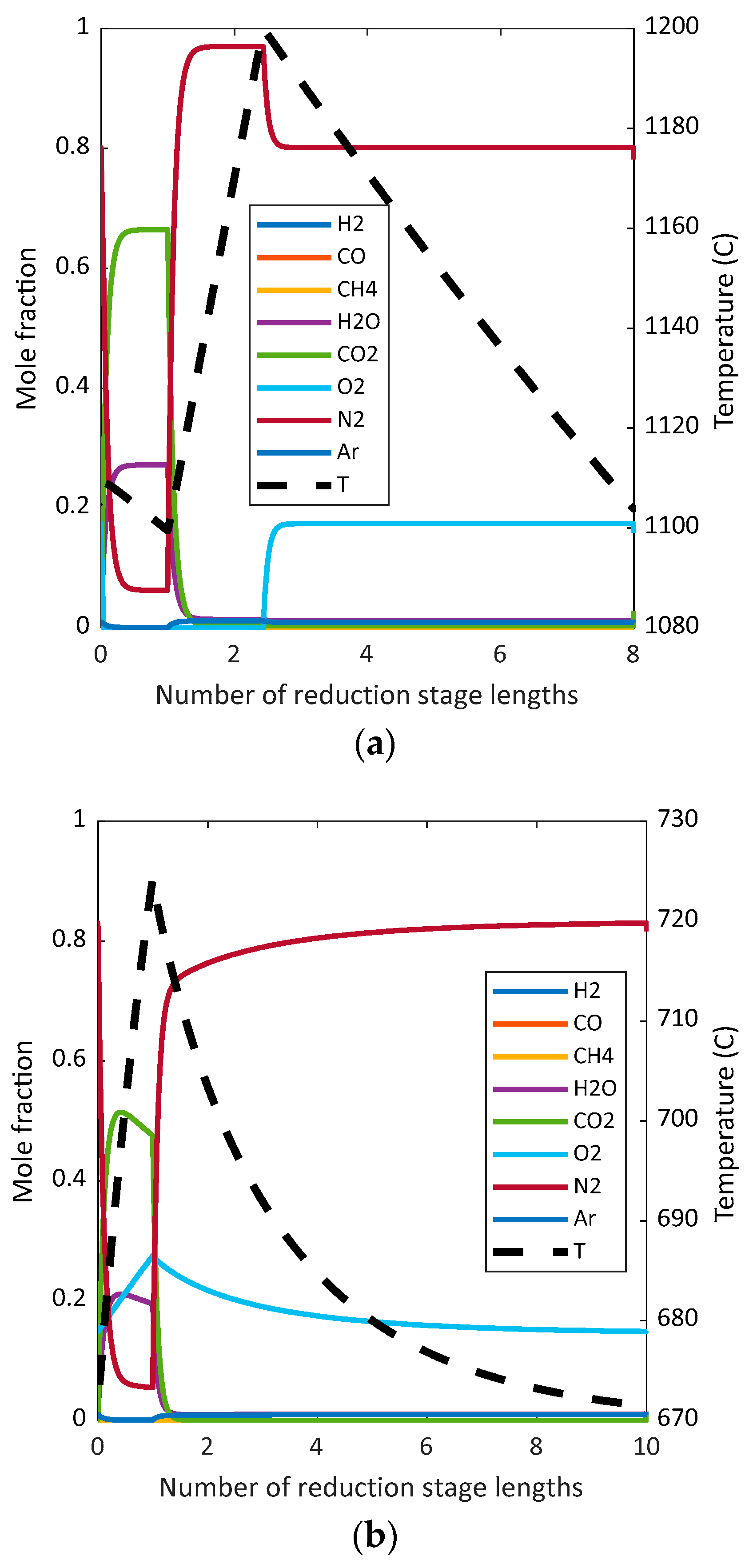
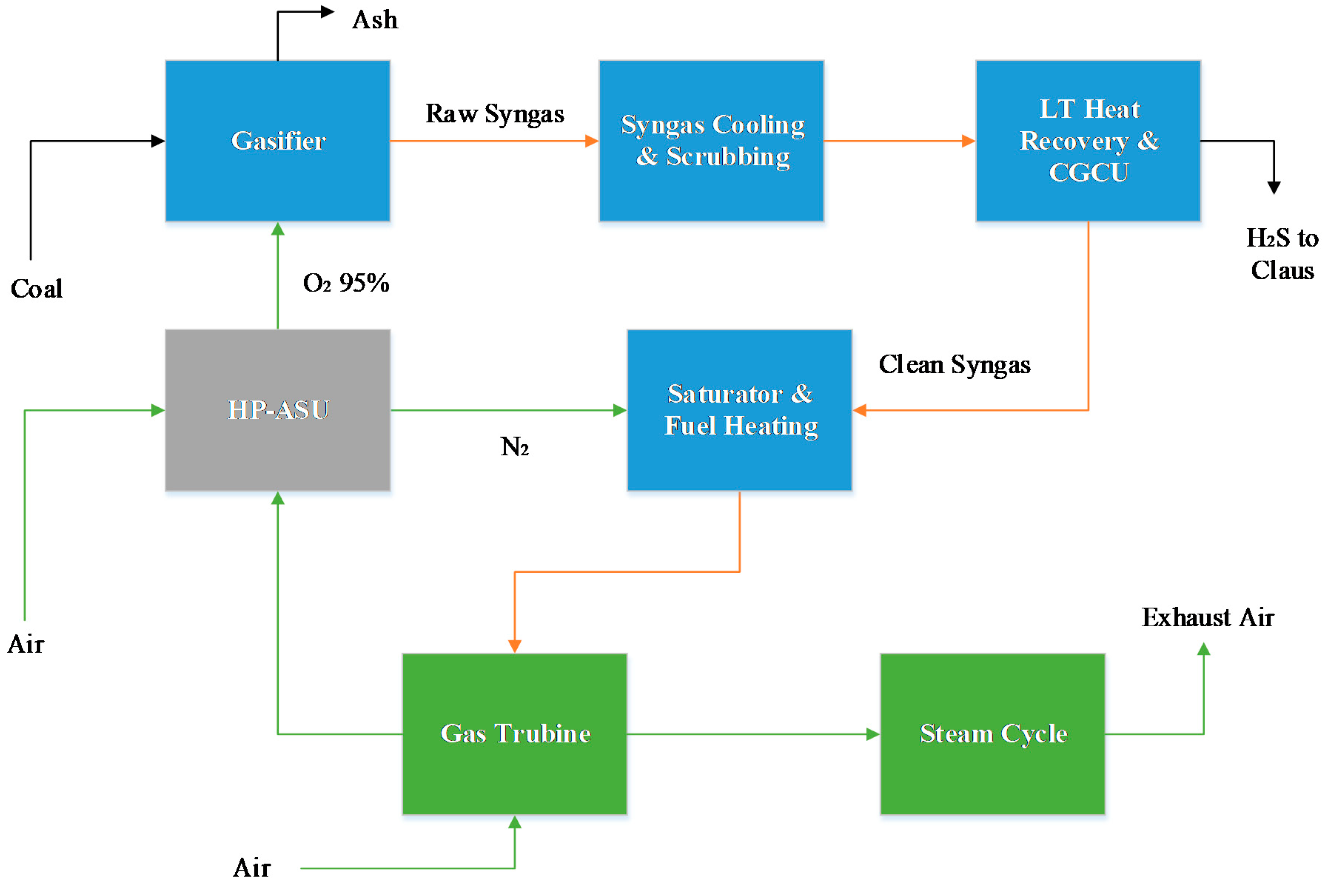
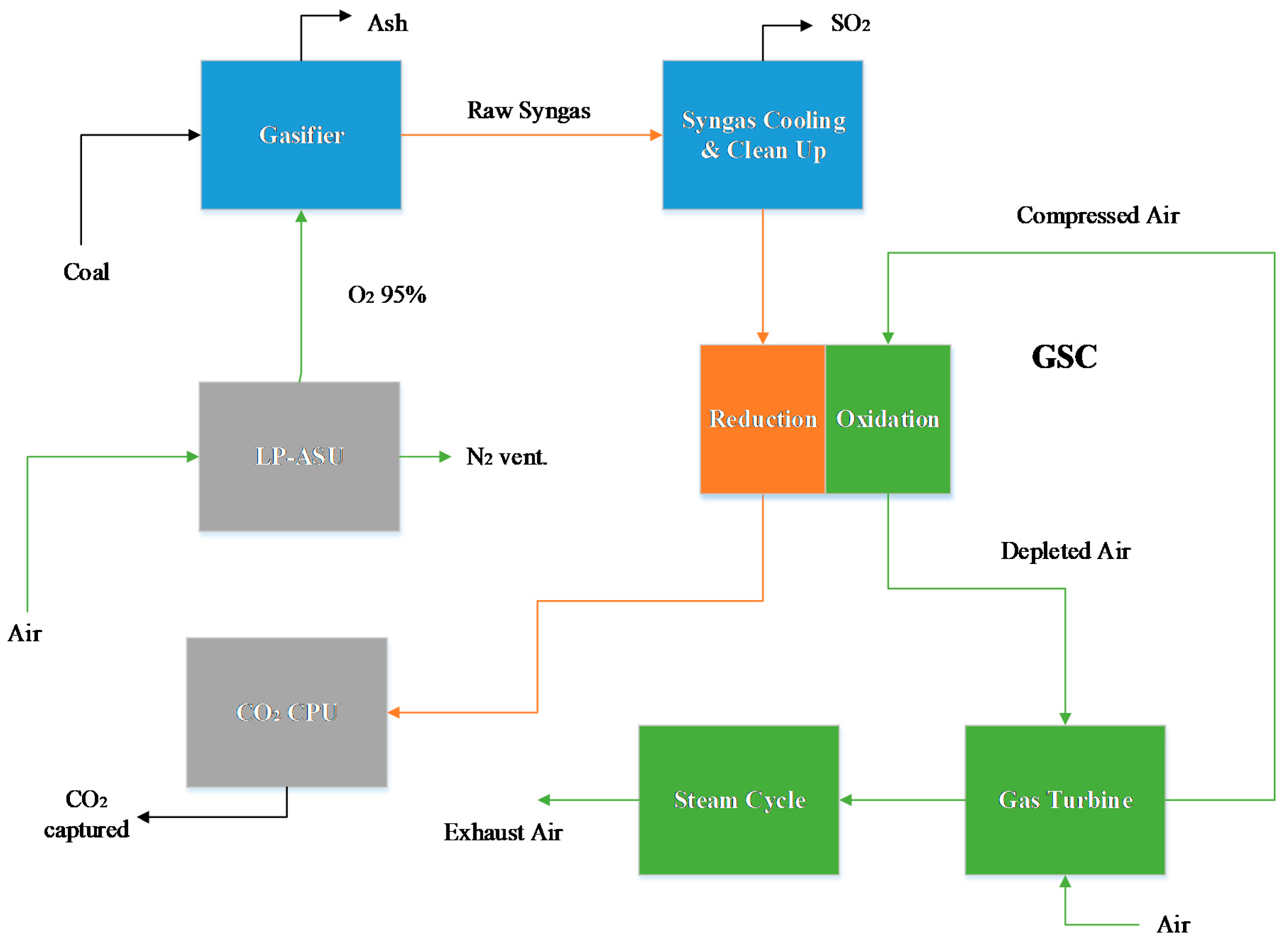
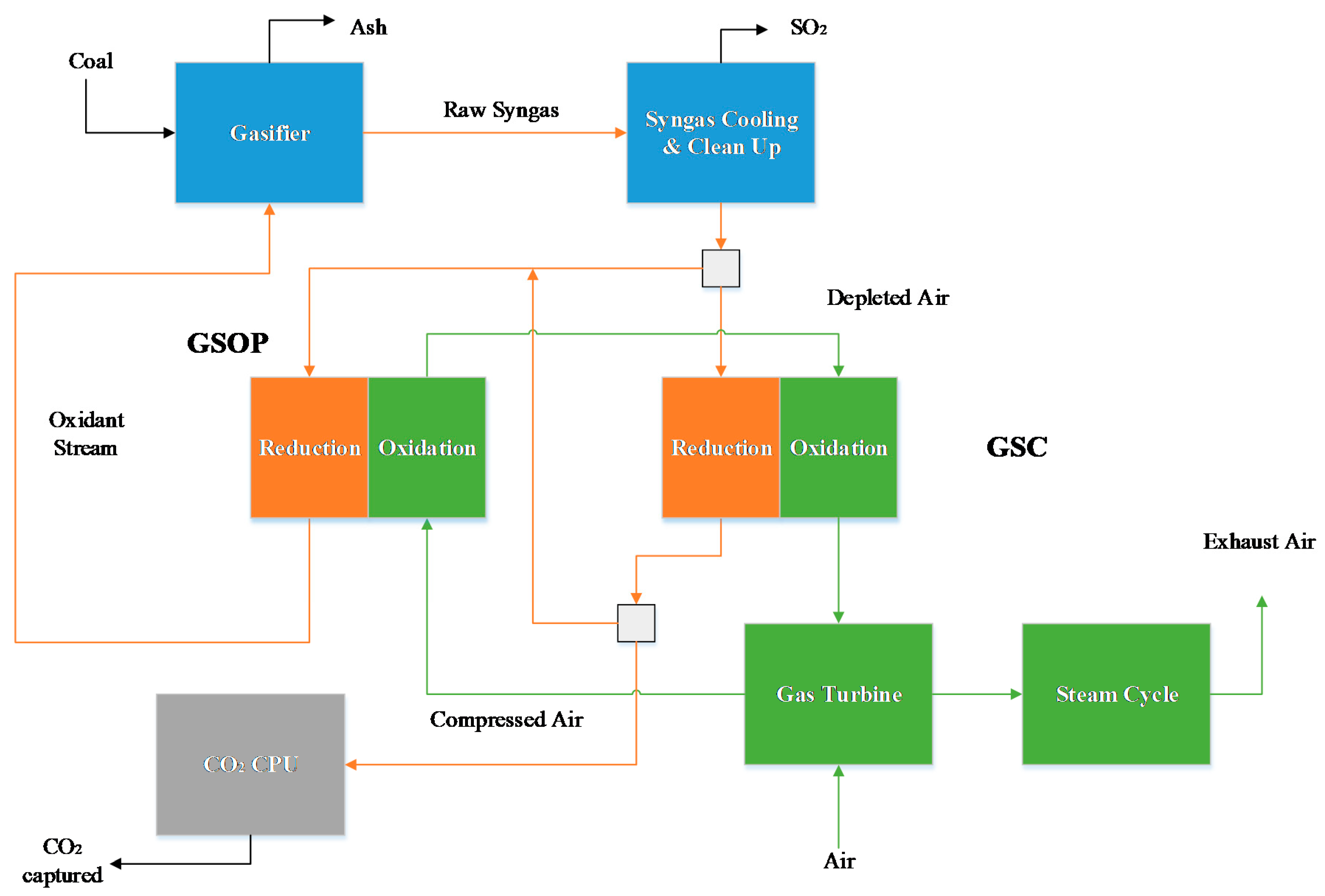
