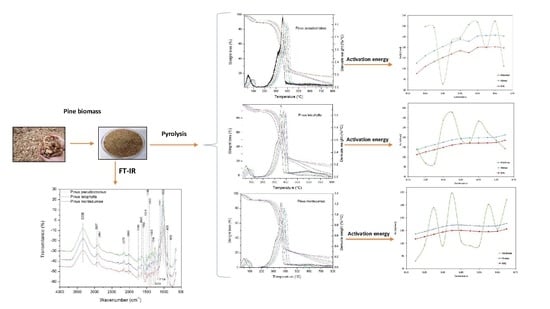
Thermal Degradation Kinetics and FT-IR Analysis on the Pyrolysis of Pinus pseudostrobus, Pinus leiophylla and Pinus montezumae as Forest Waste in Western Mexico
Abstract
1. Introduction
2. Methods
2.1. Sample Preparation and Chemical Composition Analysis
2.2. Thermogravimetric Analysis TGA-DTG
2.3. Kinetic Modeling
2.3.1. Friedman Method
2.3.2. Flynn-Wall-Ozawa Method (FWO)
2.3.3. Kissinger-Akahira-Sunose Method (KAS)
2.3.4. Frequency Factor (Z)
2.4. Fourier Transform Infrared Analysis (FT-IR)
3. Results and Discussion
3.1. Chemical Analysis
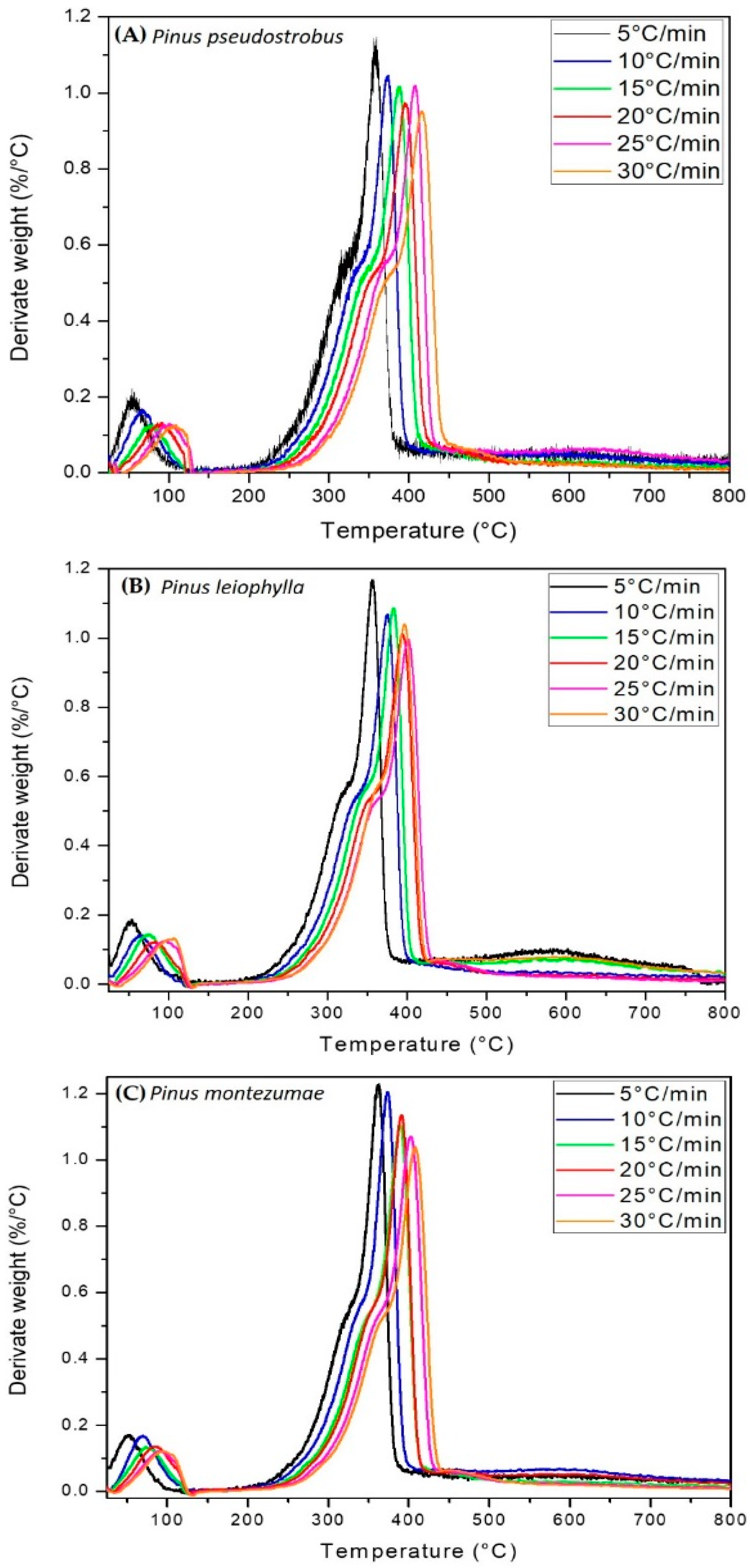
