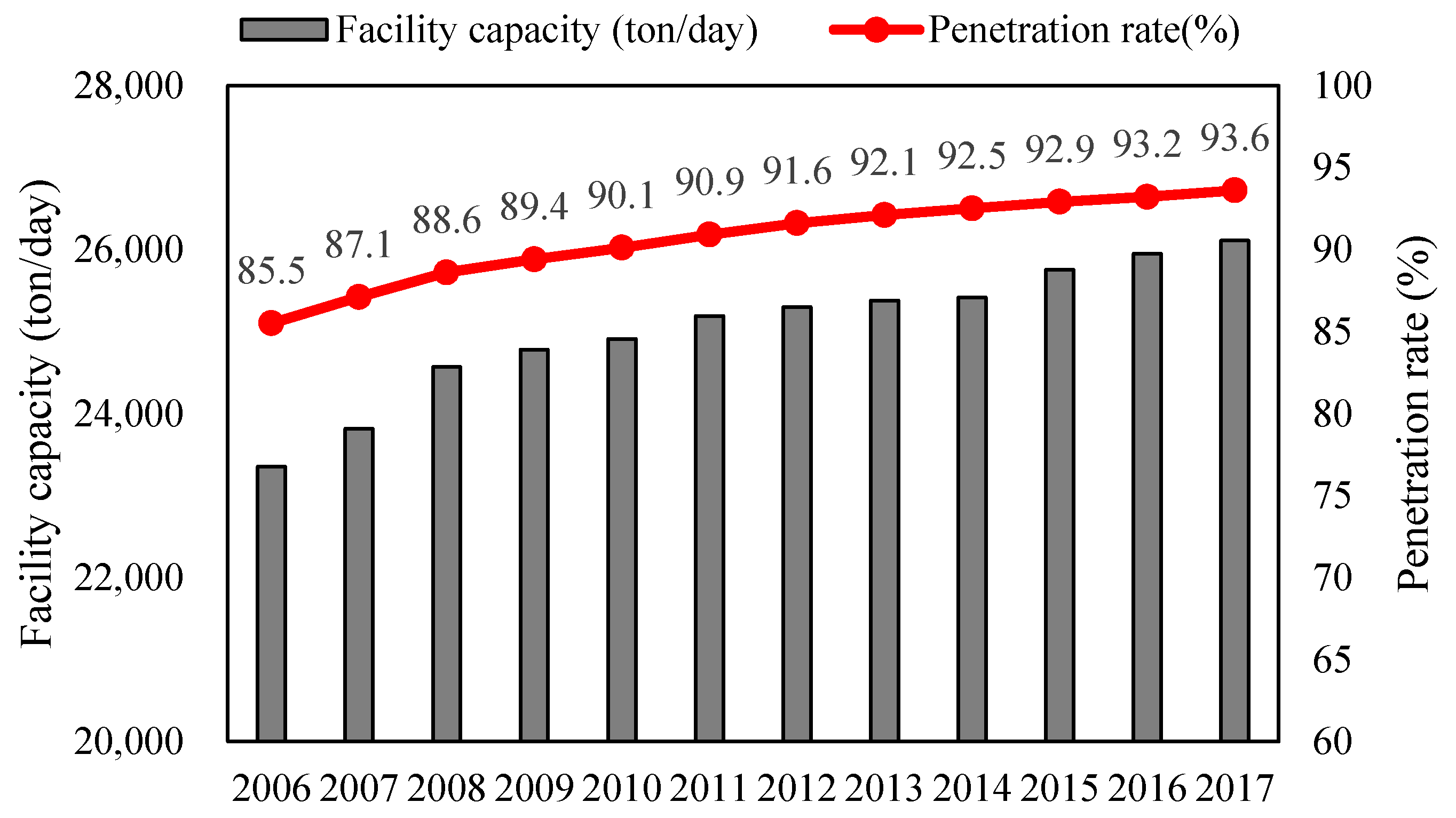
1. Introduction
2. Materials and Methods
2.1. Characteristics of Sewage Sludge Cake
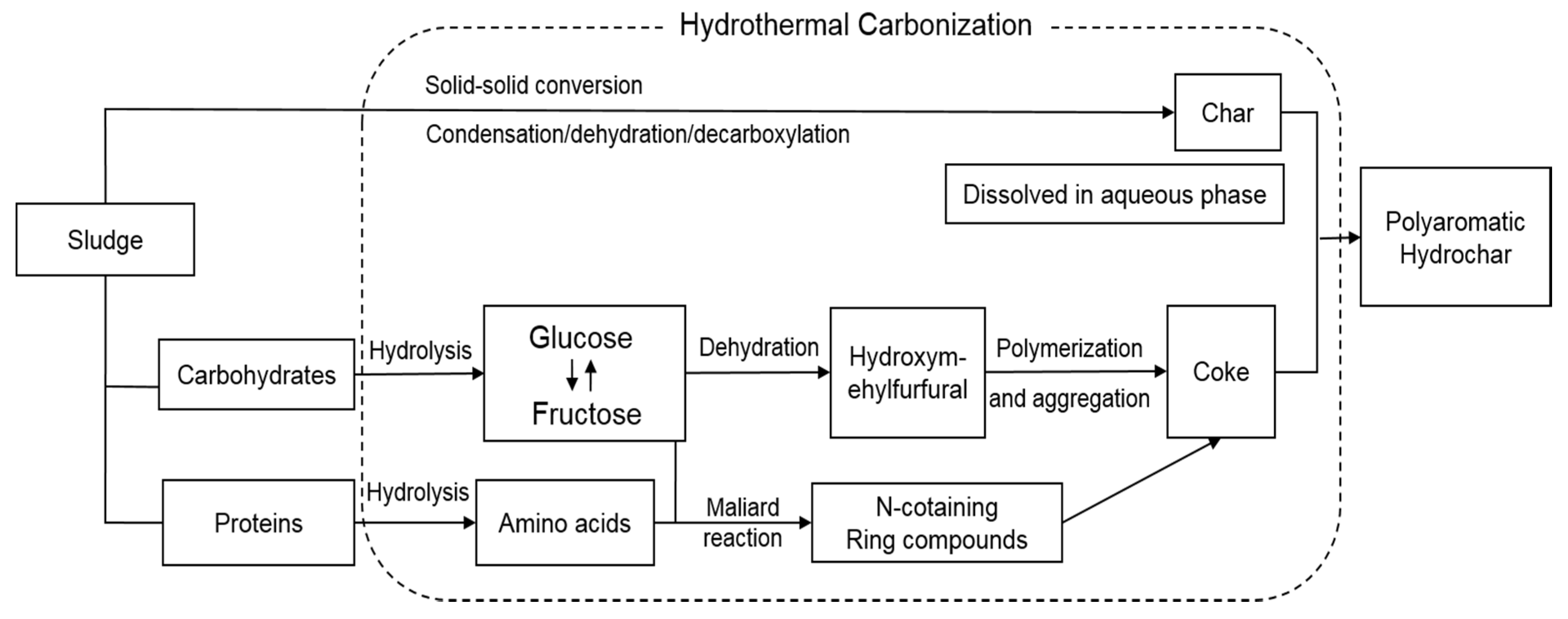
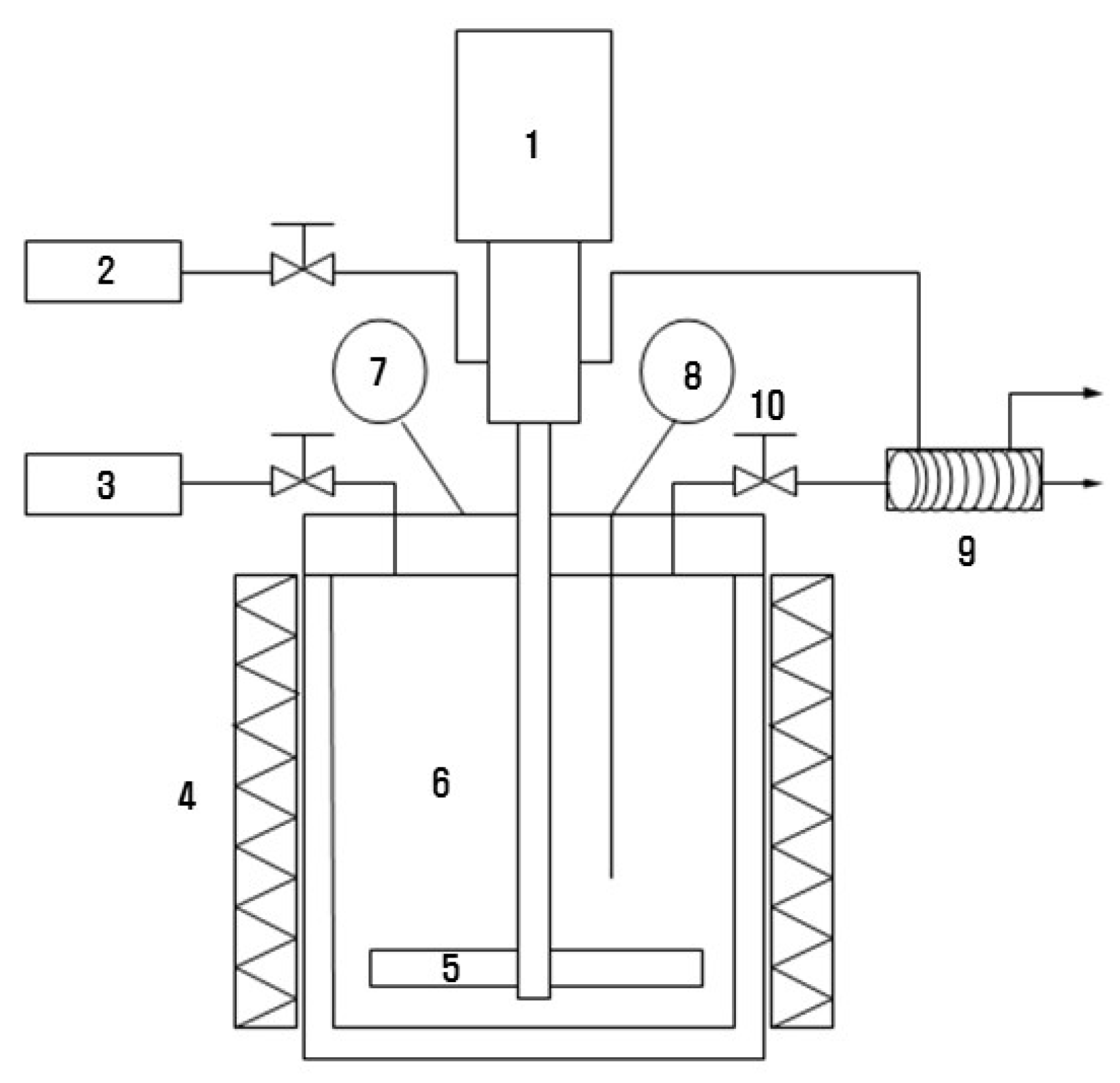
2.2. Hydrothermal Carbonization Process
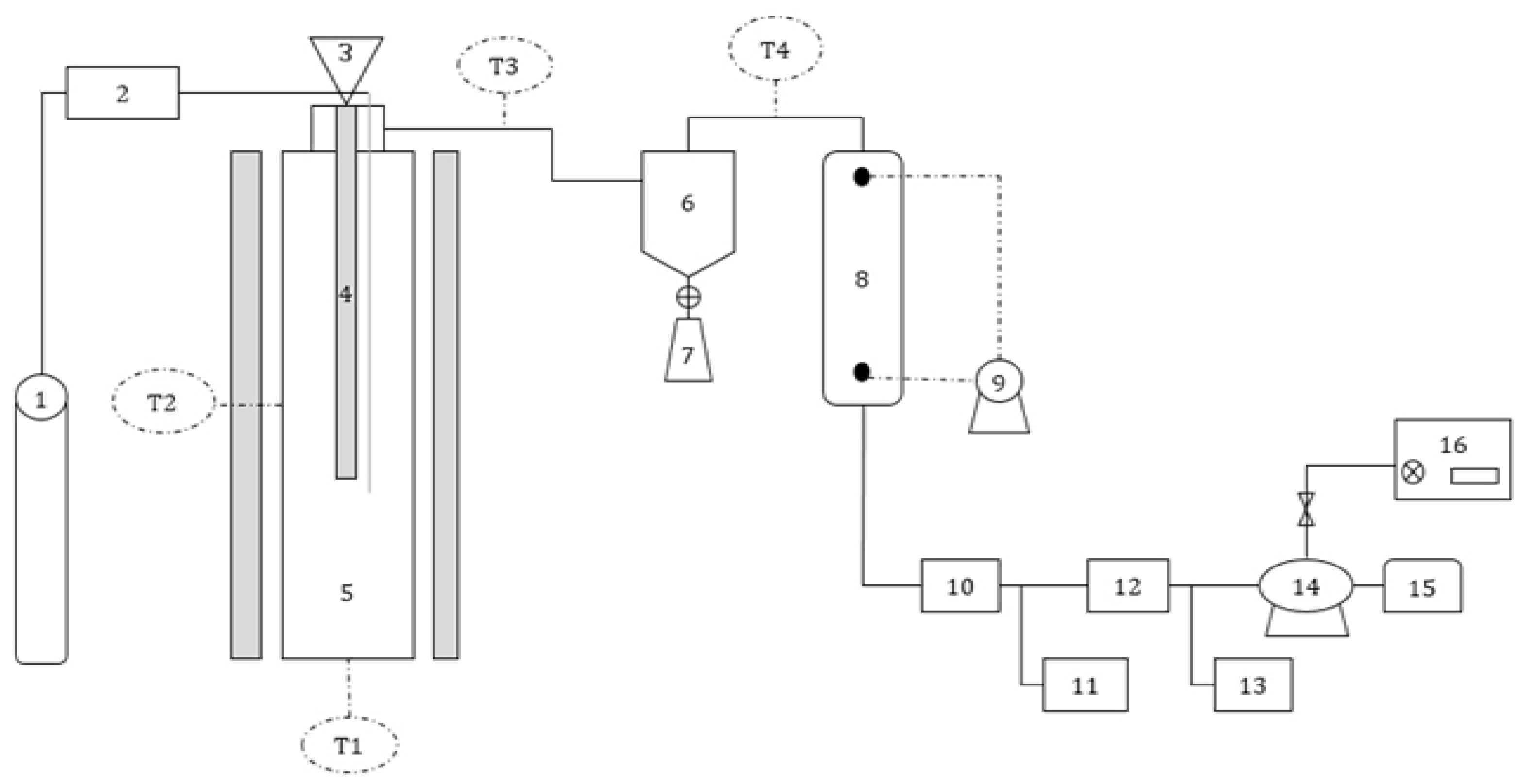
2.3. Gasification Process
3. Results and Discussion
3.1. Hydrothermal Carbonization Efficiency
3.1.1. Characteristics of Hydrothermally Treated Sludge Cake
3.1.2. Natural Drying Efficiency
3.1.3. Change in Structure with Respect to Temperature
3.2. Gasification Performance
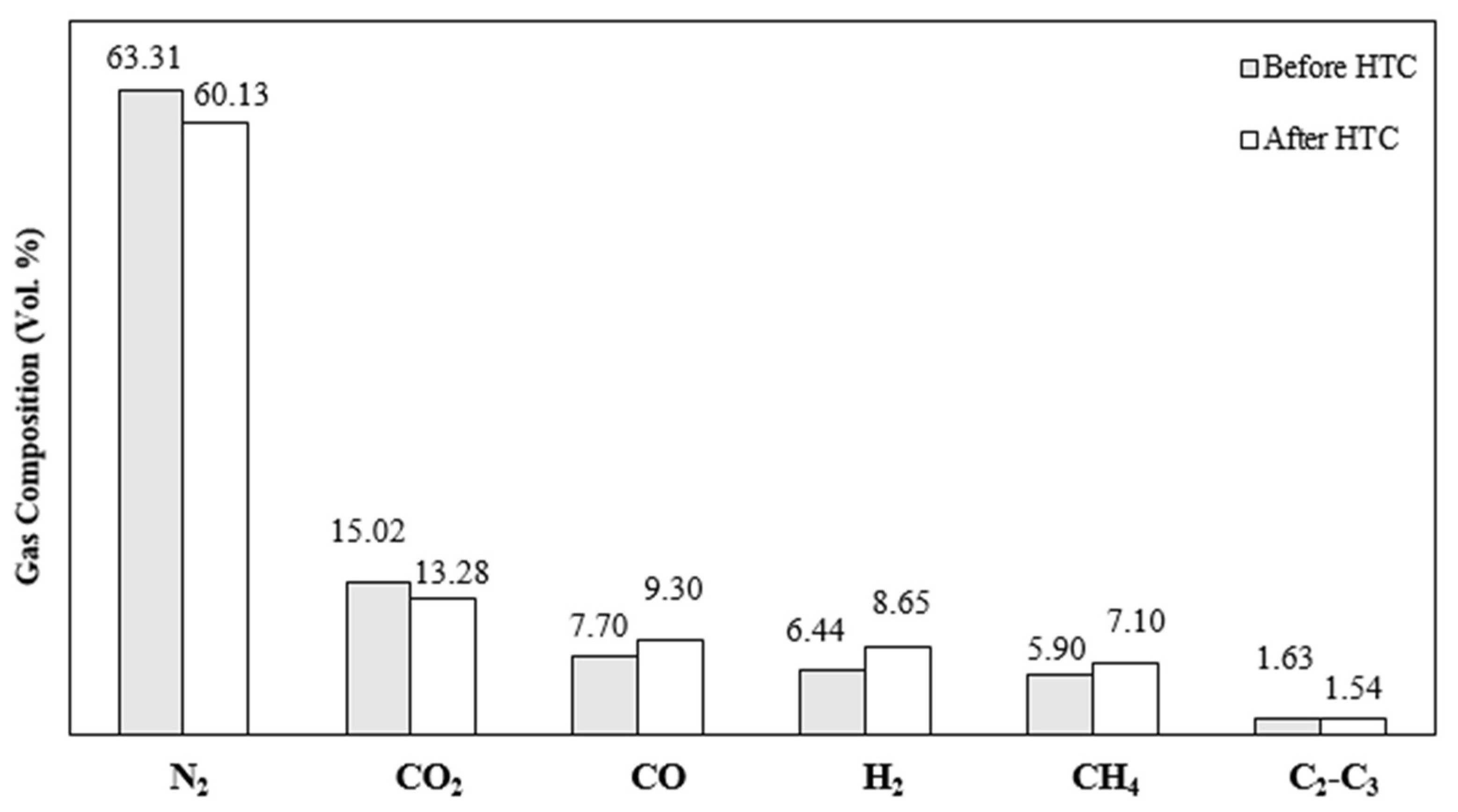
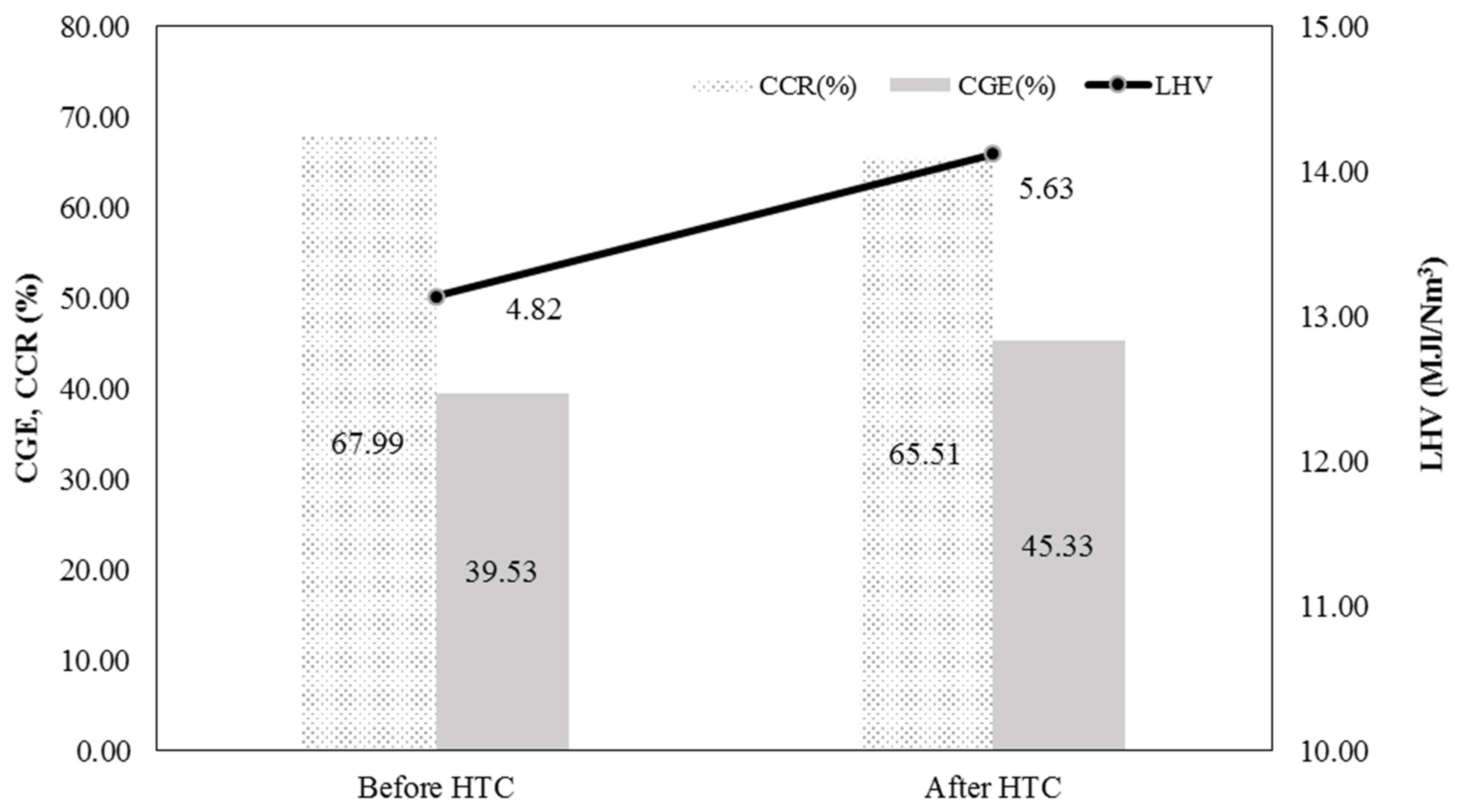
3.2.1. Syngas Efficiency
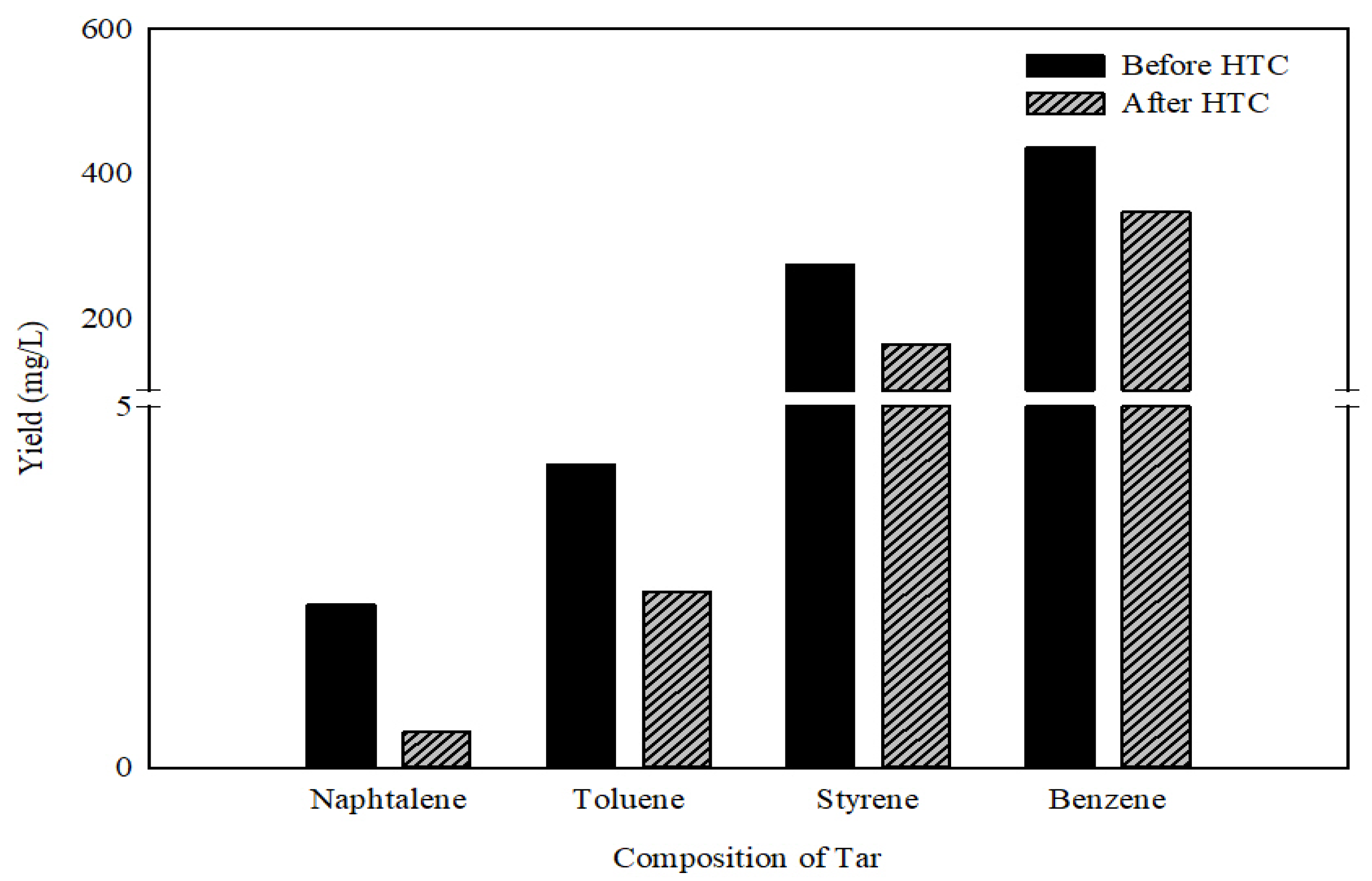
3.2.2. Gas Pollutant and Tar Characteristics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| ASTM | American Society for Testing and Materials |
| BTX | Benzene, toluene, and xylene |
| ηCCR | Carbon conversion ratio |
| ηCGE | Cold gas efficiency |
| CST | Cold solvent trapping |
| DGM | Dry gas meter |
| FC | Fixed carbon |
| FTIR | Fourier-transform infrared spectroscopy |
| HC | Hydro-char |
| HCl | Hydrochloric acid |
| HCN | Hydrogen cyanide |
| HHV | Higher heating value |
| HTC | Hydrothermal carbonization |
| IPA | Isopropyl alcohol |
| ID FAN | Induced draft fan |
| IC | Ion chromatography |
| LHV | Lower heating value |
| LHVgas | Lower heating value of product gas |
| NH3 | Ammonia |
| MC | Moisture content |
| PAHs | Polycyclic aromatic hydrocarbons |
| PCB | Polychlorinated biphenyl |
| TC | Thermocouple |
| VM | Volatile matter |
| XOCs | Xenobiotic organic compounds |
| Ygas | Yield of product gas |
References
