Investigation of Start-Up Characteristics of Thermosyphons Modified with Different Hydrophilic and Hydrophobic Inner Surfaces
Abstract
1. Introduction
2. Methodology
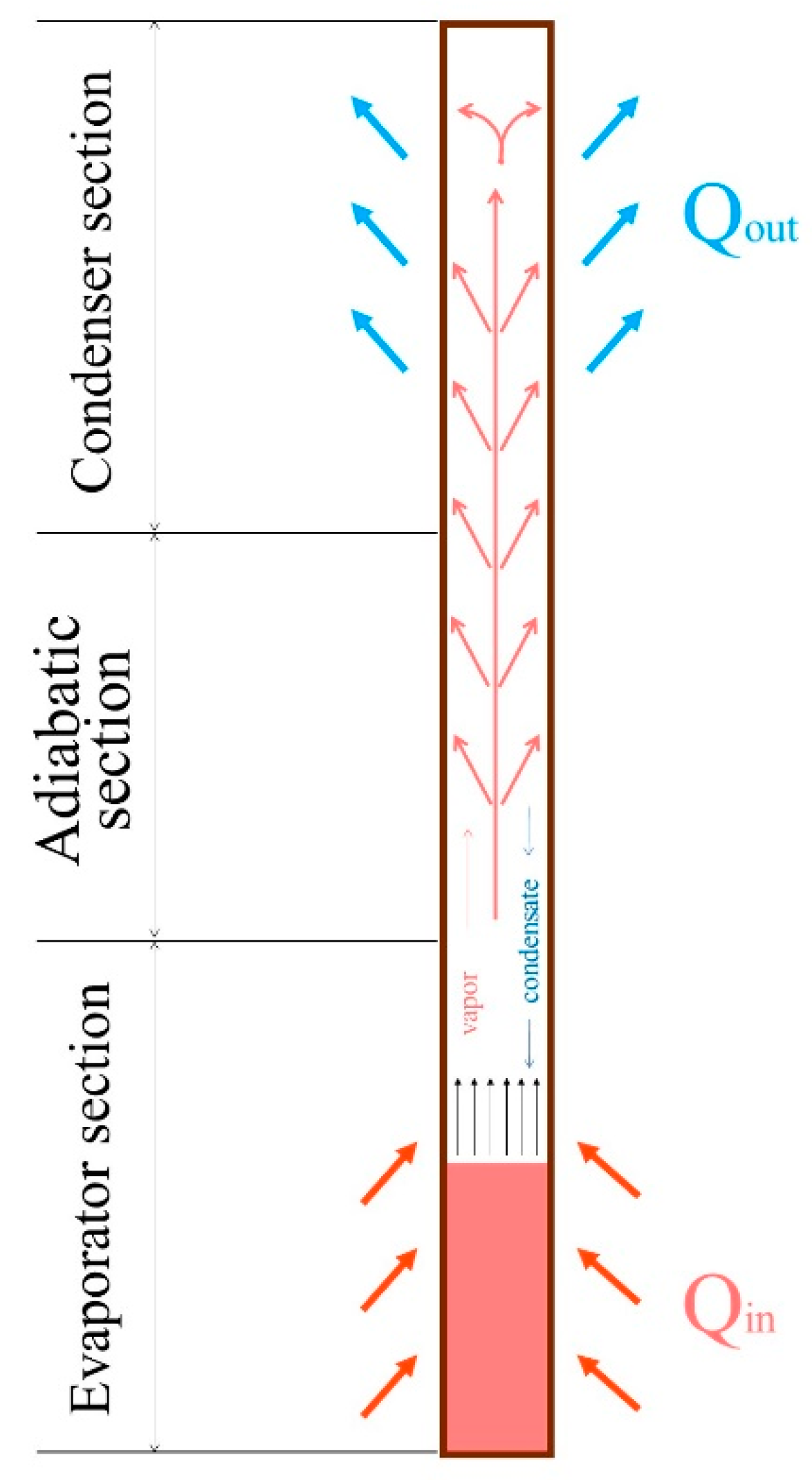
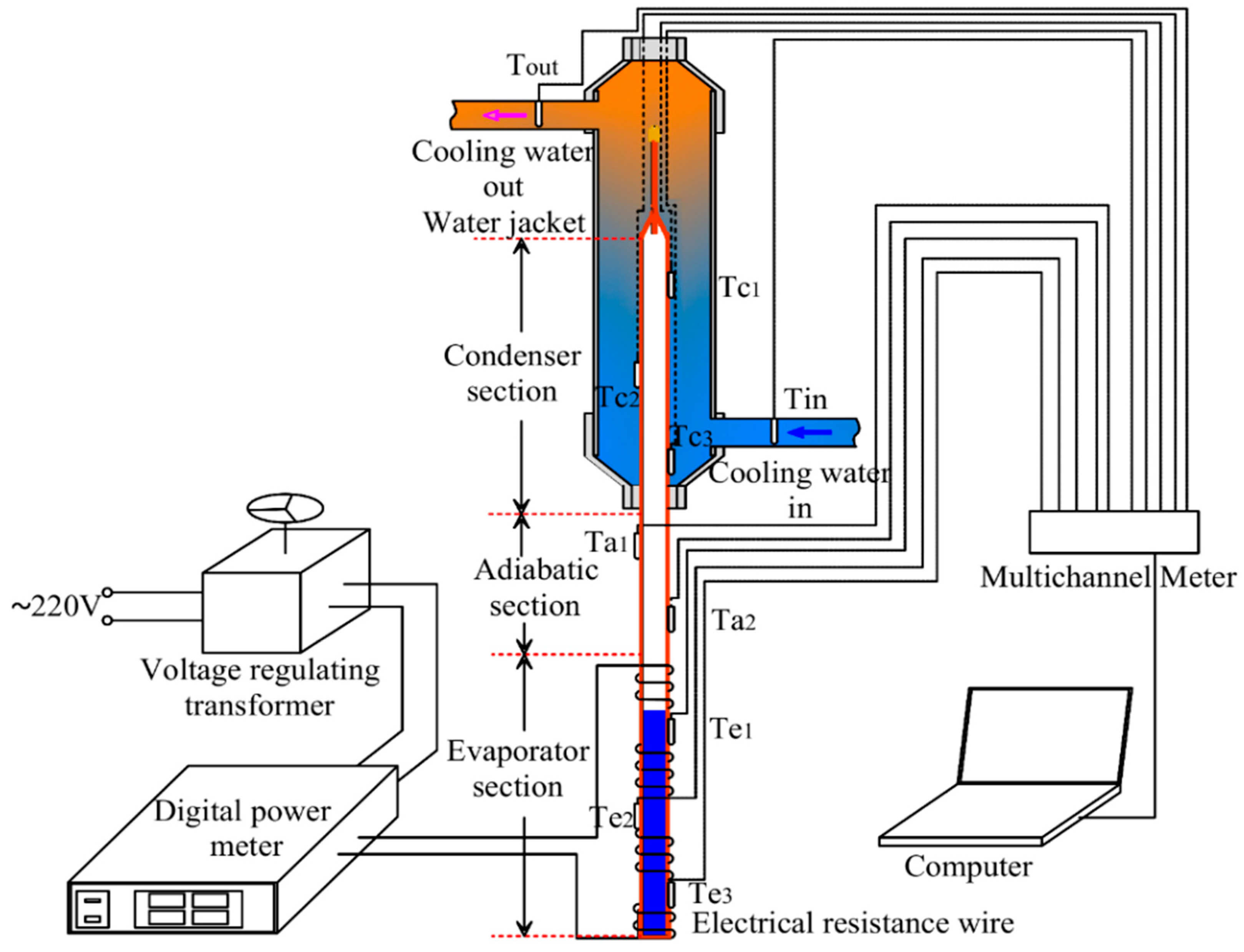
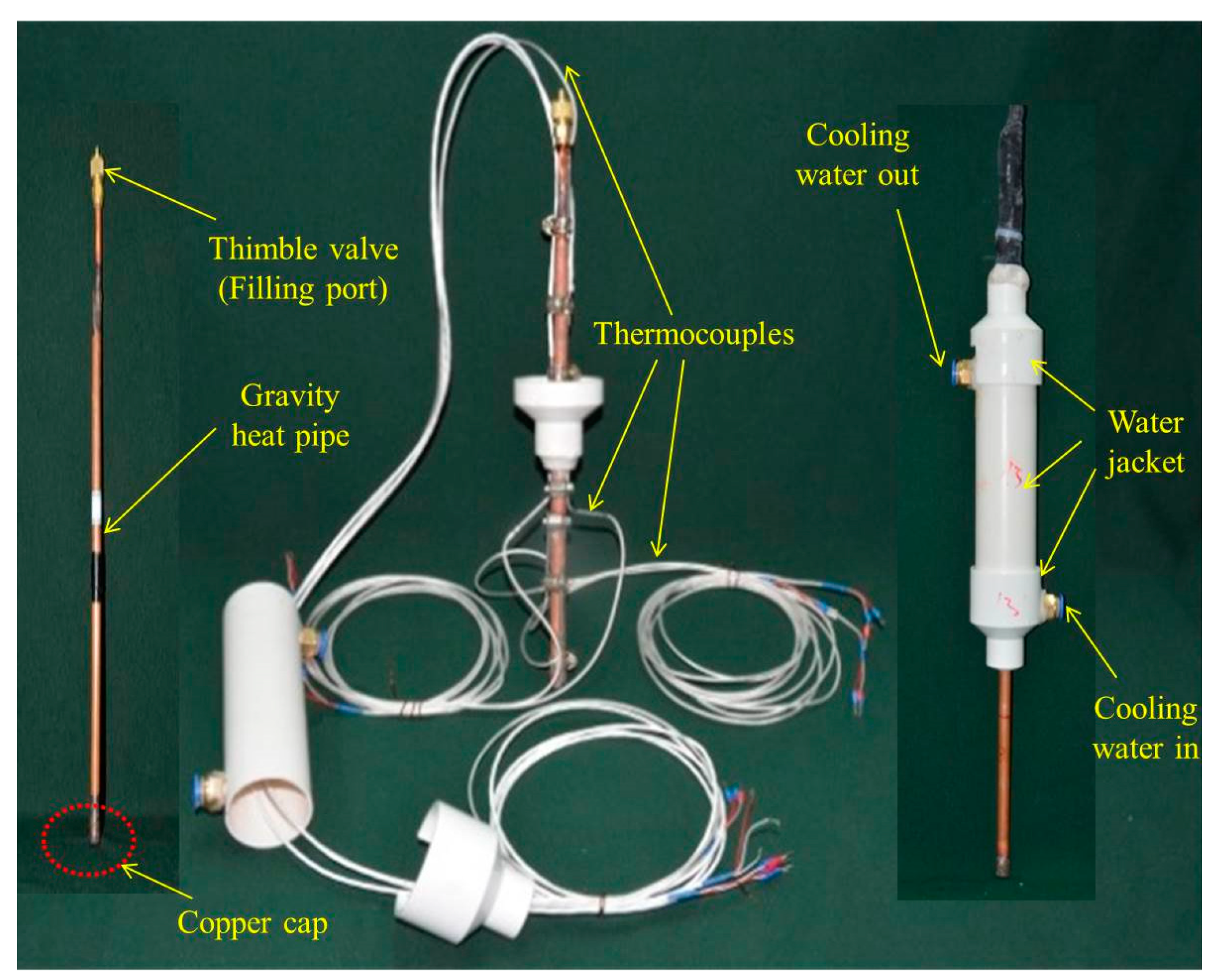
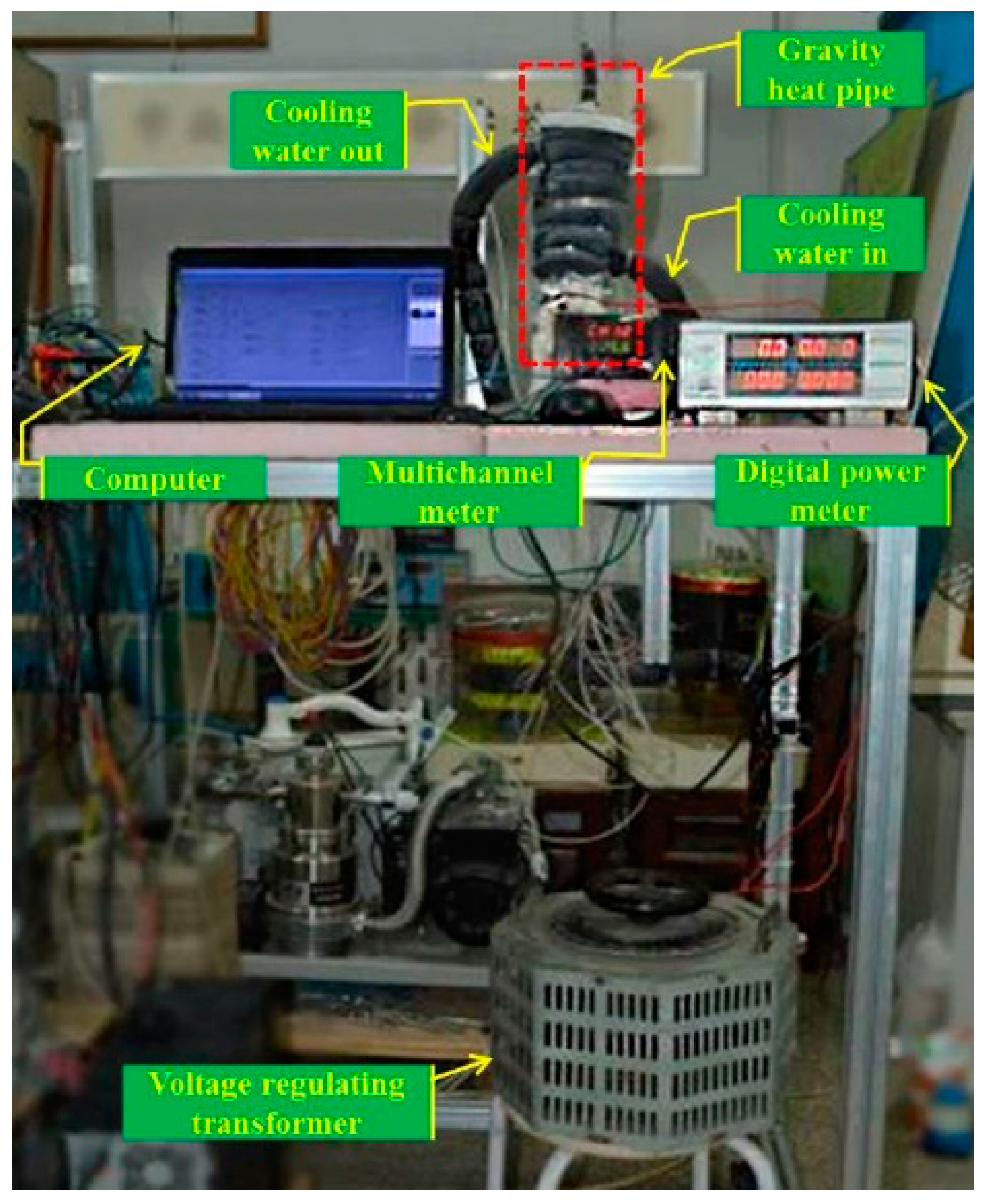
2.1. Experimental System