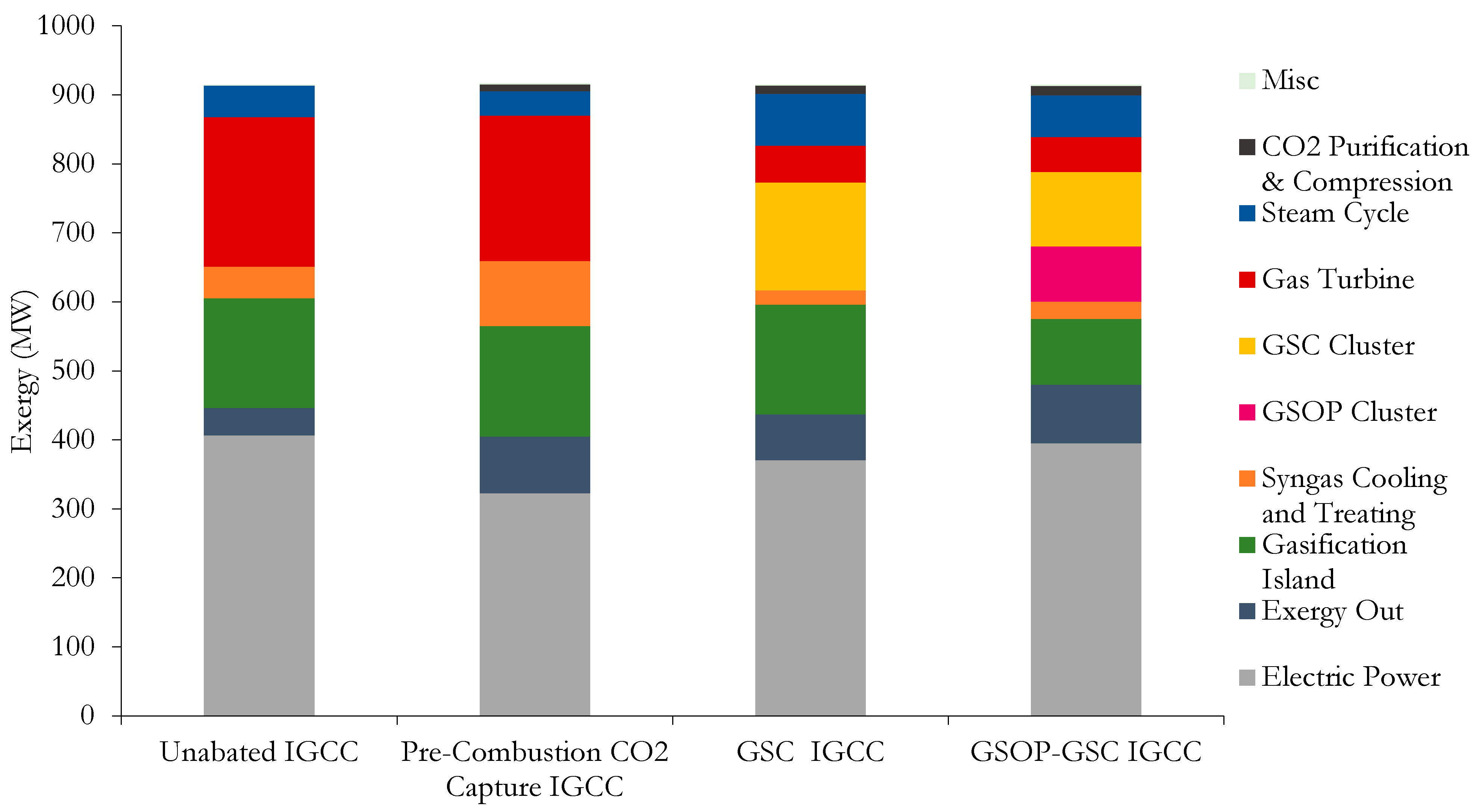In this section, the results for the power plant simulations are given. A thermal analysis, power breakdown, and emission performance is shown, and subsequently, the exergy analysis results for the different power plants are detailed.