- Kim, D.; Park, J.; Lee, J.; Lee, M. The Case Study on Performance Measurement Weighting for Efficient Value Engineering Study of Sewage Treatment Facliity. Korean J. Constr. Eng. Manag. 2016, 17, 40–47. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A. Feedstock and process influence on biodiesel produced from waste sewage sludge. J. Environ. Manag. 2018, 216, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Samolada, M.; Zabaniotou, A. Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece. Waste Manag. 2014, 34, 411–420. [Google Scholar] [CrossRef]
- Dinko, D.; Paolo, B.; Zeljko, J. Energy Recovery from Sewage Sludge: The Case Study of Croatia. Energies 2019, 12, 1927. [Google Scholar]
- Hong, S.G.B.; You, Y.J.; Lee, S.K.; Hong, T.K. Monthly Characteristics of Dewatered Sludge from Sewage Treatment Plant. J. Korean Soc. Environ. Anal. 2018, 21, 95–102. [Google Scholar]
- Park, J.-H.; Jin, M.-H.; Lee, Y.-J.; Song, G.-S.; Choi, J.W.; Lee, D.-W.; Choi, Y.-C.; Park, S.-J.; Song, K.H.; Kim, J.-G. Two-in-one fuel synthetic bioethanol-lignin from lignocellulose with sewage sludge and its air pollutants reduction effects. Energies 2019, 12, 3072. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.-K.; Im, H.-S. A Review on Fuel Properties and Liquid Biofuels Production Technologies from Sewage Sludge. J. Korean Appl. Sci. Technol. 2018, 35, 540–559. [Google Scholar]
- Eriksson, E.; Christensen, N.; Schmidt, J.E.; Ledin, A. Potential priority pollutants in sewage sludge. Desalination 2008, 226, 371–388. [Google Scholar] [CrossRef]
- Rulkens, W. Sewage sludge as a biomass resource for the production of energy: Overview and assessment of the various options. Energy Fuels 2007, 22, 9–15. [Google Scholar] [CrossRef]
- Song, U.; Lee, E.J. Environmental and economical assessment of sewage sludge compost application on soil and plants in a landfill. Resour. Conserv. Recycl. 2010, 54, 1109–1116. [Google Scholar] [CrossRef]
- Giuliano, A.; Gioiella, F.; Sofia, D.; Lotrecchiano, N. A novel methodology and technology to promote the social acceptance of biomass power plants avoiding nimby syndrome. Chem. Eng. Trans. 2018, 67, 307–312. [Google Scholar]