3.2. Thermogravimetric Analysis
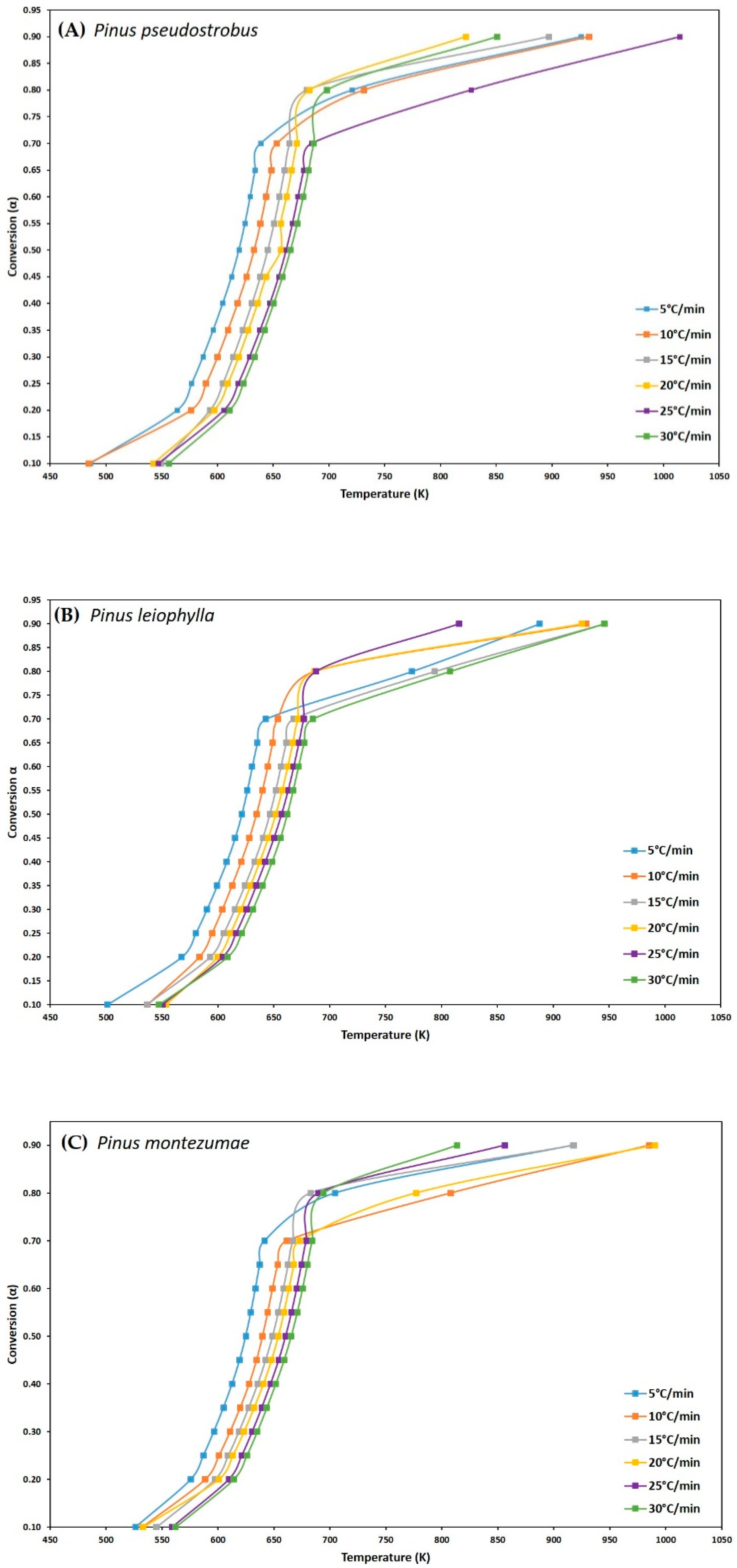
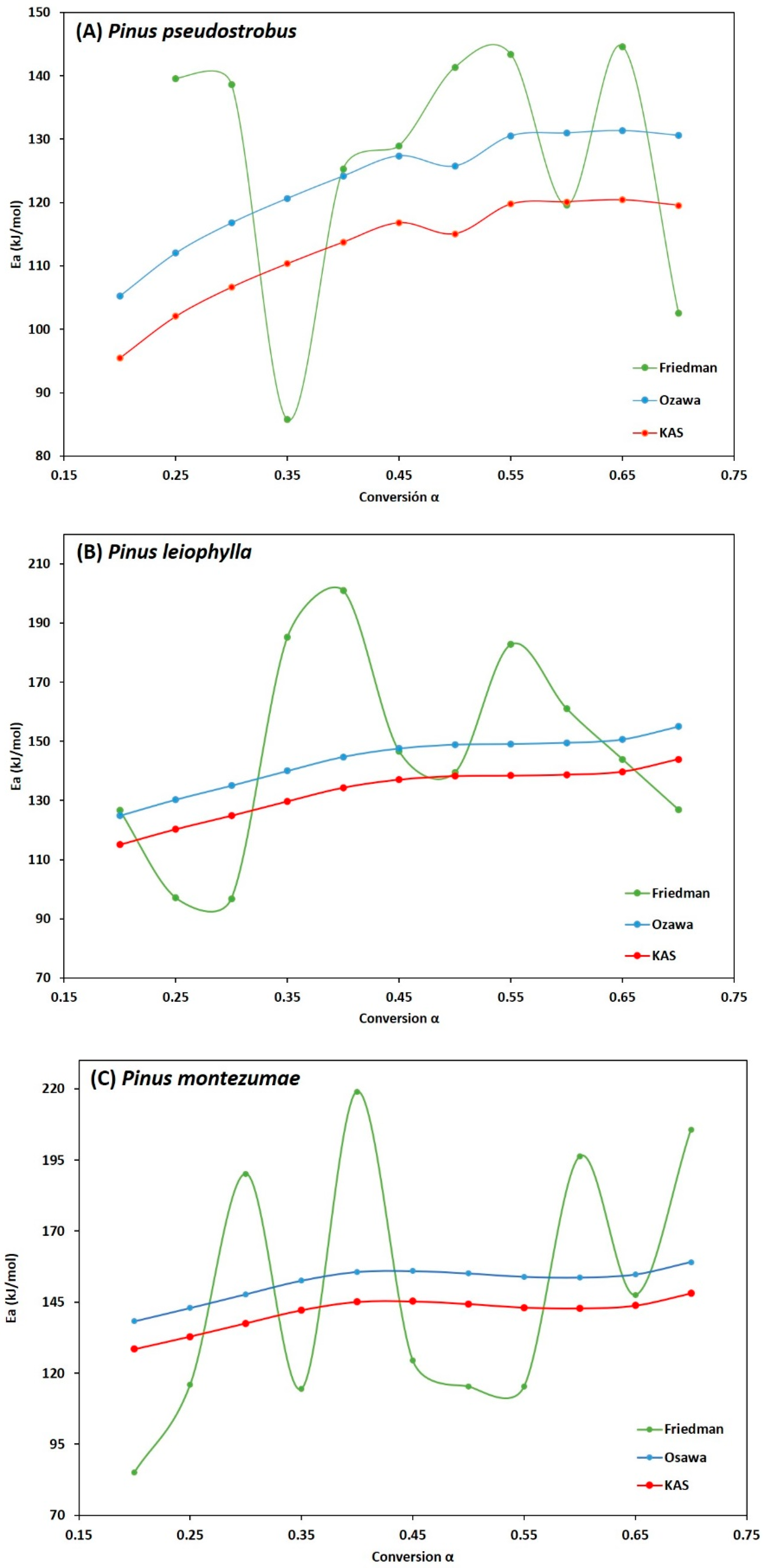
3.3. Kinetic Analysis
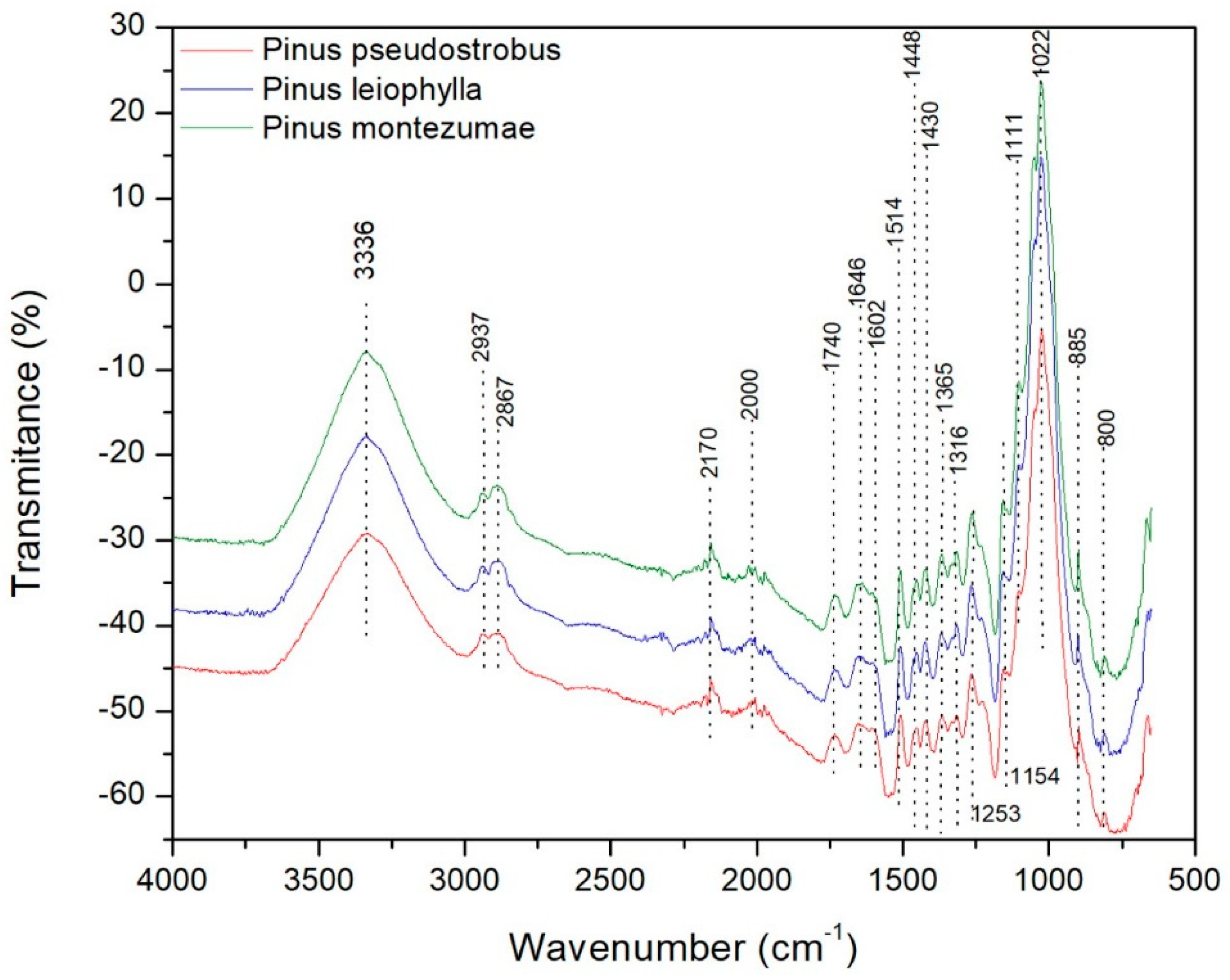
3.4. Fourier Transform Infrared Analysis (FT-IR)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manzano, F.; Alcayde, A.; Montoya, F.; Zapata, A.; Gil, C. Scientific production of renewable energies worldwide: An overview. Renew. Sustain. Energy Rev. 2013, 18, 134–143. [Google Scholar] [CrossRef]
- Varma, A.; Thakur, L.; Shankar, R.; Mondal, P. Pyrolysis of wood sawdust: Effects of process parameters on products yield and characterization of products. Waste Manag. 2019, 89, 224–235. [Google Scholar] [CrossRef]
- Saldarriaga, J.; Aguado, R.; Pablos, A.; Amutio, M.; Olazar, M.; Bilbao, J. Fast characterization of biomass fuels by thermogravimetric analysis (TGA). Fuel 2015, 140, 744–751. [Google Scholar] [CrossRef]
- Soto, N.; Machado, W.; López, D. Determinación de los parámetros cinéticos en la pirólisis del pino ciprés. Quim. Nova 2010, 33, 1500–1505. [Google Scholar] [CrossRef][Green Version]
- Davis, S.; Diegel, S.; Boundy, R. Transportation Energy Data Book; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2009; No. ORNL-6984.
