A Moving Bed Reactor for Thermochemical Energy Storage Based on Metal Oxides
Abstract
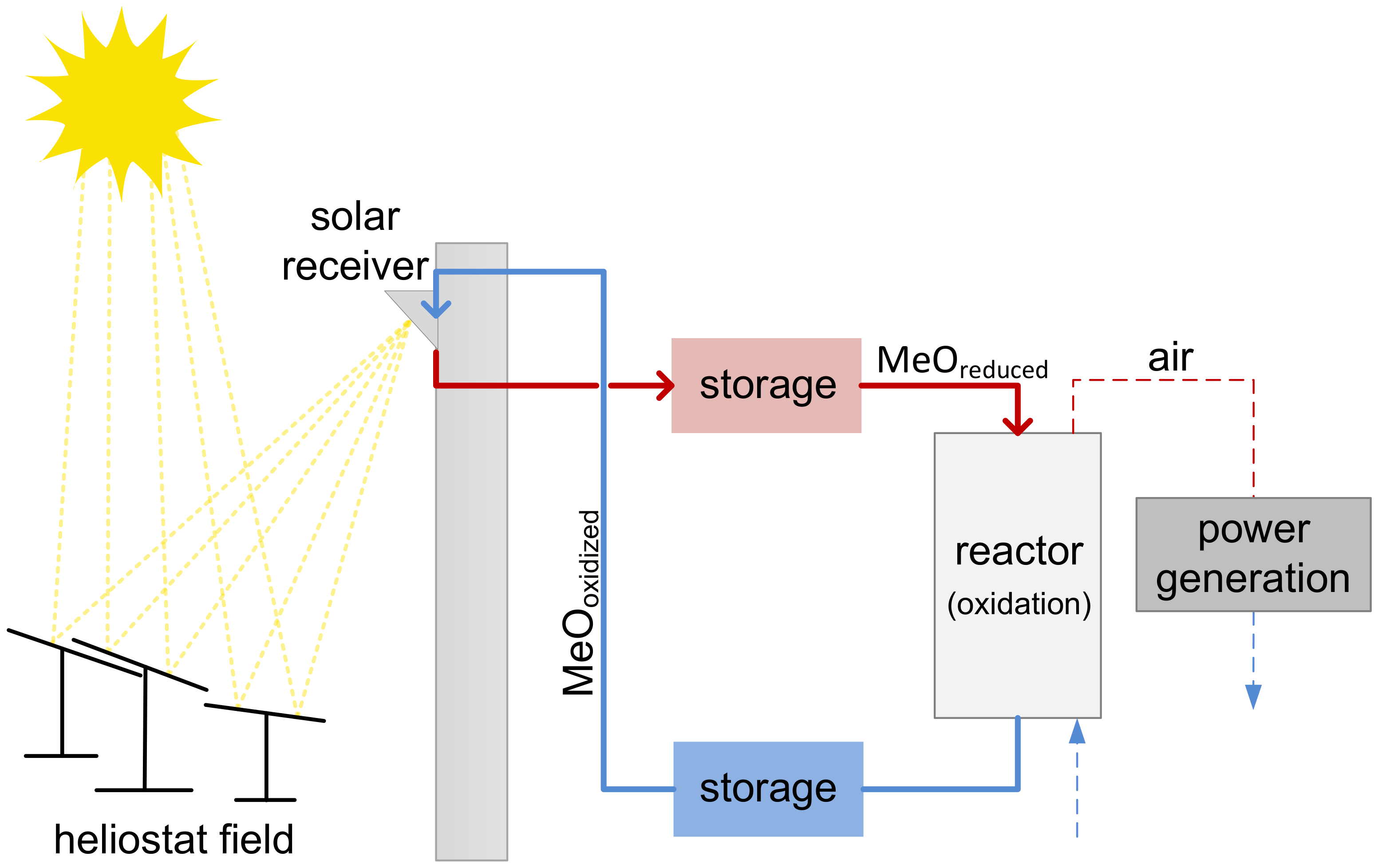
1. Introduction
2. Materials and Methods
2.1. Materials
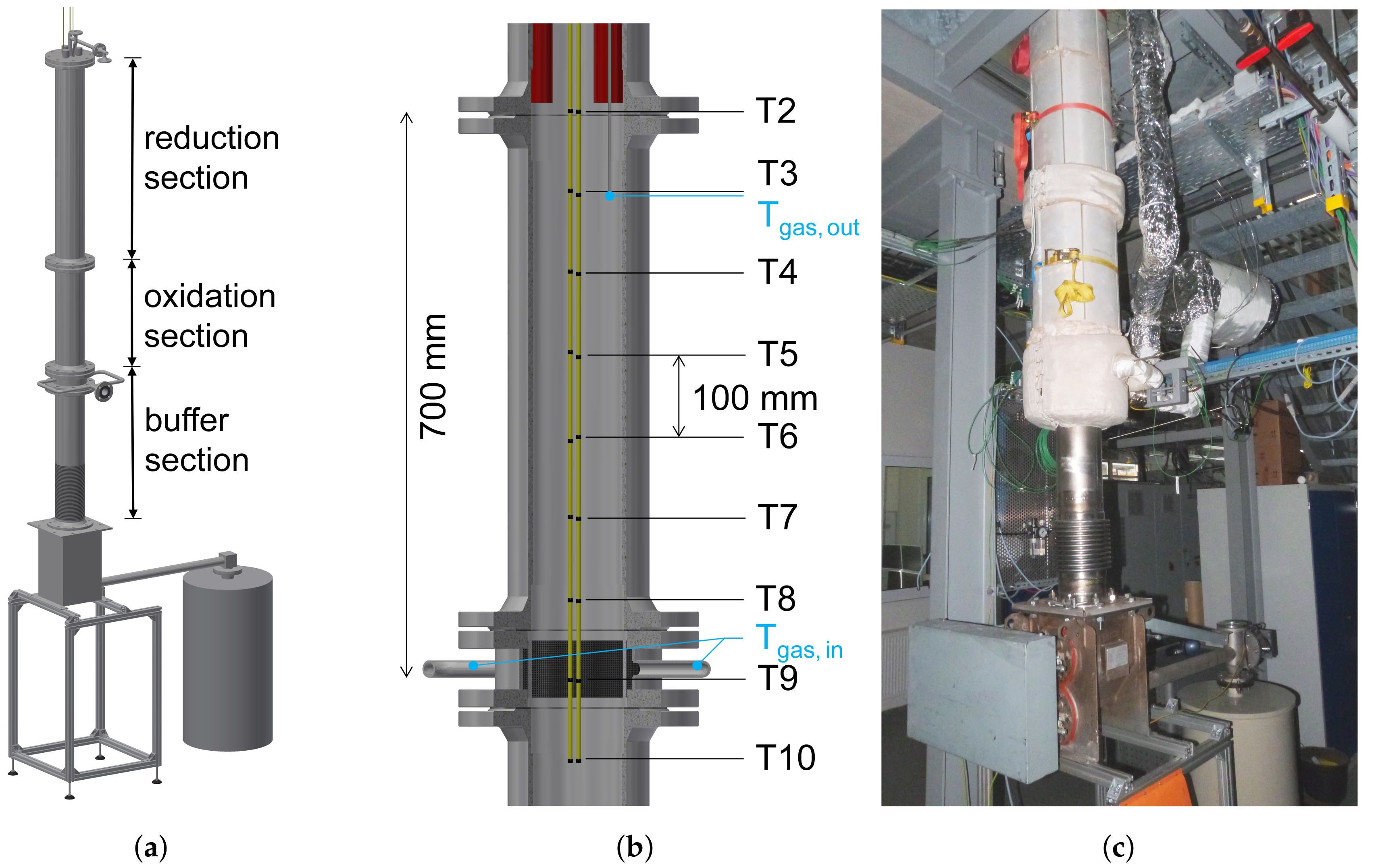
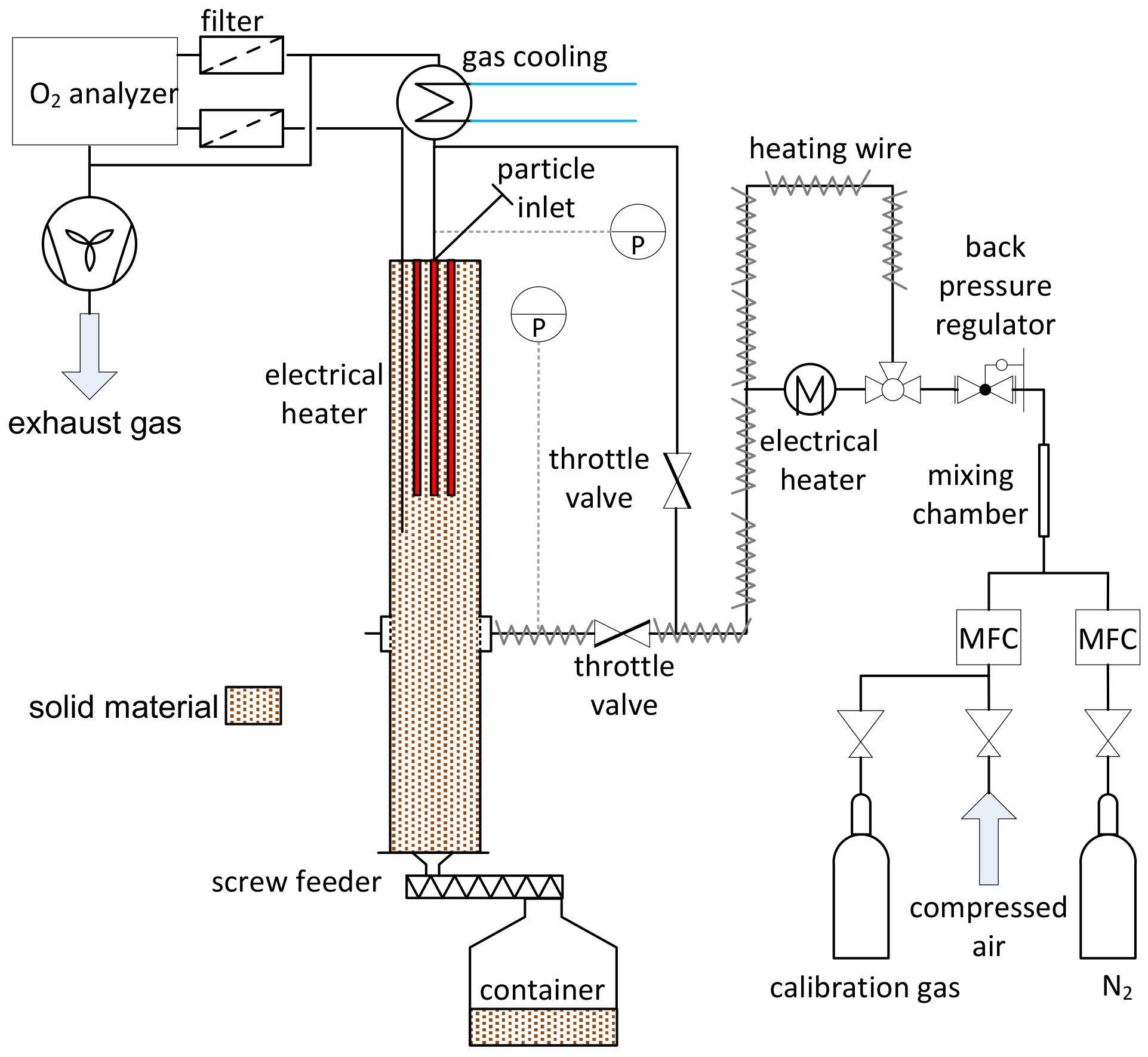
