High Temperature Pyrolysis of Municipal Plastic Waste Using Me/Ni/ZSM-5 Catalysts: The Effect of Metal/Nickel Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Catalysts
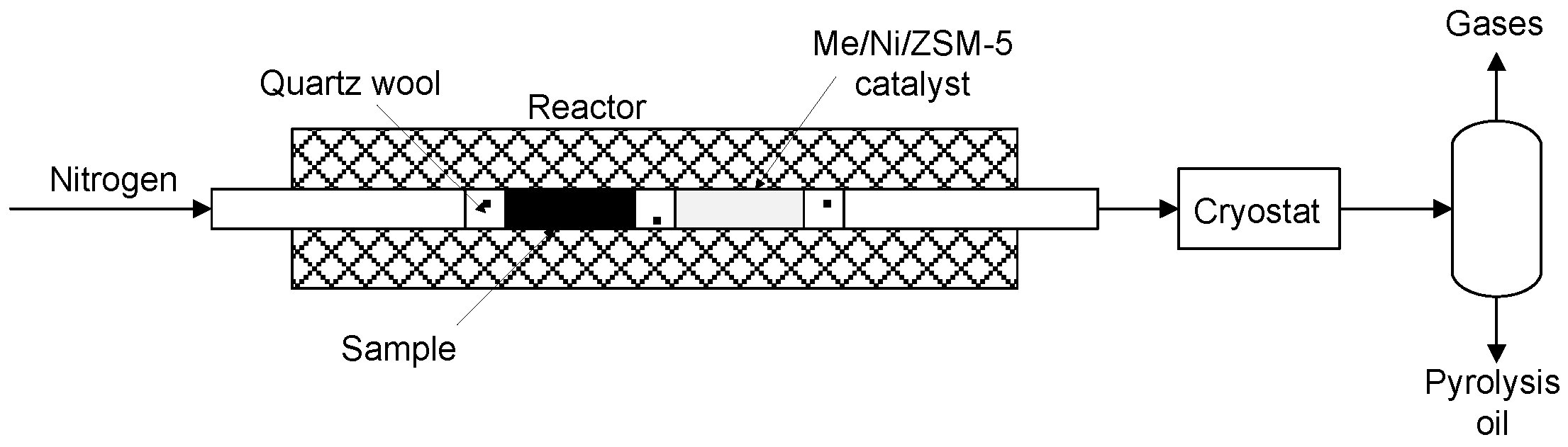
2.3. Pyrolysis Process
2.4. Product Analysis
3. Results
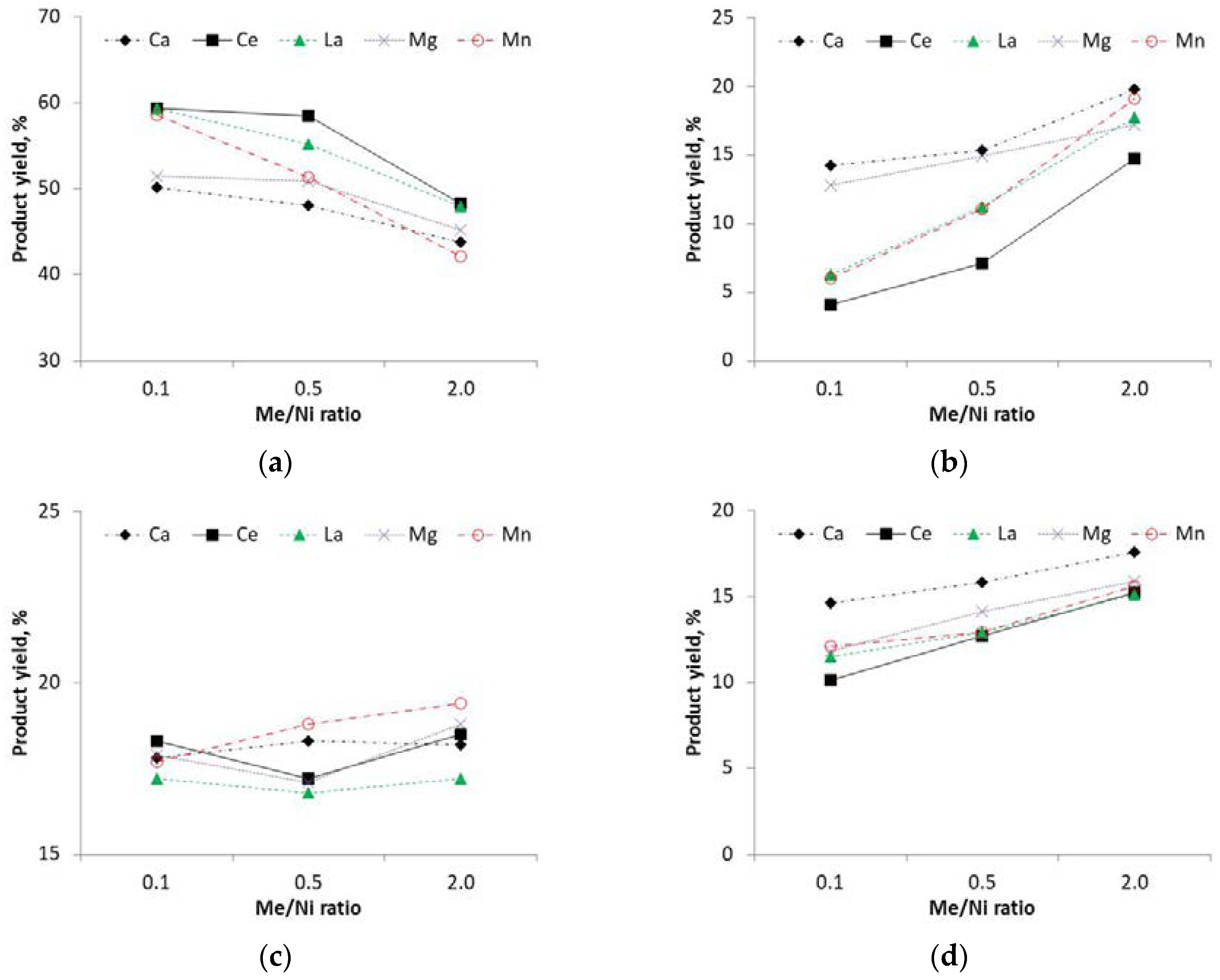
3.1. Product Yields
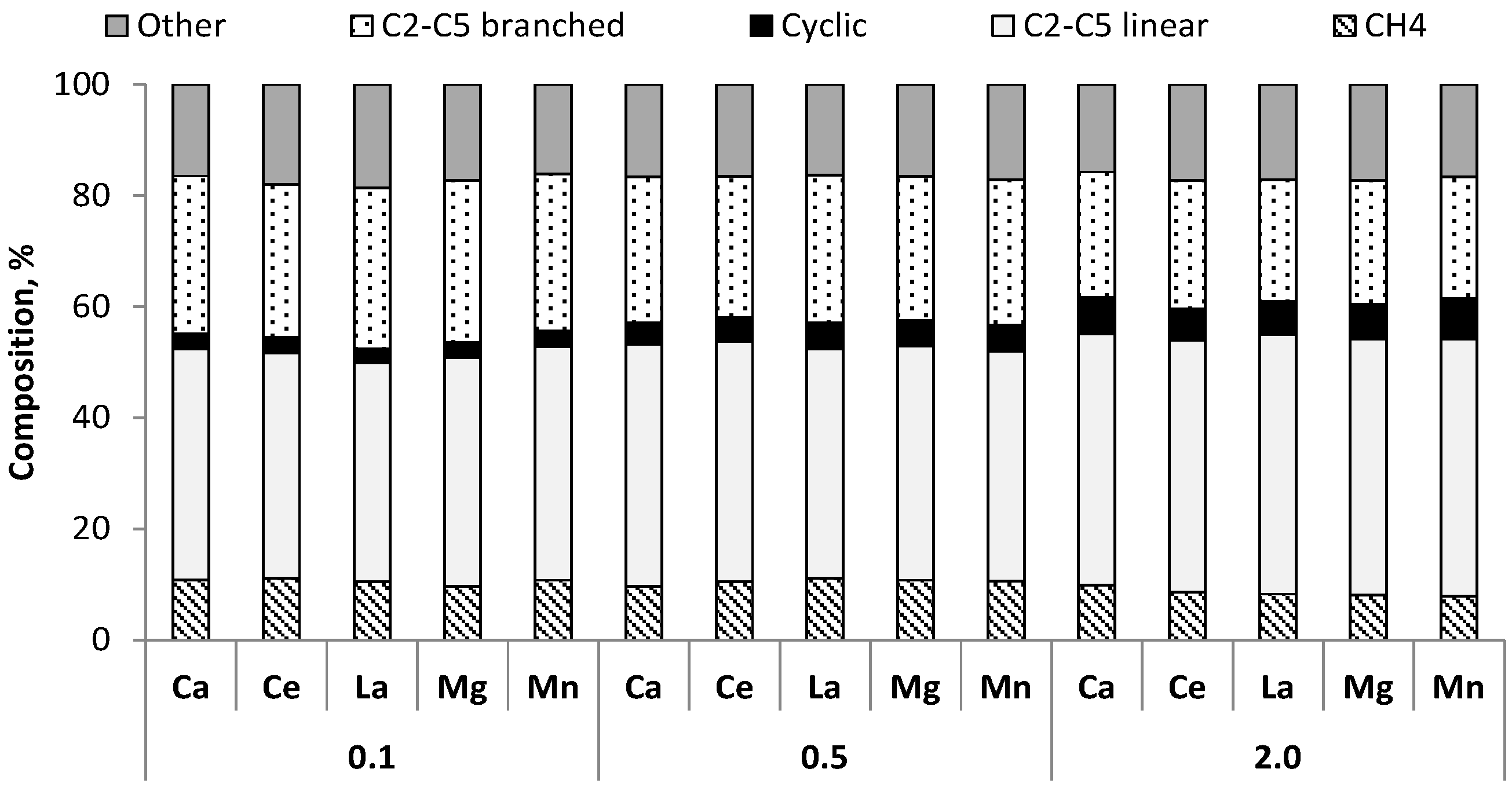
3.2. Gases
3.3. Pyrolysis Oil
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Plastics Europe. Plastics the Facts 2014/2015; Plastics Europe: Brussels, Belgium, 2015. [Google Scholar]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Conv. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Escola, J.M. Fuels from Waste Plastics by Thermal and Catalytic Processes: A Review. Ind. Engine. Chemi. Resea. 2008, 47, 7982–7992. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Wanga, J.; Cheng, G. Hydrogen-rich gas production by steam gasification of municipal solid waste (MSW) using NiO supported on modified dolomite. Int. J. Hydrogen Energy 2012, 37, 6503–6510. [Google Scholar] [CrossRef]
- Williams, P.T.; Slaney, E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour. Conserv. Recy. 2007, 51, 754–769. [Google Scholar] [CrossRef]
- Saad, M.D.J.; Williams, P.T. Catalytic dry reforming of waste plastics from different waste treatment plants for production of synthesis gases. Waste Manag. 2016, 58, 214–220. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Rehan, M.; Aburiazaiza, A.S.; Ismail, I.M.I.; Nizami, A.S. Plastic waste to liquid oil through catalytic pyrolysis using natural and synthetic zeolite catalysts. Waste Manag. 2017, 69, 66–78. [Google Scholar] [CrossRef]