3.1. Plant Power Breakdown and CO2 Emissions Performance
Table 1 shows the results of the models for the different power plants considered. It can be seen that the energy penalty associated with CO
2 capture is approximately 10% points for the precombustion CO
2 capture plant. The low thermal efficiency results in an elevated SPECCA index and a CO
2 avoided approximately 3% points below the capture rate of the plant. The benchmark plants show similar performances to [
22].
The GSC IGCC plant presents an energy penalty of approximately 4% points and a high capture rate. CO
2 emissions (lock hopper venting, coal drying, GSC mixing, and CPU vent) result in a capture rate comparable to the precombustion plant, but with a higher CO
2 avoided and lower SPECCA index due to the higher thermal efficiency. It is noteworthy to mention the lower auxiliary consumption for this plant, as no N
2 compression is required to avoid NO
x emissions. The flameless combustion in CLC has the intrinsic advantage of eliminating entirely this hazardous contaminant from these plants. Furthermore, the CO
2 compression duty is reduced relative to the precombustion CO
2 capture model, since the reduction gases are pressurized, requiring only two compression stages (and pump) to reach the delivery pressure. The efficiency results are comparable to the values obtained in [
21].
For the GSOP–GSC IGCC plant, the energy penalty is minimal: around 1.3% points. The high electrical efficiency yields a CO
2 avoidance similar to the actual capture rate of the plant (note that the carbon conversion for the Winkler gasifier is lower than the Shell gasifier used in the unabated IGCC benchmark, leading to less CO
2 production), and yields SPECCA indexes below 0.5 MJ/kg of CO
2 captured. The energy penalty imposed by air separation units and N
2 compression is very pronounced in the benchmark models (they constitute the major auxiliary consumption of these plants, approximately 80% for the unabated case). In the GSOP–GSC plant, these items are avoided and, in combination with the lower CO
2 compression duty, the total auxiliary consumption is approximately 30% of that of the precombustion CO
2 capture plant. The combination of the lower TIT reached with GSC cluster and the fact that the reduction gases’ GSC outlet is not expanded, (some of the heat is effectively recovered as useful work by raising steam in the hot gases heat recovery unit), results in a larger proportion of the power output being delivered by the steam cycle relative to the gas turbine compared to the reference plants. The precombustion CO
2 capture model shows the lowest steam power output, as a substantial amount of IP steam is extracted and delivered to the WGS unit. As shown in
Table 1, the lower TIT of the GS plants results in a higher flow rate across the turbomachinery elements for the same total heat input, while the exhaust flow is reduced due to the subtraction of O
2 in the GS clusters.
Finally, when comparing the GSOP–GSC IGCC plant to the results shown in [
10], where packed bed reactors are used, several aspects can be noted. The greater O
2 concentration difference achieved in the GSOP reactors results in an oxidizing stream with a higher oxygen purity (23.0% mol compared to 16.8% mol), and the resulting syngas has a smaller flow rate and higher heating value comparatively. As a consequence, the heating value of the syngas can be more effectively employed in heating a higher mass flow rate of air through the gas turbine, while less HP steam is generated in the SEC. A slight efficiency improvement and a shift of power output toward the topping cycle is observed. Additionally, the different GSOP cluster operation alters the relative fractions of syngas to the GS reactors. In this study, for the HGCU case, approximately 32% of syngas was routed to the GSOP, while the reduction gases’ recirculated fraction was around 19%. Overall, the syngas mass flow rate was 20% lower than [
10], with a 21% higher LHV. The CO
2 capture ratio is to some extent reduced by the fluidized bed operation of the GS clusters. Since the gasifier, GSOP, and GSC cluster are highly integrated, it is not possible to resort to a N
2 recycle from the HSRG outlet as in the GSC IGCC plant, and consequently, the high capture rate and high oxidation outlet temperatures that lead to high efficiencies cannot be achieved simultaneously. Alternative strategies such as purging with steam have been proposed to reduce the degree of mixing, reaching capture rates above 90% while sacrificing power output from the bottoming cycle, as described in [
5].