2.2. Experimental Setup
2.3. Experimental Procedure
2.4. Conversion Calculation
3. Results and Discussion
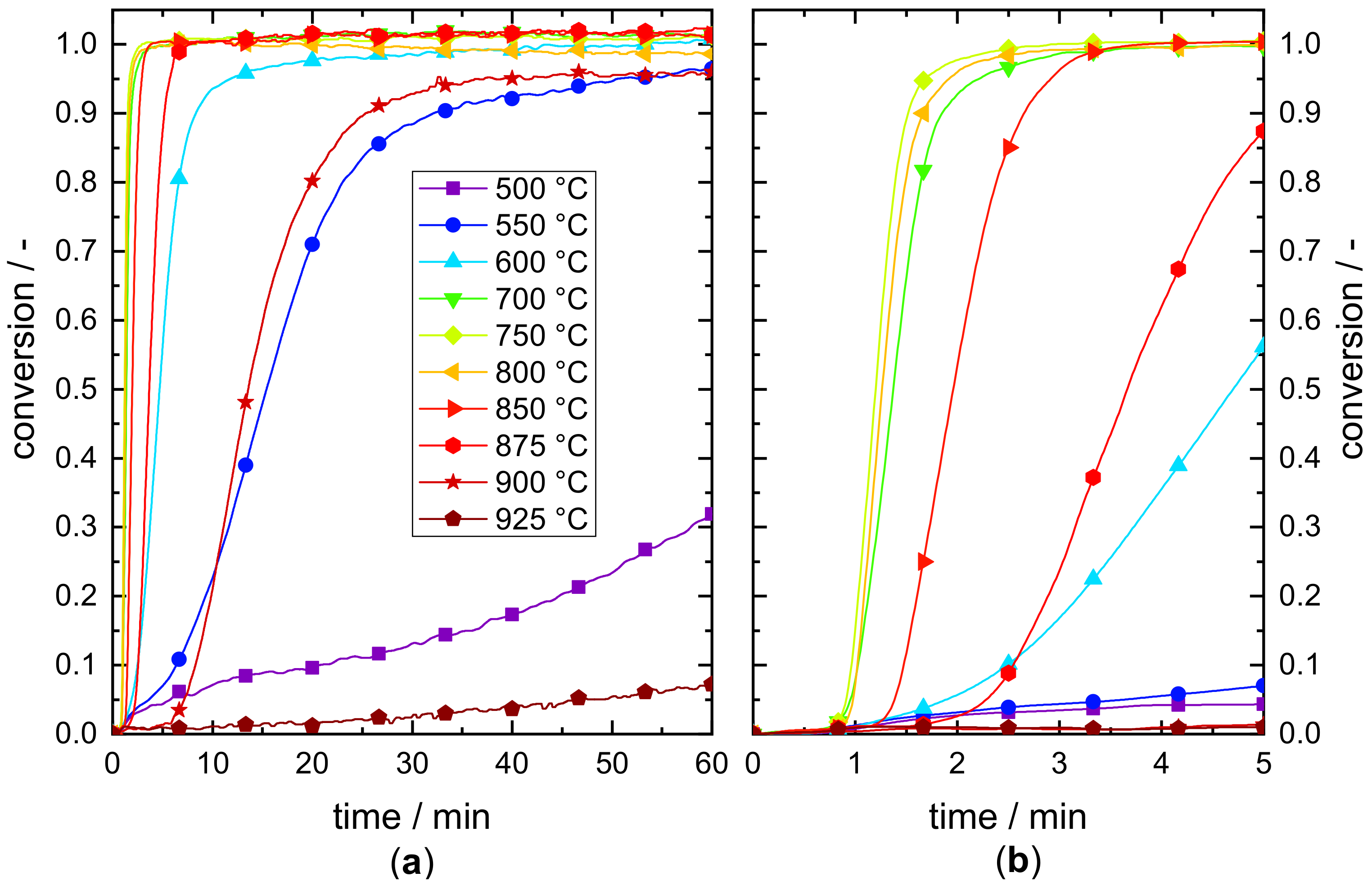
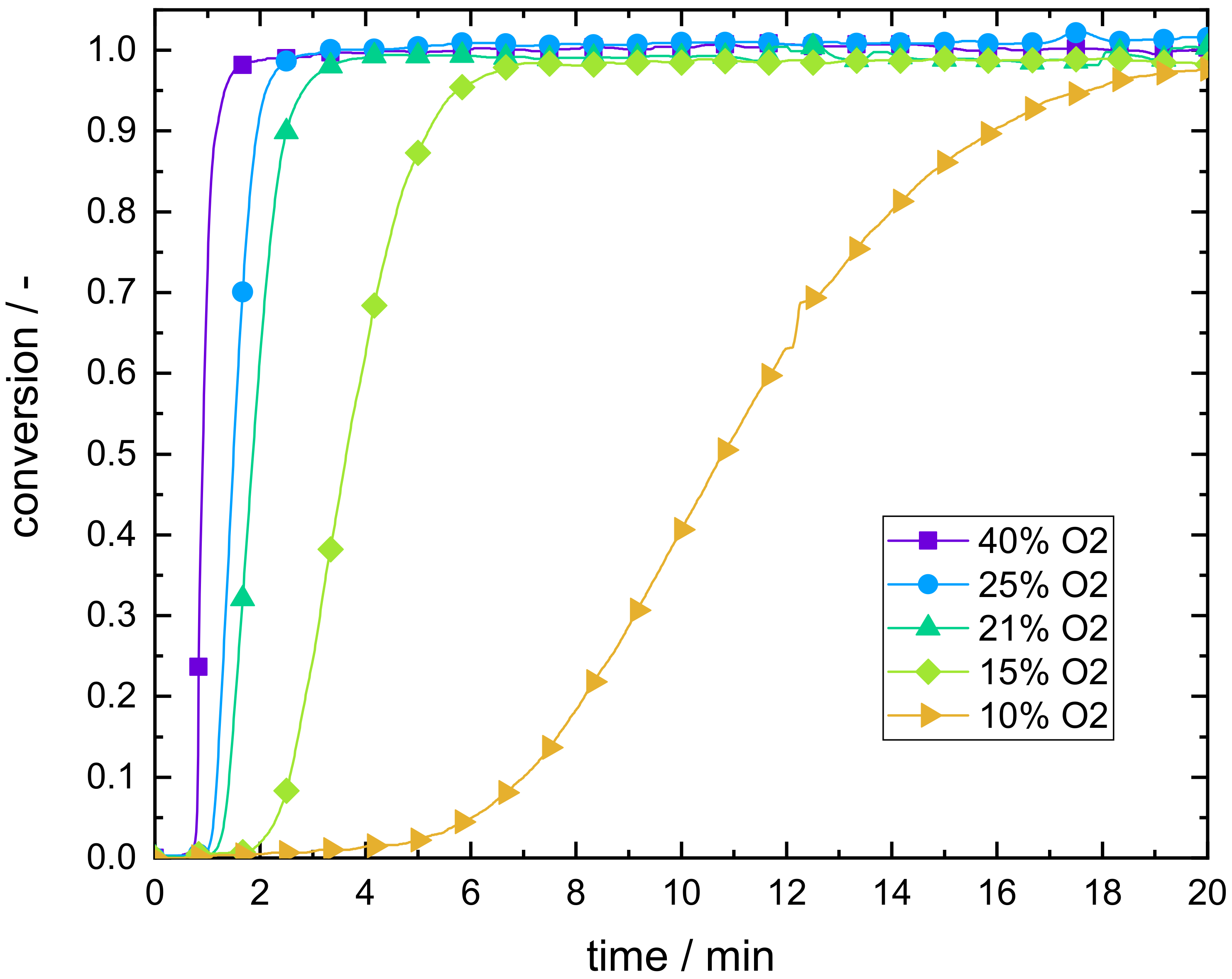
3.1. Reaction Characteristics