2.2. Data Reduction and Problem Description
2.3. Surface Modification
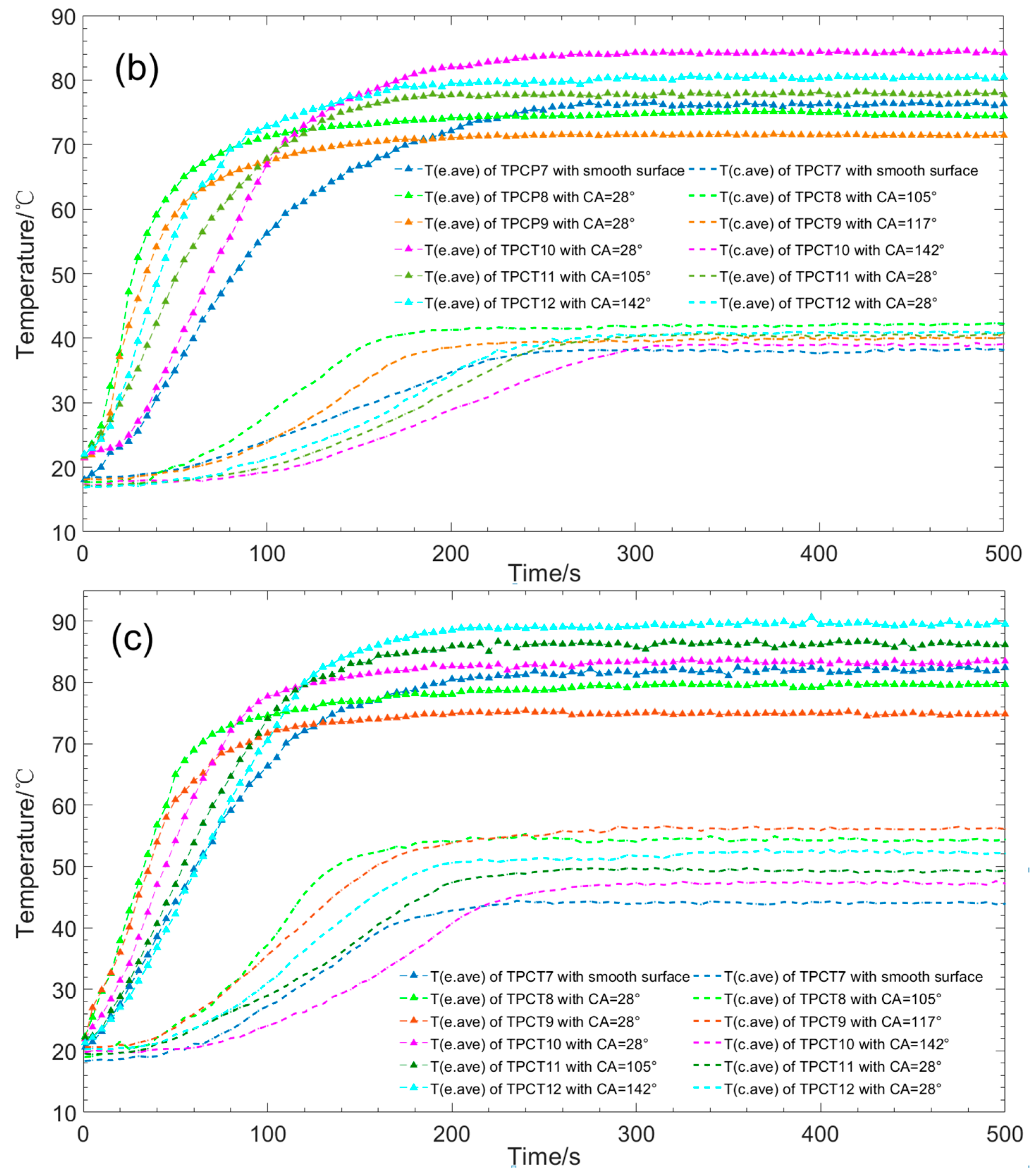
3. Results
4. Conclusions
- (1)
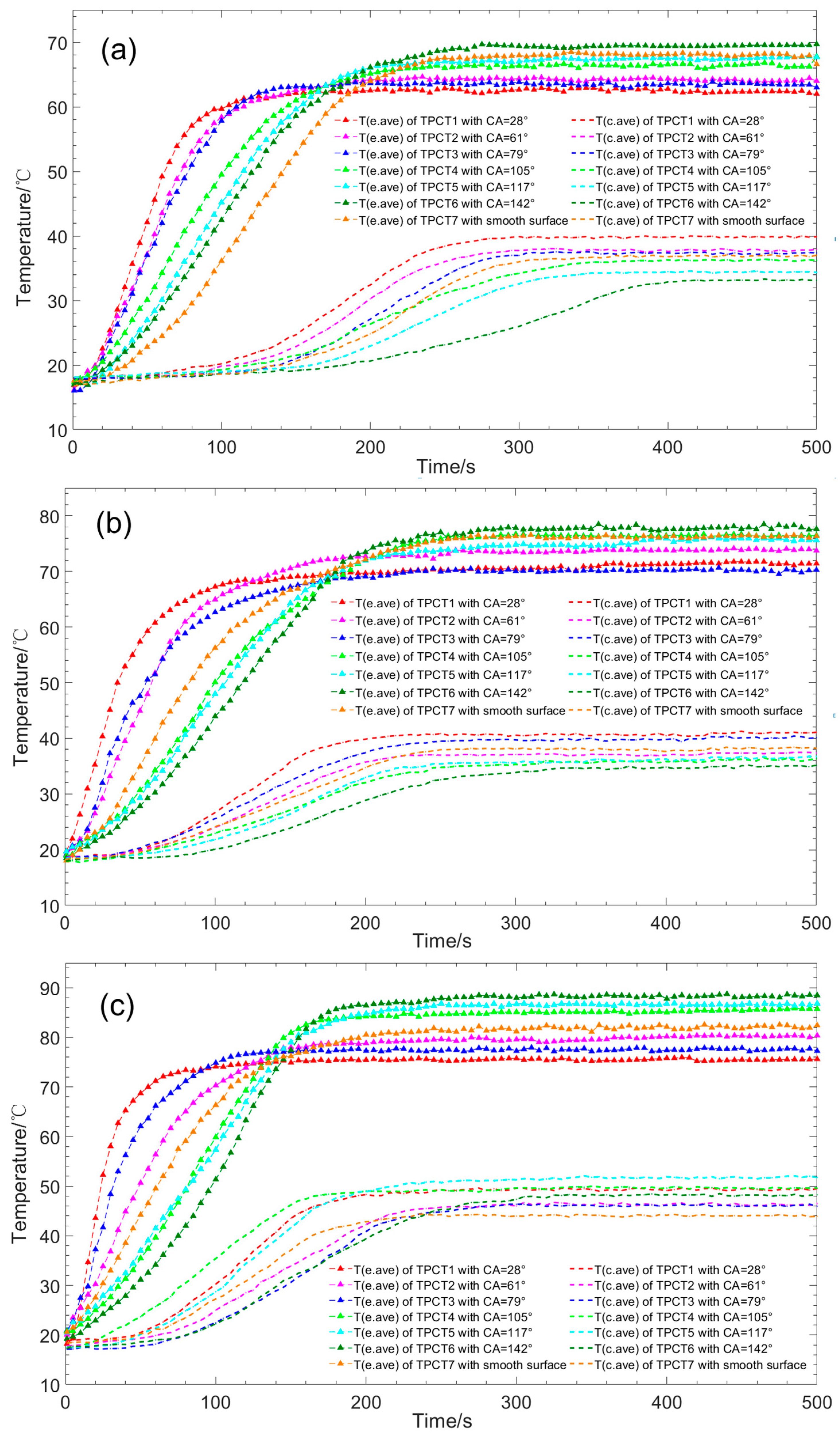
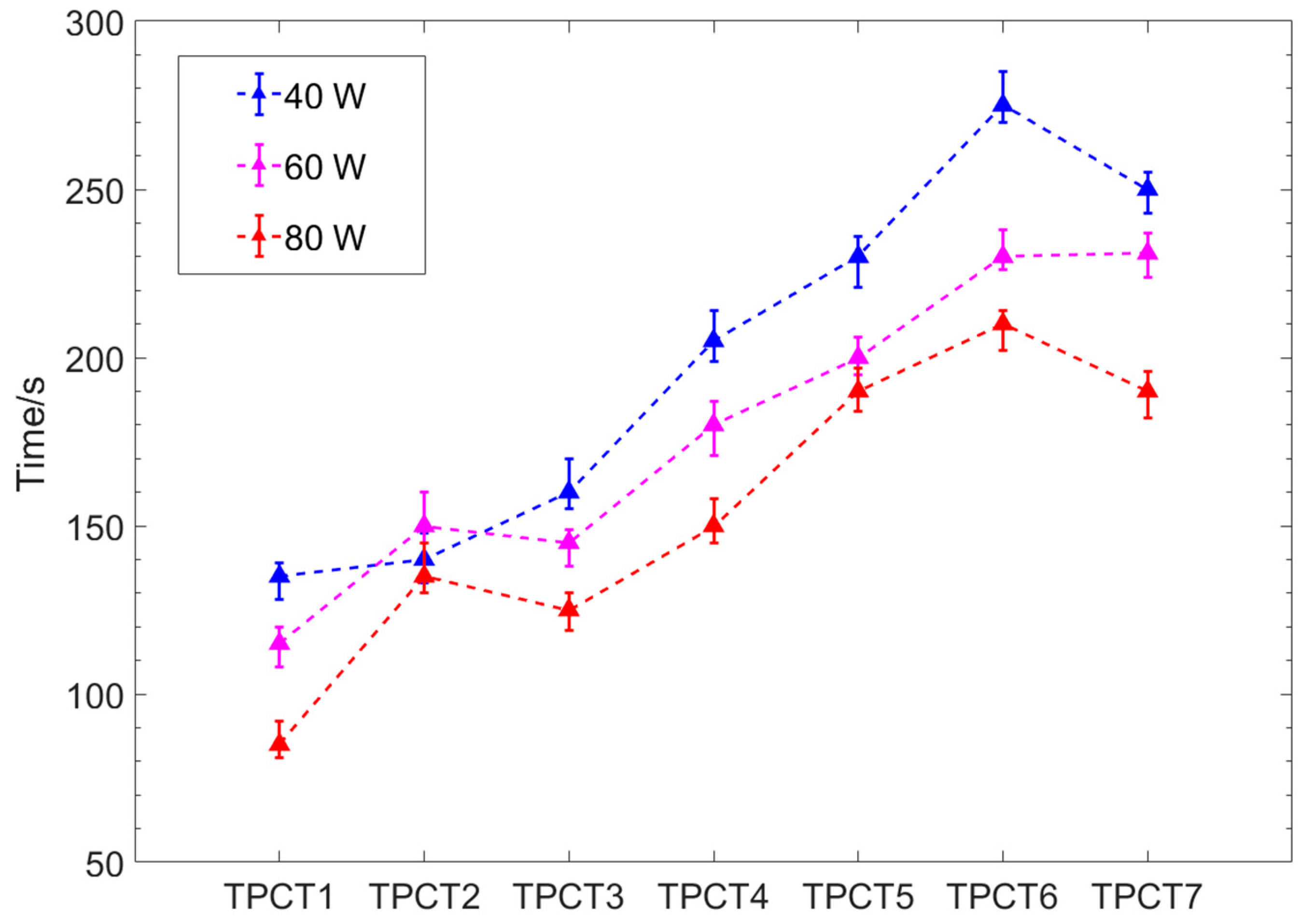
- For thermosyphons with the same wettabilities on the evaporator and the condenser sections among TPCT1–TPCT6, the introduction of hydrophilic properties inside the evaporator