- Bridgwater, A. The production of biofuels and renewable chemicals by fast pyrolysis of biomass. Int. J. Glob. Energy 2007, 27, 160–203. [Google Scholar] [CrossRef]
- Ragauskas, A.; Williams, C.; Davison, B.; Britovsek, G.; Cairney, J.; Eckert, C.; Frederick, W.; Hallett, J.; Leak, D.; Liotta, C.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Frombo, F.; Minciardi, R.; Robba, M.; Rosso, F.; Sacile, R. Planning woody biomass logistics for energy production: A strategic decision model. Biomass Bioenergy 2009, 33, 372–383. [Google Scholar] [CrossRef]
- Mullaney, H.; Farag, I.; La Claire, C.; Barrett, C. Technical, Environmental and Economic Feasibility of Bio-Oil in New Hampshire’s North Country; New Hampshire Industrial Research Center: Durham, NH, USA, 2002. [Google Scholar]
- Da Silva, J.; Alves, J.; de Araujo, W.; Andersen, S.; de Sena, R. Pyrolysis kinetic evaluation by single-step for waste wood from reforestation. Waste Manag. 2018, 72, 265–273. [Google Scholar] [CrossRef]
- Janković, B. The pyrolysis process of wood biomass samples under isothermal experimental conditions-energy density considerations: Application of the distributed apparent activation energy model with a mixture of distribution functions. Cellulose 2014, 21, 2285–2314. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.; Steele, P. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Soltes, E.; Elder, T. Pyrolysis in Organic Chemicals from Biomass. IS Goldstein; CRC Press: Florida, FL, USA, 1981. [Google Scholar]
- Branca, C.; Lannace, A.; Di Blasi, C. Devolatilization and Combustion Kinetics of Quercus cerris Bark. Energy Fuels 2007, 21, 1078–1084. [Google Scholar] [CrossRef]
- D’ Almeida, A.; Barreto, D.; Calado, V.; d’ Almeida, J. Thermal analysis of less common lignocellulose fibers. J. Therm. Anal. Calorim. 2008, 91, 405–408. [Google Scholar] [CrossRef]
- Guerrero, M.; da Silva, M.; Zaragoza, M.; Gutiérrez, J.; Velderrain, V.; Ortiz, A.; Collins, V. Thermogravimetric study on the pyrolysis kinetics of apple pomace as waste biomass. Int. J. Hydrogen. Energy 2014, 39, 16619–16627. [Google Scholar] [CrossRef]
- Hill, J. For Better Thermal Analysis and Calorimetry; International Confederation for Thermal Analysis, 1991. [Google Scholar]
- Giuntoli, J.; De Jong, W.; Arvelakis, S.; Spliethoff, H.; Verkooijen, A. Quantitative and kinetic TG-FTIR study of biomass residue pyrolysis: Dry distiller’s grains with solubles (DDGS) and chicken manure. J. Anal. Appl. Pyrol. 2009, 85, 301–312. [Google Scholar] [CrossRef]
- Zhu, H.; Yan, J.; Jiang, X.; Lai, Y.; Cen, K. Study on pyrolysis of typical medical waste materials by using TG-FTIR analysis. J. Hazard. Mater. 2008, 153, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Koreňová, Z.; Juma, M.; Annus, J.; Markoš, J. Kinetics of pyrolysis and properties of carbon black from a scrap tire. Chem. Pap. 2006, 60, 422–426. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Liu, Z.; Ma, Q.; Zhao, C.; Ma, C. Kinetic study of the pyrolysis of waste printed circuit boards subject to conventional and microwave heating. Energies 2012, 5, 3295–3306. [Google Scholar] [CrossRef]
- White, J.; Catallo, W.; Legendre, B. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrol. 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Singh, S.; Wu, C.; Williams, P. Pyrolysis of waste materials using TGA-MS and TGA-FTIR as complementary characterisation techniques. J. Anal. App. Pyrol. 2012, 94, 99–107. [Google Scholar] [CrossRef]
- Cao, R.; Naya, S.; Artiaga, R.; García, A.; Varela, A. Logistic approach to polymer degradation in dynamic TGA. Polym. Degrad. Stab. 2004, 85, 667–674. [Google Scholar] [CrossRef]
- Brown, M. Steps in a minefield: Some kinetic aspects of thermal analysis. J. Therm. Anal. Calorim. 1997, 49, 17–32. [Google Scholar] [CrossRef]
- Brown, M.; Maciejewski, M.; Vyazovkin, S.; Nomen, R.; Sempere, J.; Burnham, A.; Opfermann, J.; Strey, R.; Anderson, H.; Kemmler, A.; et al. Computational aspects of kinetic analysis: Part A: The ICTAC kinetics project-data, methods and results. Thermochim. Acta 2000, 355, 125–143. [Google Scholar] [CrossRef]
- Friedman, H. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Pol. Sym. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. B. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Kissinger, H. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Akahira, T. Trans. Joint convention of four electrical institutes. Res. Rep. Chiba Inst. Technol. 1971, 16, 22–31. [Google Scholar]