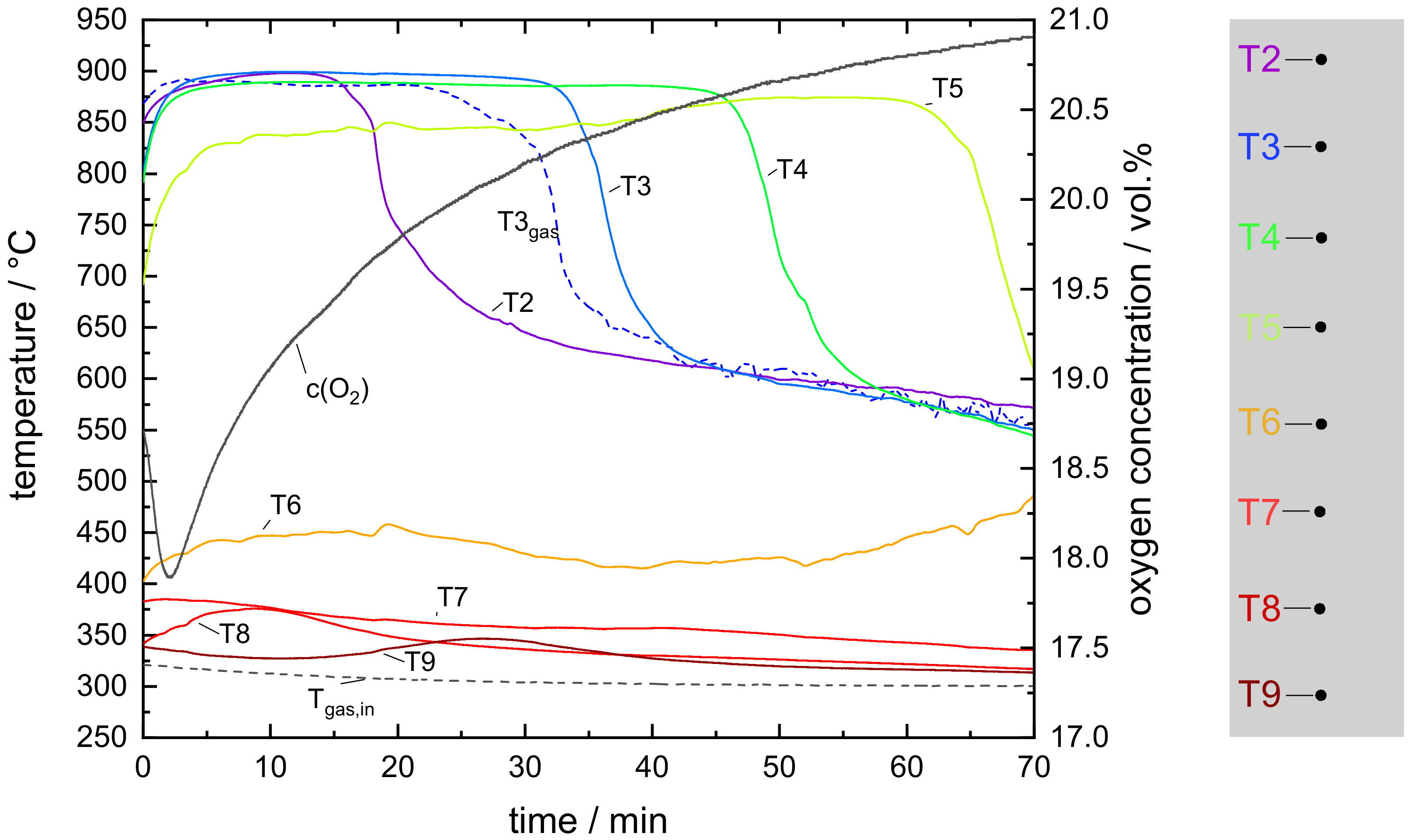
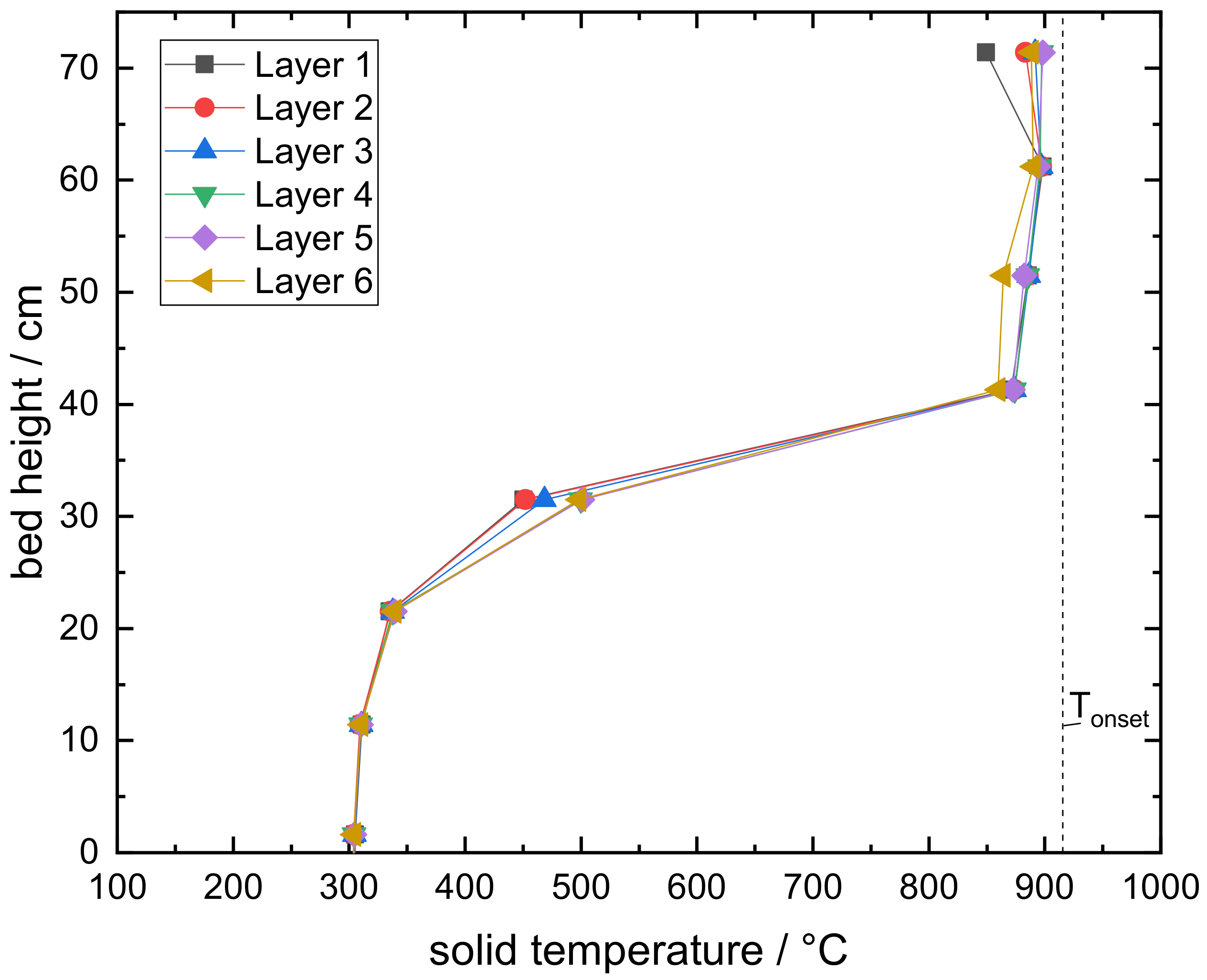
3.2. Experimental Results of a Moving Bed Reactor Operated with Mn–Fe-Oxide Particles
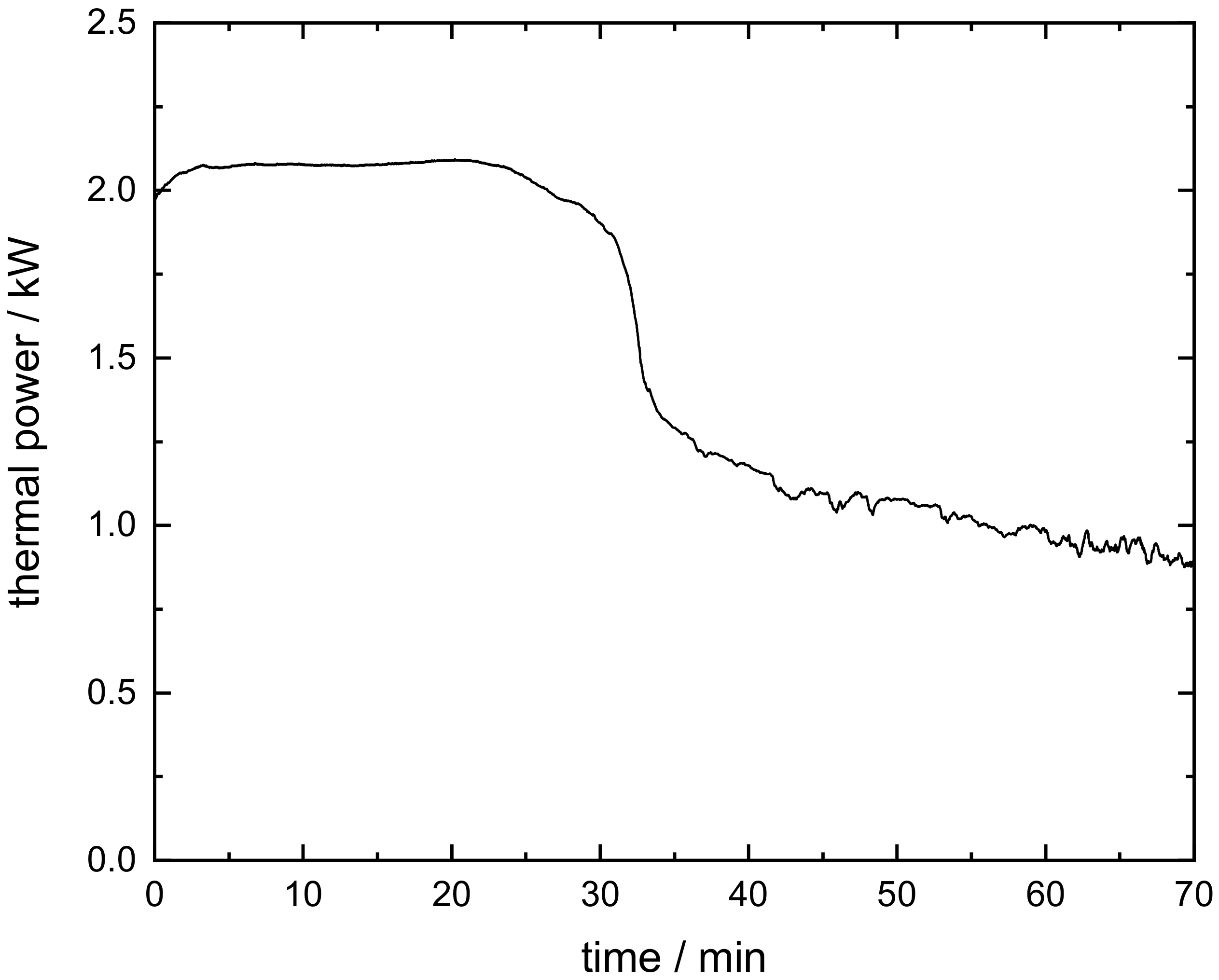
3.3. Energetic Evaluation of the Moving Bed Reactor