- Chun, Y.N.; Kim, S.C.; Yoshikawa, K. Pyrolysis gasification of dried sewage sludge in a combined screw and rotary kiln gasifier. Appl. Energy 2011, 88, 1105–1112. [Google Scholar] [CrossRef]
- Alam, M.T.; Lee, J.-S.; Lee, S.-Y.; Bhatta, D.; Yoshikawa, K.; Seo, Y.-C. Low Chlorine Fuel Pellets Production from the Mixture of Hydrothermally Treated Hospital Solid Waste, Pyrolytic Plastic Waste Residue and Biomass. Energies 2019, 12, 4390. [Google Scholar] [CrossRef]
- Sun, X.; Sumida, H.; Yoshikawa, K.; Sumida, H.; Yoshikawa, K. Effects of hydrothermal process on the nutrient release of sewage sludge. Int. J. Waste Resour. 2013, 3, 124. [Google Scholar]
- Tuan, P.-A.; Mika, S.; Pirjo, I. Sewage sludge electro-dewatering treatment—A review. Dry. Technol. 2012, 30, 691–706. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.Y. Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Reza, M.T.; Wirth, B.; Lueder, U.; Werner, M. Behavior of selected hydrolyzed and dehydrated products during hydrothermal carbonization of biomass. Bioresour. Technol. 2014, 169, 352–361. [Google Scholar] [CrossRef]
- Escala, M.; Zumbuhl, T.; Koller, C.; Junge, R.; Krebs, R. Hydrothermal Carbonization as an Energy-Efficient Alternative to Established Drying Technologies for Sewage Sludge: A Feasibility Study on a Laboratory Scale. Energy Fuels 2013, 27, 454–460. [Google Scholar] [CrossRef]
- Da Silva, A.R.G.; Giuliano, A.; Errico, M.; Rong, B.G.; Barletta, D. Economic value and environmental impact analysis of lignocellulosic ethanol production: Assessment of different pretreatment processes. Clean Technol. Environ. Policy 2019, 21, 637–654. [Google Scholar] [CrossRef]
- Seo, Y.-C.; Alam, M.T.; Yang, W.-S. Gasification of Municipal Solid Waste. Gasif. Low-Grade Feedstock 2018. [Google Scholar]
