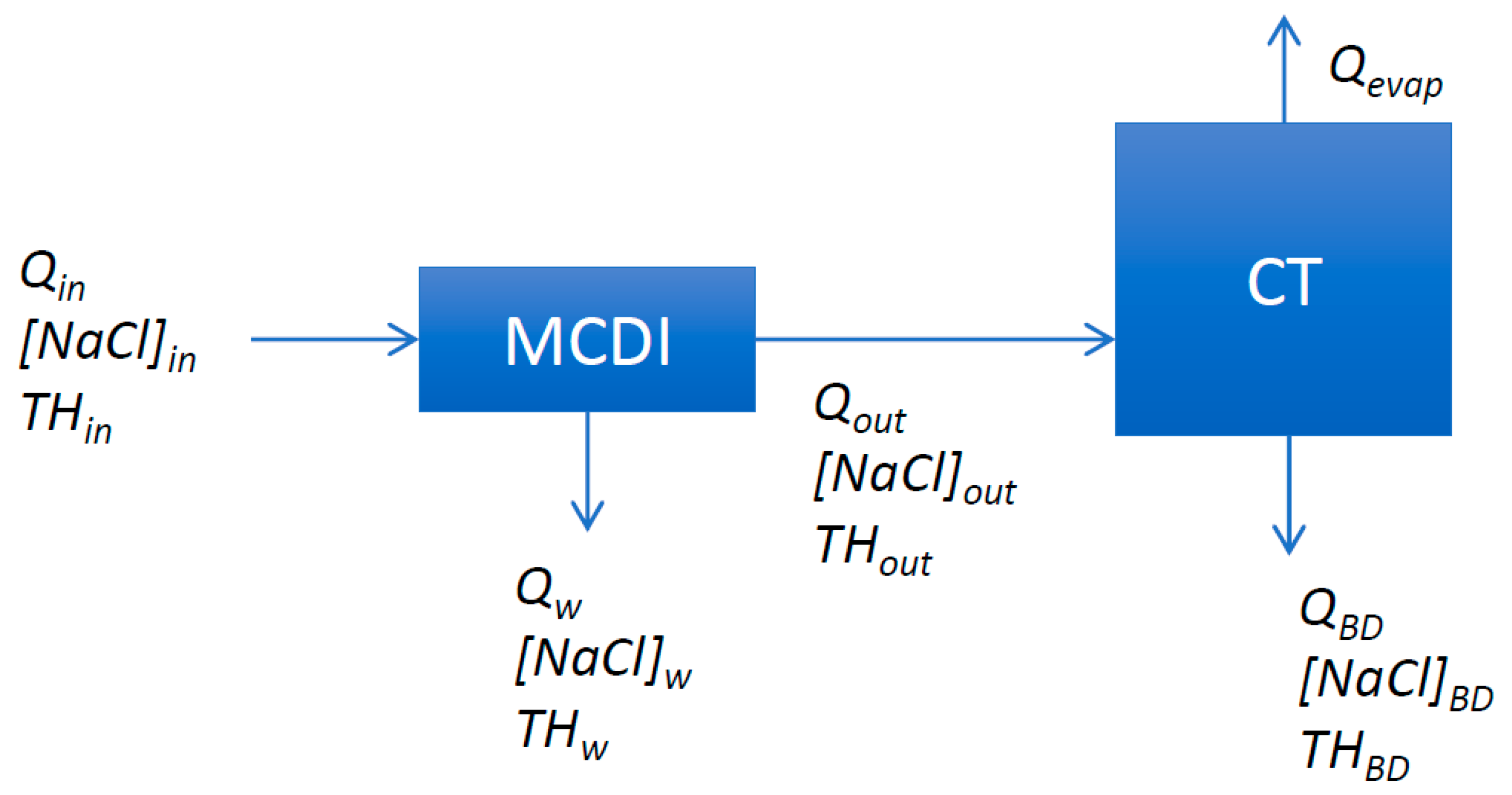
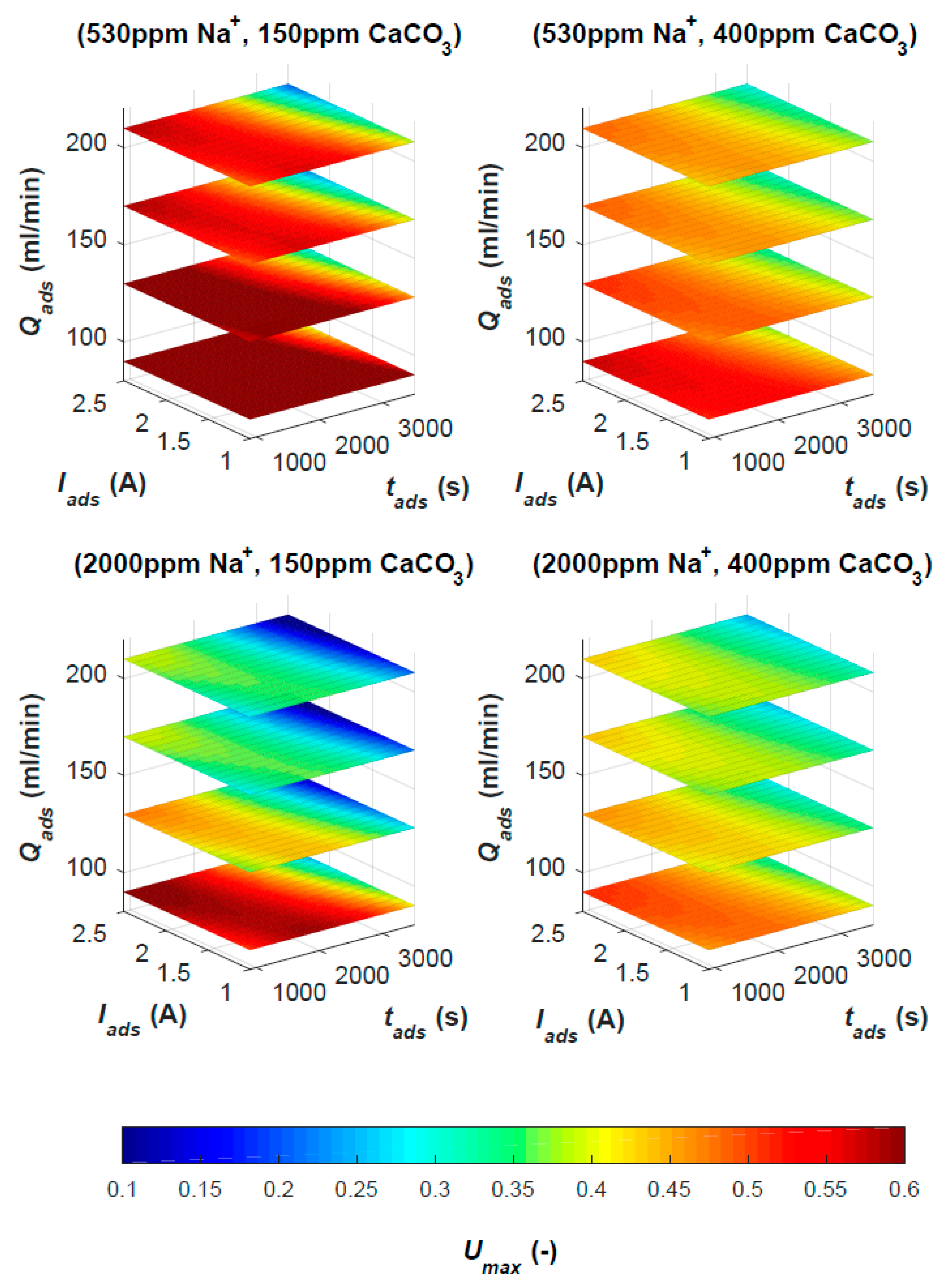
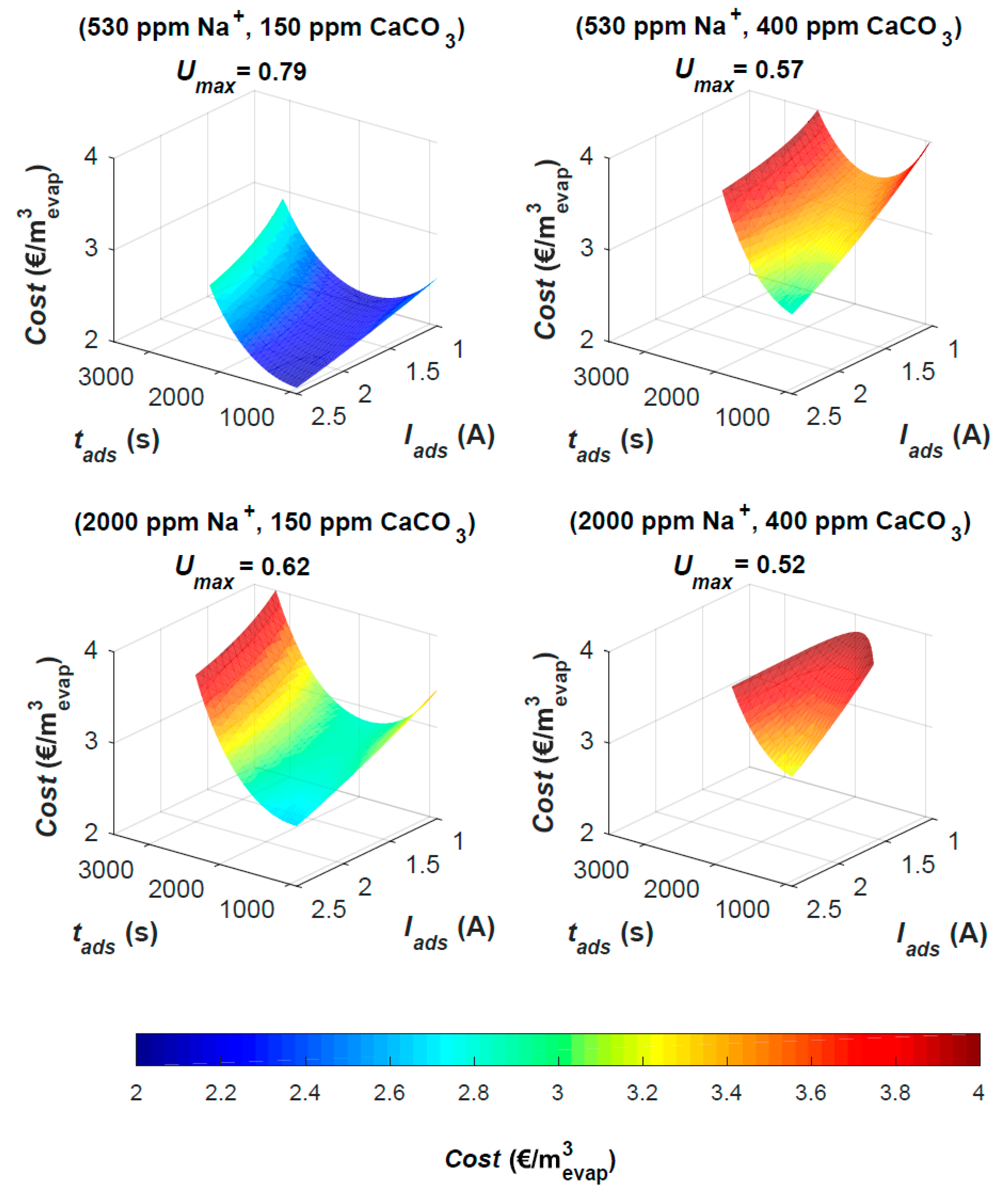
3.1. MCDI Response Surface
Measured response variables and calculated response variables are determined from MCDI lab tests (
Table 2).
The primary response data include
[NaCl]out,
THout and
WR. Comparison of
[NaCl]out and
[NaCl]in shows that feed water NaCl concentration is effectively reduced during MCDI tests. A maximal reduction of 91% in [NaCl] is found (run 20) while median removal equals only 16%. This indicates that overall removal of [NaCl] is relatively low, which is expected when aiming for partial desalination. A similar trend is found for