- Bustamante, F.; Enick, R.; Rothenberger, K.; Howard, B.; Cugini, A.; Ciocco, M. Kinetic study of the reverse water gas shift reaction in high temperature, high pressure homogeneous systems. ACS Fuel Chem. Div. Prepr. 2002, 47, 663–664. [Google Scholar]
- Garcia, L.; Benedicto, A.; Romeo, E.; Salvador, M.L.; Arauzo, J.; Bilbao, R. Hydrogen Production by Steam Gasification of Biomass Using Ni−Al Coprecipitated Catalysts Promoted with Magnesium. Energy Fuel 2002, 16, 1222–1230. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Pyrolysis–gasification of plastics, mixed plastics and real-world plastic waste with and without Ni–Mg–Al catalyst. Fuel 2010, 89, 3022–3032. [Google Scholar] [CrossRef]
- Mastral, F.J.; Esperanza, E.; García, P.; Juste, M. Pyrolysis of high-density polyethylene in a fluidised bed reactor. Influence of the temperature and residence time. J. Anal. Appl. Pyrol. 2002, 63, 1–15. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Muhammad, C.; Williams, P.T. Influence of catalyst bed temperature and properties of zeolite catalysts on pyrolysis-catalysis of a simulated mixed plastics sample for the production of upgraded fuels and chemicals. J. Energy Inst. 2019, 92, 1337–1347. [Google Scholar] [CrossRef]
- Acomb, J.C.; Wu, C.; Williams, P.T. Effect of growth temperature and feedstock:catalyst ratio on the production of carbon nanotubes and hydrogen from the pyrolysis of waste plastics. J. Anal. Appl. Pyrol. 2015, 113, 231–238. [Google Scholar] [CrossRef]
- Bober, P.; Oriňák, A.; Oriňaková, R.; Zamostný, P.; Ladomerský, J.; Fedorková, A. Hydrogen production by catalysed pyrolysis of polymer blends. Fuel 2011, 90, 2334–2339. [Google Scholar] [CrossRef]