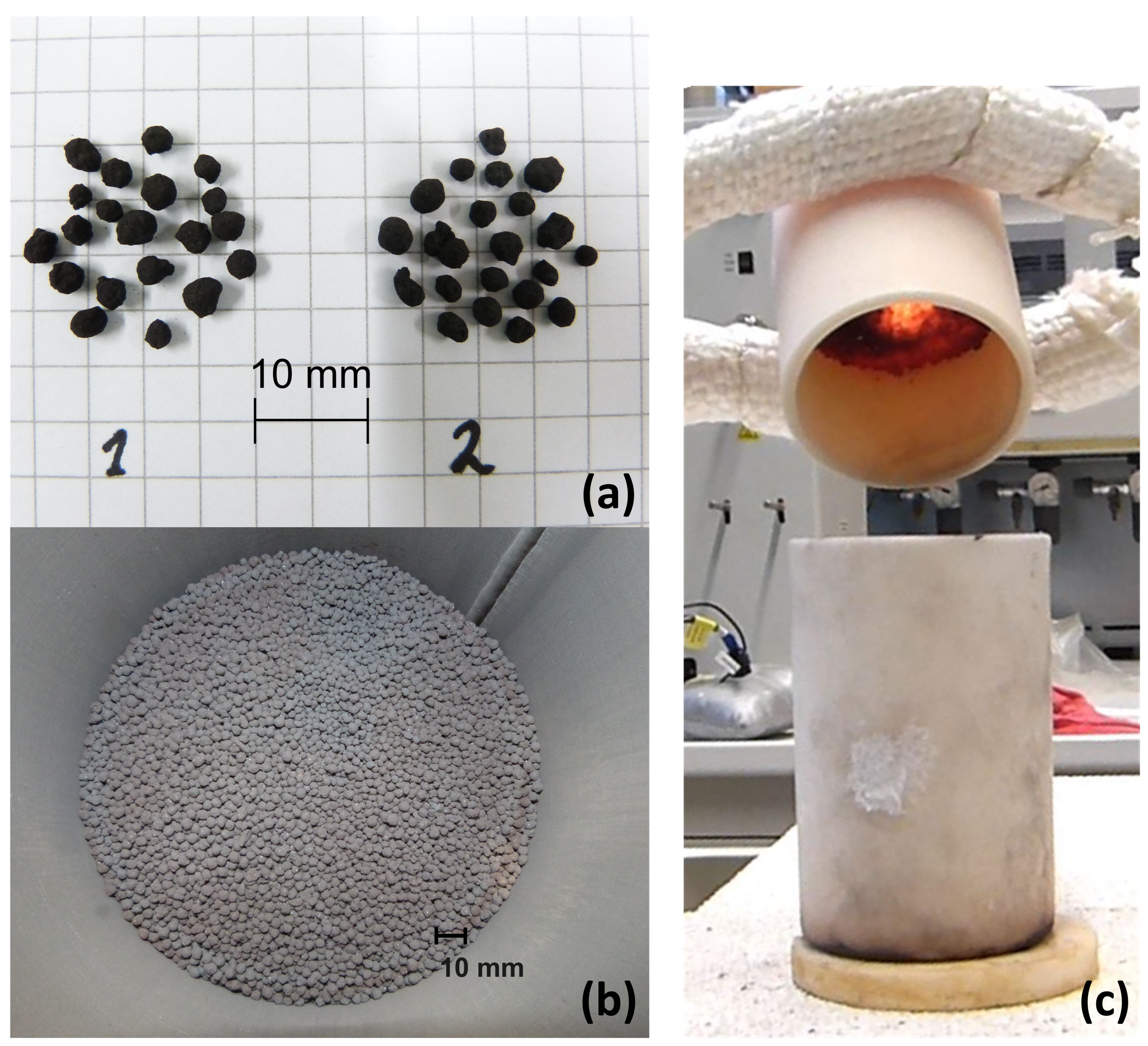
3.4. Particle Handling in a Moving Bed Reactor Based on Manganese–Iron Oxide
4. Conclusions
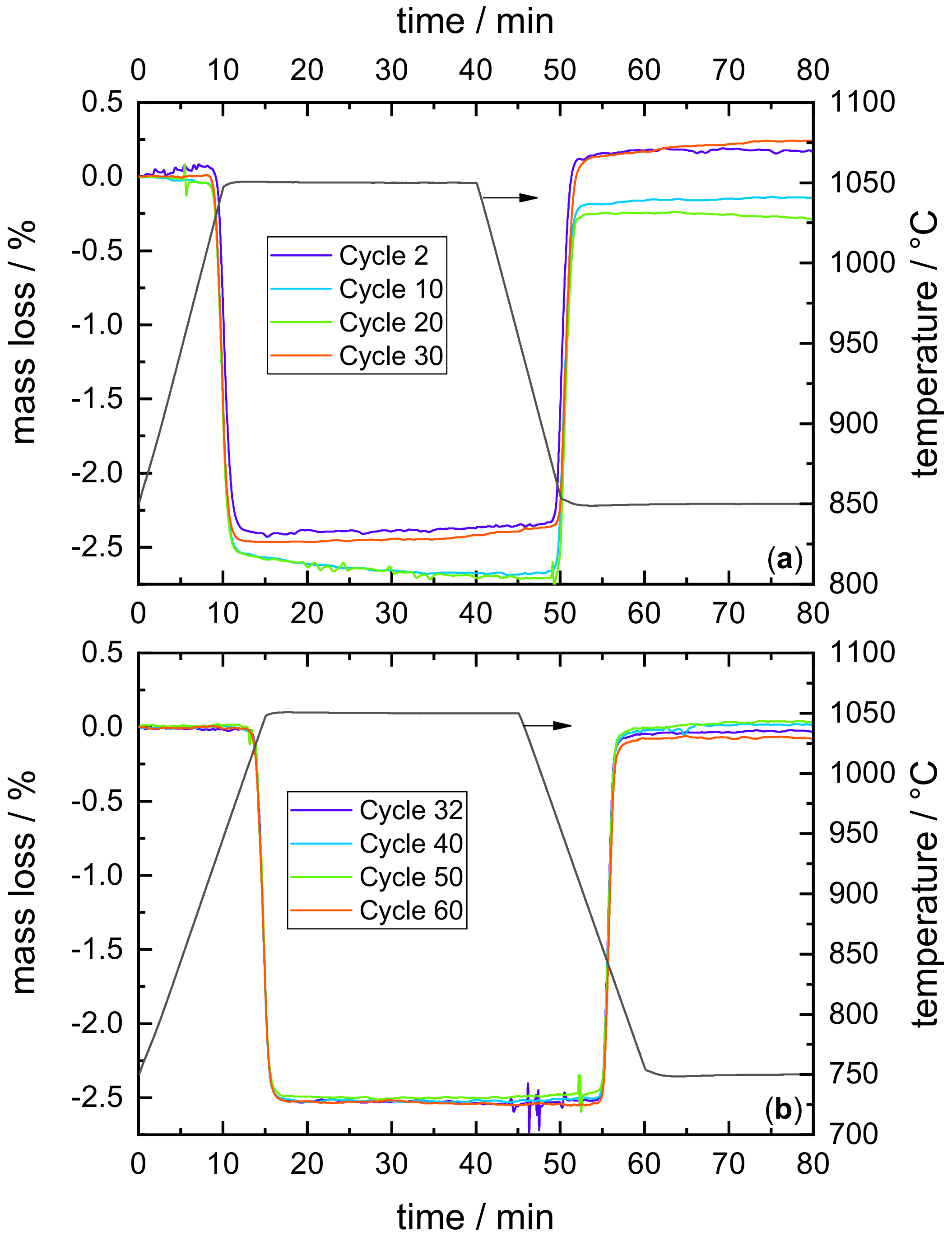
- Thermogravimetric analyses revealed that cooling rates of up to 30 K/min pose no challenge for the oxidation of the investigated manganese–iron oxide at an oxygen partial pressure of 20 kPa. Furthermore, sufficient long-term stability of the redox reaction was demonstrated for 60 consecutive cycles in TGA.
