Micro- and Macroscale Consequences of Interactions between CO2 and Shale Rocks
Abstract
1. Introduction
1.1. Shale as a Caprock
1.2. Shale as a Reservoir/Source Rock
1.3. CO2 Fluids in the Subsurface
2. Shale Rock Characteristics
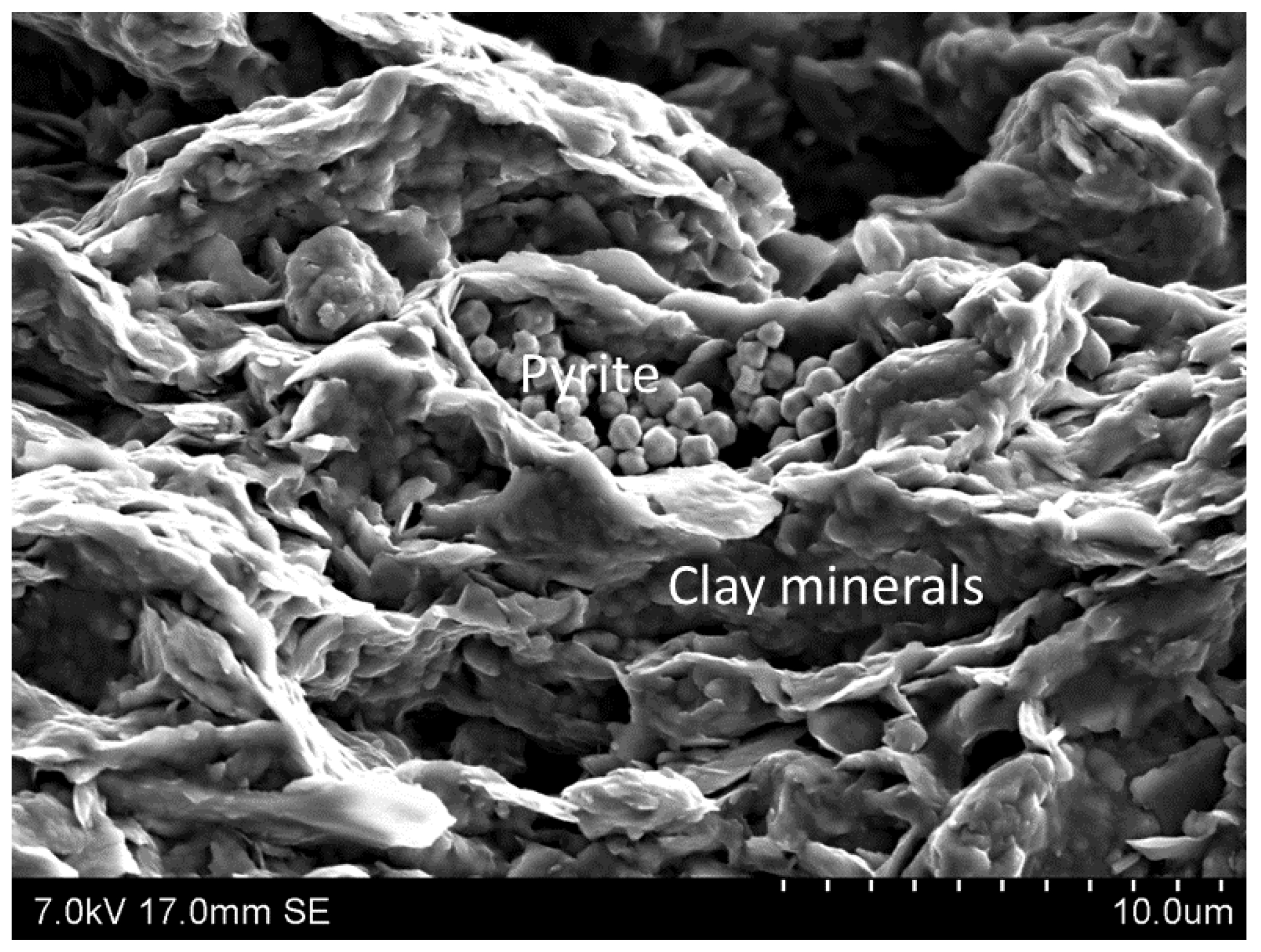
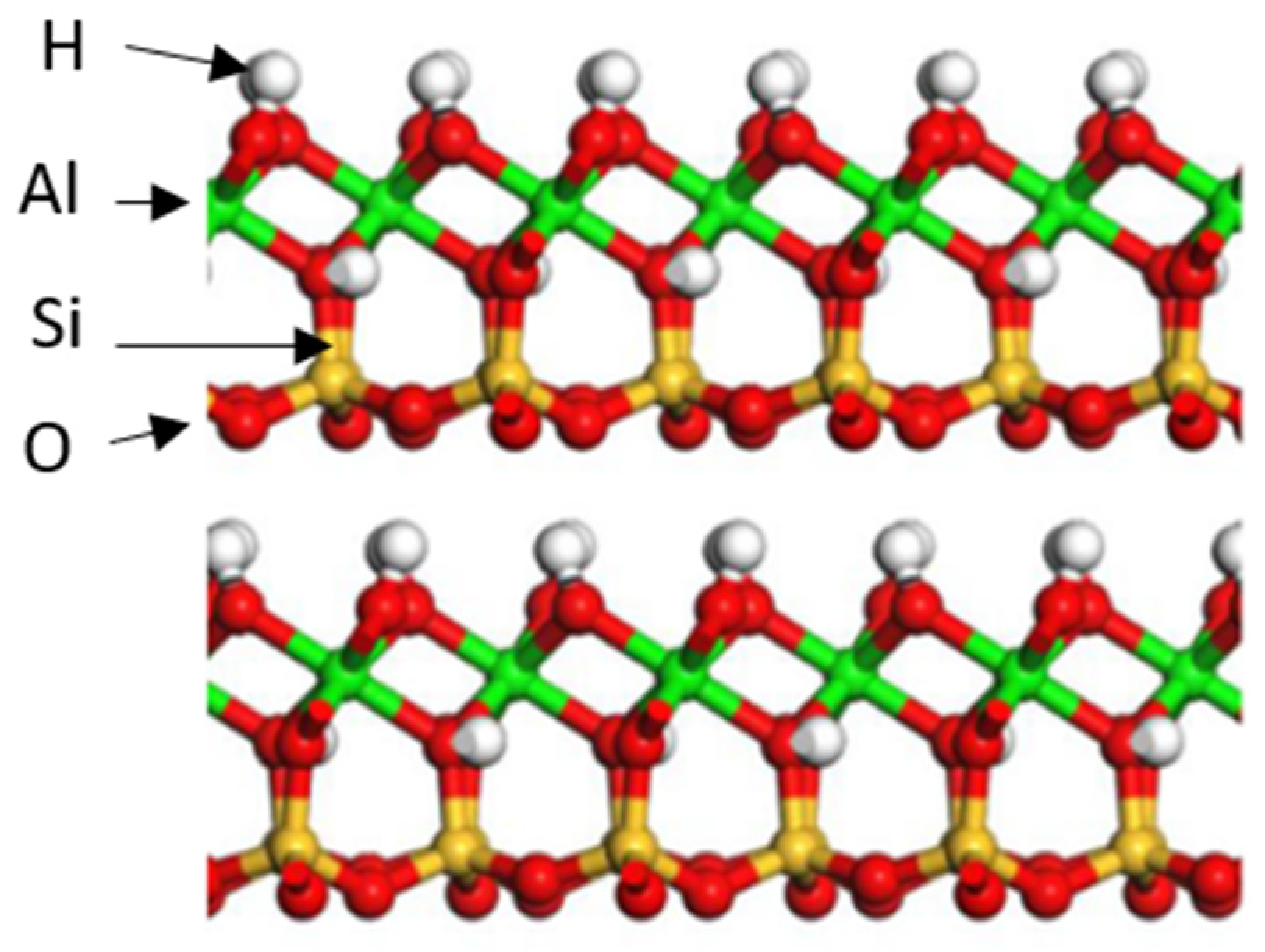
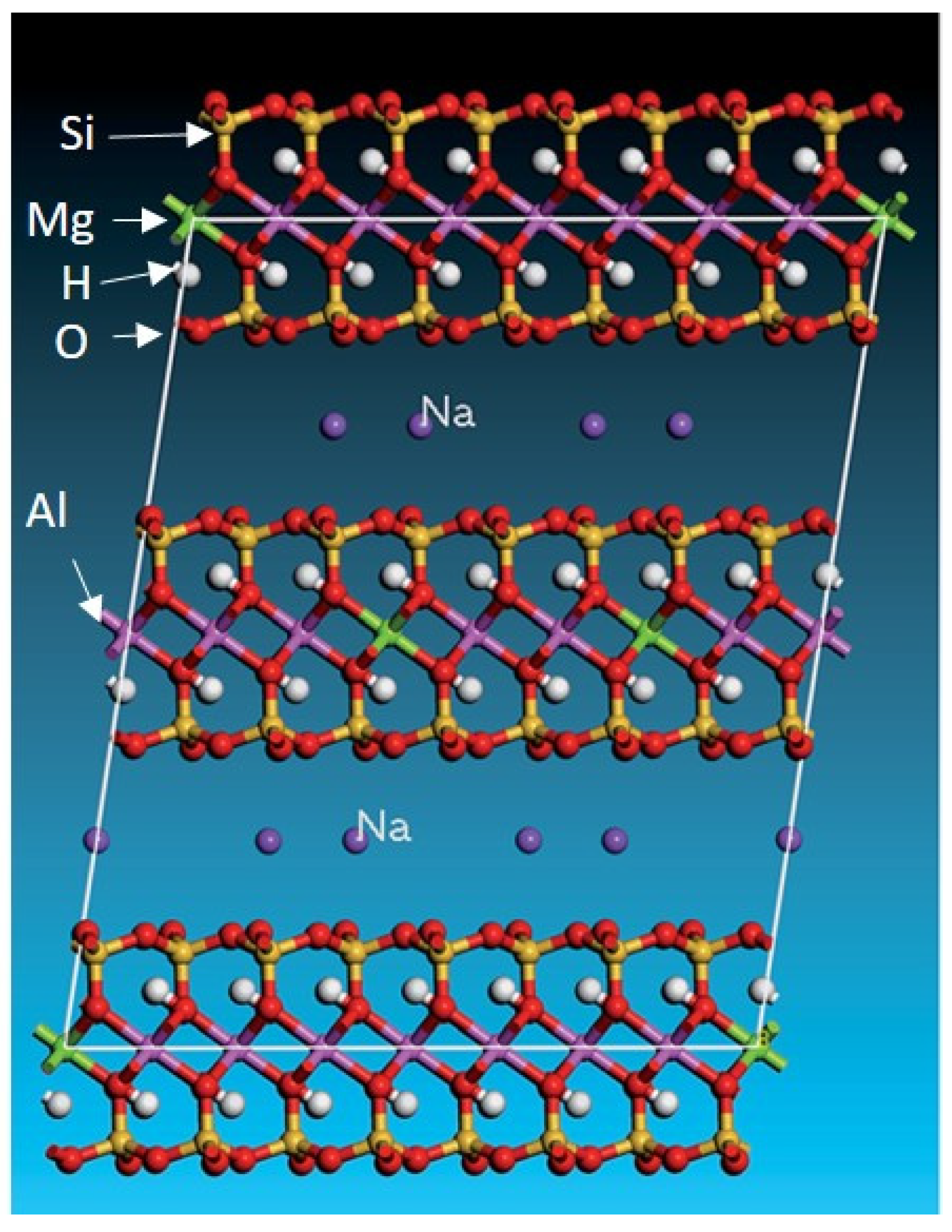
2.1. Mineralogical Properties of Shales
- Kaolinite []
- Smectite/montmorillonite []
- Illite []
- Chlorite [~]
- Quartz []
- Potassium/plagioclase feldspars [].