TH, maximal removal amounts to 88% while median removal equals 23%. Water recovery is generally high for MCDI tests (67% min. to 85% med. to 95% max.) This is desired as MCDI
WR is expected to largely affect overall water use efficiency when MCDI is used in combination with a CT. Secondary response variables include specific energy use (kWh m
−3in), estimated cost (€ m
−3in) and selectivity (-). The median specific energy use of the MCDI system amounts to 0.18 kWh m
−3, which is well within the expected range [
9,
20]. Maximal
E (0.58 kWh m
−3) is found for test run 20 in which 90% reduction in [NaCl] was obtained. Cost estimates range from 0.59 € m
−3in minimum over 0.98 € m
−3in median to 2.95 € m
−3in maximum. Cost estimates in literature are scarce and range from low, 0.11
$ m
−3 for CDI (no membranes) on low salinity feed of ≤2000 ppm assuming a 15-year module depreciation [
27], to high, 11.7 € ton
−1 for a 3 kg m
−3 [Na
+] biomass hydrolysate [
28]. It needs to be mentioned that the purpose of cost estimation is to distinguish between the economics of different process settings rather than to mirror the exact cost of the MCDI process. Selectivity is calculated for the different runs and
S is found to vary between 0.8 minimum and 1.45 maximum with 1.08 as median. Following data acquisition, least-squares multiple regression analysis and a subsequent model reduction procedure are applied resulting in a set of regression equations relating response variables to relevant factors and combinations thereof (
Table 3).