- Giuliano, A.; Catizzone, E.; Barisano, D.; Nanna, F.; Villone, A.; Bari, I.D.; Cornacchia, G.; Braccio, G. Towards Methanol Economy: A Techno-environmental Assessment for a Bio-methanol OFMSW/Biomass/Carbon Capture-based Integrated Plant. Int. J. Heat Technol. 2019, 37, 665–674. [Google Scholar] [CrossRef]
- Giuliano, A.; Poletto, M.; Barletta, D. Pure hydrogen co-production by membrane technology in an IGCC power plant with carbon capture. Int. J. Hydrog. Energy 2018, 43, 19279–19292. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, J.-S.; Yang, W.-S.; Alam, M.T.; Seo, Y.-C.; Lee, S.-Y. Gasification characteristics of biomass for tar removal by secondary oxidant injection. J. Mater. Cycles Waste Manag. 2018, 20, 823–831. [Google Scholar] [CrossRef]
- Rezaiyan, J.; Cheremisinoff, N.P. Gasification Technologies: A Primer for Engineers and Scientists; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Park, S.-W.; Lee, J.-S.; Yang, W.-S.; Kang, J.-J.; Alam, M.T.; Seo, Y.-C.; Oh, J.-H.; Gu, J.-H.; Chennamaneni, S.; Kandasamy, V. Emission Characteristics of Gaseous Pollutants from Pilot-Scale SRF Gasification Process. Waste Manag. Resour. Effic. 2019, 51–58. [Google Scholar]
- Park, S.-W.; Seo, Y.-C.; Lee, J.-S.; Yang, W.-S.; Alam, M.-T.; Lee, S.-Y. A Study on Application of Gasification and Emission Characteristics of Gaseous Pollutants for Producing Syngas Using High Calorific Waste. J. Korea Soc. Waste Manag. 2017, 34, 234–244. [Google Scholar] [CrossRef]
- Bourtsalas, A.T.; Seo, Y.; Alam, M.T.; Seo, Y.-C. The status of waste management and waste to energy for district heating in South Korea. Waste Manag. 2019, 85, 304–316. [Google Scholar] [CrossRef]
- Midilli, A.; Dogru, M.; Howarth, C.R.; Ling, M.J.; Ayhan, T. Combustible gas production from sewage sludge with a downdraft gasifier. Energy Convers. Manag. 2001, 42, 157–172. [Google Scholar] [CrossRef]