3.2. Power Plant Exergy Analysis
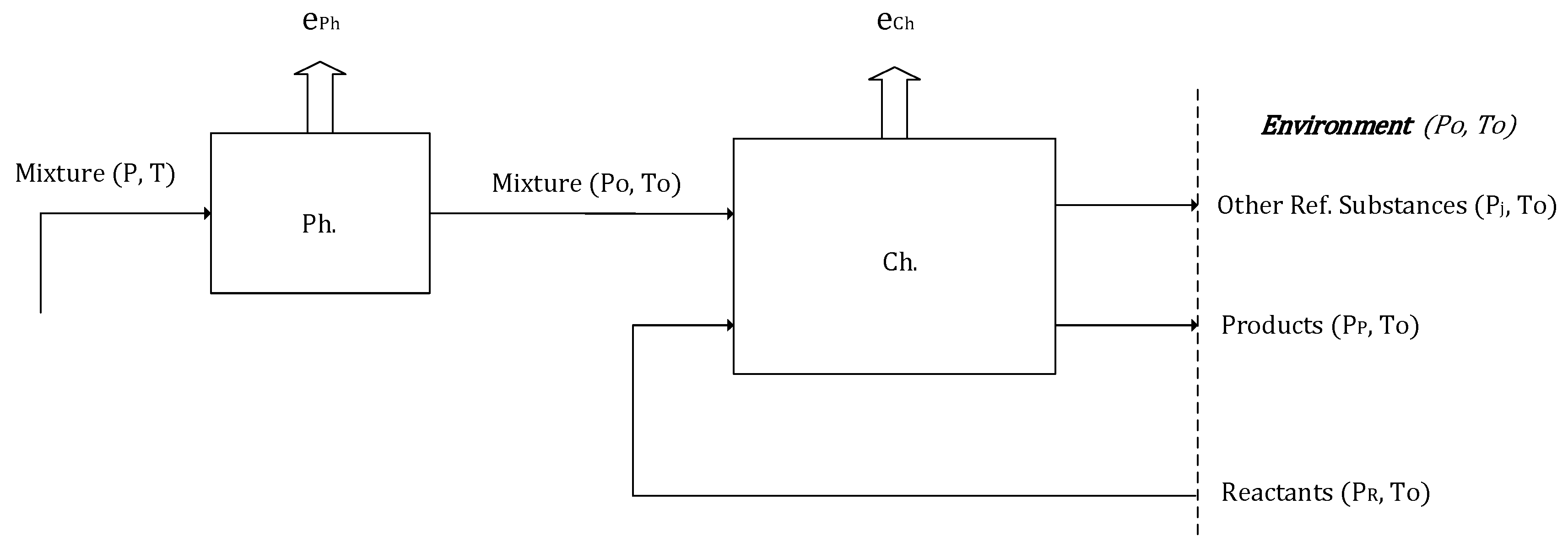
Employing the analysis procedure described in the second section, the different power plant losses were identified for each of the models employing GS technology and the benchmarks. The total exergy breakdown (in MW) is given in
Figure 7, showing the electrical power output, the total exergy destruction of each power plant block, and the exergy flows leaving the plant. The “Exergy Out” block includes the main exergy flows that leave the power plant: slag from the gasifier, the exhaust gases from the HSRG outlet, the CO
2 stream for the plants with CCS, and a group of different minor outlet streams such as water, H
2S in the precombustion capture plant, vented N
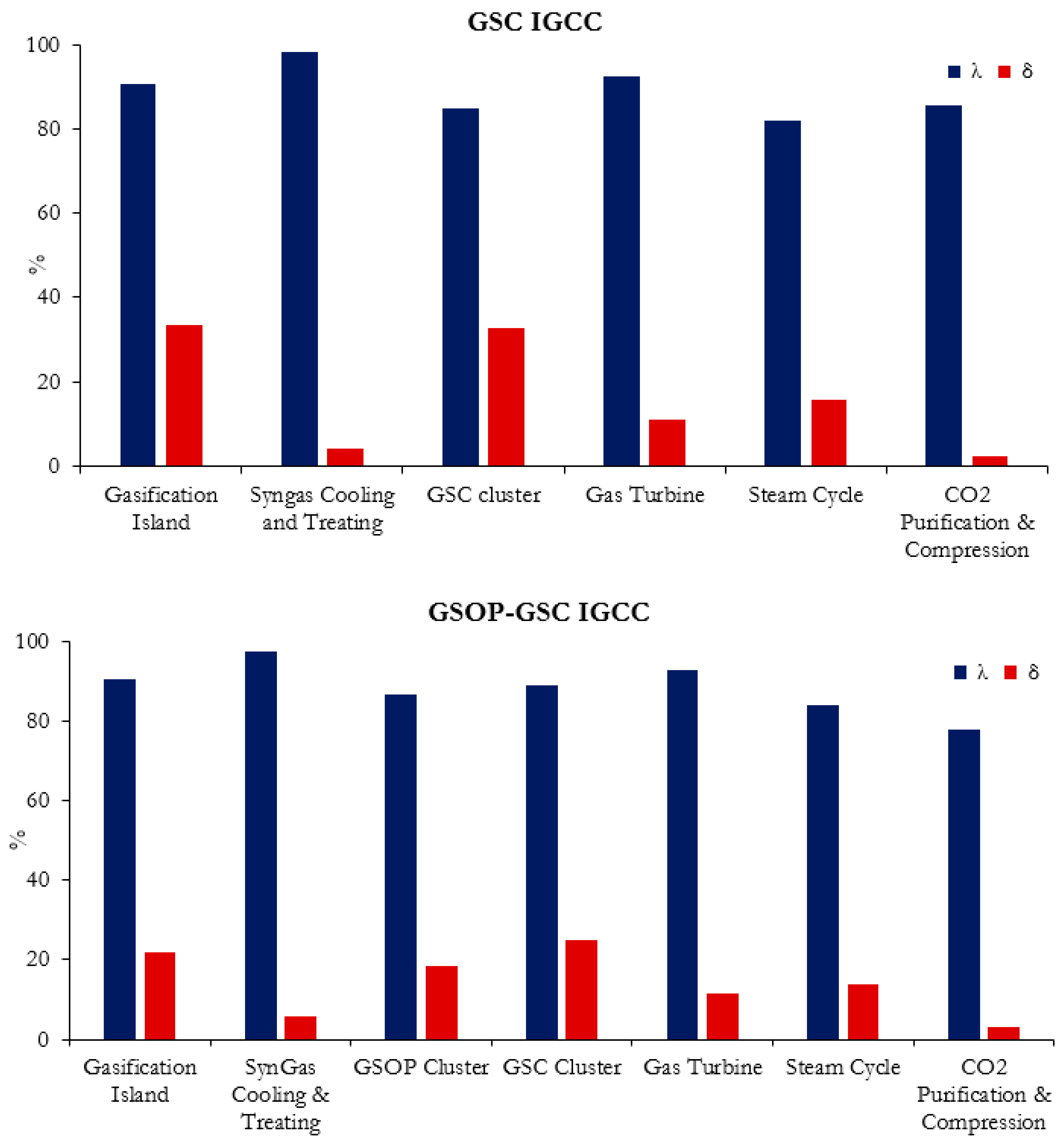
2 from the ASU, etc. The exergy utilization and exergy destruction contribution in percent is subsequently given in
Figure 8 and
Figure 9 for the benchmark and GS plants, respectively. The power plant losses are grouped in representative blocks of the different sections of the plant. The gasification island includes the gasification unit (with gas quench) and the ASU (if present). The syngas cooling and treating block include all the unit operations from syngas effluent coolers, candle filters, and syngas scrubbing, low-temperature heat recovery and desulfurization, the HGCU for the gas switching chemical looping plants, syngas boosters, fuel saturator and heating, and in the case of the precombustion model, the WGS unit. The gas turbine includes the compressor, combustion chamber, and turbine. For the plants with GS technology, lacking a combustion chamber or ASU, the GS clusters are shown separately. The steam cycle elements include the main HSRG, the condenser, the deaerator feedwater tank and pumps, the steam turbine, and the hot reduction gas recovery unit for the GS plants. The CO
2 compression and purification unit comprises the reduction gases condenser for water knock out, the two flash vessel purification units for the GS plants, and the CO
2 pump. For the precombustion CO
2 capture model, the five-stage intercooled compressor is accounted for here. The detailed summary of the different process units considered in each power plant block with their respective exergy losses (total and percentage) is provided in
Table A6 in
Appendix A.
Taking a close look at the benchmark models’ exergy breakdown, it can be seen that most of the exergy destruction occurs in the gasifier and gas turbine (combustion chamber primarily), where the chemical degradation of the fuel takes place. Similar losses are found between the two models due to the similar assumptions made for these plant items. The high-exergy utilization of the gasifier island (>90%) is due to the large amount of incoming exergy to this plant section, although the exergy loss contribution surpasses 30%. The gas turbine presents a low-exergy utilization (around 70%) with a high overall loss contribution (approximately 45%). With regard to the precombustion CO