The equations for the primary response variables [NaCl]
out and
THout have a good fit (
R2adj.,
R2 = 0.99 and 0.95), while that for WR is less good (
R2adj. = 0.83). Post-hoc testing of residuals shows that the assumption of normality is satisfied (Shapiro–Wilk’s (SW) W test:
[NaCl]out,
W = 0.96,
p = 0.40;
THout,
W = 0.98,
p = 0.76;
WR,
W = 0.95,
p = 0.17). The effect of different factors on response variables is quantified by the pareto order of their standardized effects (
Table 3). It can be seen that
[NaCl]out depends largely on the
[NaCl]in. Besides this effect,
Qads, Iads and
tads have a smaller but relevant influence on desalination. Low
[NaCl]out occurs for intermediate
tads, high
Iads and low
Qads. From factor signs, it can be seen that
[NaCl]out is lowest at low flow rates. This is in accordance with previous findings [
30], ion removal rate increases with increasing flowrate, but the effect of shorter contact time due to increased flow rate is larger.
THout depends largely on
THin and is also lowest at intermediate
tads, high
Iads and low
Qads. Trends for
THout are highly similar to those for
[NaCl]out. In addition, less hardness is removed from brackish water compared to sweet water, since
THout depends also on
[NaCl]in. Maximal
WR is achieved at low
Iads and short
tads, which conflicts with the desired parameter settings required for low
THout and
[NaCl]out. For the secondary response variables, only the reduced equation for
Cost has a good fit (
R2adj. = 0.95). The equation (
Table 3) indicates that
Cost is depending of
Qads and
Q2ads only. Flowrate is directly related to required electrode surface, which confirms the notion that MCDI cost is largely determined by equipment cost [
23]. Residuals analysis shows a systematic underprediction of cost at low flowrate and overprediction at high flowrate causing the normality assumption not to be satisfied (SW,
W = 0.77,
p < 0.001). A power law of
Q and
Cost is fit and used instead for further evaluation (
Cost = 146,
Qads−0.997,
R2 = 0.99). The model equation for E has a less good fit (
R2adj. = 0.82) and residuals normality was not satisfied (SW,
W = 0.91,
p = 0.013). The model equation for
S consists of an intercept only. This is indicative of a small but significant selectivity for Ca
2+ removal (
= 1.11;
t (4.9);
p < 0.001). The equations for
S and
E are not further used in the analysis of the combined MCDI-CT system.
3.3. MCDI on Real Feed Water
MCDI tests are performed on real CT feed water samples (BC Canal, GT Canal, Eumes River and STP effluent). Treated STP effluent is included as a possible alternative source of cooling water [
31]. The selected feed water types have a distinct composition (
Table 5). BC Canal water has a relatively low [Na
+] and a medium to high
TH ([Ca
2+] = 125.7 ppm, [Mg
2+] = 15 ppm). GT Canal feed water
TH is highly similar to BC Canal ([Ca
2+] = 114 ppm, [Mg
2+] = 43 ppm) while being higher in [Na
+] (300 ppm Na
+). Eumes river feed water is very low in
TH ([Ca
2+] < 0.05 ppm, [Mg
2+] < 10 ppm) with a relatively high [Na
+] and high pH compared to the other water types. STP effluent has a low sodium concentration (22.2 ppm) and relative low
TH ([Ca
2+] = 23.9 ppm, [Mg
2+] = 3.3 ppm). In addition, total organic carbon (TOC) is not considered specifically in this study but could contribute to membrane fouling. Indicative
TOC values for the cooling water samples (
Table 5) are 70 mg C/L (BC Canal), 4.4 mg C/L (Eumes river) and <15 mg C/L (STP effluent).