- Uemura, Y.; Sellappah, V.; Trinh, T.H.; Hassan, S.; Tanoue, K.-I. Torrefaction of empty fruit bunches under biomass combustion gas atmosphere. Bioresour. Technol. 2017, 243, 107–117. [Google Scholar] [CrossRef]
- Asses, N.; Farhat, A.; Cherif, S.; Hamdi, M.; Bouallagui, H. Comparative study of sewage sludge co-composting with olive mill wastes or green residues: Process monitoring and agriculture value of the resulting composts. Process. Saf. Environ. Prot. 2018, 114, 25–35. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, J.; Yang, W.; Kang, J.; Sung, J.; Alam, M.T.; Seo, Y.; Rao, C.S.; Saravanakumar, A.; Kumar, K.V. For Waste to Energy, Assessment of Fluff Type Solid Refuse Fuel by Thermal Characteristics Analyses. Procedia Environ. Sci. 2016, 35, 498–505. [Google Scholar] [CrossRef]
- Yang, W.-S.; Lee, J.-S.; Park, S.-W.; Kang, J.-J.; Alam, M.T.; Seo, Y.-C. Gasification applicability study of polyurethane solid refuse fuel fabricated from electric waste by measuring syngas and nitrogenous pollutant gases. J. Mater. Cycles Waste Manag. 2016, 18, 509–516. [Google Scholar] [CrossRef]
- Zhao, P.; Shen, Y.; Ge, S.; Chen, Z.; Yoshikawa, K. Clean solid biofuel production from high moisture content waste biomass employing hydrothermal treatment. Appl. Energy 2014, 131, 345–367. [Google Scholar] [CrossRef]
- Kang, J.; Lee, J.; Yang, W.; Park, S.; Alam, M.T.; Back, S.; Choi, H.; Seo, Y.; Yun, Y.; Gu, J. A study on environmental assessment of residue from gasification of polyurethane waste in e-waste recycling process. Procedia Environ. Sci. 2016, 35, 639–642. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Wu, W.; Chen, W.-H. Gasification performances of raw and torrefied biomass in a downdraft fixed bed gasifier using thermodynamic analysis. Fuel 2014, 117, 1231–1241. [Google Scholar] [CrossRef]
- Ma, Z.; Ye, J.; Zhao, C.; Zhang, Q. Gasification of rice husk in a downdraft gasifier: The effect of equivalence ratio on the gasification performance, properties, and utilization analysis of byproducts of char and tar. Bioresources 2015, 10, 2888–2902. [Google Scholar] [CrossRef]