- The oxidation of the manganese–iron oxide caused two distinct temperature sections during the moving bed experiment: one section with nearly isothermal conditions and one section with a temperature gradient similar to a moving bed operation based on inert storage material. A thermal power of 2 kW was transferred to the gas flow during stationary temperature conditions.
- The manganese–iron oxide particles were oxidized to an extent of 80.2% during moving bed operation, based on the preceding partial reduction of 77.1%. An indirect heat transfer in the section of nearly isothermal condition could increase the oxidation conversion because the particle temperature could be regulated to a level of higher reaction rates.
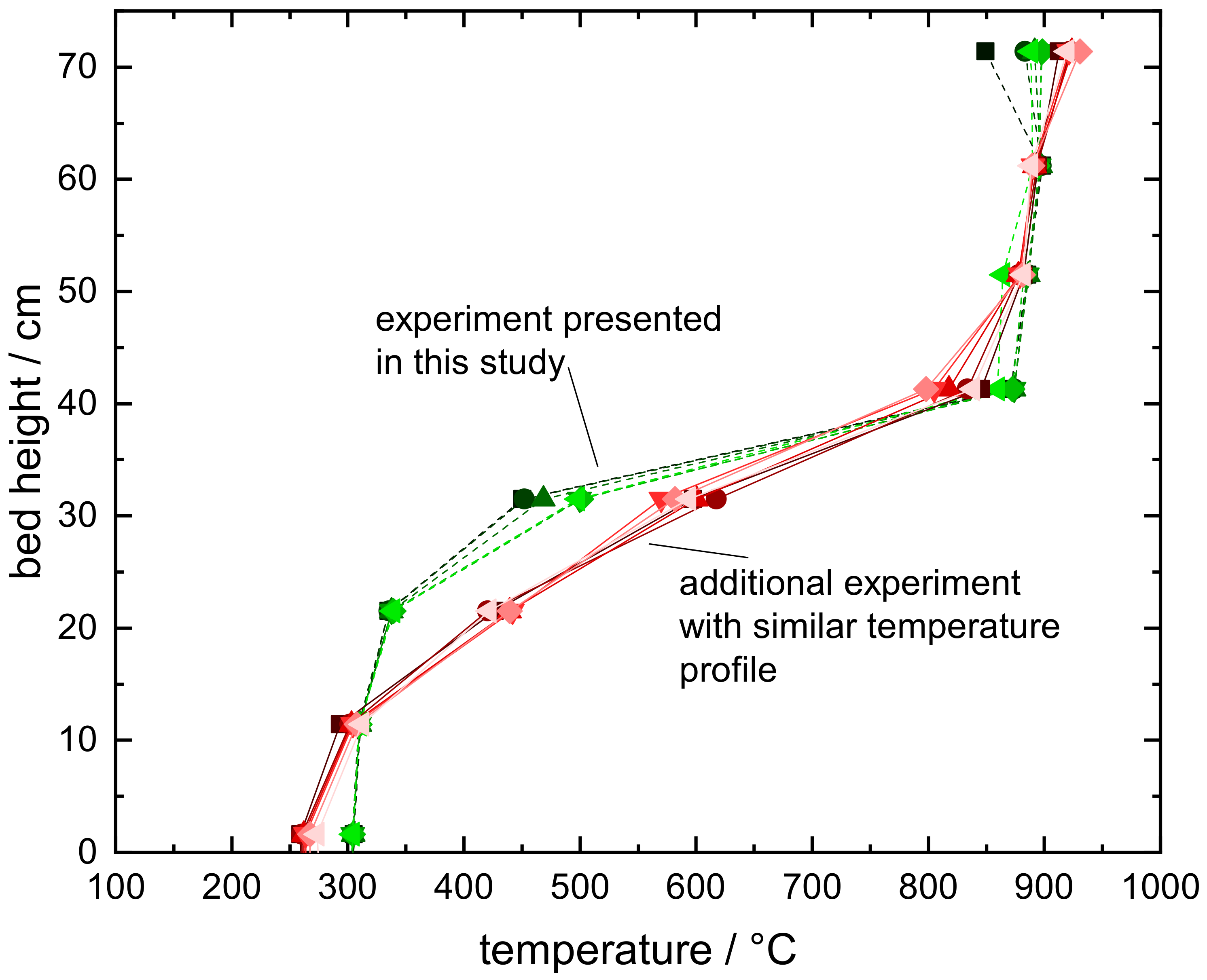
- The flowability of the manganese–iron-oxide particles was limited at high temperatures in the moving bed reactor. Additional tests revealed an insufficient flowability between 850 and 1050 . However, the underlying mechanism needs to be further addressed for an application of this manganese–iron oxide compound in a continuously operated system.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Cycle Stability of the Redox Reaction of Mn–Fe Oxide
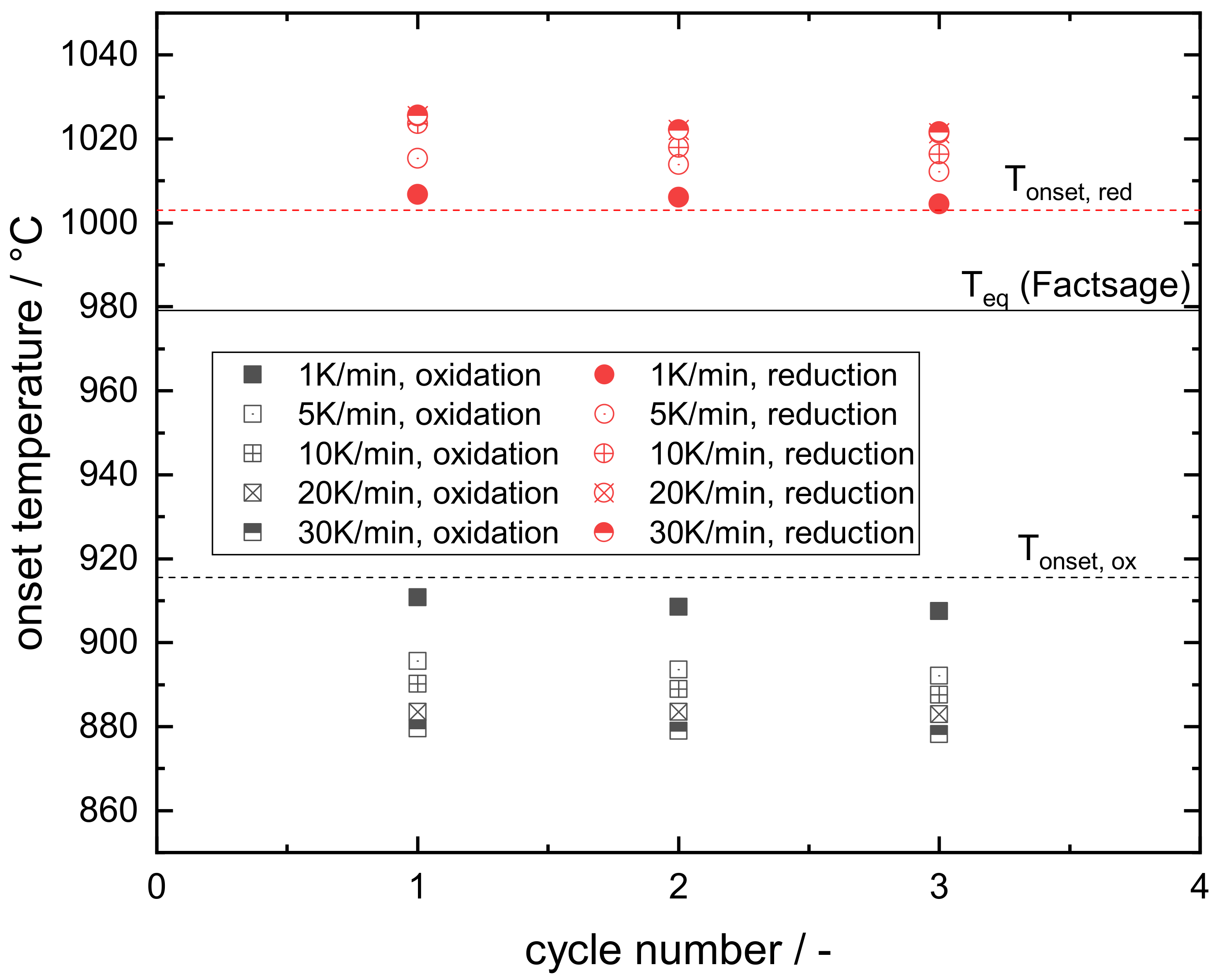
Appendix B. Determination of the Effective Onset Temperature of the Investigated Mn–Fe Oxide
Appendix C. Reproducibility of the Characteristic Isothermal Bed Segment
References
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Doroodchi, E.; Nellore, R.; Moghtaderi, B. A review on high-temperature thermochemical energy storage based on metal oxides redox cycle. Energy Conv. Manag. 2018, 168, 421–453. [Google Scholar] [CrossRef]
- Block, T.; Schmücker, M. Metal oxides for thermochemical energy storage: A comparison of several metal oxide systems. Solar Energy 2016, 126, 195–207. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Flamant, G. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715. [Google Scholar] [CrossRef]
- Schrader, A.J.; Muroyama, A.P.; Loutzenhiser, P.G. Solar electricity via an Air Brayton cycle with an integrated two-step thermochemical cycle for heat storage based on Co3O4/CoO redox reactions: Thermodynamic analysis. Sol. Energy 2015, 118, 485–495. [Google Scholar] [CrossRef]
- Falcone, P.K.; Noring, J.E.; Hacket, C.E. Evaluation and application of solid thermal energy carriers in a high temperature solar central receiver system. In Proceedings of the IECEC 17th Intersociety Energy Conversion Engineering Conference, Los Angeles, CA, USA, 8–12 August 1982. [Google Scholar]
- Siegel, N.; Kolb, G. Design and on-sun testing of a solid particle receiver prototype. In Proceedings of the ASME 2008 2nd International Conference on Energy Sustainability, ASMEDC, Jacksonville, FL, USA, 10–14 August 2008; Volume 2. [Google Scholar] [CrossRef]