2.2. The Petrophysical and Mechanical Characteristics of Shale
2.3. Water Sensitivity of Shale Rocks
- -
- Dehydrated state (d001 ~ 9.6–10.7 Å; 0W: no complete water layer);
- -
- Mono-hydrated state (d001 ~ 11.8–12.9 Å; 1W: one complete water layer);
- -
- Bi-hydrated state (d001 ~ 14.5–15.8 Å; 2W: two complete water layers);
- -
- Tri-hydrated state (d001 ~ 18.0–19.5 Å; 3W: three complete water layers);
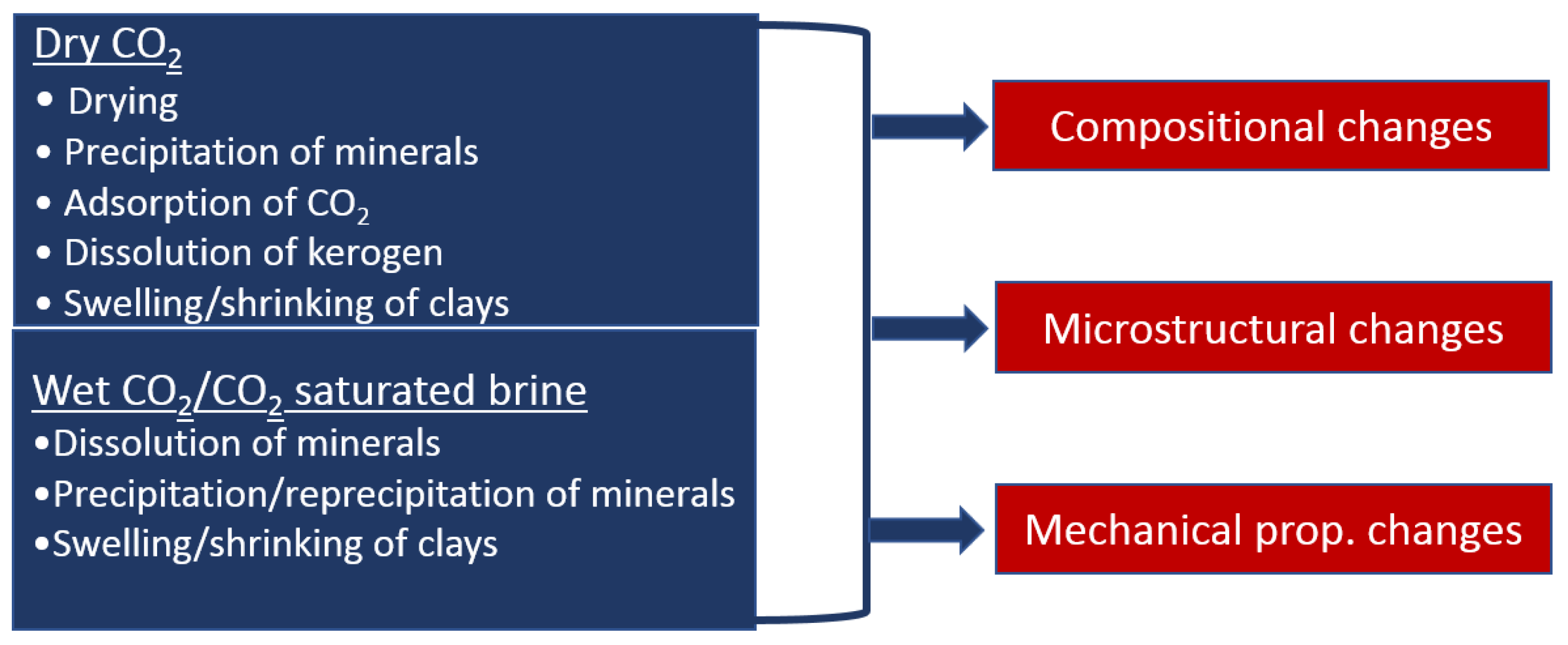
3. Interactions between CO2 and Shale
3.1. Processes at the CO2/Brine Interface
3.2. Shale in Contact with Dry CO2
3.2.1. Drying
3.2.2. Dissolution of Organic Matter
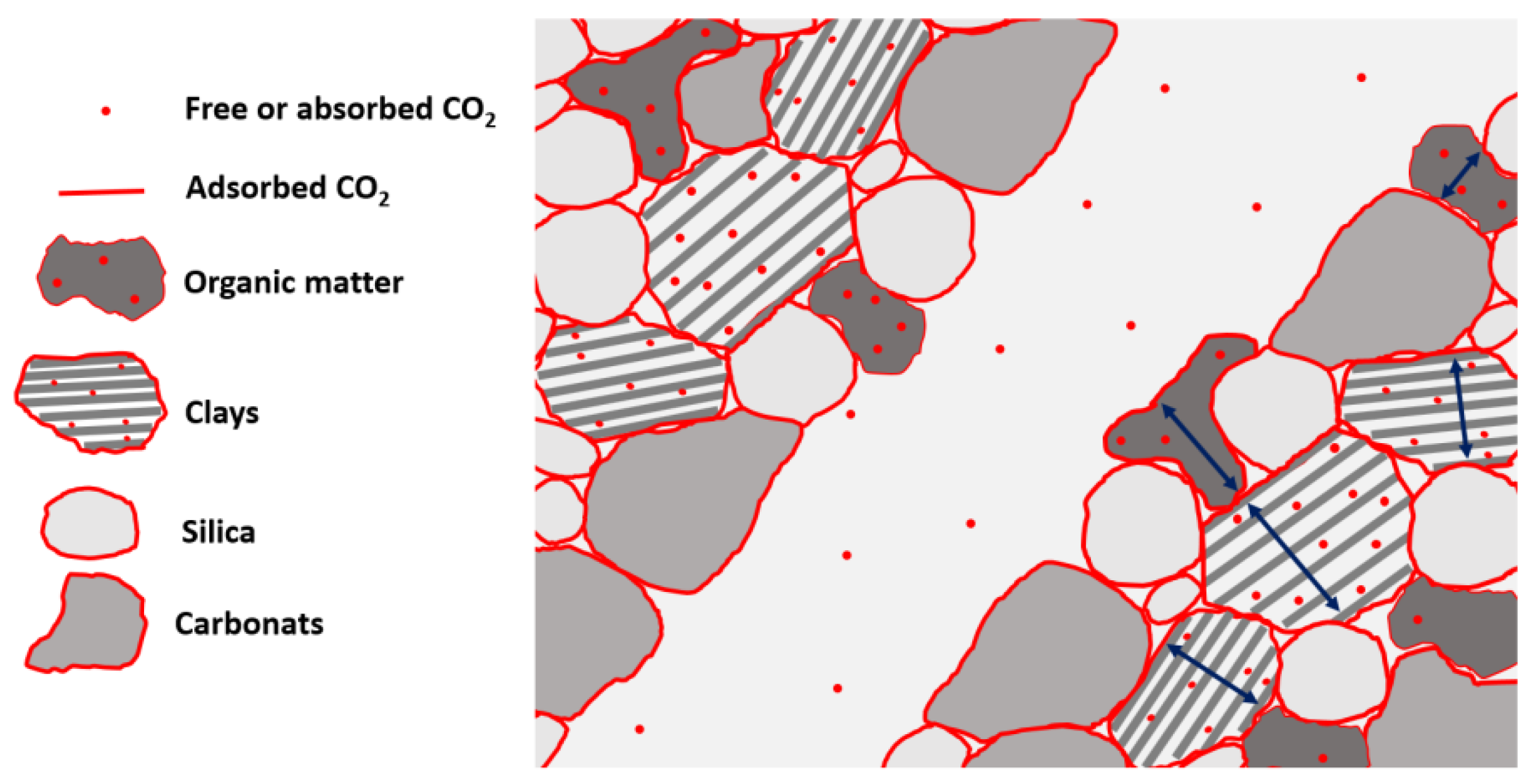
3.2.3. Sorption of CO2 on Shale Rocks
3.3. Shale in Contact with CO2-Saturated Brine or Wet CO2
3.3.1. Dissolution of Minerals
3.3.2. Precipitation and Reprecipitation of Minerals
4. Effect of CO2 on Petrophysical Parameters of Shale
5. The Effect of CO2 on the Mechanical Properties of Shale Rock
5.1. Effect of CO2 on Shale Volume Expansion (swelling) and Brittleness Index
5.2. Effect of CO2 on Shale Strength, Stiffness, and Poisson’s Ratio
5.2.1. Exposure to Dry CO2
5.2.2. Shale Samples Exposed to CO2–Water or CO2–Brine
5.3. Stress Thresholds for Crack Initiation and Crack Damage
5.4. Tensile Strength and Surface Energy
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jia, B.; Tsau, J.S.; Barati, R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs. Fuel 2019, 236, 404–427. [Google Scholar] [CrossRef]
- Ledley, T.S.; Sundquist, E.T.; Hall, D.K.; Fellows, J.D.; Killeen, T.L. Climate Change and Greenhouse Gases, EOS, Transaction. Am. Geophys. Union 1999, 80, 453–458. [Google Scholar] [CrossRef]
- Bernstein, L.; Bosch, P.; Canziani, O.; Chen, Z.; Christ, R.; Riahi, K. Climate Change, Synthesis Report, Summary for Policymakers; A report of the intergovernmental Panel on Climate Change; IPCC: Valencia, Spain, 2007; p. 22. [Google Scholar]
- Otheim, T.L. Monitoring CO2 Sequestrqtion in Basalt with Elastic Waves. Ph.D. Thesis, Boise State University, Boise, ID, USA, 2012; p. 134. [Google Scholar]
- Torp, T.A.; Gale, J. Demonstrating storage of CO2 in geological reservoirs: The Sleipner and SACS projects. Energy 2004, 29, 1361–1369. [Google Scholar] [CrossRef]
- Gislason, S.R.; Wolff-Boenisch, D.; Stefansson, A.; Oelkers, E.H.; Gunnlaugsson, E.; Sigurdardottir, H.; Sigfusson, B.; Broecker, W.S.; Matter, J.M.; Stute, M.; et al. Mineral sequestration of carbon dioxide in basalt: A pre-injection overview of the CarbFix project. Int. J. Greenh. Gas Control 2010, 4, 537–545. [Google Scholar] [CrossRef]
- Lu, J.; Kharaka, Y.K.; Thordsen, J.J.; Horita, J.; Karamalidis, A.; Griffith, C.; Hakala, J.A.; Ambats, G.; Cole, D.R.; Phelps, T.J.; et al. CO2–rock–brine interactions in Lower Tuscaloosa Formation at Cranfield CO2 sequestration site, Mississippi, U.S.A. Chem. Geol. 2012, 291, 269–277. [Google Scholar] [CrossRef]
- Maldal, T.; Tappel, I.M. CO2 underground storage for Snohvit gas field development. Energy 2004, 29, 1403–1411. [Google Scholar] [CrossRef]
- Mathieson, A.; Midgely, J.; Wright, I.; Saoula, N.; Ringrose, P. In Salah CO2 Storage JIP: CO2 sequestration monitoring and verification technologies applied at Krechba, Algeria. Energy Procedia 2011, 4, 3596–3603. [Google Scholar] [CrossRef]
- Bennaceur, K.; Gielen, D.; Kerre, T.; Tam, C. CO2 Capture and Storage: A Key Carbon Abatement Option; OECD Publishing: Paris, France, 2008. [Google Scholar]
- Wilson, E.J.; Gerard, D. Carbon Capture and Sequestration: Integrating Technology, Monitoring, Regulation; WileyBlackwell: Hoboken, NJ, USA, 2007. [Google Scholar]
- Metz, B.; Davidson, O.; De Coninck, H.; Loos, M.; Meyer, L. Carbon dioxide capture and storage: Intergovernmental panel on climate change. In Chapter 5: Underground Geological Storage; IPCC, Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Michael, K.; Golab, A.; Shulakova, V.; Ennis-King, J.; Allinson, G.; Sharma, S.; Aiken, T. Geological storage of CO2 in saline aquifers-A review of the experience from existing storage operations. Int. J. Greenh. Gas Control 2010, 4, 659–667. [Google Scholar] [CrossRef]
- Song, J.; Zhang, D.X. Comprehensive Review of Caprock-Sealing Mechanisms for Geologic Carbon Sequestration. Environ. Sci. Technol. 2013, 47, 9–22. [Google Scholar] [CrossRef]
- Liu, D.Q.; Li, Y.L.; Agarwal, R.K. Numerical simulation of long-term storage of CO2 in Yanchang shale reservoir of the Ordos basin in China. Chem. Geol. 2016, 440, 288–305. [Google Scholar] [CrossRef]
- Krevor, S.C.M.; Pini, R.; Zuo, L.; Benson, S.M. Relative permeability and trapping of CO2 and water in sandstone rocks at reservoir conditions. Water Resour. Res. 2012, 48. [Google Scholar] [CrossRef]
- Pruess, K.; Nordbotten, J. Numerical Simulation Studies of the Long-term Evolution of a CO2 Plume in a Saline Aquifer with a Sloping Caprock. Transp. Porous Media 2011, 90, 135–151. [Google Scholar] [CrossRef]
- Barati, R.; Liang, J.-T. A review of fracturing fluid systems used for hydraulic fracturing of oil and gas wells. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Gawel, K.; Lavrov, A.; Torsæter, M. Emerging methods and materials for shale gas operations. In M4ShaleGas Report; M4ShaleGas Consortium, European Union: Brussels, Belgium, 2016. [Google Scholar]
- Middleton, R.S.; Carey, J.W.; Currier, R.P.; Hyman, J.D.; Kang, Q.; Karra, S.; Jiménez-Martínez, J.; Porter, M.L.; Viswanathan, H.S. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef]
- Sinal, M.L.; Lancaster, G. Liquid CO Fracturing: Advantages And Limitations. J. Can. Pet. Technol. 1987, 26, 6. [Google Scholar] [CrossRef]
- Eshkalak, M.O.; Al-shalabi, E.W.; Sanaei, A.; Aybar, U.; Sepehrnoori, K. Enhanced Gas Recovery by CO2 Sequestration versus Re-fracturing Treatment in Unconventional Shale Gas Reservoirs. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAE, 10–13 November 2014; p. 18. [Google Scholar]
- Laboureur, L.; Ollero, M.; Touboul, D. Lipidomics by Supercritical Fluid Chromatography. Int. J. Mol. Sci. 2015, 16, 13868–13884. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Kang, Y.; Sun, Z.D.; Wang, X.C.; Hu, Y.Q. Effects of supercritical CO2 adsorption on the mechanical characteristics and failure mechanisms of shale. Energy 2019, 173, 870–882. [Google Scholar] [CrossRef]