- Ma, J.; Chen, M.; Yang, T.; Liu, Z.; Jiao, W.; Li, D.; Gai, C. Gasification performance of the hydrochar derived from co-hydrothermal carbonization of sewage sludge and sawdust. Energy 2019, 173, 732–739. [Google Scholar] [CrossRef]
- Zainal, Z.; Rifau, A.; Quadir, G.; Seetharamu, K. Experimental investigation of a downdraft biomass gasifier. Biomass Bioenergy 2002, 23, 283–289. [Google Scholar] [CrossRef]
- Tamosiunas, A.; Gimzauskaite, D.; Aikas, M.; Uscila, R.; Praspaliauskas, M.; Eimontas, J. Gasification of waste cooking oil to synagas by thermal arc plasma. Energies 2019, 12, 2612. [Google Scholar] [CrossRef]
- Meng, Q.M.; Chen, X.P.; Zhuang, Y.M.; Liang, C. Effect of temperature on controlled air oxidation of plastic and biomass in a packed-bed reactor. Chem. Eng. Technol. 2013, 36, 220–227. [Google Scholar] [CrossRef]
- Gai, C.; Chen, M.; Liu, T.; Peng, N.; Liu, Z. Gasification characteristics of hydrochar and pyrochar derived from sewage sludge. Energy 2016, 113, 957–965. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, J.-S.; Yang, W.-S.; Alam, M.T.; Seo, Y.-C. A Comparative Study of the Gasification of Solid Refuse Fuel in Downdraft Fixed Bed and Bubbling Fluidized Bed Reactors. Waste Biomass Valorization 2018, 1–12. [Google Scholar] [CrossRef]
- Shin, M.-S.; Lee, H.-D.; Jeon, Y.-W. Evaluation of drying performances by hydrothermal reaction of sewage sludge and food wastes. J. Korea Org. Resour. Recycl. Assoc. 2017, 25, 47–55. [Google Scholar]
- Fonts, I.; Azuara, M.; Gea, G.; Murillo, M. Study of the pyrolysis liquids obtained from different sewage sludge. J. Anal. Appl. Pyrolysis 2009, 85, 184–191. [Google Scholar] [CrossRef]