- Flamant, G.; Gauthier, D.; Benoit, H.; Sans, J.L.; Garcia, R.; Boissière, B.; Ansart, R.; Hemati, M. Dense suspension of solid particles as a new heat transfer fluid for concentrated solar thermal plants: On-sun proof of concept. Chem. Eng. Sci. 2013, 102, 567–576. [Google Scholar] [CrossRef]
- Agrafiotis, C.; de Oliveira, L.; Roeb, M.; Sattler, C. A solar receiver-storage modular cascade based on porous ceramic structures for hybrid sensible/thermochemical solar energy storage. In SolarPACES, AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1734, ISBN 978-0-7354-1386-3. [Google Scholar] [CrossRef]
- Jafarian, M.; Arjomandi, M.; Nathan, G.J. Thermodynamic potential of molten copper oxide for high temperature solar energy storage and oxygen production. Appl. Energy 2017, 201, 69–83. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Schmücker, M.; Sattler, C. Exploitation of thermochemical cycles based on solid oxide redox systems for thermochemical storage of solar heat. Part 1: Testing of cobalt oxide-based powders. Sol. Energy 2014, 102, 189–211. [Google Scholar] [CrossRef]
- Imponenti, L.; Albrecht, K.J.; Kharait, R.; Sanders, M.D.; Jackson, G.S. Redox cycles with doped calcium manganites for thermochemical energy storage to 1000 °C. Appl. Energy 2018, 230, 1–18. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Improving the thermochemical energy storage performance of the Mn2O3/Mn3O4 redox couple by the incorporation of iron. ChemSusChem 2015, 8, 1947–1954. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Understanding redox kinetics of iron-doped manganese oxides for high temperature thermochemical energy storage. J. Phys. Chem. C 2016, 120, 27800–27812. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Cassayre, L. High-temperature thermochemical energy storage based on redox reactions using Co-Fe and Mn-Fe mixed metal oxides. J. Solid State Chem. 2017, 253, 6–14. [Google Scholar] [CrossRef]
- Wokon, M.; Block, T.; Nicolai, S.; Linder, M.; Schmücker, M. Thermodynamic and kinetic investigation of a technical grade manganese-iron binary oxide for thermochemical energy storage. Solar Energy 2017, 153, 471–485. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Moya, J.; Bayón, A.; Jana, P.; de la Peña O’Shea, V.A.; Romero, M.; Gonzalez-Aguilar, J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Thermochemical energy storage at high temperature via redox cycles of Mn and Co oxides: Pure oxides versus mixed ones. Solar Energy Mat. Solar Cells 2014, 123, 47–57. [Google Scholar] [CrossRef]
- Block, T.; Knoblauch, N.; Schmücker, M. The cobalt-oxide/iron-oxide binary system for use as high temperature thermochemical energy storage material. Thermochim. Acta 2014, 577, 25–32. [Google Scholar] [CrossRef]
- Wong, B. Thermochemical Heat Storage for Concentrated Solar Power; Technical Report; Phase II Final Report for the period September 30, 2008 through April 30, 2011; U.S. Department of Energy: Washington, DC, USA, 2011.