- Qu, H.Y.; Liu, J.S.; Pan, Z.J.; Connell, L. Impact of thermal processes on CO2 injectivity into a coal seam. In Proceedings of the 9th World Congress on Computational Mechanics and 4th Asian Pacific Congress on Computational Mechanics, Sydney, Australia, 19–23 July 2010; Volume 10. [Google Scholar]
- Pettijohn, F.J. Sedimentary Rocks, 3rd ed.; Harper & Row: New York, NY, USA, 1975. [Google Scholar]
- Bates, R.L.; Jackson, J.A. Glossary of Geology (Second Edition); American Geological Institute: Fall Church, VA, USA, 1980. [Google Scholar]
- Blatt, H. Determination of mean sediment thickness in the crust: A sedimentologic method. Bull. Geol. Soc. Am. 1970, 81, 8. [Google Scholar] [CrossRef]
- Clarke, F.W. Data of geochemistry. US Geol. Survey Bull. 1924, 770, 841. [Google Scholar]
- Garrels, R.M.; Mackenzie, F.T. Evolution of Sedimentary Rocks; Norton: New York, NY, USA, 1971. [Google Scholar]
- Fjær, E.; Holt, R.M.; Horsrud, P.; Raaen, A.M.; Risnes, R. Petroleum Related Rock Mechanics; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Bhuiyan, M.H.; Kolstø, M.I.; Holt, R.M. Effects of Stress and Strain on Wave Velocities in Compacted Sand-kaolinite and Kaolinite-smectite. In Proceedings of the 73rd EAGE Conference and Exhibition Incorporating SPE EUROPEC 2011, Vienna, Austria, 23–26 May 2011. [Google Scholar]
- Marion, D.; Nur, A.; Yin, H.; Han, D. Compressional Velocity and Porosity in Sand-Clay Mixtures. Geophysics 1992, 57, 554–563. [Google Scholar] [CrossRef]
- Sattler, F.R.; Barnes, D.A. Geological Characterization and Assessment of Confining Layer Potential of the Upper Ordovician Utica Shale, Michigan Basin, USA. In Paleozoic Stratigraphy and Resources of the Michigan Basin; Grammar, G.M., Harrison, W.B., III, Barnes, D.A., Eds.; The Geological society of America: Colorado, USA, 2017; Volume 531, pp. 35–54. [Google Scholar]
- Blatt, H.; Middleton, G.; Murray, R. Origin of Sedimentary Rocks, 2nd ed.; Prentice-Hall Inc.: Flgewood Cliffs, NJ, USA, 1980. [Google Scholar]
- Eslinger, E.; Pevear, D. Clay Minerals for Petroleum Geologists and Engineers. In SEPM Short Course Notes no. 1988; The Society of Economic Paleontogists and Mineralogists: Tulsa, OK, USA, 1998. [Google Scholar]
- Dashtian, H.; Haimeng, W.; Sahimi, M. Nucleation of Salt Crystals in Clay Minerals: Molecular Dynamics Simulation. J. Phys. Chem. Lett. 2017, 8, 3166–3172. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jiang, Z.; Feng, X.; Zhang, N.; Xu, X. Relation between molecular structure of smectite and liquefaction of mudstone. RSC Adv. 2015, 5, 23481–23488. [Google Scholar] [CrossRef]
- Drever, J.I. The Geochemistry of Natural Waters; Printice Hall Inc.: Englewood Cliffs, NJ, USA, 1982. [Google Scholar]
- Bryant, W.R.; Bennettt, R.H.; Burkett, R.J.; Rack, F.R. Microfabric and physical properties characteristics of a consolidated clay section: ODP Site 697, Weddell Sea. In Microstructure of Fine-Grained Sediments; from Mud to Shale; Bennett, R.H., Bryant, W.R., Hulbert, M.H., Eds.; Springer: New York, NY, USA, 1991. [Google Scholar]
- Burst, J.F. Diagenesis of Gulf Coast clayey sediments and its possible relation to petroleum migration. AAPG Bull. 1969, 53, 21. [Google Scholar]
- Dewhurst, D.N.; Sarout, J.; Delle Piane, C.; Siggins, A.F.; Raven, M.D. Empirical strength prediction for preserved shales. Mar. Pet. Geol. 2015, 67, 512–525. [Google Scholar] [CrossRef]
- Hedberg, H.D. Gravitational compaction of clays and shales. Am. J. Sci. 1936, 31, 47. [Google Scholar] [CrossRef]
- Mondol, N.H. Porosity and permeability development in mechanically compacted silt-kaolinite mixtures. In Proceedings of the SEG Houston 2009 International Exposotion and Annual Meeting, Houston, TX, USA, 25–30 October 2009. [Google Scholar]
- Neuzil, C.E. How Permeable are clay and shales. Water Resour. Res. 1994, 30, 6. [Google Scholar] [CrossRef]
- Salama, A.; Amin, M.F.E.; Kumar, K.; Sun, S. Flow and Transport in Tight and Shale Formations: A Review. Geofluids 2017, 2017, 1–21. [Google Scholar] [CrossRef]
- Kozeny, J. Ueber kapillare Leitung des Wassers im Boden. Sitzungsber Akad. Wiss 1927, 136, 36. [Google Scholar]
- Carman, P.C. Fluid flow through granular beds. Inst. Chem. Eng. 1937, 15, 17. [Google Scholar] [CrossRef]
- Bethke, C.M. Modeling subsurface flow in sedimentary basins. Geol. Rundsch. 1989, 78, 26. [Google Scholar] [CrossRef]
- Bredehoeft, J.D.; Neuzil, C.E.; Milly, P.C.D. Regional flow in the Dakota Aquifer: A Study of the role of confining layers. US Geol. Surv. Water Supply Pap. 1983, 2237, 45. [Google Scholar]
- Rudolph, D.L.; Cherry, J.A.; Farvolden, R.N. Graoundwater flow and solute transport in fractured lacustrine clay near Mexico City. Water Resour. Res. 1991, 27, 15. [Google Scholar] [CrossRef]
- Keller, C.K.; van der Kamp, G.; Cherry, J.A. Hydrogeology of two Saskatchewan tills, I, Fractures, bulk permeability, and spatial variability of downward flow. J. Hydrol. 1988, 101, 25. [Google Scholar] [CrossRef]
- Kampman, N.; Burnside, N.M.; Shipton, Z.K.; Chapman, H.J.; Nicholl, J.A.; Ellam, R.M.; Bickle, M.J. Pulses of carbon dioxide emissions from intracrustal faults following climatic warming. Nat. Geosci. 2012, 5, 7. [Google Scholar] [CrossRef]
- Bond, C.E.; Wightman, R.; Ringrose, P.S. The influence of fracture anisotropy on CO2flow. Geophys. Res. Letters 2013, 40, 1284–1289. [Google Scholar] [CrossRef]
- Ingram, G.M.; Urai, J.L. Top-seal leakage through faults and fractures: The role of mudrock properties. In Muds and Mudsstone: Physical and Fluid Flow Properties; Aplin, A.C., Fleet, A.J., Macquaker, J.H.S., Eds.; Geological Society of London: London, UK, 1999; Volume 158, pp. 125–135. [Google Scholar]
- Lewicki, J.L.; Birkholzer, J.; Tsang, C.-F. Natural and industrial analogues for leakage of CO2 from storage reservoirs: Identification of features, events, and processes and lessons learned. Environ. Geol. 2006, 52, 457–467. [Google Scholar] [CrossRef]
- Chong, Z.; Li, X.; Hou, P.; Wu, Y.; Zhang, J.; Chen, T.; Liang, S. Numerical investigation of bedding plane parameters of transversely isotropic shale. Rock Mech. Rock Eng. 2017, 50, 22. [Google Scholar] [CrossRef]
- Al-Bazali, T.M.; Zhang, H.; Chenevert, M.E.; Sharma, M.M. Measurement of the sealing capacity of shale caprocks. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 9–12 October 2005. [Google Scholar]