- Pintor, L.; Carrillo, A.; Herrera, R.; López, P.; Rutiaga, J. Physical and chemical properties of timber byproducts from Pinus leiophylla, P. montezumae and P. pseudostrobus for a bioenergetic use. Wood Res. 2017, 62, 849–862. [Google Scholar]
- Téllez, C.; Ochoa, H.; Sanjuan, R.; Rutiaga, J. Componentes químicos del duramen de Andira inermis (W. Wright) DC. (Leguminosae). Rev. Chapingo Ser. Cienc. For. Ambient. 2010, 16, 87–93. [Google Scholar]
- UNE-EN 14775. Solid Biofuels. Method for the Ash Content Determination; CONFEMADERA, AENOR, Grupo 9: Madrid, España, 2010; 10p. (In Spanish) [Google Scholar]
- Vyazovkin, S.; Burnham, A.; Criado, J.; Pérez, L.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Damartzis, T.; Vamvuka, D.; Sfakiotakis, S.; Zabaniotou, A. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour. Technol. 2011, 102, 6230–6238. [Google Scholar] [CrossRef]
- Flynn, J. The isoconversional method for determination of energy of activation at constant heating rates: Corrections for the Doyle approximation. J. Therm. Anal. Calorim. 1983, 27, 95–102. [Google Scholar] [CrossRef]
- Sadhukhan, A.; Gupta, P.; Saha, R. Modelling of pyrolysis of large wood particles. Bioresour. Technol. 2009, 100, 3134–3139. [Google Scholar] [CrossRef] [PubMed]
- Min, F.; Zhang, M.; Chen, Q. Non-isothermal kinetics of pyrolysis of three kinds of fresh biomass. J. China Univ. Min. Technol. 2007, 17, 105–111. [Google Scholar] [CrossRef]
- Capart, R.; Khezami, L.; Burnham, A. Assessment of various kinetic models for the pyrolysis of a microgranular cellulose. Thermochim. Acta 2004, 417, 79–89. [Google Scholar] [CrossRef]
- Vlaev, L.; Markovska, I.; Lyubchev, L. Non-isothermal kinetics of pyrolysis of rice husk. Thermochim. Acta 2003, 406, 1–7. [Google Scholar] [CrossRef]
- Galwey, A.; Brown, M. Application of the Arrhenius equation to solid state kinetics: Can this be justified? Thermochim. Acta 2002, 386, 91–98. [Google Scholar] [CrossRef]
- Steinfeld, J.; Francisco, J.; Hase, W. Chemical Kinetics and Dynamics, 2nd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Atkins, P.W. Physical Chemistry, 5th ed.; W.H. Freeman: New York, NY, USA, 1994. [Google Scholar]
- Flynn, J. The ‘temperature integral’—Its use and abuse. Thermochim. Acta 1997, 300, 83–92. [Google Scholar] [CrossRef]
- Liu, M.; Gao, L.; Zhao, Q.; Wang, Y.; Yang, X.; Cao, S. Thermal degradation process and kinetics of poly (dodecamethyleneisophthalamide). Chem. J. Internet. 2003, 5, 43. [Google Scholar]
- Biagini, E.; Guerrini, L.; Nicolella, C. Development of a variable activation energy model for biomass devolatilization. Energy Fuels 2009, 23, 3300–3306. [Google Scholar] [CrossRef]
- Kantarelis, E.; Yang, W.; Blasiak, W.; Forsgren, C.; Zabaniotou, A. Thermochemical treatment of E-waste from small household appliances using highly pre-heated nitrogen-thermogravimetric investigation and pyrolysis kinetics. Appl. Energy 2011, 88, 922–929. [Google Scholar] [CrossRef]
- Flynn, J.; Wall, L. General treatment of the thermogravimetry of polymers. J. Res. Nat. Bur. Stand. 1966, 70, 487–523. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Chaudhri, M. Analysis of dynamic kinetic data from solid-state reactions. J. Therm. Anal. Calorim. 1980, 18, 247–261. [Google Scholar] [CrossRef]
- Doyle, C. Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1961, 5, 285–292. [Google Scholar] [CrossRef]
- Chao, M.; Li, W.; Wang, X. Influence of antioxidant on the thermal–oxidative degradation behavior and oxidation stability of synthetic ester. Thermochim. Acta 2014, 591, 16–21. [Google Scholar] [CrossRef]
- Coats, A.; Redfern, J. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Lyon, R. An integral method of nonisothermal kinetic analysis. Thermochim. Acta 1997, 297, 117–124. [Google Scholar] [CrossRef]
- Obernberger, I.; Thek, G. The Pellet Handbook, 1st ed.; Earthscan: London, UK; Washington, DC, USA, 2010. [Google Scholar]
- Van Lith, S.; Alonso, V.; Jensen, P.; Frandsen, F.; Glarborg, P. Release to the gas phase of inorganic elements during wood combustion. Part 1: Development and evaluation of quantification methods. Energy Fuels 2006, 20, 964–978. [Google Scholar] [CrossRef]