2 capture IGCC model, substantial losses are incurred upon by adding the shift reaction step and the acid gas removal unit (AGRU), increasing the loss contribution of this block by around 10% points, and duplicating the exergy losses in this section relative to the unabated IGCC plant. A large portion of the steam is supplied to the WGS unit; therefore, the steam cycle losses are somewhat lower comparatively. Since the precombustion CO
2 capture IGCC plant delivers a pure CO
2 stream at 150 bar, it can be seen in
Figure 7 that the exergy flow leaving the system is twice the amount of the unabated IGCC model, where the only source of exergy flow leaving the plant are the exhaust gases from the HSRG and to a lesser extent the slag and captured sulfur in the absorption treating unit.
On the other hand, for the GSC IGCC plant, it can be observed that the losses due to the GT are greatly reduced as the combustion chamber is removed, and the exergy utilization of this block significantly increases by more than 20% points. The turbomachinery elements show a different distribution of losses due to the different TIT with respect to the reference plant. The combustor losses are taken up to a great extent by the GSC cluster, with an exergy utilization and loss contribution of approximately 84% and 33%, respectively. While the gasification island remains unchanged, the effect of employing an HGCU system reduces the losses of the syngas cooling and treating section by half, relative to the unabated IGCC model. As the power ratio in the GS chemical looping plants shifts toward the steam cycle, it is observed that this item takes a greater portion of the overall exergy destruction. The extra heat recovery unit for the GSC reduction outlet and the N2 cooler also contribute significantly to this increase, reducing the exergy utilization and increasing the loss contribution relative to the unabated benchmark plants by around 3% points and 5% points, respectively. Since the exhaust gases are cooled down and recycled to the GT compressor, and all the exergy in the sulfur is assumed to be lost (as full H2S combustion takes place during absorption and regeneration), the fraction of exergy leaving the plant is reduced compared to the precombustion CO2 capture model, given that both have similar CO2 exergy output. In addition, the condensation of the reduction gases for the GSC-IGCC plant slightly increases the losses due to CO2 purification and compression, compensating for the lower compression requirements of the CO2 CPU compared to intercooled compressor of the precombustion CO2 capture model, and resulting in around 7% points higher exergy utilization, but overall with a slightly higher exergy loss contribution of around 1% point.