MCDI tests with real CT feed water are used to reevaluate the RSM model. Since both Eumes river and STP effluent water compositions are far outside the factor ranges of the RSM (
Table 1) they are not used for comparison with model predictions. RSM ranges for
THin and NaCl are based on the average composition of BC and GT Canal water types. Despite of seasonal variation the current samples (
Table 5) have a similar composition (
EC, [Ca
2+]) when compared to the ranges used for RSM ([Ca
2+]: 56–180 ppm,
EC: 1.2 mS/cm–4.6 mS/cm), the current BC Canal water sample however has a relative low [Na
+]
in. For GT Canal and BC Canal feed water types,
Umax,
WR and
THout are calculated from test results and from RSM equations (predicted value and 95% confidence interval, Statistica prediction and profiling tool) and compared (
Figure 4).
Comparison results for GT Canal water shows that
Umax and
WR are well predicted by RSM. Prediction of parameter
THout is less accurate and the inverse effect on
Umax is notable (e.g., for 120-900-2.5). Average difference between RSM predicted and test results derived parameters is <5% for GT Canal water. Comparison of results for BC Canal shows a larger deviation; specifically,
THout is overestimated by RSM. On average, RSM underestimates
Umax by 20%,
WR by 6% and overestimates
THout by 89% for BC Canal water. Overestimation of
THout is attributed to the relatively low [Na
+]
in in BC channel water (60.1 ppm) compared to the RSM factor range (
Table 1) making the RSM model less predictive.
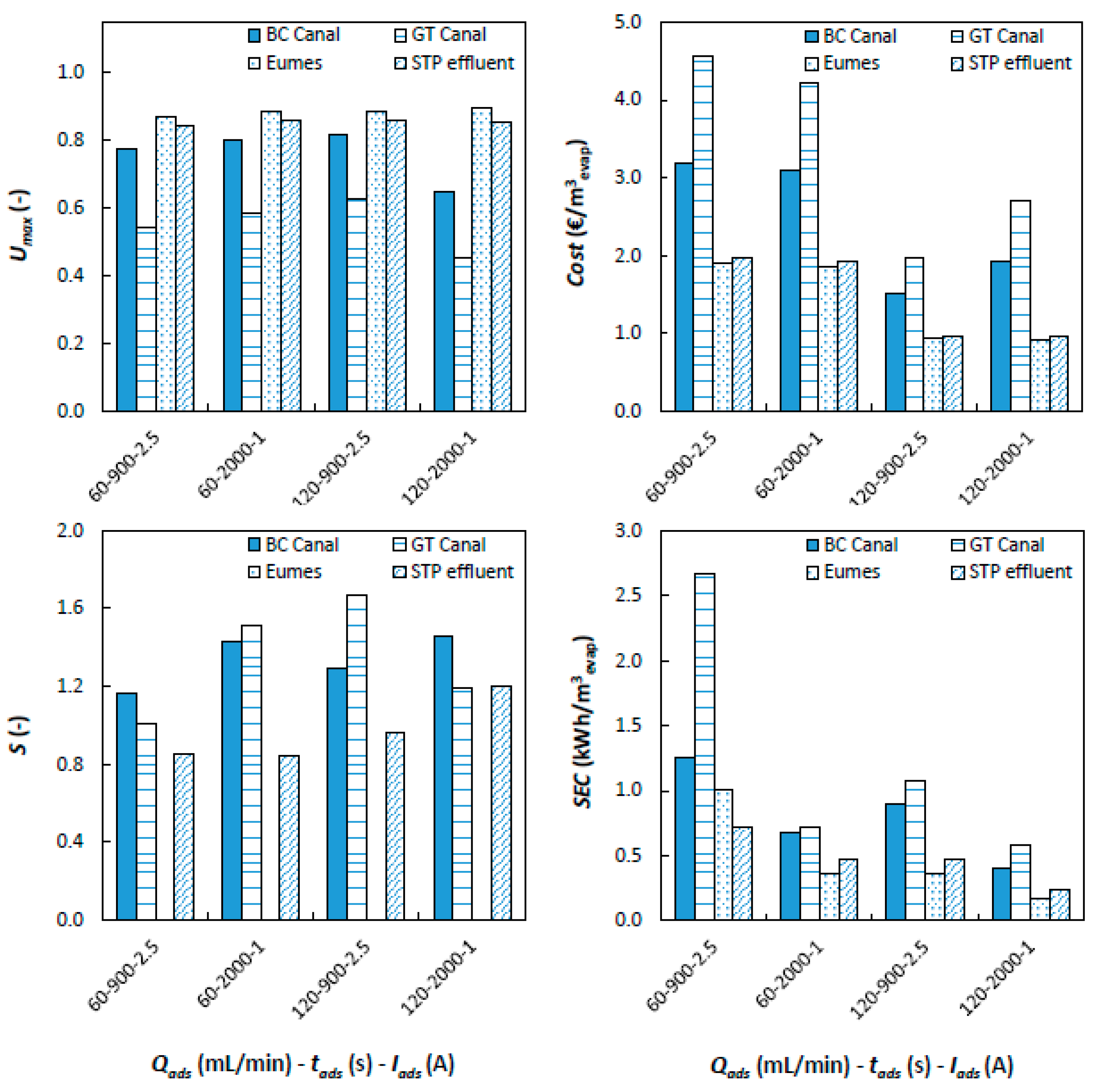
MCDI tests with real CT feed water are further used to evaluate the effect of feed water composition on MCDI-CT process parameters (
SEC, selectivity,
Umax and
Cost). Desired properties of the MCDI process in relation to process efficiency are low cost, low energy consumption, high
Umax and high selectivity (
S) for bivalent ion removal (e.g., Ca
2+, Mg
2+). Process parameters are determined for the 4 selected water types and various MCDI process conditions and plotted for comparison (
Figure 5).
Umax is found to be highest for Eumes river water followed by STP effluent, BC Canal and GT Canal feed water types. This expected as
Umax is inversely correlated with feed water EC and
TH following their effect on
THout. Variation of
Umax among process conditions is small, except for BC Canal and GT Canal feed water where ((
tads,
Iads):(2000 s, 1 A))
Umax is lower in comparison to other test conditions. This is also mirrored in