- Werkelin, J.; Lindberg, D.; Boström, D.; Skrifvars, B.; Hupa, M. Ash-forming elements in four Scandinavian wood species part 3: Combustion of five spruce samples. Biomass Bioenergy 2011, 35, 725–733. [Google Scholar] [CrossRef]
- Miles, T. Alkali Deposits Found in Biomass Power Plants; NREL Report 443-8142; Vol 1. Sand 96-8225; National Techrucd Momation Service (NTIS): Springfield, VA, USA, 1995. [Google Scholar]
- Du, Z.; Sarofim, A.; Longwell, J. Activation energy distribution in temperature-programmed desorption: Modeling and application to the soot oxygen system. Energy Fuels 1990, 4, 296–302. [Google Scholar] [CrossRef]
- Yahiaoui, M.; Hadoun, H.; Toumert, I.; Hassani, A. Determination of kinetic parameters of Phlomis bovei de Noé using thermogravimetric analysis. Bioresour. Technol. 2015, 196, 441–447. [Google Scholar] [CrossRef]
- Vamvuka, D.; Kakaras, E.; Kastanaki, E.; Grammelis, P. Pyrolysis characteristics and kinetics of biomass residuals mixtures with lignite. Fuel 2003, 82, 1949–1960. [Google Scholar] [CrossRef]
- Meesri, C.; Moghtaderi, B. Lack of synergetic effects in the pyrolytic characteristics of woody biomass/coal blends under low and high heating rate regimes. Biomass Bioenergy 2002, 23, 55–66. [Google Scholar] [CrossRef]
- Jeguirim, M.; Trouvé, G. Pyrolysis characteristics and kinetics of Arundo donax using thermogravimetric analysis. Bioresour. Technol. 2009, 100, 4026–4031. [Google Scholar] [CrossRef] [PubMed]
- Gašparovič, L.; Koreňová, Z.; Jelemenský, Ľ. Kinetic study of wood chips decomposition by TGA. Chem. Pap. 2010, 64, 174–181. [Google Scholar] [CrossRef]
- Quan, C.; Li, A.; Gao, N. Thermogravimetric analysis and kinetic study on large particles of printed circuit board wastes. Waste Manag. 2009, 29, 2353–2360. [Google Scholar] [CrossRef]
- Kumar, A.; Wang, L.; Dzenis, Y.; Jones, D.; Hanna, M. Thermogravimetric characterization of corn stover as gasification and pyrolysis feedstock. Biomass Bioenergy 2008, 32, 460–467. [Google Scholar] [CrossRef]
- Wang, G.; Li, W.; Li, B.; Chen, H. TG study on pyrolysis of biomass and its three components under syngas. Fuel 2008, 87, 552–558. [Google Scholar] [CrossRef]
- Mishra, R.; Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour. Technol. 2018, 251, 63–74. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim. Acta 1999, 340, 53–68. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Aguado, R.; Artetxe, M.; Bilbao, J.; Olazar, M. Kinetic study of lignocellulosic biomass oxidative pyrolysis. Fuel 2012, 95, 305–311. [Google Scholar] [CrossRef]
- Domínguez, J.; Santos, T.; Rigual, V.; Oliet, M.; Alonso, M.; Rodriguez, F. Thermal stability, degradation kinetics, and molecular weight of organosolv lignins from Pinus radiata. Ind. Crop. Prod. 2018, 111, 889–898. [Google Scholar] [CrossRef]
- Vyazovkin, S. Computational aspects of kinetic analysis.: Part C. The ICTAC Kinetics Project-the light at the end of the tunnel? Thermochim. Acta 2000, 355, 155–163. [Google Scholar] [CrossRef]
- Aboyade, A.; Hugo, J.; Carrier, M.; Meyer, E.; Stahl, R.; Knoetze, J.; Görgens, J. Non-isothermal kinetic analysis of the devolatilization of corn cobs and sugar cane bagasse in an inert atmosphere. Thermochim. Acta 2011, 517, 81–89. [Google Scholar] [CrossRef]
- Gai, C.; Dong, Y.; Zhang, T. The kinetic analysis of the pyrolysis of agricultural residue under non-isothermal conditions. Bioresour. Technol. 2013, 127, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kuan, W.; Chiueh, P.; Lo, S. A sequential method to analyze the kinetics of biomass pyrolysis. Bioresour. Technol. 2011, 102, 9241–9246. [Google Scholar] [CrossRef]
- Turmanova, S.; Genieva, S.; Dimitrova, A.; Vlaev, L. Non-isothermal degradation kinetics of filled with rise husk ash polypropene composites. Express Polym. Lett. 2008, 2, 133–146. [Google Scholar] [CrossRef]
- Kondo, T. Hydrogen Bonds in Cellulose and Cellulose. Derivatives, Polysaccharides II—Structural Diversity and Functional Versatility; Dumitriu, S., Ed.; Marcel Dekker: New York, NY, USA, 2005; Chapter 3. [Google Scholar]
- Popescu, C.; Popescu, M.; Vasile, C. Structural analysis of photodegraded lime wood by means of FT-IR and 2D IR correlation spectroscopy. Int. J. Biol. Macromol. 2011, 48, 667–675. [Google Scholar] [CrossRef]
- Baeza, J.; Freer, J. Chemical characterization of wood and its components. In Wood and Cellulosic Chemistry; Hon, D.-S., Shiraishi, N., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2001; Chapter 8; pp. 275–384. [Google Scholar]