- Muradov, N.Z.; Veziroǧlu, T.N. From hydrocarbon to hydrogen–carbon to hydrogen economy. Int. J. Hydrogen Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Goodman, D.W. Methane Decomposition: Production of Hydrogen and Carbon Filaments. Catalysis 2006, 19, 164–183. [Google Scholar]
- Nakagawa, K.; Yamagishi, M.; Nishimoto, H.; Ikenaga, N.; Suzuki, T.; Kobayashi, T. Oxidized Diamond as a Simultaneous Production Medium of Carbon Nanomaterials and Hydrogen for Fuel Cell. Chem. Mater. 2003, 15, 4571–4575. [Google Scholar] [CrossRef]
- Yao, D.; Chunfei, W.; Haiping, Y.; Hu, Q.; Nahil, M.; Chen, H.; Williams, P.T. Hydrogen production from catalytic reforming of the aqueous fraction of pyrolysis bio-oil with modified Ni–Al catalysts. Int. J. Hydrogen Energy 2014, 39, 14642–14652. [Google Scholar] [CrossRef]
- Liu, W.W.; Hu, C.W.; Yang, Y.; Tong, D.M.; Zhu, L.F.; Zhang, R.N.; Zhao, B.H. Study on the effect of metal types in (Me)-Al-MCM-41 on the mesoporous structure and catalytic behavior during the vapor-catalyzed co-pyrolysis of pubescens and LDPE. Appl. Catal. B Environ. 2013, 129, 202–213. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Chen, H.; Williams, P.T. Investigation of nickel-impregnated zeolite catalysts for hydrogen/syngas production from the catalytic reforming of waste polyethylene. Appl. Catal. B Environ. 2018, 227, 477–487. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.P.; Zhao, X.Y.; Wei, F.; Zhu, C.; Wei, X.Y. Extension of catalyst lifetime by doping of Ce in Ni-loaded acid-washed Shengli lignite char for biomass catalytic gasification. Catal. Sci. Technol. 2017, 7, 5741–5749. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Liu, J.; Chen, D. Catalytic characteristics of innovative Ni/slag catalysts for syngas production and tar removal from biomass pyrolysis. Int. J. Hydrogen Energy 2019, 44, 11848–11860. [Google Scholar] [CrossRef]