Cost (€ m
−3evap.), which is inversely correlated with flowrate and
Umax and to a much lesser extent energy consumption (kWh m
−3evap) as can be seen for BC and GT Canal feed water. Energy consumption (kWh m
−3evap) is high for BC Canal and GT Canal water types when compared to STP effluent and Eumes feed water. Selective removal of bivalent ions (Ca
2+ and Mg
2+) is a desired MCDI feature. Selectivity is found to some extend for BC Canal water (
= 1.35; Standard Deviation (
SD)
= 0.3;
N = 4) and GT Canal water (
= 1.34;
SD = 0.13;
N = 4). No selectivity was found for STP effluent (
= 0.97;
SD = 0.16;
N = 4) while S could not be calculated for Eumes river water due to the lack of hardness ions. Preferential removal of bivalent ions is common in MCDI; it is observed in both synthetic feed mixtures [
19,
32] and real feed water [
9]. This phenomenon is the result of diffusion kinetics and adsorption equilibria in MCDI and is attributed to the preferential storage of multivalent ions in ion exchange membranes [
32,
33]. Overall comparison shows that high flowrate (120 mL min
−1) results in minimal
Cost (€ m
−3evap.) for all studied feed water types. Feed water with high
THin (GT Canal, BC Canal) is preferably treated using short
tads while applying high
Iads. In addition, the gain in water use efficiency in relation to the base scenario where no pretreatment is in place needs to be considered. For BC Canal and GT Canal water utilization without treatment (
Umax = 0.33) is relatively low making pretreatment of possible interest while for STP effluent (
Umax = 0.76) and Eumes river (
Umax = 0.99), utilization is already high, and therefore, pretreatment is not useful. Application of MCDI on BC Canal and GT Canal water types results in a relatively high estimated cost per m
3 evaporate. This is partly due to the estimation procedure neglecting in part scale up effects. It generally indicates that the studied MCDI-CT scheme is currently only useful when CT feed water is costly or when legislative boundaries are present that limit water uptake. Legislative constraints on abstraction volumes have been reported to limit energy production in Southern Europe and the US [
2]. This is specifically critical in countries where thermoelectric power generation is dominant, and regional water scarcity is a significant concern [
1]. BC Canal and GT Canal water feed water cases are currently not severely impacted.