- Muan, A.; Somiya, S. The system iron oxide-manganese oxide in air. Am. J. Sci. 1962, 260, 230–240. [Google Scholar] [CrossRef]
- Wickham, D.G. The chemical composition of spinels in the system Fe3O4-Mn3O4. J. Inorg. Nucl. Chem. 1969, 31, 313–320. [Google Scholar] [CrossRef]
- Crum, J.V.; Riley, B.J.; Vienna, J.D. Binary phase diagram of the manganese oxide-iron oxide system. J. Am. Ceram. Soc. 2009, 92, 2378–2384. [Google Scholar] [CrossRef]
- Kjellqvist, L.; Selleby, M. Thermodynamic Assessment of the Fe-Mn-O system. J. Ph. Equilib. Diffus. 2010, 31, 113–134. [Google Scholar] [CrossRef]
- Kang, Y.B.; Jung, I.H. Thermodynamic modeling of oxide phases in the Fe–Mn–O system. J. Phys. Chem. Solids 2016, 98, 237–246. [Google Scholar] [CrossRef]
- Wokon, M.; Kohzer, A.; Linder, M. Investigations on thermochemical energy storage based on technical grade manganese-iron oxide in a lab-scale packed bed reactor. Sol. Energy 2017, 153, 200–214. [Google Scholar] [CrossRef]
- Preisner, N.C.; Block, T.; Linder, M.; Leion, H. Stabilizing particles of manganese-iron oxide with additives for thermochemical energy storage. Energy Technol. 2018, 6, 2154–2165. [Google Scholar] [CrossRef]
- Alonso, E.; Pérez-Rábago, C.; Licurgo, J.; Fuentealba, E.; Estrada, C.A. First experimental studies of solar redox reactions of copper oxides for thermochemical energy storage. Sol. Energy 2015, 115, 297–305. [Google Scholar] [CrossRef]
- Neises, M.; Tescari, S.; de Oliveira, L.; Roeb, M.; Sattler, C.; Wong, B. Solar-heated rotary kiln for thermochemical energy storage. Sol. Energy 2012, 86, 3040–3048. [Google Scholar] [CrossRef]
- Tescari, S.; Sundarraj, P.; Moumin, G.; Duarte, J.P.R.; Agrafiotis, C.; de Oliveira, L.; Willsch, C.; Roeb, M.; Sattler, C. Solar rotary kiln for continuous treatment of particle material: Chemical experiments from micro to milli meter particle size. In Proceedings of the SOLARPACES 2019: International Conference on Concentrating Solar Power and Chemical Energy Systems, Daegu, Korea, 1–4 October 2019. [Google Scholar]
- Jackson, G.S.; Imponenti, L.; Albrecht, K.J.; Miller, D.C.; Braun, R.J. Inert and reactive oxide particles for high-temperature thermal energy capture and storage for concentrating solar power. J. Sol. Energy Eng. 2019, 141. [Google Scholar] [CrossRef]
- Alonso, E.; Gómez, F.; Gonzalez-Aguilar, J.; Romero, M. Experimental analysis of Mn3O4/MnO reduction in a packed-bed type solar reactor: Oxygen partial pressure influence. In Proceedings of the 2011 SolarPACES Conference, Granada, Spain, 20–23 September 2011. [Google Scholar]
- Oles, A.S.; Jackson, G.S. Modeling of a concentrated-solar, falling-particle receiver for ceria reduction. Solar Energy 2015, 122, 126–147. [Google Scholar] [CrossRef]
- Schrader, A.J.; Dominicis, G.D.; Schieber, G.L.; Loutzenhiser, P.G. Solar electricity via an Air Brayton cycle with an integrated two-step thermochemical cycle for heat storage based on Co3O4/CoO redox reactions III: Solar thermochemical reactor design and modeling. Sol. Energy 2017, 150, 584–595. [Google Scholar] [CrossRef]
- Nie, F.; Cui, Z.; Bai, F.; Wang, Z. Properties of solid particles as heat transfer fluid in a gravity driven moving bed solar receiver. Sol. Energy Mater. Solar Cells 2019, 200, 110007. [Google Scholar] [CrossRef]
- Álvarez de Miguel, S. Analysis of redox reactions in a fluidized/fixed bed reactor for thermochemical energy storage in solar thermal power plants. Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 2017. [Google Scholar]
- Cui, Z.; Shao, W.; Chen, Z.; Cheng, L. Mathematical model and numerical solutions for the coupled gas–solid heat transfer process in moving packed beds. Appl. Energy 2017, 206, 1297–1308. [Google Scholar] [CrossRef]
- Negri, E.D.; Alfano, O.M.; Chiovetta, M.G. Moving-bed reactor model for the direct reduction of hematite. parametric study. Ind. Eng. Chem. Res 1995, 34, 4266–4276. [Google Scholar] [CrossRef]
- Takenaka, Y.; Kimura, Y.; Narita, K.; Kaneko, D. Mathematical model of direct reduction shaft furnace and its application to actual operations of a model plant. Comput. Chem. Eng. 1986, 10, 67–75. [Google Scholar] [CrossRef]
- Tong, A.; Sridhar, D.; Sun, Z.; Kim, H.R.; Zeng, L.; Wang, F.; Wang, D.; Kathe, M.V.; Luo, S.; Sun, Y.; et al. Continuous high purity hydrogen generation from a syngas chemical looping 25 kWth sub-pilot unit with 100% carbon capture. Fuel 2013, 103, 495–505. [Google Scholar] [CrossRef]
- Zeng, L.; Tong, A.; Kathe, M.; Bayham, S.; Fan, L.S. Iron oxide looping for natural gas conversion in a countercurrent moving bed reactor. Appl. Energy 2015, 157, 338–347. [Google Scholar] [CrossRef]
- Chen, C.; Lee, H.H.; Chen, W.; Chang, Y.C.; Wang, E.; Shen, C.H.; Huang, K.E. Study of an iron-based oxygen carrier on the moving bed chemical looping system. Energy Fuels 2018, 32, 3660–3667. [Google Scholar] [CrossRef]
| Parameter | Symbol | Value/Correlation | Unit | Reference |
|---|---|---|---|---|
| Particle diameter | 2–3 | as received | ||
| Bulk density | 1400 | measured | ||
| Reaction enthalpy, based on oxidized phase | 188 | measured | ||
| Specific heat capacity of (Mn0.7Fe0.3)2O3 + 20% ZrO2 ( 30 to 580 ) | measured | |||
| True density of (Mn0.7Fe0.3)2O3 + 20% ZrO2 | 5204 | measured via He-pycnometry | ||
| Total porosity | 0.73 | − | calculated | |
| Bulk porosity | 0.48 | − | calculated | |
| Intra-particle porosity | 0.48 | − | measured via Hg-intrusion-porosimetry |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preisner, N.C.; Linder, M. A Moving Bed Reactor for Thermochemical Energy Storage Based on Metal Oxides. Energies 2020, 13, 1232. https://doi.org/10.3390/en13051232
Preisner NC, Linder M. A Moving Bed Reactor for Thermochemical Energy Storage Based on Metal Oxides. Energies. 2020; 13(5):1232. https://doi.org/10.3390/en13051232
Chicago/Turabian StylePreisner, Nicole Carina, and Marc Linder. 2020. "A Moving Bed Reactor for Thermochemical Energy Storage Based on Metal Oxides" Energies 13, no. 5: 1232. https://doi.org/10.3390/en13051232
APA StylePreisner, N. C., & Linder, M. (2020). A Moving Bed Reactor for Thermochemical Energy Storage Based on Metal Oxides. Energies, 13(5), 1232. https://doi.org/10.3390/en13051232