section not only significantly shortens the start-up time but also decreases the start-up temperature. At the same input power, the start-up time of a thermosyphon with CA < 90° is shorter than that with CA > 90°. The start-up time of TPCT with CA = 28° has the shortest start-up time, while under the input powers of 40 W, 60 W and 80 W it is 46%, 50% and 55% shorter than that of TPCT with a smooth surface, respectively. The start-up time becomes longer with the increase of CA of the evaporator sections.
- (2)
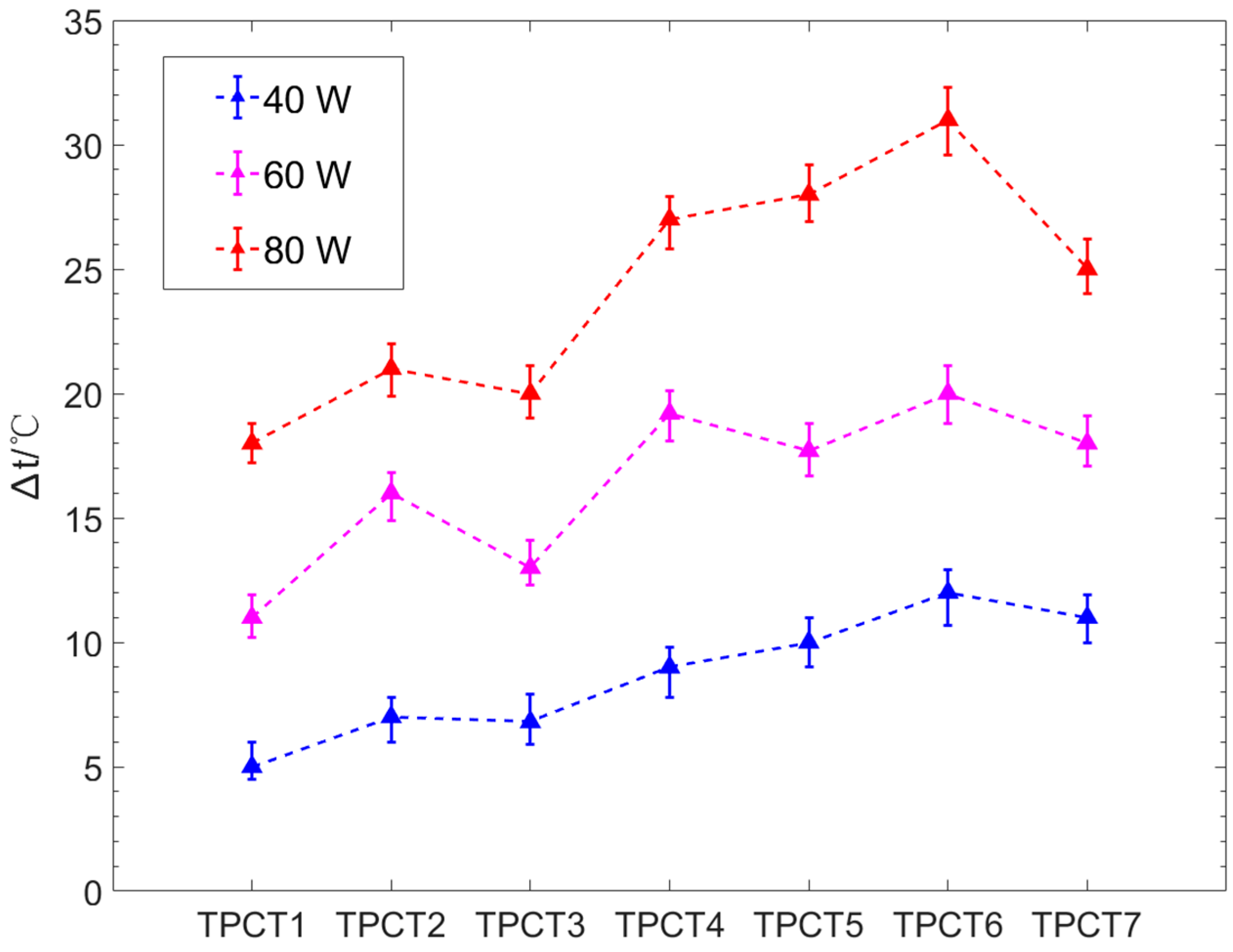
- As the CAs on the evaporator sections of TPCT1–TPCT6 increase, the wall superheat degree gradually increases. The TPCT with CA = 28° has a minimum superheat degree at input powers of 40 W, 60 W and 80 W, and the wall superheat degree is 55%, 39% and 28% lower than that of TPCT with smooth surfaces, respectively. In addition, the superheat degree of the hydrophobic evaporator section increases more obviously than that of the hydrophilic with increasing input power. In the experiment, the TPCT with CA = 142° has the highest superheat degree among TPCT1–TPCT6.