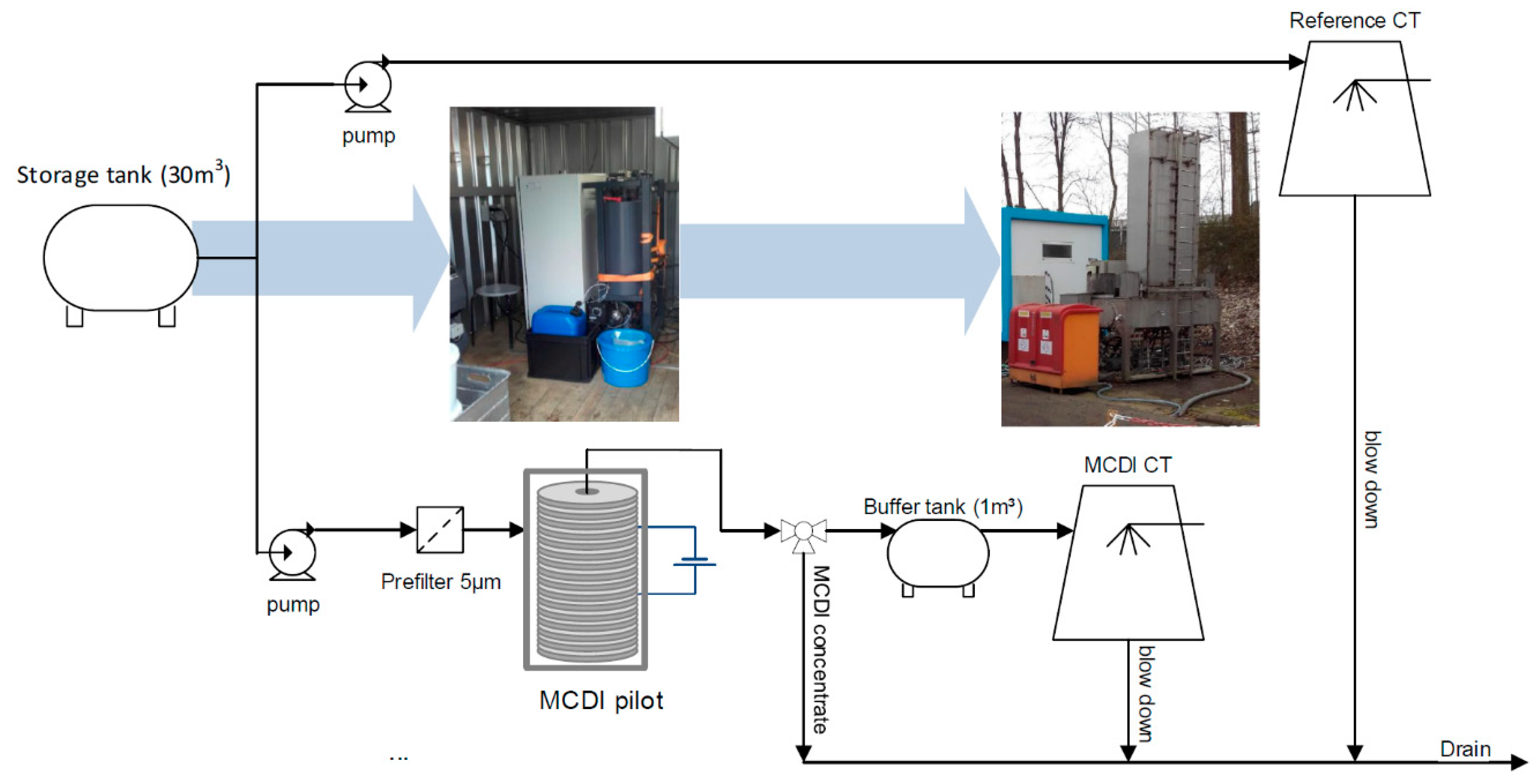
3.4. MCDI-CT Pilot Test
The main purpose of the MCD-CT pilot test is to assess the effect of MCDI treated BC Canal feed water on cooling tower performance and acid consumption. BC Canal water was selected as single feed water source for pilot testing in view of relevance, availability and pilot duration (3 months). Equal ambient conditions for comparison are realized by simultaneously feeding one of Merades cooling tower circuits with untreated BC Canal water (reference) and the other one with MCDI treated BC canal water (
Figure 6).