- Santamaria, L.; Arregi, A.; Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Effect of La2O3 promotion on a Ni/Al2O3 catalyst for H2 production in the in-line biomass pyrolysis-reforming. Fuel 2020, 262, 116593. [Google Scholar] [CrossRef]
- Nahil, M.A.; Wu, C.; Williams, P.T. Influence of metal addition to Ni-based catalysts for the co-production of carbon nanotubes and hydrogen from the thermal processing of waste polypropylene. Fuel Process. Technol. 2015, 130, 46–53. [Google Scholar] [CrossRef]
- Uemichi, Y.; Makino, Y.; Kanazuka, T. Degradation of polyethylene to aromatic hydrocarbons over metal-supported activated carbon catalysts. J. Anal. Appl. Pyrol. 1989, 14, 331–344. [Google Scholar] [CrossRef]
- Khobragade, M.; Majhi, S.; Pant, K.K. Effect of K and CeO2 promoters on the activity of Co/SiO2 catalyst for liquid fuel production from syngas. Appl. Energy 2012, 94, 385–394. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Ni/CeO2/ZSM-5 catalysts for the production of hydrogen from the pyrolysis–gasification of polypropylene. Int. J. Hydrogen Energy 2009, 34, 6242–6252. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Aromatic fuel oils produced from the pyrolysis-catalysis of polyethylene plastic with metal-impregnated zeolite catalysts. J. Energy Inst. 2019, 92, 195–202. [Google Scholar] [CrossRef]
- Fricke, R.; Kosslick, H.; Lischke, G.; Richter, M. Incorporation of Gallium into Zeolites: Syntheses, Properties and Catalytic Application. Chem. Rev. 2000, 100, 2303–2406. [Google Scholar] [CrossRef]