- Lohr, C.D.; Hackley, P.C. Using mercury injection pressure analyses to estimate sealing capacity of the Tuscaloosa marine shale in Mississippi, USA: Implications for carbon dioxide sequestration. Int. J. Greenh. Gas Control 2018, 78, 375–387. [Google Scholar] [CrossRef]
- Busch, A.; Amann, A.; Bertier, P.; Waschbusch, M.; Krooss, B.M. The Significance of caprock seal ingintegrity for CO2 storage. In Proceedings of the SPE International Conference on CO2 Capture, Storage, and Utilization, Orleans, LA, USA, 10–12 November 2010. [Google Scholar]
- Chiquet, P.; Broseta, D. Capillary entry pressure of shaly caprock by Carbon Dioxide. In Proceedings of the SPE Europec/EAGE Annual Conference, Madrid, Spain, 13–16 June 2005. [Google Scholar]
- Edlmann, K.; Haszeldine, S.; McDermott, C.I. Experimental investigation into the sealing capability of naturally fractured shale caprocks to supercritical carbon dioxide flow. Environ. Earth Sci. 2013, 70, 3393–3409. [Google Scholar] [CrossRef]
- Al-Bazali, T.M.; Zhang, J.; Chenevert, M.E.; Sharma, M.M. Capillary entry pressure of oil-based muds in shales: The key to the success of oil-based muds. Energy Source Part A 2008, 30, 297–308. [Google Scholar] [CrossRef]
- Grunau, H.R. A WORLDWIDE LOOK AT THE CAP-ROCK PROBLEM. J. Pet. Geol. 1987, 10, 245–265. [Google Scholar] [CrossRef]
- Mokhtari, M.; Alqahtani, A.A.; Tutuncu, A.N.; Yin, X. Stress dependent permeability anisotropy and wettablitlity of shale resources. In Proceedings of the Unconventional Resource Technology Conference, Denver, CO, USA, 12–14 August 2013. [Google Scholar]
- Odusina, E.; Sondergeld, C.; Rai, C. An NMR study on shale wettability. In Proceedings of the Canadian Society for unconventional Gas, Calgery, AB, Canada, 15–17 November 2011. [Google Scholar]
- Wang, D.; Butler, R.; Zhang, J.; Seright, R. Wettability survery in Bakken shale with surfactant-formulation imbibition. SPE Reserv. Eval. Eng. 2012, 15, 695–705. [Google Scholar] [CrossRef]
- Passey, Q.R.; Bohacs, K.M.; Esch, W.L.; Klimentidis, R.; Sinha, S. From oil-prone source rock to Gas producing shale reservoir-geologic and petrophysical chanracterization of unconventional Shale-gas reservoir. In Proceedings of the CPS/SPE International Oil & Gas Conference and Exhibition, Beijing, China, 8–10 June 2010. [Google Scholar]
- Borysenko, A.; Clennell, B.; Sedev, R.; Burgar, I.; Ralston, J.; Raven, M.; Dewhurst, D.; Liu, K.Y. Experimental investigations of the wettability of clays and shales. J. Geophys. Res. Solid Earth 2009, 114. [Google Scholar] [CrossRef]
- Agofack, N.; Cerasi, P.; Stroisz, A.; Rørheim, S. Sorption of CO2 and integrity of a caprock shale. In Proceedings of the 53rd ARMA Symposium, New York, NY, USA, 23–26 June 2019; p. 9. [Google Scholar]
- Espinoza, D.N.; Jung, H.; Major, J.R.; Sun, Z.; Ramos, M.J.; Eichhubl, P.; Balhoff, M.T.; Choens, R.C.; Dewers, T.A. CO2 charged brines changed rock strength and stiffness at Crystal Geyser, Utah: Implications for leaking subsurface CO2 storage reservoirs. Int. J. Greenh. Gas Control 2018, 73, 16–28. [Google Scholar] [CrossRef]
- Lyu, Q.; Ranjith, P.G.; Long, X.P.; Ji, B. Experimental Investigation of Mechanical Properties of Black Shales after CO2-Water-Rock Interaction. Materials 2016, 9, 633. [Google Scholar] [CrossRef]
- Jin, Z.; Li, W.; Jin, C.; Hambleton, J.; Cusatis, G. Anisotropic elastic, strength, and fracture properties of Marcellus shale. Int. J. Rock Mech. Min. Sci. 2018, 109, 124–137. [Google Scholar] [CrossRef]
- Fjær, E.; Nes, O.-M. The Impact of Heterogeneity on the Anisotropic Strength of an Outcrop Shale. Rock Mech. Rock Eng. 2014, 47, 1603–1611. [Google Scholar] [CrossRef]
- Cheng, P.; Bestehorn, M.; Firoozabadi, A. Effect of permeability anisotropy on buoyancy-driven flow for CO2 sequestration in saline aquifers. Water Resour. Res. 2012, 48. [Google Scholar] [CrossRef]
- Armitage, P.J.; Faulkner, D.R.; Worden, R.H.; Aplin, A.C.; Butcher, A.R.; Iliffe, J. Experimental measurement of, and controls on, permeability and permeability anisotropy of caprocks from the CO2storage project at the Krechba Field, Algeria. J. Geophys. Res. 2011, 116. [Google Scholar] [CrossRef]
- Al Ismail, M.; Raeece, J.S.; Hol, S.; Zoback, M. The Effect of CO2 adsorption on permeability anisotropy inthe Eagle Frod shale. In Proceedings of the Unconventional Resource Technology Conference, Denver, CO, USA, 25–27 August 2014. [Google Scholar]
- Taheri, A.; Wessel-Berg, D.; Torsæter, O.; Soroush, M. The Effect of Anisotropy and heterogeneity on Co2 dissolution in deep saline aquifers. In Proceedings of the Carbon Management Technology Conference, Orlando, FL, USA, 7–9 February 2012. [Google Scholar]
- Lu, Y.Y.; Ao, X.; Tang, J.R.; Jia, Y.Z.; Zhang, X.W.; Chen, Y.T. Swelling of shale in supercritical carbon dioxide. J. Nat. Gas Sci. Eng. 2016, 30, 268–275. [Google Scholar] [CrossRef]
- Ferrage, E. Investigation of the interlayer organization of water and ions in smectite from the combined use of diffraction experiments and molecular simulations. A review of methodology, applications, and perspectives. Clays Clay Miner. 2016, 64, 27. [Google Scholar] [CrossRef]
- Du, J.; Hu, L.; Meegoda, J.N.; Zhang, G. Shale softening: Observations, phenomenological behavior, and mechanisms. Appl. Clay Sci. 2018, 161, 290–300. [Google Scholar] [CrossRef]
- Brochard, L.; Vandamme, M.; Pellenq, R.J.-M. Poromechanics of microporous media. J. Mech. Phys. Solids 2012, 60, 17. [Google Scholar] [CrossRef]
- Perrier, L.; Pijaudier-Cadot, G.; Gregoire, D. Poromechanics of adsorption-induced swelling in microporous materials: A new poromechanical model taking into account strain effects on adsorption. Continuum. Mech. Thermodyn. 2015, 27, 15. [Google Scholar] [CrossRef]
- Sechrist, F. Effect of Carbon Dioxide on Evaporation of Water. Nature 1963, 199, 899–900. [Google Scholar] [CrossRef]
- Byck, H.T. Effect of Dissolved CO2 on the PH of Water. Science 1932, 75, 224. [Google Scholar] [CrossRef] [PubMed]
- Spycher, N.; Pruess, K. CO2-H2O mixtures in the geological sequestration of CO2. II. Partitioning in chloride brines at 12-100°C and up to 600 bar. Geochimica et Cosmochimica Acta 2005, 69, 3309–3320. [Google Scholar] [CrossRef]
- Kaszuba, J.; Yardley, B.; Andreani, M. Experimental perspectives of mineral dissolution and precipitation due to carbon dioxide-water-rock interactions. Rev. Mineral. Geochem. 2013, 77, 153–188. [Google Scholar] [CrossRef]