When analyzing the GSOP–GSC plant and comparing it to the GSC IGCC model, a great reduction in the gasifier island total exergy destruction is observed. Although the exergy utilization is similar, a reduction in the exergy loss contribution of more than 10% points is achieved. This is due to several factors: the most obvious one is the removal of the ASU with a high auxiliary consumption. Secondly, the gasification taking place with a hot oxidant stream delivered by the GSOP and occurring at lower temperature relative to the entrained flow shell gasification plants results in a better overall conversion of coal into syngas, reducing the amount of heating value in the original fuel, which is downgraded into steam. The syngas cooling and treating losses are similar to the GSC IGCC plant: although a bigger syngas booster is required to overcome the pressure losses in the Winkler gasifier–GSOP loop, in the GSC IGCC plant, a high irreversibility occurs during the pressure letdown of the syngas from the shell gasifier to GSC pressure, which suggests that a syngas expander after HGCU can be an effective way to minimize these losses. The losses that are caused by the conversion of syngas are now distributed between GSOP and GSC clusters, which increase the temperature of the compressed air sequentially with the heat released in the oxidation stage, resulting in a similar exergy utilization as in the cluster of the GSC IGCC plant. An overall greater exergy loss between both clusters relative to the GSC IGCC plant is observed, since the recirculation of some reduction gases from the GSC reduction outlet to the GSOP results in a mixing of streams at significantly different temperatures. The GT shows a minor improvement as the compressor inlet is at a lower temperature than the recirculated (cooled) gases in the GSC IGCC model. The steam cycle has a lower exergy loss contribution, since the stack must not be further cooled and recycled; therefore, a higher portion of exergy leaves the system as hot HSRG exhaust. Although the capture rate is approximately 10% points lower, the exergy leaving the system is substantially higher. This is not only due to the aforementioned stack losses but primarily because of the larger exergy content of the slag leaving the gasification system. Notably, increasing the carbon conversion of the Winkler gasifier would lead to interesting efficiency benefits, as this exergy stream corresponds to around 3% of the total.
A relevant result observed from this study is that more exergy is destroyed in the hot reduction gases heat recovery unit than in the main HRSG (although the flow in the former is approximately one-sixth of the latter). These losses are due to the high temperature difference between the steam that is generated and hot reduction gases, despite the lower overall heat transferred compared to the HSRG. Alternative process line-ups could be conceived based on this insight. For instance, operation of the GT at a lower pressure ratio (to achieve a sufficiently high turbine outlet temperature (TOT) for steam superheating in the main HSRG) and syngas preheating with reduction gases outlet could be a possibility to be explored in future works. [
21] shows the benefit of adding a recuperator between the hot gases and compressed air for a GSC IGCC plant with extra firing. Alternatively, a reduction gases expander could be employed, although a higher power demand and number of recompression stages for CO
2 capture will lessen the attractiveness of this option. If the reduction gases have an increased flow due to a larger presence of water, which is condensed before CO
2 compression (for instance in a configuration where syngas cooling is accomplished with a water quench), then the expansion of the reduction gases expander will likely be interesting, and the greater power output will compensate for the increased compression stages of the CO
2. The improved benefits of a hot oxidant stream for gasification are manifest from the GSC-GSOP IGCC model, which suggests that the exergy contained in the reduction gases outlet of the GSC could be effectively invested in a coal pregasification step to enhance the CGE. Preheating to 300 °C of a coal–water slurry feed in an entrained flow gasifier showed substantial efficiency improvement [
35]. These alternative configurations are left for future work.
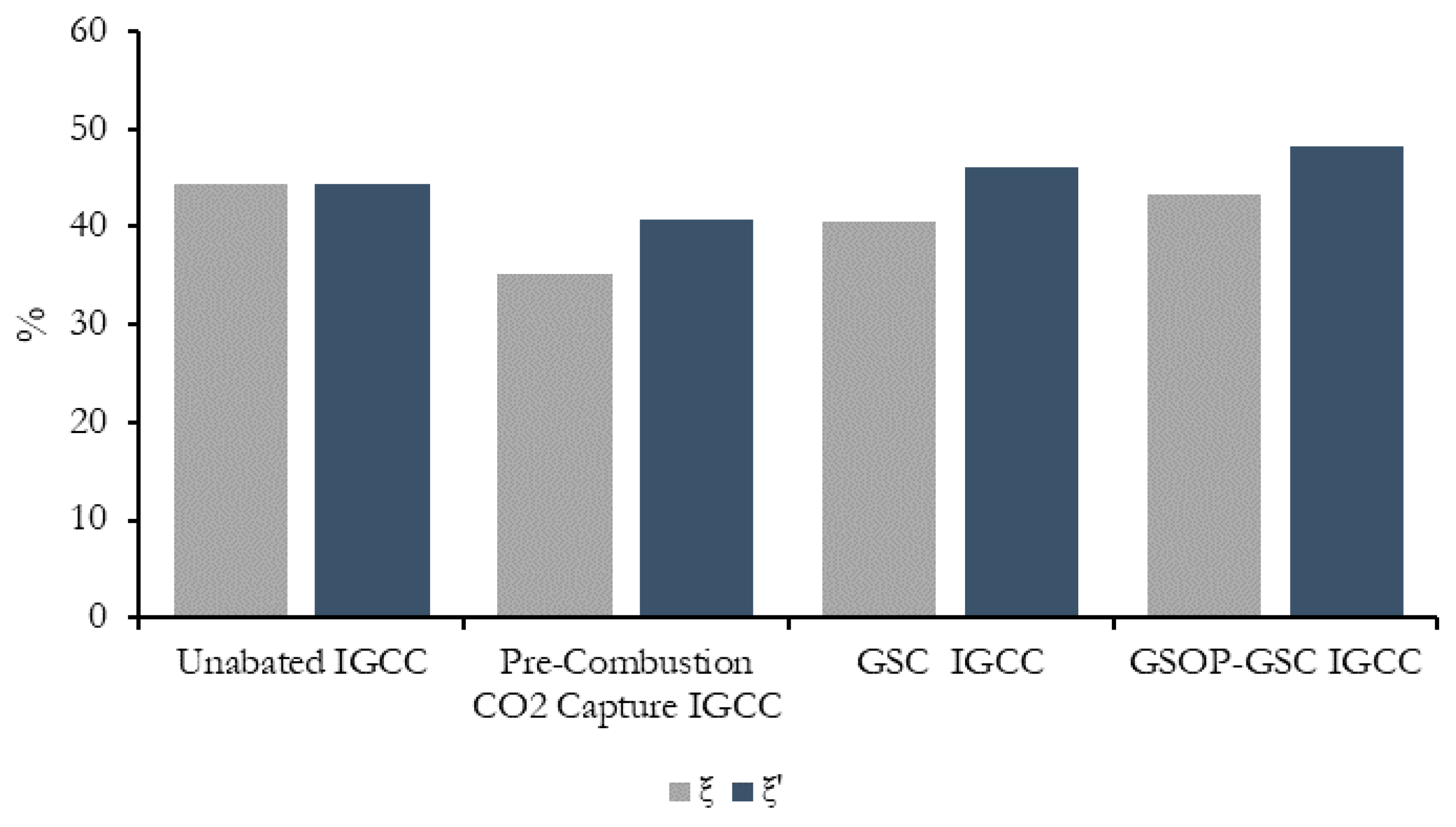
The overall exergy efficiency, based on the definitions presented in Equation (6), is compared in
Figure 10, when the exergy-contained CO
2 captured stream is considered as a useful effect, and when only the net power output is assumed. Since the chemical exergy of fuels predicted by Equation (3) is typically above the lower heating value, this latter exergy efficiency is around 3% points below the respective thermal efficiencies given in
Table 1. Interestingly, considering the former exergy efficiency definition, the GSC IGCC and the GSOP–GSC plants with CO
2 capture can outperform the unabated IGCC benchmark by around 1.5% and 3.5% points, respectively.