- Popescu, C.; Popescu, M.; Vasile, C. Characterization of fungal degraded lime wood by FT-IR and 2D IR correlation spectroscopy. Microchem. J. 2010, 95, 377–387. [Google Scholar] [CrossRef]
- Xu, C.; Etcheverry, T. Hydro-liquefaction of woody biomass in sub-and super-critical ethanol with iron-based catalysts. Fuel 2008, 87, 335–345. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, H.; Oh, S.; Kim, Y.; Kim, U.; Choi, J. Investigation of structural modification and thermal characteristics of lignin after heat treatment. Int. J. Biol. Macromol. 2014, 66, 57–65. [Google Scholar] [CrossRef]
- Popescu, M.; Froidevaux, J.; Navi, P.; Popescu, C. Structural modifications of Tilia cordata wood during heat treatment investigated by FT-IR and 2D IR correlation spectroscopy. J. Mol. Struct. 2013, 1033, 176–186. [Google Scholar] [CrossRef]




| Property | Technique | Parameter Measured | Acronym |
|---|---|---|---|
| Mass. | Thermogravimetric analysis. | Sample mass. | TGA |
| Derivative thermogravimetry. | First derivative of mass. | DTG | |
| Temperature. | Differential thermal analysis. | Temperature difference between sample and inert reference material. | DTA |
| Derivative differential thermal analysis. | First derivative of DTA curve. | ||
| Heat. | Differential scanning calorimetry. | Heat supplied to sample or reference. | DSC |
| Pressure. | Thermomanometry. | Pressure. | |
| Dimensions. | Thermodilatometry. | Coefficient of linear or volumetric expansion. | |
| Mechanical properties. | Thermomechanical analysis. | TMA | |
| Electrical properties. | Thermoelectrical analysis. | Electrical resistance. | TEA |
| Magnetic properties. | Thermomagnetic analysis. | ||
| Acoustic properties. | Thermoacoustic analysis. | Acoustic waves. | TAA |
| Optical properties. | Thermoptical analysis. | TOA |
| Symbol | Mechanism | g(α) | f(α) |
|---|---|---|---|
| D1 | Diffusion One-way transport | α2 | 1/2α |
| D2 | Two-way transport | α + (1 − α)ln(1 − α) | [− ln(1 − α)]−1 |
| D3 | Three-way transport | [1 − (1 − α)1/3]2 | (3/2)(1 − α)2/3[1 − (1 − α)1/3]−1 |
| G-B | Ginstling-Brounshtein equation | 1 − (2/3)α − (1 − α)2/3 | (3/2)[(1 − α) − 1/3 − 1]−1 |
| Zh | Zhuravlev equation | [(1 − α)−1/3 − 1]2 | (3/2)(1 − α)4/3[(1 − α)−1/3 − 1]−1 |
| A2 | Random nucleation and nuclei growth Bi-dimensional | [−ln(1 − α)]1/2 | 2(1 − α)[−ln(1 − α)]1/2 |
| A3 | Tree-dimensional | [−ln(1 − α)]1/3 | 3(1 − α)[−ln(1 − α)]2/3 |
| P-T1 | Prout-Tompkins (m = 0.5) | ln[(1 + α1/2)/(1 − α1/2)] | (1 − α) α1/2 |
| P-T2 | Prout-Tompkins (m = 1) | ln(α/(1 − α)] | (1 − α) α |
| F1 | Chemical reaction First-order | −ln(1 − α) | 1 − α |
| F2 | Second-order | (1 − α)−1 − 1 | (1 − α)2 |
| R1 | Limiting surface reaction between both phase One dimension. | α | 1 |
| R2 | Two dimension | 1 − (1 − α)1/2 | 2(1 − α)1/2 |
| R3 | Three dimension | 1 − (1 − α)1/3 | 3(1 − α)2/3 |
| Parameter | P. pseudostrobus | P. leiophylla | P. montezumae |
|---|---|---|---|
| Ash (%) | 0.19 (±0.1) | 0.23 (±0.06) | 0.13 (±0.01) |
| Elemental ash composition of forest residues the three conifers (%) | |||
| Ca | 25.48 (±0.5) | 42.46 (±0.98) | 42.44 (±0.54) |
| K | 12.23 (±0.77) | 13.16 (±0.78) | 21.1 (±0.66) |
| Mg | 10.82 (±0.44) | 21.64 (±0.31) | 13.08 (±0.47) |
| P | 9.35 (±0.54) | 5.37 (±0.20) | 4.06 (±0.16) |
| S | 1.70 (±0.21) | 3.8 (±0.18) | 2.59 (±0.09) |
| Na | 2.17 (±0.37) | 5.74 (±0.61) | 2.33 (±0.29) |
| Si | 17.46 (±0.70) | 4.57 (±0.43) | 4.89 (±0.61) |
| Al | 12.70 (±0.69) | 3.22 (±0.51) | 6.26 (±0.28) |
| Fe | 7.01 (±0.33) | no detected | 3.21 (±0.24) |
| Ti | 0.5 (±0.1) | no detected | no detected |
| Conversion (α) | Ea, Friedman | R2 | Ea, FWO | R2 | Ea, KAS | R2 | |
|---|---|---|---|---|---|---|---|
| 0.20 | 139.60 | 0.9698 | 105.24 | 0.9822 | 95.48 | 0.9784 | |
| 0.25 | 138.62 | 0.9693 | 112.02 | 0.9873 | 102.04 | 0.9846 | |
| 0.30 | 85.72 | 0.9873 | 116.79 | 0.9885 | 106.65 | 0.9861 | |
| 0.35 | 125.29 | 0.9936 | 120.66 | 0.9876 | 110.36 | 0.985 | |
| PP | 0.40 | 128.95 | 0.9925 | 124.18 | 0.9864 | 113.75 | 0.9837 |
| 0.45 | 141.33 | 0.9806 | 127.37 | 0.9863 | 116.80 | 0.9835 | |
| 0.50 | 143.34 | 0.9471 | 125.76 | 0.9888 | 115.082 | 0.9866 | |
| 0.55 | 119.56 | 0.9896 | 130.52 | 0.9891 | 119.75 | 0.9869 | |
| 0.60 | 144.58 | 0.9942 | 130.97 | 0.9897 | 120.11 | 0.9876 | |