- Spycher, N.; Pruess, K.; Ennis-King, J. CO2-H2O mixtures in the geological sequestration of CO2. I. Assessment and calculation of mutual solubilities from 12 to 100°C and up to 600 bar. Geochimica et Cosmochimica Acta 2003, 67, 3015–3031. [Google Scholar] [CrossRef]
- Zettlitzer, M.; Moeller, F.; Morozova, D.; Lokay, P.; Würdemann, H. Re-establishment of the proper injectivity of the CO2-injection well Ktzi 201 in Ketzin, Germany. Int. J. Greenh. Gas Control 2010, 4, 952–959. [Google Scholar] [CrossRef]
- Baumann, G.; Henninges, J.; De Lucia, M. Monitoring of saturation changes and salt precipitation during CO2 injection using pulsed neutron-gamma logging at the Ketzin pilot site. Int. J. Greenh. Gas Control 2014, 28, 134–146. [Google Scholar] [CrossRef]
- Roels, S.M.; El Chatib, N.; Nicolaides, C.; Zitha, P.L.J. Capillary-Driven Transport of Dissolved Salt to the Drying Zone During CO2 Injection in Homogeneous and Layered Porous Media. Transp. Porous Media 2016, 111, 411–424. [Google Scholar] [CrossRef]
- Miri, R.; van Noort, R.; Aagaard, P.; Hellevang, H. New insights on the physics of salt precipitation during injection of CO2 into saline aquifers. Int. J. Greenh. Gas Control 2015, 43, 10–21. [Google Scholar] [CrossRef]
- Miri, R.; Hellevang, H. Salt precipitation during CO2 storage-A review. Int. J. Greenh. Gas Control 2016, 51, 136–147. [Google Scholar] [CrossRef]
- Hellevang, H.; Miri, R.; Haile, B.G. New insights into the mechanisms controlling the rate of crystal growth. Cryst. Growth Des. 2014, 14, 6451–6458. [Google Scholar] [CrossRef]
- André, L.; Peysson, Y.; Azaroual, M. Well injectivity during CO2 storage operations in deep saline aquifers – Part 2: Numerical simulations of drying, salt deposit mechanisms and role of capillary forces. Int. J. Greenh. Gas Control 2014, 22, 301–312. [Google Scholar] [CrossRef]
- Bacci, G.; Korre, A.; Durucan, S. An experimental and numerical investigation into the impact of dissolution/precipitation mechanisms on CO2 injectivity in the wellbore and far field regions. Int. J. Greenh. Gas Control 2011, 5, 579–588. [Google Scholar] [CrossRef]
- Nooraiepour, M.; Fazeli, H.; Miri, R.; Hellevang, H. Effect of CO2 Phase States and Flow Rate on Salt Precipitation in Shale Caprocks—A Microfluidic Study. Environ. Sci. Technol. 2018, 52, 6050–6060. [Google Scholar] [CrossRef]
- Mugridge, S.J.; Young, H.R. Disintegration of shale by cyclic wetting and drying and frost action. Can. J. Earth Sci. 1983, 20, 568–576. [Google Scholar] [CrossRef]
- Eckert, C.A.; Knutson, B.L.; Debenedetti, P.G. Supercritical fluids as solvents for chemical and materials processing. Nature 1996, 383, 313–318. [Google Scholar] [CrossRef]
- Wu, T.; Xue, Q.; Li, X.; Tao, Y.; Jin, Y.; Ling, C.; Lu, S. Extraction of kerogen from oil shale with supercritical carbon dioxide: Molecular dynamics simulations. J. Supercrit. Fluids 2016, 107, 499–506. [Google Scholar] [CrossRef]
- Marcus, Y. Some Advances in Supercritical Fluid Extraction for Fuels, Bio-Materials and Purification. Processes 2019, 7, 156. [Google Scholar] [CrossRef]
- Bondar, E.; Koel, M. Application of supercritical fluid extraction to organic geochemical studies of oil shales. Fuel 1998, 77, 211–213. [Google Scholar] [CrossRef]
- Allawzi, M.; Al-Otoom, A.; Allaboun, H.; Ajlouni, A.; Al Nseirat, F. CO2 supercritical fluid extraction of Jordanian oil shale utilizing different co-solvents. Fuel Process. Technol. 2011, 92, 2016–2023. [Google Scholar] [CrossRef]
- Merey, S.; Sinayuc, C. Analysis of carbon dioxide sequestration in shale gas reservoirs by using experimental adsorption data and adsorption models. J. Nat. Gas Sci. Eng. 2016, 36, 1087–1105. [Google Scholar] [CrossRef]
- Kang, S.M.; Fathi, E.; Ambrose, R.J.; Akkutlu, I.Y.; Sigal, R.F. Carbon dioxide storage capacity of organic-rich shales. SPE J. 2011, 16, 842–855. [Google Scholar] [CrossRef]
- Gensterblum, Y.; Busch, A.; Krooss, B.M. Molecular concept and experimental evidence of competitive adsorption of H2O, CO2 and CH4 on organic material. Fuel 2014, 115, 581–588. [Google Scholar] [CrossRef]
- Chareonsuppanimit, P.; Mohammad, S.A.; Robinson, R.L.; Gasem, K.A.M. High-pressure adsorption of gases on shales: Measurements and modeling. Int. J. Coal Geol. 2012, 95, 34–46. [Google Scholar] [CrossRef]
- Eliebid, M.; Mahmoud, M.; Elkatatny, S.; Abouelresh, M.; Shawabkeh, R. Adsorption Role in Shale Gas Recovery and the Feasibility of CO2 in Shale Enhanced Gas Recovery: A Study on Shale Gas from Saudi Arabia. In SPE Kuwait Oil & Gas Show and Conference; Society of Petroleum Engineers: Kuwait City, Kuwait, 2017; p. 12. [Google Scholar]
- Busch, A.; Bertier, P.; Gensterblum, Y.; Rother, G.; Spiers, C.J.; Zhang, M.; Wentinck, H.M. On sorption and swelling of CO2 in clays. Geomech. Geophys. Geo Energy Geo Resour. 2016, 2, 111–130. [Google Scholar] [CrossRef]
- Lutynski, M.; Waszczuk, P.; Slomski, P.; Szczepanski, J. CO2 sorption of Pomeranian gas bearing shales—The effect of clay minerals. Energy Procedia. 2017, 125, 457–466. [Google Scholar] [CrossRef]
- Heller, R.; Zoback, M. Adsorption of methane and carbon dioxide on gas shale and pure mineral samples. J. Unconv. Oil Gas Resour 2014, 8, 14–24. [Google Scholar] [CrossRef]
- Nuttal, B.C.; Eble, C.; Bustin, R.M.; Drahovzal, J.A. Analysis of Devonian black shales in kentucky for potential carbon dioxide sequestration and enhanced natural gas production. Greenh. Gas Control Technol. 2005, 7, 4. [Google Scholar]
- Pathak, M.; Huang, H.; Meakin, P.; Deo, M. Molecular investigation of the interactions of carbon dioxide and methane with kerogen: Application in enhanced shale gas recovery. J. Nat. Gas Sci. Eng. 2018, 51, 1–8. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Furmaniak, S.; Gauden, P.A.; Terzyk, A.P. Carbon dioxide adsorption-induced deformation of microporous carbons. J. Phys. Chem. C 2010, 114, 5126–5133. [Google Scholar] [CrossRef]
- Giesting, P.; Guggenheim, S.; Koster van Groos, A.F.; Busch, A. X-ray Diffraction Study of K- and Ca-Exchanged Montmorillonites in CO2 Atmospheres. Environ. Sci. Technol. 2012, 46, 5623–5630. [Google Scholar] [CrossRef]
- Rother, G.; Ilton, E.S.; Wallacher, D.; Hauβ, T.; Schaef, H.T.; Qafoku, O.; Rosso, K.M.; Felmy, A.R.; Krukowski, E.G.; Stack, A.G.; et al. CO2 Sorption to Subsingle Hydration Layer Montmorillonite Clay Studied by Excess Sorption and Neutron Diffraction Measurements. Environ. Sci. Technol. 2013, 47, 205–211. [Google Scholar] [CrossRef]