- Jouraiphy, A.; Amir, S.; El Gharous, M.; Revel, J.-C.; Hafidi, M. Chemical and spectroscopic analysis of organic matter transformation during composting of sewage sludge and green plant waste. Int. Biodeterior. Biodegrad. 2005, 56, 101–108. [Google Scholar] [CrossRef]
- ZHANG, J.-H.; LIN, Q.-M.; ZHAO, X.-R. The hydrochar characters of municipal sewage sludge under different hydrothermal temperatures and durations. J. Integr. Agric. 2014, 13, 471–482. [Google Scholar] [CrossRef]
- Wang, L.; Li, A. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: The dewatering performance and the characteristics of products. Water Res. 2015, 68, 291–303. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, T.; Ma, K.; Xu, G.; Hu, Y.; Chen, D. Effect of hydrothermal temperature on the steam gasification performance of sewage sludge: Syngas quality and tar formation. Energy Fuels 2018, 32, 6834–6838. [Google Scholar] [CrossRef]
- Seggiani, M.; Vitolo, S.; Puccini, M.; Bellini, A. Cogasification of sewage sludge in an updraft gasifier. Fuel 2012, 93, 486–491. [Google Scholar] [CrossRef]




| Reaction Type | Chemical Reaction |
|---|---|
| Carbon reactions | |
| R1 | C + CO2 ↔ 2CO +172.0 kJ/mol |
| R2 | C + H2O ↔ CO + H2 +131.0 kJ/mol |
| R3 | C + 2H2 ↔ CH4 −74.8 kJ/mol |
| R4 | C + 0.5O2 ↔ CO −11.0 kJ/mol |
| Oxidation reactions | |
| R5 | C + O2 ↔ CO2 −394.0 kJ/mol |
| R6 | CO + 0.5O2 ↔ CO2 −284.0 kJ/mol |
| R7 | CH4 + 2O2 ↔ CO2 + 2H2O −803.0 kJ/mol |
| R8 | H2 + 0.5O2 ↔ H2O −242.0 kJ/mol |
| Shift reaction | |
| R9 | CO + H2O ↔ CO2 + H2 −41.2 kJ/mol |
| Methanation reactions | |
| R10 | 2CO + 2H2 ↔ CH4 + CO −247.0 kJ/mol |
| R11 | CO + 3H2 ↔ CH4 + H2O −206.0 kJ/mol |
| R14 | CO2 + 4H2 ↔ CH4 + 2H2O −165.0 kJ/mol |
| Steam-reforming reactions | |
| R12 | CH4 + H2O ↔ CO +3H2 +206.0 kJ/mol |
| R13 | CH4 + 0.5O2 ↔ CO + 2H2 −36.0 kJ/mol |
| Analysis | Target | Equipment | Method |
|---|---|---|---|
| Elemental analysis | C, H, O, N, S | EA 1112 - Thermo Fisher Scientific (Waltham, Massachusetts, USA) EA 1110 - CE Instrument (Wigan, Greater Manchester, England) | ASTM D 5373 ASTM D 7359-08 |