- (3)
- For thermosyphons with combined hydrophilic and hydrophobic properties, the start-up time of the evaporator section with CA < 90° and the condenser section with CA > 90° is less than the evaporator section with CA > 90° and the condenser section with CA < 90°. With the increase of CA on the condenser sections of TPCT8–TPCT10, the start-up process of TPCT8 is faster than those of TPCT9 and TPCT10 with the same CA = 28° on the evaporator section. The experimental results and data analysis demonstrate that the start-up time of the TPCT8 with CA = 28° on the evaporator section and CA = 105° on the condenser section is the shortest among TPCT7–TPCT12.
- (4)
- In this paper, the surfaces with CAs of 28°, 61°, 79°, 105°, 117° and 142° are fabricated inside the evaporator and the condenser sections of the thermosyphons. The experimental results show that the TPCT with CA = 28° on both the evaporator and the condenser section has the best start-up characteristics considering start-up time and wall superheat degree, which reflect the optimal wettabilitiy inside the inner surface of thermosyphons for industrial reference. Further research directions should extend to the preparation and investigation of superhydrophobic and superhydrophilic surfaces in the analysis of the thermal performance and start-up characteristics.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Specific heat values (J/kg·K) | |
| m | Mass flow(kg/s) |
| Q | Heat load (W) |
| T | Temperature (°C) |
| Current (A) | |
| Voltage (V) |
Greek symbols
| Wall superheat degree (°C) | |
| Efficiency of the thermosyphon |
Subscripts
| a | Adiabatic section |
| ave | Average |
| c | Condenser section |
| e | Evaporator section |
| in | Cooling water inlet/ Input power |
| out | Cooling water outlet |
| sat | Saturated |
| w | Wall/water |
Acronyms
| CA | Contact angle |
| MCGS | Monitor and Control Generated System |
| SEM | Scanning electron microscopy |
| TPCP | Two-phase closed thermosyphon |
References
- Chang, Y.; Cheng, C.; Wang, J.; Chen, S. Heat pipe for cooling of electronic equipment. Energy Convers. Manag. 2008, 49, 3398–3404. [Google Scholar] [CrossRef]
- Jafari, D.; Franco, A.; Filippeschi, S.; Di Marco, P. Two-phase closed thermosyphons: A review of studies and solar applications. Renew. Sustain. Energy Rev. 2016, 53, 575–593. [Google Scholar] [CrossRef]
- Ziapour, B.M.; Khalili, M.B. PVT type of the two-phase loop mini tube thermosyphon solar water heater. Energy Convers. Manag. 2016, 129, 54–61. [Google Scholar] [CrossRef]
- Jouhara, H.; Merchant, H. Experimental investigation of a thermosyphon based heat exchanger used in energy efficient air handling units. Energy 2012, 39, 82–89. [Google Scholar] [CrossRef]
- Gaugler, R.S. Heat Transfer Device. U.S. Patent 2,350,348, 6 June 1994. [Google Scholar]
- Faghri, A. Heat Pipe Science and Technology; Taylor & Francis: Philadelphia, PA, USA, 1995. [Google Scholar]
- Shabgard, H.; Allen, M.J.; Sharifi, N.; Benn, S.P.; Faghri, A.; Bergman, T.L. Heat pipe heat exchangers and heat sinks: Opportunities, challenges, applications, analysis, and state of the art. Int. J. Heat Mass Transf. 2015, 89, 138–158. [Google Scholar] [CrossRef]
- Amatachaya, P.; Srimuang, W. Comparative heat transfer characteristics of a flat two-phase closed thermosyphon (FTPCT) and a conventional two-phase closed thermosyphon (CTPCT). Int. Commun. Heat Mass Transfer. 2010, 37, 293–298. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, P.; Zhou, Y.; Zhang, Y.; Zhang, Y. Heat transfer characteristics of two-phase closed thermosyphons modified with inner surfaces of various wettabilities. Int. Commun. Heat Mass Transfer. 2019, 103, 100–109. [Google Scholar] [CrossRef]
- Lataoui, Z.; Jemni, A. Experimental investigation of a stainless steel two-phase closed thermosyphon. Appl. Therm. Eng. 2017, 121, 721–727. [Google Scholar] [CrossRef]