- Chang, C.S.; Lee Gen, M.D. Effects of hydrogen pretreatment on the acidic and catalytic properties of gallium-supported H-ZSM-5 in n-hexane aromatization. Appl. Catal. A 1995, 123, 7–21. [Google Scholar] [CrossRef]



| SBJH, m2/g | Smicro, m2/g | BET, m2/g | Average Grain Diameter, μm | |
|---|---|---|---|---|
| 0.1 Ce/Ni/ZSM-5 | 94.2 | 325 | 464 | 0.74 |
| 0.1 La/Ni/ZSM-5 | 95.1 | 320 | 461 | 0.56 |
| 0.1 Mg/Ni/ZSM-5 | 92.6 | 315 | 465 | 0.69 |
| 0.1 Ca/Ni/ZSM-5 | 92.4 | 318 | 460 | 0.62 |
| 0.1 Mn/Ni/ZSM-5 | 91.9 | 322 | 466 | 0.64 |
| 0.5 Ce/Ni/ZSM-5 | 92.7 | 311 | 445 | 0.83 |
| 0.5 La/Ni/ZSM-5 | 90.9 | 305 | 408 | 0.63 |
| 0.5 Mg/Ni/ZSM-5 | 89.5 | 288 | 404 | 0.57 |
| 0.5 Ca/Ni/ZSM-5 | 93.8 | 210 | 419 | 0.73 |
| 0.5 Mn/Ni/ZSM-5 | 92.4 | 281 | 395 | 0.87 |
| 2.0 Ce/Ni/ZSM-5 | 91.1 | 280 | 359 | 1.16 |
| 2.0 La/Ni/ZSM-5 | 93.5 | 285 | 362 | 0.96 |
| 2.0 Mg/Ni/ZSM-5 | 92.6 | 257 | 355 | 1.04 |
| 2.0 Ca/Ni/ZSM-5 | 91.4 | 195 | 350 | 1.28 |
| 2.0 Mn/Ni/ZSM-5 | 95.2 | 258 | 361 | 0.76 |
| Catalyst Surface Area | Catalyst Grain Surface | |||||||
|---|---|---|---|---|---|---|---|---|
| Me, % | Ni, % | O, % | Si/Al | Me, % | Ni, % | O, % | Si/Al | |
| 0.1 Ce/Ni/ZSM-5 | 0.41 | 3.83 | 60.98 | 30.09 | 0.39 | 3.85 | 61.04 | 30.81 |
| 0.1 La/Ni/ZSM-5 | 0.42 | 3.86 | 58.57 | 30.89 | 0.42 | 3.81 | 59.74 | 31.63 |
| 0.1 Mg/Ni/ZSM-5 | 0.48 | 4.12 | 58.63 | 32.44 | 0.46 | 4.07 | 61.00 | 30.55 |
| 0.1 Ca/Ni/ZSM-5 | 0.41 | 3.89 | 59.93 | 30.33 | 0.40 | 4.02 | 58.78 | 29.61 |
| 0.1 Mn/Ni/ZSM-5 | 0.44 | 4.10 | 57.31 | 29.52 | 0.44 | 4.27 | 59.92 | 30.79 |
| 0.5 Ce/Ni/ZSM-5 | 2.11 | 3.89 | 60.53 | 30.53 | 2.03 | 4.02 | 59.35 | 30.94 |