- Giesting, P.; Guggenheim, S.; Koster van Groos, A.F.; Busch, A. Interaction of carbon dioxide with Na-exchanged montmorillonite at pressures to 640bars: Implications for CO2 sequestration. Int. J. Greenh. Gas Control 2012, 8, 73–81. [Google Scholar] [CrossRef]
- Ho, T.A.; Wang, Y.; Criscenti, L.J. Chemo-mechanical coupling in kerogen gas adsorption/desorption. Phys. Chem. Chem. Phys. 2018, 20, 12390–12395. [Google Scholar] [CrossRef]
- Zhang, M.; de Jong, S.M.; Spiers, C.J.; Busch, A.; Wentinck, H.M. Swelling stress development in confined smectite clays through exposure to CO 2. Int. J. Greenh. Gas Control 2018, 74, 49–61. [Google Scholar] [CrossRef]
- Daniel, B.; Shaw, C.E.W. The mineralogical composition of shales. J. Sediment. Res. 1965, 35, 213–222. [Google Scholar]
- Lahann, R.; Mastalerz, M.; Rupp, J.A.; Drobniak, A. Influence of CO2 on New Albany Shale composition and pore structure. Int. J. Coal Geol. 2013, 108, 2–9. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Golubev, S.V.; Schott, J.; Castillo, A. Calcite, dolomite and magnesite dissolution kinetics in aqueous solutions at acid to circumneutral pH, 25 to 150 °C and 1 to 55 atm pCO2: New constraints on CO2 sequestration in sedimentary basins. Chem. Geol. 2009, 265, 20–32. [Google Scholar] [CrossRef]
- Golubev, S.V.; Bénézeth, P.; Schott, J.; Dandurand, J.L.; Castillo, A. Siderite dissolution kinetics in acidic aqueous solutions from 25 to 100 °C and 0 to 50 atm pCO2. Chem. Geol. 2009, 265, 13–19. [Google Scholar] [CrossRef]
- Luquot, L.; Gouze, P. Experimental determination of porosity and permeability changes induced by injection of CO2 into carbonate rocks. Chem Geol. 2009, 265, 148–159. [Google Scholar] [CrossRef]
- Rathnaweera, T.D.; Ranjith, P.G.; Perera, M.S.A. Experimental investigation of geochemical and mineralogical effects of CO2 sequestration on flow characteristics of reservoir rock in deep saline aquifers. Sci. Rep. 2016, 6, 19362. [Google Scholar] [CrossRef]
- Jung, H.B.; Um, W.; Cantrell, K.J. Effect of oxygen co-injected with carbon dioxide on Gothic shale caprock–CO2–brine interaction during geologic carbon sequestration. Chem. Geol. 2013, 354, 1–14. [Google Scholar] [CrossRef]
- Luo, X.; Ren, X.; Wang, S. Supercritical CO2-water-shale Interactions under Supercritical CO2 Stimulation Conditions. Energy Procedia 2018, 144, 182–185. [Google Scholar] [CrossRef]
- Armitage, P.J.; Faulkner, D.R.; Worden, R.H. Caprock corrosion. Nat. Geosci. 2013, 6, 79. [Google Scholar] [CrossRef]
- Sanguinito, S.; Goodman, A.; Tkach, M.; Kutchko, B.; Culp, J.; Natesakhawat, S.; Fazio, J.; Fukai, I.; Crandall, D. Quantifying dry supercritical CO2-induced changes of the Utica Shale. Fuel 2018, 226, 54–64. [Google Scholar] [CrossRef]
- Goodman, A.; Sanguinito, S.; Kutchko, B.; Natesakhawat, S.; Cvetic, P.; Allen, A.J. Shale pore alteration: Potential implications for hydrocarbon extraction and CO2 storage. Fuel 2020, 265, 116930. [Google Scholar] [CrossRef]
- Kutchko, B.; Sanguinito, S.; Natesakhawat, S.; Cvetic, P.; Culp, J.T.; Goodman, A. Quantifying pore scale and matrix interactions of SCCO2 with the Marcellus shale. Fuel 2020, 266, 116928. [Google Scholar] [CrossRef]
- Black, J.R.; Haese, R.R. Chlorite dissolution rates under CO2 saturated conditions from 50 to 120°C and 120 to 200bar CO2. Geochimica et Cosmochimica Acta 2014, 125, 225–240. [Google Scholar] [CrossRef]
- Choi, B.-Y.; Shinn, Y.-J.; Park, Y.-C.; Park, J.; Kwon, Y.-K.; Kim, K.-Y. Simulation of CO2 injection in a small-scale pilot site in the Pohang Basin, Korea: Effect of dissolution rate of chlorite on mineral trapping. Int. J. Greenh. Gas Control 2017, 59, 1–12. [Google Scholar] [CrossRef]
- Fu, Q.; Lu, P.; Konishi, H.; Dilmore, R.; Xu, H.; Seyfried, W.E.; Zhu, C. Coupled alkali-feldspar dissolution and secondary mineral precipitation in batch systems: 1. New experiments at 200 °C and 300 bars. Chem. Geol. 2009, 258, 125–135. [Google Scholar] [CrossRef]
- Gaus, I.; Azaroual, M.; Czernichowski-Lauriol, I. Reactive transport modelling of the impact of CO2 injection on the clayey cap rock at Sleipner (North Sea). Chem. Geol. 2005, 217, 319–337. [Google Scholar] [CrossRef]
- Knauss, K.G.; Wolery, T.J. The dissolution kinetics of quartz as a function of pH and time at 70°C. Geochimica et Cosmochimica Acta 1988, 52, 43–53. [Google Scholar] [CrossRef]
- Knauss, K.G.; Johnson, J.W.; Steefel, C.I. Evaluation of the impact of CO2, co-contaminant gas, aqueous fluid and reservoir rock interactions on the geologic sequestration of CO2. Chem. Geol. 2005, 217, 339–350. [Google Scholar] [CrossRef]
- Kampman, N.; Busch, A.; Bertier, P.; Snippe, J.; Hangx, S.; Pipich, V.; Di, Z.; Rother, G.; Harrington, J.F.; Evans, J.P.; et al. Observational evidence confirms modelling of the long-term integrity of CO2-reservoir caprocks. Nat. Commun. 2016, 7, 12268. [Google Scholar] [CrossRef]
- Tian, H.; Xu, T.; Wang, F.; Patil, V.; Sun, Y.; Yue, G. A Numerical Study of Mineral Alteration and Self-Sealing Efficiency of a Caprock for CO2 Geological Storage. Acta Geotech 2014, 9, 87–100. [Google Scholar] [CrossRef]
- Pan, Y.; Hui, D.; Luo, P.Y.; Zhang, Y.; Zhang, L.; Sun, L. Influences of subcritical and supercritical CO2 treatment on the pore structure characteristics of marine and terrestrial shales. J. CO2 Util. 2018, 28, 152–167. [Google Scholar] [CrossRef]
- Gaus, I. Role and impact of CO2–rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Mouzakis, K.M.; Navarre-Sitchler, A.K.; Rother, G.; Banuelos, J.L.; Wang, X.Y.; Kaszuba, J.P.; Heath, J.E.; Miller, Q.R.S.; Alvarado, V.; McCray, J.E. Experimental Study of Porosity Changes in Shale Caprocks Exposed to CO2-Saturated Brines I: Evolution of Mineralogy, Pore Connectivity, Pore Size Distribution, and Surface Area. Environ. Eng. Sci. 2016, 33, 725–735. [Google Scholar] [CrossRef]
- Niu, Q.H.; Cao, L.W.; Sang, S.X.; Zhou, X.Z.; Liu, S.Q. Experimental study of permeability changes and its influencing factors with CO2 injection in coal. J. Nat. Gas Sci. Eng. 2019, 61, 215–225. [Google Scholar] [CrossRef]
- Rezaee, R.; Saeedi, A.; Iglauer, S.; Evans, B. Shale alteration after exposure to supercritical CO2. Int. J. Greenh. Gas Control 2017, 62, 91–99. [Google Scholar] [CrossRef]