| Proximate analysis | Moisture volatiles, fixed carbon, ash | TGA-701 - LECO (St. Joseph, Michigan, USA) | ASTM D 3172 |
| Heating value analysis | Higher heating value | AC-600 - LECO (St. Joseph, Michigan, USA) | ASTM D 4809 |
| Analysis | Component | Sample | |||
|---|---|---|---|---|---|
| Sludge Cake | HTC-180 | HTC-200 | HTC-220 | ||
| Proximate (wt.%) | FC | 7.54 | 8.30 | 8.67 | 8.96 |
| VC | 70.30 | 68.60 | 66.32 | 65.12 | |
| AC | 22.16 | 23.10 | 25.01 | 25.92 | |
| Elementary (wt.%) | C | 42.54 | 43.91 | 45.03 | 45.58 |
| H | 7.62 | 7.34 | 7.00 | 6.68 | |
| N | 3.89 | 2.59 | 2.01 | 1.84 | |
| S | 0.36 | 0.20 | 0.17 | 0.15 | |
| O | 23.43 | 22.86 | 20.78 | 19.83 | |
| Heating value (MJ/kg) | HHV | 21.33 | 21.48 | 21.74 | 21.64 |
| LHV | 19.59 | 19.80 | 20.15 | 20.12 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.Y.; Park, S.W.; Alam, M.T.; Jeong, Y.O.; Seo, Y.-C.; Choi, H.S. Studies on the Gasification Performance of Sludge Cake Pre-Treated by Hydrothermal Carbonization. Energies 2020, 13, 1442. https://doi.org/10.3390/en13061442
Lee SY, Park SW, Alam MT, Jeong YO, Seo Y-C, Choi HS. Studies on the Gasification Performance of Sludge Cake Pre-Treated by Hydrothermal Carbonization. Energies. 2020; 13(6):1442. https://doi.org/10.3390/en13061442
Chicago/Turabian StyleLee, Sang Yeop, Se Won Park, Md Tanvir Alam, Yean Ouk Jeong, Yong-Chil Seo, and Hang Seok Choi. 2020. "Studies on the Gasification Performance of Sludge Cake Pre-Treated by Hydrothermal Carbonization" Energies 13, no. 6: 1442. https://doi.org/10.3390/en13061442
APA StyleLee, S. Y., Park, S. W., Alam, M. T., Jeong, Y. O., Seo, Y.-C., & Choi, H. S. (2020). Studies on the Gasification Performance of Sludge Cake Pre-Treated by Hydrothermal Carbonization. Energies, 13(6), 1442. https://doi.org/10.3390/en13061442