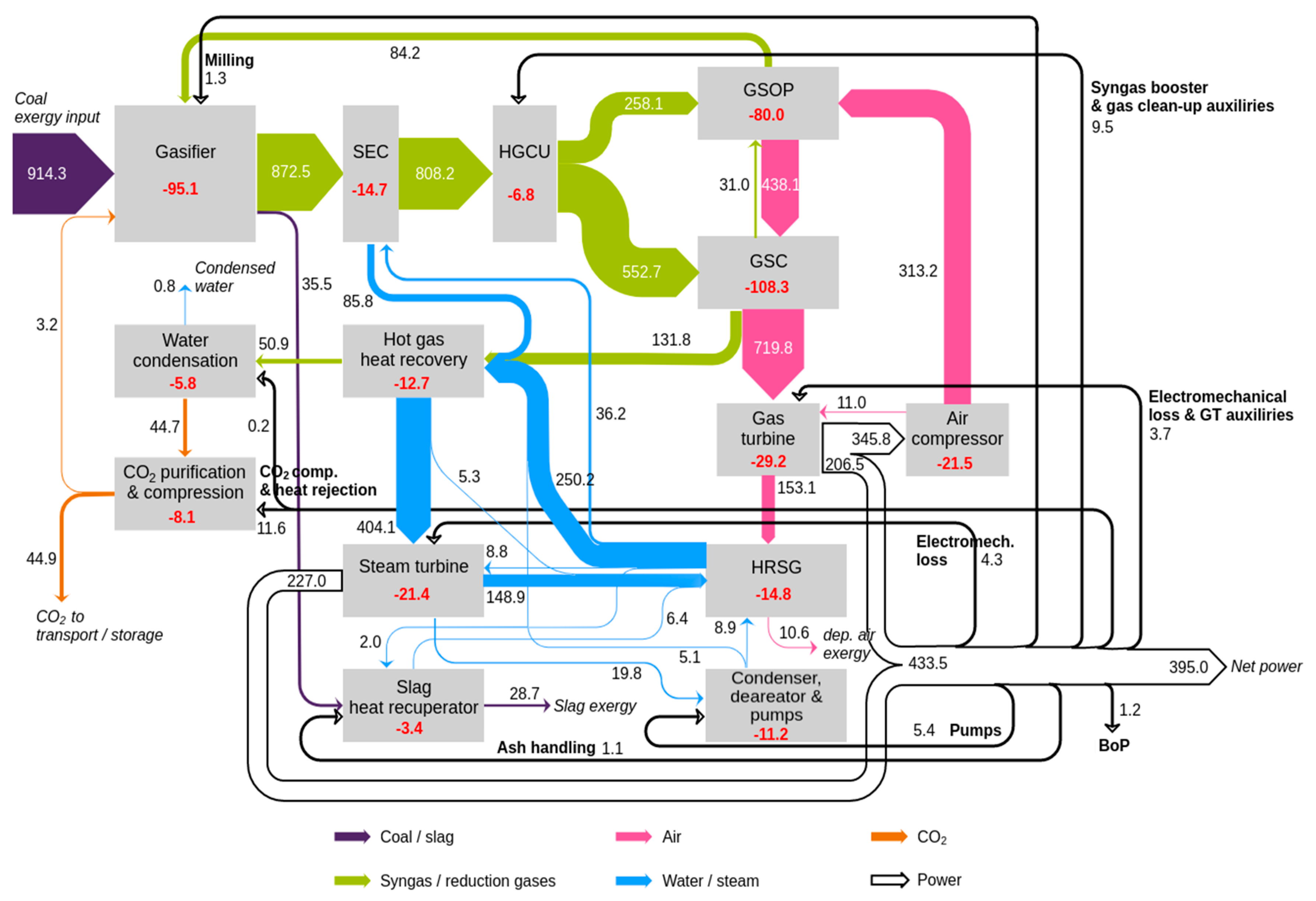
Lastly, a comprehensive tool to visualize the different flows of exergy across the plant is their representation in a Sankey flow diagram, in which the different nodes of the plant are interconnected with exergy arrows with a size that is proportional to the magnitude of these streams. In this way, an insight of the proportion of exergy flows that are exchanged between the different subsystems is given. Since GS plants involving chemical looping are less known compared to the reference plants, in this work, such diagrams are shown in
Figure 11 and
Figure 12 for the GSC IGCC and GSOP–GSC IGCC plants, respectively. A detailed breakdown per item of the different power plant models is presented in
Table A6 in
Appendix A.