- Wu, W.; Zoback, M.D.; Kohli, A.H. The impacts of effective stress and CO2 sorption on the matrix permeability of shale reservoir rocks. Fuel 2017, 203, 179–186. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, J.P.; Jiang, Y.D.; Xian, X.F.; Liu, Q.L. Physical and structural changes in shale associated with supercritical CO2 exposure. Fuel 2016, 184, 289–303. [Google Scholar] [CrossRef]
- Zou, Y.S.; Li, S.H.; Ma, X.F.; Zhang, S.C.; Li, N.; Chen, M. Effects of CO2-brine-rock interaction on porosity/permeability and mechanical properties during supercritical-CO2 fracturing in shale reservoirs. Int. J. Greenh. Gas Control 2018, 49, 157–168. [Google Scholar]
- Pearce, J.K.; Dawson, G.K.W.; Blach, T.P.; Bahadur, J.; Melnichenko, Y.B.; Golding, S.D. Impure CO2 reaction of feldspar, clay, and organic matter rich cap-rocks: Decreases in the fraction of accessible mesopores measured by SANS. Int. J. Coal Geol. 2018, 185, 79–90. [Google Scholar] [CrossRef]
- Li, X.; Elsworth, D. Geomechanics of CO2 enhanced shale gas recovery. J. Nat. Gas Sci. Eng. 2015, 26, 1607–1619. [Google Scholar] [CrossRef]
- Liu, F.; Lu, P.; Griffith, C.; Hedges, S.W.; Soong, Y.; Hellevang, H.; Zhu, C. CO2-brine-caprock interaction: Reactivity experiments on Eau Claire shale and a review of relevant literature. Int. J. Greenh. Gas Control 2012, 7, 153–167. [Google Scholar] [CrossRef]
- Okamoto, I.; Li, X.C.; Ohsumi, T. Effect of supercritical CO2 as the organic solvent on cap rock sealing performance for underground storage. Energy 2005, 30, 2344–2351. [Google Scholar] [CrossRef]
- Jeon, P.R.; Choi, J.; Yun, T.S.; Lee, C.-H. Sorption equilibrium and kinetics of CO2 on clay minerals from subcritical to supercritical conditions: CO2 sequestration at nanoscale interfaces. Chem. Eng. J. 2014, 255, 705–715. [Google Scholar] [CrossRef]
- De Jong, S.M.; Spiers, C.J.; Busch, A. Development of swelling strain in smectite clays through exposure to carbon dioxide. Int. J. Greenh. Gas Control 2014, 24, 149–161. [Google Scholar] [CrossRef]
- Schaef, H.T.; Ilton, E.S.; Qafoku, O.; Martin, P.F.; Felmy, A.R.; Rosso, K.M. In situ XRD study of Ca2+ saturated montmorillonite (STX-1) exposed to anhydrous and wet supercritical carbon dioxide. Int. J. Greenh. Gas Control 2012, 6, 220–229. [Google Scholar] [CrossRef]
- Rohmer, J.; Pluymakers, A.; Renard, F. Mechano-chemical interactions in sedimentary rocks in the context of CO2 storage: Weak acid, weak effects? Earth Sci. Rev. 2016, 157, 25. [Google Scholar] [CrossRef]
- Heath, J.E.; Dewers, T.A.; McPherson, B.J.O.L.; Petrusak, R.; Chidsey, T.C.; Rinehart, A.J.; Mozley, P.S. Pore networks in continental and marine mudstones: Characteristics and controls on sealing behavior. Geosphere 2011, 7, 429–454. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, S.; Bahadur, J.; Elsworth, D.; Wang, Y.; Hu, G.; Liang, Y. Changes in pore structure of coal caused by coal-to-gas bioconversion. Sci. Rep. 2017, 7, 3840. [Google Scholar] [CrossRef]
- Bakhshian, S.; Hosseini, S.A. Prediction of CO2 adsorption-induced deformation in shale nanopores. Fuel 2019, 241, 767–776. [Google Scholar] [CrossRef]
- Bakhshian, S.; Shi, Z.; Sahimi, M.; Tsotsis, T.T.; Jessen, K. Image-based modeling of gas adsorption and deformation in porous media. Sci. Rep. 2018, 8, 8249. [Google Scholar] [CrossRef]
- Ghassemzadeh, J.; Xu, L.F.; Tsotsis, T.T.; Sahimi, M. Statistical mechanics and molecular simulation of adsorption in microporous materials: Pillared clays and carbon molecular sieve membranes. J. Phys. Chem. B 2000, 104, 3892–3905. [Google Scholar] [CrossRef]
- Zhang, J.; Clennell, M.B.; Liu, K.; Pervukhina, M.; Chen, G.; Dewhurst, D.N. Methane and Carbon Dioxide Adsorption on Illite. Energy Fuels 2016, 30, 10643–10652. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Cui, X.J.; Bustin, R.M.; Chikatamarla, L. Adsorption-induced coal swelling and stress: Implications for methane production and acid gas sequestration into coal seams. J. Geophys. Res. Solid Earth 2007, 112. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Viete, D.R. Effects of gaseous and super-critical carbon dioxide saturation on the mechanical properties of bituminous coal from the Southern Sydney Basin. Appl. Energy 2013, 110, 73–81. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Choi, S.K.; Airey, D. The effects of sub-critical and super-critical carbon dioxide adsorption-induced coal matrix swelling on the permeability of naturally fractured black coal. Energy 2011, 36, 6442–6450. [Google Scholar] [CrossRef]
- Lyu, Q.; Long, X.P.; Ranjith, P.G.; Kang, Y. Unconventional Gas: Experimental Study of the Influence of Subcritical Carbon Dioxide on the Mechanical Properties of Black Shale. Energies 2016, 9, 516. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, J.P.; Xian, X.F.; Jiang, Y.D.; Lu, Z.H.; Tan, J.Q.; Liu, G.J. Experimental study of the effects of sub- and super-critical CO2 saturation on the mechanical characteristics of organic-rich shales. Energy 2017, 132, 84–95. [Google Scholar] [CrossRef]
- Zhang, S.; Xian, X.; Zhou, J.; Zhang, L. Mechanical behaviour of Longmaxi black shale saturated with different fluids: An experimental study. RSC Adv. 2017, 7, 10. [Google Scholar] [CrossRef]
- Lyu, Q.; Long, X.P.; Ranjith, P.G.; Tan, J.Q.; Kang, Y.; Wang, Z.H. Experimental investigation on the mechanical properties of a low-clay shale with different adsorption times in sub-/super-critical CO2. Energy 2018, 147, 1288–1298. [Google Scholar] [CrossRef]
- Tang, J.R.; Lu, Y.Y.; Chen, Y.T.; Zhang, X.W.; Ao, X.; Jia, Y.Z.; Li, Q. Experimental study of damage of shale mechanical properties under supercritical CO2. Rock Soil. Mech. 2018, 39, 797–802. [Google Scholar]
- Ao, X.; Lu, Y.Y.; Tang, J.R.; Chen, Y.T.; Li, H.L. Investigation on the physics structure and chemical properties of the shale treated by supercritical CO2. J. CO2 Util. 2017, 20, 274–281. [Google Scholar] [CrossRef]
- Al-Ameri, W.A.; Abdulraheem, A.; Mahmoud, M. Long-Term Effects of CO2 Sequestration on Rock Mechanical Properties. J. Energy Resour. Technol. 2016, 138. [Google Scholar] [CrossRef]
- Choi, C.-S.; Song, J.-J. Swelling and mechanical property change of shale and sandstone in supercritical CO2. In Proceedings of the 7th Asian Rock Mechanics Symposium, Seoul, Korea, 15–19 October 2012. [Google Scholar]