| 0.65 | 102.50 | 0.9801 | 131.34 | 0.989 | 120.41 | 0.9867 | |
| 0.70 | 122.88 | 0.9194 | 130.58 | 0.9806 | 119.55 | 0.9767 | |
| Average | 126.58 | 0.9748 | 123.22 | 0.9868 | 112.72 | 0.9841 | |
| 0.20 | 126.63 | 0.9487 | 124.86 | 0.9990 | 115.09 | 0.9989 | |
| 0.25 | 97.06 | 0.9795 | 130.28 | 0.9992 | 120.31 | 0.9991 | |
| 0.30 | 96.81 | 0.9546 | 135.02 | 0.9980 | 124.88 | 0.9976 | |
| 0.35 | 185.14 | 0.9589 | 139.99 | 0.9965 | 129.70 | 0.9959 | |
| PL | 0.40 | 201.07 | 0.9971 | 144.75 | 0.9950 | 134.32 | 0.9941 |
| 0.45 | 146.74 | 0.9624 | 147.59 | 0.9951 | 137.03 | 0.9942 | |
| 0.50 | 139.45 | 0.9891 | 148.89 | 0.9965 | 138.23 | 0.9960 | |
| 0.55 | 182.75 | 0.9907 | 149.14 | 0.9974 | 138.40 | 0.9970 | |
| 0.60 | 161.10 | 0.9883 | 149.53 | 0.9976 | 138.71 | 0.9972 | |
| 0.65 | 143.94 | 0.9666 | 150.64 | 0.9968 | 139.73 | 0.9963 | |
| 0.70 | 126.93 | 0.9261 | 154.96 | 0.9806 | 143.94 | 0.9774 | |
| Average | 146.15 | 0.9692 | 143.24 | 0.9956 | 132.76 | 0.9948 | |
| 0.20 | 85.16 | 0.9534 | 138.33 | 0.9872 | 128.45 | 0.9850 | |
| 0.25 | 115.97 | 0.9878 | 142.98 | 0.9927 | 132.89 | 0.9915 | |
| 0.30 | 190.20 | 0.9962 | 147.80 | 0.9951 | 137.57 | 0.9943 | |
| 0.35 | 114.47 | 0.9802 | 152.57 | 0.9961 | 142.19 | 0.9955 | |
| PM | 0.40 | 218.99 | 0.9919 | 155.63 | 0.9970 | 145.12 | 0.9965 |
| 0.45 | 124.45 | 0.9934 | 155.93 | 0.9977 | 145.31 | 0.9974 | |
| 0.50 | 115.32 | 0.9916 | 155.09 | 0.9980 | 144.38 | 0.9977 | |
| 0.55 | 115.27 | 0.9935 | 153.93 | 0.9977 | 143.12 | 0.9973 | |
| 0.60 | 196.31 | 0.9759 | 153.69 | 0.9976 | 142.81 | 0.9972 | |
| 0.65 | 147.50 | 0.9763 | 154.74 | 0.9977 | 143.79 | 0.9974 | |
| 0.70 | 205.71 | 0.9712 | 159.129 | 0.9864 | 148.12 | 0.9844 | |
| Average | 148.12 | 0.9828 | 151.80 | 0.9948 | 141.25 | 0.9940 |
| β | 5 | 10 | 15 | 20 | 25 | 30 | ||
|---|---|---|---|---|---|---|---|---|
| PP | FRIEDMAN | Z | 6.28 × 109 | 6.48 × 109 | 4.46 × 109 | 4.73 × 109 | 3.87 × 109 | 3.72 × 109 |
| OFW | 1.69 × 108 | 1.89 × 108 | 1.71 × 108 | 1.79 × 108 | 1.50 × 108 | 1.55 × 108 | ||
| KAS | 1.95 × 107 | 2.29 × 107 | 2.15 × 107 | 2.30 × 107 | 1.98 × 107 | 2.08 × 107 | ||
| PL | FRIEDMAN | Z | 1.06 × 1014 | 8.90 × 1013 | 6.24 × 1013 | 6.16 × 1013 | 5.62 × 1013 | 4.71 × 1013 |
| OFW | 9.04 × 109 | 9.66 × 109 | 8.24 × 109 | 8.69 × 109 | 8.62 × 109 | 8.10 × 109 | ||
| KAS | 1.07 × 109 | 1.19 × 109 | 1.06 × 109 | 1.13 × 109 | 1.14 × 109 | 1.09 × 109 | ||
| PM | FRIEDMAN | Z | 2.65 × 1015 | 1.78 × 1015 | 1.59 × 1015 | 1.50 × 1015 | 1.22 × 1015 | 1.09 × 1015 |
| OFW | 3.88 × 1010 | 3.58 × 1010 | 3.64 × 1010 | 3.82 × 1010 | 3.45 × 1010 | 3.34 × 1010 | ||
| KAS | 4.61 × 109 | 4.47 × 109 | 4.66 × 109 | 4.97 × 109 | 4.59 × 109 | 4.50 × 109 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado Flores, J.J.; Rutiaga Quiñones, J.G.; Ávalos Rodríguez, M.L.; Alcaraz Vera, J.V.; Espino Valencia, J.; Guevara Martínez, S.J.; Márquez Montesino, F.; Alfaro Rosas, A. Thermal Degradation Kinetics and FT-IR Analysis on the Pyrolysis of Pinus pseudostrobus, Pinus leiophylla and Pinus montezumae as Forest Waste in Western Mexico. Energies 2020, 13, 969. https://doi.org/10.3390/en13040969
Alvarado Flores JJ, Rutiaga Quiñones JG, Ávalos Rodríguez ML, Alcaraz Vera JV, Espino Valencia J, Guevara Martínez SJ, Márquez Montesino F, Alfaro Rosas A. Thermal Degradation Kinetics and FT-IR Analysis on the Pyrolysis of Pinus pseudostrobus, Pinus leiophylla and Pinus montezumae as Forest Waste in Western Mexico. Energies. 2020; 13(4):969. https://doi.org/10.3390/en13040969
Chicago/Turabian StyleAlvarado Flores, José Juan, José Guadalupe Rutiaga Quiñones, María Liliana Ávalos Rodríguez, Jorge Víctor Alcaraz Vera, Jaime Espino Valencia, Santiago José Guevara Martínez, Francisco Márquez Montesino, and Antonio Alfaro Rosas. 2020. "Thermal Degradation Kinetics and FT-IR Analysis on the Pyrolysis of Pinus pseudostrobus, Pinus leiophylla and Pinus montezumae as Forest Waste in Western Mexico" Energies 13, no. 4: 969. https://doi.org/10.3390/en13040969
APA StyleAlvarado Flores, J. J., Rutiaga Quiñones, J. G., Ávalos Rodríguez, M. L., Alcaraz Vera, J. V., Espino Valencia, J., Guevara Martínez, S. J., Márquez Montesino, F., & Alfaro Rosas, A. (2020). Thermal Degradation Kinetics and FT-IR Analysis on the Pyrolysis of Pinus pseudostrobus, Pinus leiophylla and Pinus montezumae as Forest Waste in Western Mexico. Energies, 13(4), 969. https://doi.org/10.3390/en13040969