- Moradikazerouni, A.; Afrand, M.; Alsarraf, J.; Mahian, O.; Wongwises, S.; Tran, M.-D. Comparison of the effect of five different entrance channel shapes of a micro-channel heat sink in forced convection with application to cooling a supercomputer circuit board. Appl. Therm. Eng. 2019, 150, 1078–1089. [Google Scholar] [CrossRef]
- Moradikazerouni, A.; Afrand, M.; Alsarraf, J.; Wongwises, S.; Asadi, A.; Nguyen, T.K. Investigation of a computer CPU heat sink under laminar forced convection using a structural stability method. Int. J. Heat Mass Transf. 2019, 134, 1218–1226. [Google Scholar] [CrossRef]
- Rahimi, M.; Asgary, K.; Jesri, S. Thermal characteristics of a resurfaced condenser and evaporator closed two-phase thermosyphon. Int. Commun. Heat Mass Transf. 2010, 37, 703–710. [Google Scholar] [CrossRef]
- Singh, R.R.; Selladurai, V.; Ponkarthik, P.; Solomon, A.B. Effect of anodization on the heat transfer performance of flat thermosyphon. Exp. Therm. Fluid Sci. 2015, 68, 574–581. [Google Scholar] [CrossRef]
- Solomon, A.B.; Daniel, V.A.; Ramachandran, K.; Pillai, B.; Singh, R.R.; Sharifpur, M.; Meyer, J. Performance enhancement of a two-phase closed thermosiphon with a thin porous copper coating. Int. Commun. Heat Mass Transf. 2017, 82, 9–19. [Google Scholar] [CrossRef]
- Solomon, A.B.; Mathew, A.; Ramachandran, K.; Pillai, B.; Karthikeyan, V. Thermal performance of anodized two phase closed thermosyphon (TPCT). Exp. Therm. Fluid Sci. 2013, 48, 49–57. [Google Scholar] [CrossRef]
- Noie, S. Heat transfer characteristics of a two-phase closed thermosyphon. Appl. Therm. Eng. 2005, 25, 495–506. [Google Scholar] [CrossRef]
- Gedik, E. Experimental investigation of the thermal performance of a two-phase closed thermosyphon at different operating conditions. Energy Build. 2016, 127, 1096–1107. [Google Scholar] [CrossRef]
- Ma, Y.; Shahsavar, A.; Moradi, I.; Rostami, S.; Moradikazerouni, A.; Yarmand, H.; Zulkifli, N.W.B.M. Using finite volume method for simulating the natural convective heat transfer of nano-fluid flow inside an inclined enclosure with conductive walls in the presence of a constant temperature heat source. Phys. A Stat. Mech. Appl. 2019, 123035. [Google Scholar] [CrossRef]
- Vo, D.D.; Alsarraf, J.; Moradikazerouni, A.; Afrand, M.; Salehipour, H.; Qi, C. Numerical investigation of γ-AlOOH nano-fluid convection performance in a wavy channel considering various shapes of nanoadditives. Powder Technol. 2019, 345, 649–657. [Google Scholar] [CrossRef]
- Sun, Q.; Qu, J.; Yuan, J.; Wang, H. Start-up characteristics of MEMS-based micro oscillating heat pipe with and without bubble nucleation. Int. J. Heat Mass Transf. 2018, 122, 515–528. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, H.; Yan, X.; Wang, X.; Ye, F.; Ma, C. Experimental study of start-up performance of sodium-potassium heat pipe. J. Eng. Thermophys. 2014, 35, 2508–2512. [Google Scholar]
- Guo, Q.; Guo, H.; Yan, X.; Wang, X.; Ye, F.; Ma, C. Effect of the evaporator length on start-up performance fo sodium-potassium alloy heat pipe. J. Eng. Thermophys. 2016, 37, 1717–1720. [Google Scholar]
- Wang, X.; Xin, G.; Tian, F.; Cheng, L. Start-up behavior of gravity heat pipe with small diameter. CIESC J. 2012, 63, 94–98. [Google Scholar]
- Huang, J.; Wang, L.; Shen, J.; Liu, C. Effect of non-condensable gas on the start-up of a gravity loop thermosyphon with gas–liquid separator. Exp. Therm. Fluid Sci. 2016, 72, 161–170. [Google Scholar] [CrossRef]
- Joung, W.; Yu, T.; Lee, J. Experimental study on the loop heat pipe with a planar bifacial wick structure. Int. J. Heat Mass Transf. 2008, 51, 1573–1581. [Google Scholar] [CrossRef]
- Singh, R.; Akbarzadeh, A.; Mochizuki, M. Operational characteristics of a miniature loop heat pipe with flat evaporator. Int. J. Therm. Sci. 2008, 47, 1504–1515. [Google Scholar] [CrossRef]
- Ji, X.; Wang, Y.; Xu, J.; Huang, Y. Experimental study of heat transfer and start-up of loop heat pipe with multiscale porous wicks. Appl. Therm. Eng. 2017, 117, 782–798. [Google Scholar] [CrossRef]