| 0.5 La/Ni/ZSM-5 | 2.04 | 3.83 | 58.19 | 31.83 | 1.93 | 3.76 | 59.16 | 31.82 |
| 0.5 Mg/Ni/ZSM-5 | 1.97 | 3.85 | 57.10 | 30.62 | 1.92 | 3.84 | 57.51 | 30.78 |
| 0.5 Ca/Ni/ZSM-5 | 2.05 | 3.90 | 60.27 | 30.24 | 1.92 | 3.73 | 58.97 | 32.15 |
| 0.5 Mn/Ni/ZSM-5 | 2.05 | 3.88 | 57.41 | 31.74 | 1.99 | 3.79 | 57.10 | 30.24 |
| 2.0 Ce/Ni/ZSM-5 | 7.70 | 3.87 | 58.35 | 31.07 | 7.37 | 3.67 | 58.06 | 31.91 |
| 2.0 La/Ni/ZSM-5 | 7.72 | 3.83 | 56.55 | 32.35 | 7.14 | 3.52 | 57.83 | 31.87 |
| 2.0 Mg/Ni/ZSM-5 | 8.38 | 3.84 | 56.79 | 31.17 | 7.55 | 3.82 | 58.84 | 30.93 |
| 2.0 Ca/Ni/ZSM-5 | 8.51 | 3.93 | 59.63 | 31.48 | 8.02 | 4.05 | 60.40 | 30.46 |
| 2.0 Mn/Ni/ZSM-5 | 8.03 | 3.86 | 57.42 | 30.36 | 7.55 | 3.77 | 58.21 | 30.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-asadi, M.; Miskolczi, N. High Temperature Pyrolysis of Municipal Plastic Waste Using Me/Ni/ZSM-5 Catalysts: The Effect of Metal/Nickel Ratio. Energies 2020, 13, 1284. https://doi.org/10.3390/en13051284
Al-asadi M, Miskolczi N. High Temperature Pyrolysis of Municipal Plastic Waste Using Me/Ni/ZSM-5 Catalysts: The Effect of Metal/Nickel Ratio. Energies. 2020; 13(5):1284. https://doi.org/10.3390/en13051284
Chicago/Turabian StyleAl-asadi, Mohammed, and Norbert Miskolczi. 2020. "High Temperature Pyrolysis of Municipal Plastic Waste Using Me/Ni/ZSM-5 Catalysts: The Effect of Metal/Nickel Ratio" Energies 13, no. 5: 1284. https://doi.org/10.3390/en13051284
APA StyleAl-asadi, M., & Miskolczi, N. (2020). High Temperature Pyrolysis of Municipal Plastic Waste Using Me/Ni/ZSM-5 Catalysts: The Effect of Metal/Nickel Ratio. Energies, 13(5), 1284. https://doi.org/10.3390/en13051284