To allow comparison of both cooling tower circuits, a fixed MCDI product water quality (conductivity setpoint) is produced in each test run by allowing variable current (0–110 A) and fixed flow rate during adsorption phases, yielding a water recovery of 73%–88%. The process was controlled by the setpoint of the conductivity, i.e., either at 25% or 50% reduction of the incoming conductivity. This build in control strategy uses a variable current and adsorption time depending on the product water conductivity. The operating conditions of the pilot therefore differed from the optimal conditions determined from lab tests (
Figure 5). In the pilot, specific flowrate was high (10 L/m
2h), adsorption time was intermediate (1700 s) and time averaged current density was relatively low (1 A/m
2) for 50% desalination compared to lab tests where following ranges for specific flowrate (5–10 L/hm
2), adsorption time (900–2000 s) and current density (1.5 A/m
2 to 3.5 A/m
2) were used. In practice, removal ratios differed quite significantly in the first 3 test runs due to software issues (
Table 6). BC Channel feed water quality is seasonal and both conductivity (0.70–0.87 mS/cm) and [Ca
2+] (88–108 ppm) are lower compared to previous samples (
Table 5). The specific energy consumption was low (0.08–0.12 kWh m
−3 produced) in part due to the relative high capacity of the MCDI system used (design flowrate = 0.2–1.8 m
3 h
−1, applied flowrate = 0.42 m
3 h
−1) but comparable to values found in literature [
9,
22]. The feed water was also found to have a significant fouling potential previously undetected during lab tests. A steady and consistent increase in hydraulic impedance (10
8 Pa s m
−3) of the MCDI cell resulted in an increase in pressure drop over the cell by 2.8 bar in 24 h (at
Qads = 0.42 m
3 h
−1). Consequently, the MCDI system passes through an automatic cleaning cycle each 4 h. This type of fouling behavior (spacer fouling) is expectedly caused by small particles and colloidal matter (e.g., clay particles) present in the feed water, which adhere to the spacer fabric, indicating the applied pretreatment (5 µm bag filtration) is insufficient for BC canal feed water. This indicates that fouling prevention remains an important aspect of MCDI operation despite the common notion that MCDI is less vulnerable to fouling compared to other membrane processes as also indicated recently by Choi and coworkers [
34]. Overall the MCDI pilot was able to deliver the required volume for each of 6 test runs with Merades CT pilot (
Table 6).
The resulting
Umax of the MCD-pilot at operational conditions (
Table 6) is lower for MCDI treated water when compared to the reference untreated water. This is deceptive however since a different operational pH is applied in all test runs (no acid dosing). CaCO
3 scaling is highly pH dependent, and comparison of CT test conditions therefore requires taking into account both pH control (acid dosage) and water use efficiency explicitly. An extrapolation from test data of CaCO
3 scaling, acid dosage and operational pH is performed using ENGIE lab proprietary cooling water simulation software (
Table 7).
Comparing acid dosage and feed water savings for a given pH shows that MCDI technology allows a clear reduction of water consumption (74%–80%) when CT is operated at higher pH meanwhile strongly reducing acid dosage (63%–80%). MCDI pretreatment reduces acid consumption when operating at low pH but also increases feed water usage by 10%–30%. Under these conditions MCDI pretreatment is less useful. Comparison between operation at pH 8 without MCDI and pH 8.5 with MCDI shows 50% reduction in acid use for comparable Umax. It is concluded that the usefulness of MCDI for CT pretreatment depends strongly on operational conditions and feed water type. Specifically, CT feed water with a high hardness benefits from MCDI pretreatment. In addition, monitoring of total viable count (weekly) indicates that MCDI technology has no impact on biological growth in CT recirculation water, suggesting that nutrients (e.g., TOC) required for growth are not extensively removed by MCDI. LPR corrosion measurements show that MCDI treated BC Canal water is more corrosive ( = 2.0 mills/year, N = 25) in comparison to non-treated water ( = 1.4 mills year−1, N = 25). However, the ability to operate at higher elevated COC and pH counteracts this effect.