- Huang, B.; Huang, H.; Liang, T. System dynamics model and startup behavior of loop heat pipe. Appl. Therm. Eng. 2009, 29, 2999–3005. [Google Scholar] [CrossRef]
- Dai, C.; Yang, L.; Xie, J.; Wang, T. Nutrient diffusion control of fertilizer granules coated with a gradient hydrophobic film. Colloids Surf. A Physicochem. Eng. Asp. 2020, 588, 124361. [Google Scholar] [CrossRef]
- Anosov, A.; Smirnova, E.Y.; Sharakshane, A.; Nikolayeva, E.; Zhdankina, Y.S. Increase in the current variance in bilayer lipid membranes near phase transition as a result of the occurrence of hydrophobic defects. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183147. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Wang, G. A predictive model of nucleate pool boiling on heated hydrophilic surfaces. Int. J. Heat Mass Transfer. 2013, 65, 789–797. [Google Scholar] [CrossRef]
- Phan, H.T.; Caney, N.; Marty, P.; Colasson, S.; Gavillet, J. Surface wettability control by nanocoating: The effects on pool boiling heat transfer and nucleation mechanism. Int. J. Heat Mass Transfer. 2009, 52, 5459–5471. [Google Scholar] [CrossRef]
- Hsu, C.; Chen, P. Surface wettability effects on critical heat flux of boiling heat transfer using nanopartical coatings. Int. J. Heat Mass Transfer. 2012, 55, 3713–3719. [Google Scholar] [CrossRef]
- Leese, H.; Bhurtun, V.; Lee, K.P.; Mattia, D. Wetting behavior of hydrophilic and hydrophobic nanostructured porous anodic alumina. Colloids Surf. A Physicochem. Eng. Asp. 2013, 420, 53–58. [Google Scholar] [CrossRef]
- Shi, B.; Wang, Y.; Chen, K. Pool boiling heat transfer enhancement with copper nanowire arrays. Appl. Therm. Eng. 2015, 75, 115–121. [Google Scholar] [CrossRef]
- Bankoff, S. Ebullition from solid surfaces in the absence of a pre-existing gaseous phase. Trans. Am. Mech. Eng. 1957, 79, 735–740. [Google Scholar]
- Zheng, X.; Ji, X.; Wang, Y.; Xu, J. Study of the pool boiling heat transfer on the superhydrophilic and Superhydrophobic surface. Prog. Chem. Ind. 2016, 12, 3793–3798. [Google Scholar]
- Thiagarajan, S.J.; Yang, R.; King, C.; Narumanchi, S. Bubble dynamics and nucleate pool boiling heat transfer on microporous copper surfaces. Int. J. Heat Mass Transfer 2015, 89, 1297–1315. [Google Scholar] [CrossRef]
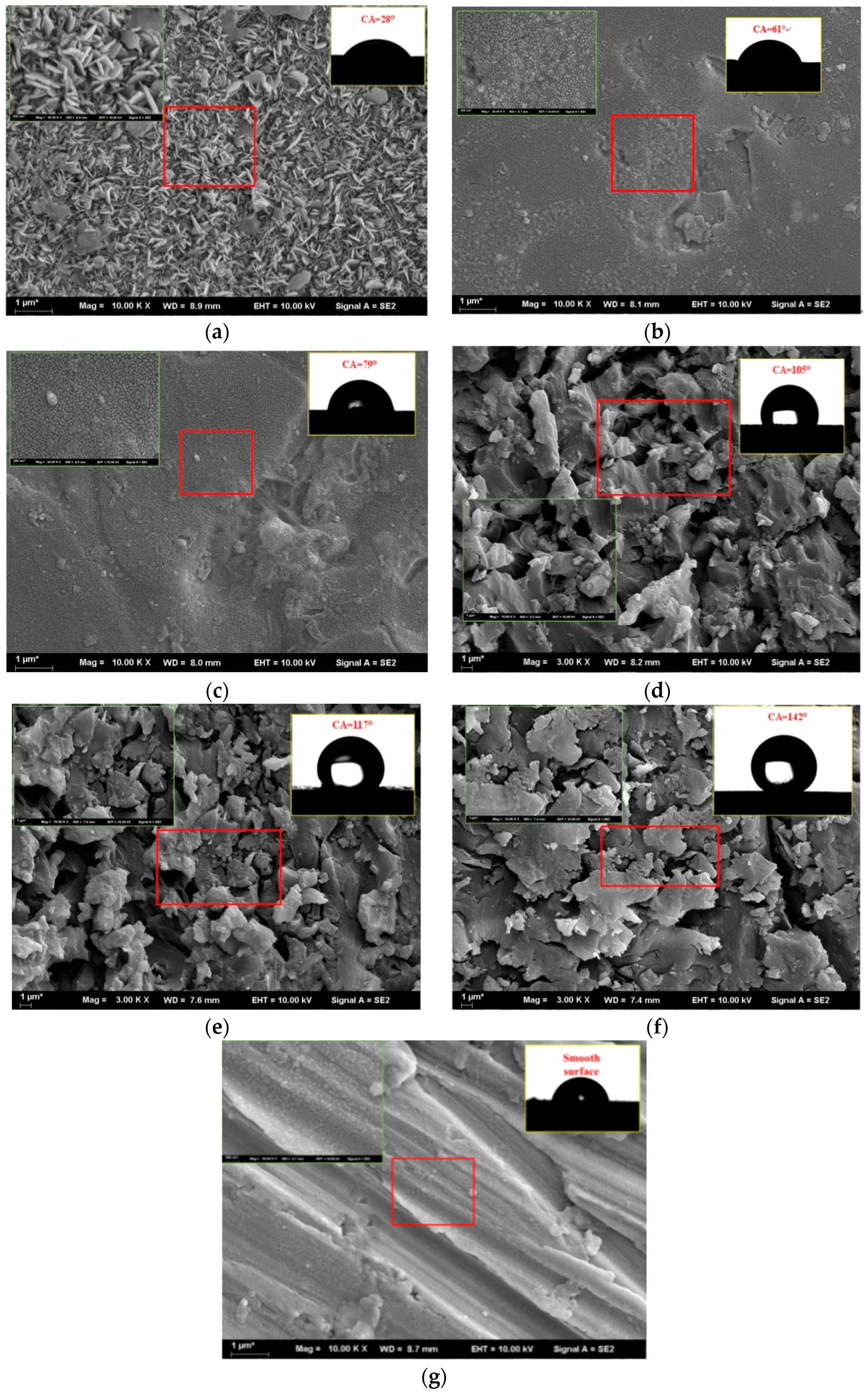

| Input Power (W) | Temperature of Cooling Water (°C) | Flow Rate of Cooling Water |
|---|---|---|
| 40 | 18 | 20 |
| 60 | 18 | 30 |
| 80 | 18 | 40 |
| TPCTs | Evaporator | Adiabatic | Condenser |
|---|---|---|---|
| TPCT1 | 28° | 28° | |
| TPCT2 | 61° | 61° | |
| TPCT3 | 79° | 79° | |
| TPCT4 | 105° | 105° | |
| TPCT5 | 117° | 117° | |
| TPCT6 | 142° | 142° | |
| TPCT7 | smooth surface | smooth surface | smooth surface |
| TPCT8 | 28° | smooth surface | 105° |
| TPCT9 | 28° | smooth surface | 117° |
| TPCT10 | 28° | smooth surface | 142° |
| TPCT11 | 105° | smooth surface | 28° |
| TPCT12 | 142° | smooth surface | 28° |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhao, Z.; Jiang, P.; Yang, S.; Li, S.; Chen, X. Investigation of Start-Up Characteristics of Thermosyphons Modified with Different Hydrophilic and Hydrophobic Inner Surfaces. Energies 2020, 13, 765. https://doi.org/10.3390/en13030765
Ma X, Zhao Z, Jiang P, Yang S, Li S, Chen X. Investigation of Start-Up Characteristics of Thermosyphons Modified with Different Hydrophilic and Hydrophobic Inner Surfaces. Energies. 2020; 13(3):765. https://doi.org/10.3390/en13030765
Chicago/Turabian StyleMa, Xiaolong, Zhongchao Zhao, Pengpeng Jiang, Shan Yang, Shilin Li, and Xudong Chen. 2020. "Investigation of Start-Up Characteristics of Thermosyphons Modified with Different Hydrophilic and Hydrophobic Inner Surfaces" Energies 13, no. 3: 765. https://doi.org/10.3390/en13030765
APA StyleMa, X., Zhao, Z., Jiang, P., Yang, S., Li, S., & Chen, X. (2020). Investigation of Start-Up Characteristics of Thermosyphons Modified with Different Hydrophilic and Hydrophobic Inner Surfaces. Energies, 13(3), 765. https://doi.org/10.3390/en13030765