- Lyu, Q.; Long, X.P.; Ranjith, P.G.; Tan, J.Q.; Kang, Y.; Luo, W.B. A Damage Constitutive Model for the Effects of CO2-Brine-Rock Interactions on the Brittleness of a Low-Clay Shale. Geofluids 2018, 2018, 7321961. [Google Scholar] [CrossRef]
- Agofack, N.; Lozovyi, S.; Bauer, A. Effect of CO2 on P- and S-wave velocities at seismic and ultrasonic frequencies. Int. J. Greenh. Gas Control 2018, 78, 12. [Google Scholar] [CrossRef]
- Miedzinska, D.; Lutynski, M. CO2-CH4 sorption induced swelling of gas shales: An experimental study on the Silurian shales from the Baltic Basin, Poland. Physicochem. Probl. Miner. Process. 2018, 54, 415–427. [Google Scholar]
- Haghi, R.K.; Chapoy, A.; Peirera, L.M.C.; Yang, J.H.; Tohidi, B. pH of CO2 saturated water and CO2 saturated brines: Experimental measurements and modelling. Int. J. Greenh. Gas Control 2017, 66, 190–203. [Google Scholar] [CrossRef]
- Izgec, O.; Demiral, B.; Bertin, H.; Akin, S. CO2 injection into saline carbonate aquifer formations II: Comparison of numerical simulations to experiments. Transp. Porous Media 2008, 73, 57–74. [Google Scholar] [CrossRef]
- Wong, R.C.K. Swelling and softening behaviour of La Biche shale. Can. Geotech. J. 1998, 35, 206–221. [Google Scholar] [CrossRef]
- Chenevert, M.E. Shale Alteration by Water Adsorption. J. Pet. Technol. 1970, 22, 1–41. [Google Scholar] [CrossRef]
- Hucka, V.; Das, B. Brittleness Determination of Rocks by Different Methods. Int. J. Rock Mech. Min. Sci. 1974, 11, 389–392. [Google Scholar] [CrossRef]
- Ranathunga, A.S.; Perera, M.S.A.; Ranjith, P.G. Influence of CO2 adsorption on the strength and elastic modulus of low rank Australian coal under confining pressure. Int. J. Coal Geol. 2016, 167, 9. [Google Scholar] [CrossRef]
- Ojala, I.O. The effect of CO2 on the mechanical properties of reservoir and cap rock. Energy Procedia 2011, 4, 5392–5397. [Google Scholar] [CrossRef]
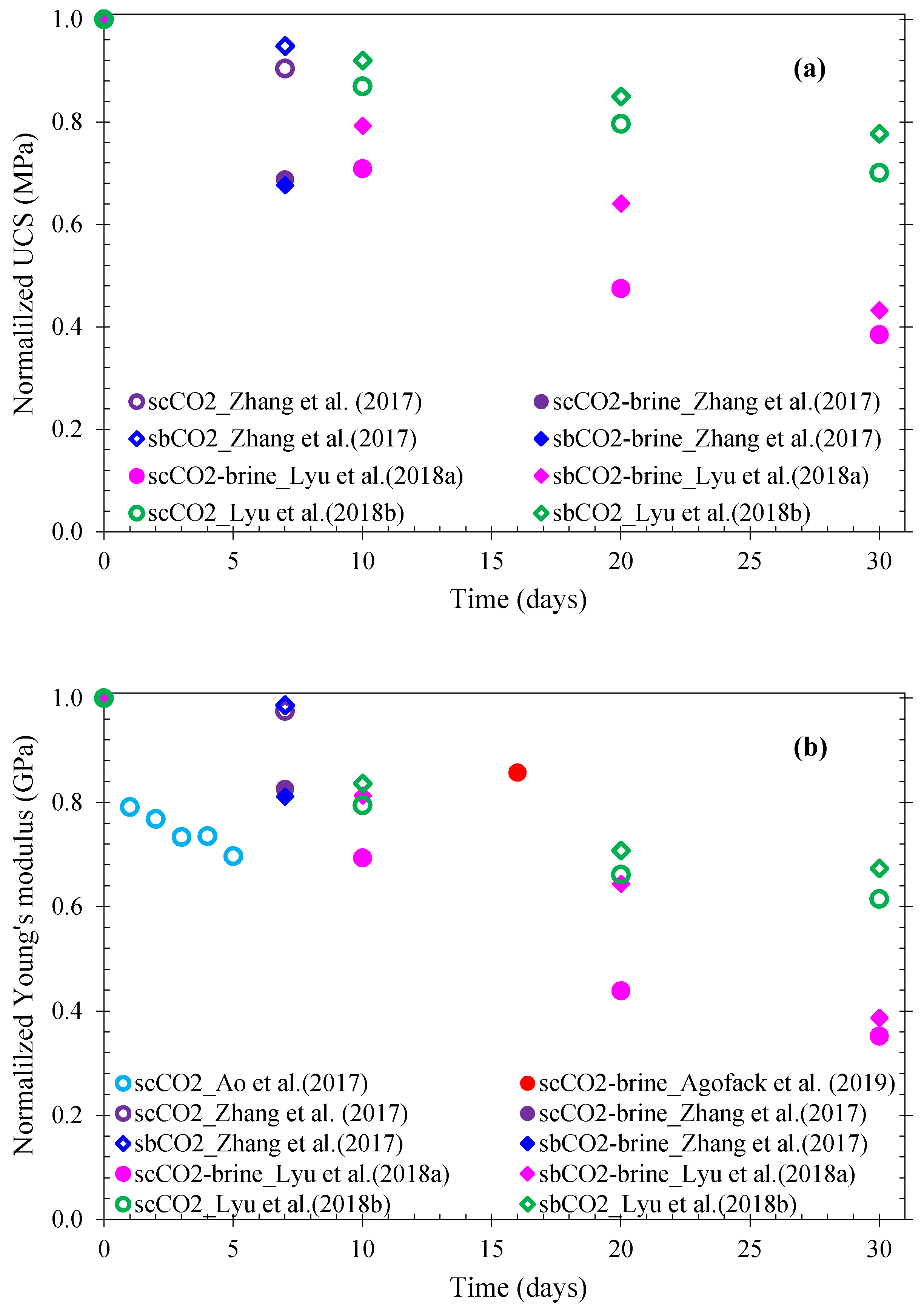
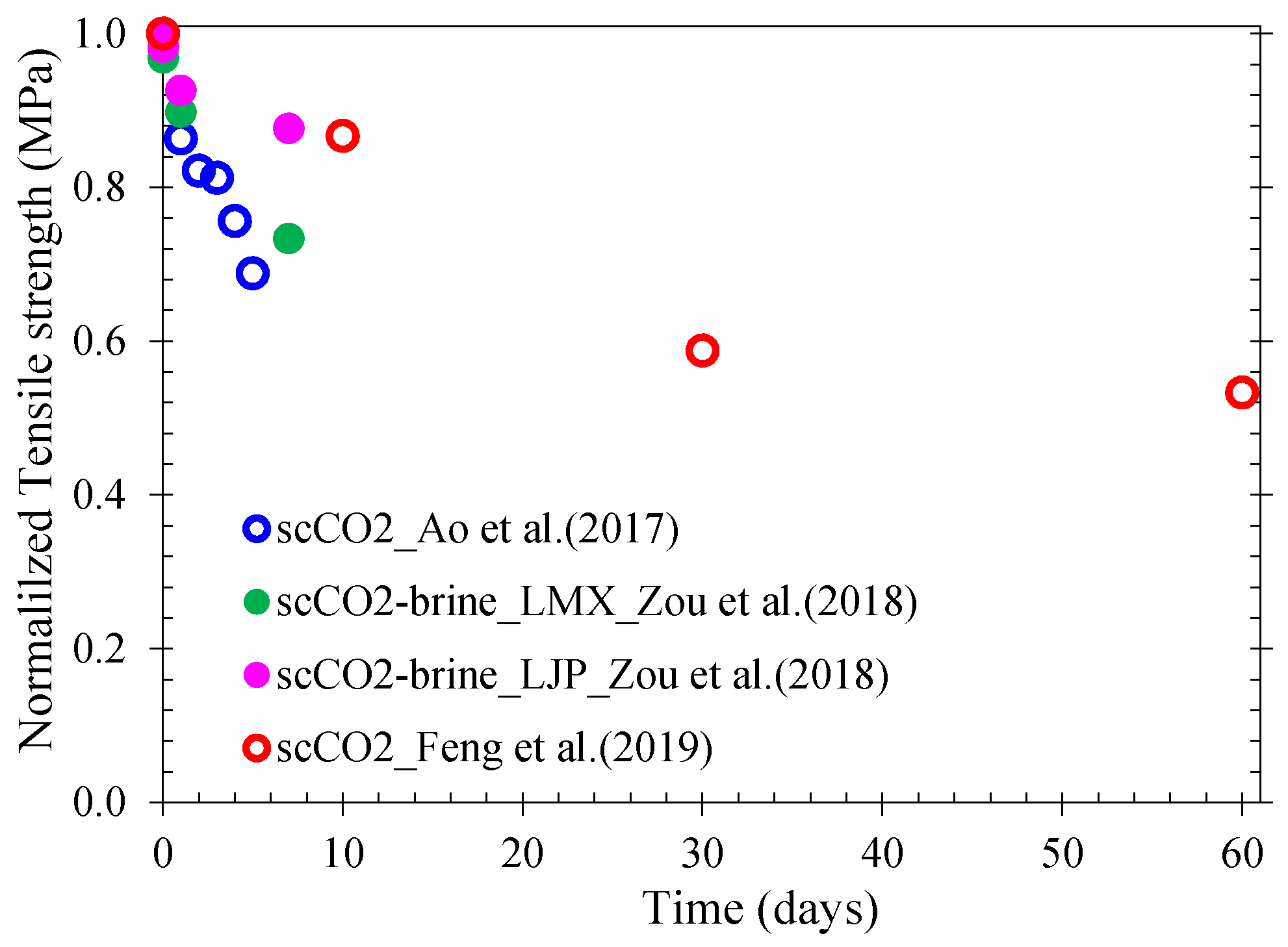
| Reference | Shale | Sample Size (mm) and Bedding | Exposure Medium | Test Procedures | Exposure Temperature, Pressure, and Duration | Measured Parameters |
|---|---|---|---|---|---|---|
| Choi et al. [173] | - | D:38; L:70 | Water, brine, CO2, CO2–water, CO2–brine |
| 80–100 °C, 10 MPa For 14 days | Swelling, UCS (unconfined compressive strength); Acoustic Emission (AE) for stress thresholds (ST) for crack initiation and crack damage; ultrasonic P-wave velocity |
| Al-Ameri et al. [172] | Overburden shale samples (no indication of the origin and its composition) | D:37; L:53 | scCO2 |
| 100 °C; 13.8 MPa For 30 days | Dynamic Young modulus. |
| Lyu et al. [166] | Outcrop shale (from Sichuan basin, China), clay fraction: 40%, TOC: 3.35%, initial water content: 15% | D:38; L:78 | CO2 |
| 22 °C; 40 °C; 7 MPa For 0, 10, and 20 days | UCS, Young modulus; Brittleness index; AE |
| Lyu et al. [72,169,174] | D:30; L:60 | Dry gCO2–water scCO2–water, sbCO2–brine, scCO2–brine | Procedure not clearly stated, but seems to be similar to that of Lyu et al. [166] | 40 °C; 7 MPa; 9 MPa For 10, 20, and 30 days. | ||
| Zhang et al. [168] | Outcrop shale (from Sichuan basin, China), TOC: 7.88%, more than 70% of quartz, dolomite, calcite, 8.1% clay. | D:50 L:100 | dry, water, brine, CO2–brine, |
| 40 °C 6, 12 MPa For 7 days. | |
| Yin et al. [167] | Organic-rich shale (from Sichuan basin, China) | D:50 L:100 ┴ to bedding | gCO2-water scCO2-water |
| 38 °C 4, 6, 8, 12, and 16 MPa For 10 days | UCS; Young’s modulus, AE |
| Ao et al. [171] | Similar to that of Zhang et al. [168] | D:50 L:100 and D:50 L:25 for tensile strength test. ┴ to bedding | scCO2 |
| 35 °C 15 MPa For 1, 2, 3, 4 and 5 days. | Triaxial compressive strength, elastic modulus, tensile strength measurement on treated and untreated samples |
| Zou et al. [147] | Lujiaping (LJP) and the Longmaxi (LMX) shales of Sichuan Basin | D:25 L:8 | CO2–brine (pH = 3.02–3.34) |
| 40–120 °C 10, 20, and 30 MPa For 0, 1, and 7 days | Tensile strength, Porosity, Permeability, Frictional coefficient. |
| Feng et al. [24] | Outcrop shale (from Sichuan basin, China), TOC: 3.8%, moisture content: 1.8% | D:50 L:25 Different bedding orientations | scCO2 |
| 40 °C; 10 MPa For 10, 30, and 60 days. | Tensile strength as a function of saturation time and of bedding orientation |
| Agofack et al. [70] | Draupne shale (from Draupne formation, Norway) with more than 60% clay Porosity: 12% TOC: 6–8% Water content: 6.9% | D:15 L:30 ┴ to bedding | Brine scCO2–brine |
| 40 °C; 10 MPa For 12 days. | Undrained bulk modulus, Young modulus, Poisson’s ratio, Skempton coefficient, maximum deviatoric stress, ultrasonic P-wave velocity continuously measured |
| Fluid | Temp. (°C) | Pressure (MPa) | Exposure Time (Days) | Peak Strength (MPa) | Crack Initiation (MPa) | Crack Damage (MPa) | Reference |
|---|---|---|---|---|---|---|---|
| CO2 | 40 | 7 | 10 | 40.41 | 12.77 | 26.52 | Lyu et al. [72] |
| 20 | 31.33 | 15.76 | 21.93 | ||||
| 30 | 25.63 | 19.79 | 22.09 | ||||
| 9 | 10 | 39.05 | 18.86 | 31.57 | |||
| 20 | 30.71 | 16.40 | 26.48 | ||||
| 30 | 19.99 | 14.29 | 19.13 | ||||
| CO2-water | 38 | 4 | 10 | 184.23 | 104.00 | 167.38 | Yin et al. [167] |
| 6 | 141.19 | 101.40 | 148.96 | ||||
| 8 | 134.69 | 93.80 | 133.82 | ||||
| 12 | 132.50 | 64.33 | 128.62 | ||||
| 16 | 161.39 | 124.24 | 154.50 | ||||
| CO2 | 40 | 6 | 7 | 209.63 | 151.59 | 176.78 | Zhang et al. [168] |
| 12 | 197.99 | 152.92 | 175.13 | ||||
| CO2–brine | 40 | 6 | 7 | 147.68 | 51.88 | 90.85 | |
| 12 | 150.64 | 74.95 | 132.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuiyan, M.H.; Agofack, N.; Gawel, K.M.; Cerasi, P.R. Micro- and Macroscale Consequences of Interactions between CO2 and Shale Rocks. Energies 2020, 13, 1167. https://doi.org/10.3390/en13051167
Bhuiyan MH, Agofack N, Gawel KM, Cerasi PR. Micro- and Macroscale Consequences of Interactions between CO2 and Shale Rocks. Energies. 2020; 13(5):1167. https://doi.org/10.3390/en13051167
Chicago/Turabian StyleBhuiyan, Mohammad H., Nicolaine Agofack, Kamila M. Gawel, and Pierre R. Cerasi. 2020. "Micro- and Macroscale Consequences of Interactions between CO2 and Shale Rocks" Energies 13, no. 5: 1167. https://doi.org/10.3390/en13051167
APA StyleBhuiyan, M. H., Agofack, N., Gawel, K. M., & Cerasi, P. R. (2020). Micro- and Macroscale Consequences of Interactions between CO2 and Shale Rocks. Energies, 13(5), 1167. https://doi.org/10.3390/en13051167





